Abstract
We have investigated the role of cellular redox state on the regulation of cell cycle in hypoxia and shown that whereas cells expressing mutant thioredoxin (Trx) or a normal level of Trx undergo increased apoptosis, cells overexpressing Trx are protected against apoptosis. We show that hypoxia activates p53 and Chk1/Chk2 proteins in cells expressing normal or mutant Trx but not in cells overexpressing Trx. We also show that the activity of ribonucleotide reductase decreases in hypoxia in cells expressing redox-inactive Trx. Although hypoxia has been shown to induce reactive oxygen species (ROS) generation in the mitochondria resulting in enhanced p53 expression, our data demonstrate that hypoxia-induced p53 expression and phosphorylation are independent of ROS. Furthermore, hypoxia induces oxidation of Trx, and this oxidation is potentiated in the presence of 6-aminonicotinamide, an inhibitor of glucose-6-phosphate dehydrogenase. Taken together our study shows that Trx redox state is modulated in hypoxia independent of ROS and is a critical determinant of cell cycle regulation.
Hypoxia is a broad term used to describe a range of oxygen concentrations that are lower than those normally experienced by living cells. Regions of hypoxia are found not only in disease states, but also during normal development. Indeed, mammalian embryos develop for a significant time in an entirely hypoxic environment. Within tumors, hypoxic regions form in areas that are relatively distant from the vasculature. It has been known for many years that well oxygenated cells and tissues are more sensitive to the lethal effects of ionizing radiation than those experiencing hypoxic conditions (1, 2). Recent clinical studies have unequivocally demonstrated that hypoxia in solid tumors is a major problem for radiotherapy, and that low oxygenation accelerates malignant progression and metastasis, yielding a poor prognosis for successful treatment (3–5). Radiation therapy is only one-third as effective in treating hypoxic cells compared with well oxygenated cells (6–8) because oxygen is required for the formation of reactive oxygen species (ROS)2 that have been implicated as cytotoxic agents in radiotherapy. In addition, oxygen is required for the effectiveness of chemotherapeutic drugs, which depend on the formation of ROS as a cytocidal mechanism. Decreased proliferation or arrest of cell cycle in hypoxia reduces the uptake and effectiveness of phase-specific cytotoxic anticancer drugs (8, 9). In addition, hypoxia acts as a prognostic factor for survival that is independent of other factors, including tumor grade or treatment modality. Many studies have shown that the less oxygenated a tumor, the more negative the prognosis (10). Therefore, elucidating the mechanisms of cell-growth arrest associated with hypoxia is critical for developing an effective therapeutic strategy.
Regulation of the cell cycle relies on the integrity of genetic information and requires a balance of growth and division. This balance is maintained by different feedback controls and termed checkpoints that respond to various cellular conditions (11–13). Checkpoint pathways mediating cell cycle arrest in G1 or G2 phases are thought to operate through inhibition of cyclin-dependent kinases required for major cell cycle transitions at the onset of S phase or mitosis (11–13). Regulation of the G1/S checkpoint control of cell cycle progression in hypoxia has been extensively studied (14–16). Several in vitro studies concluded that hypoxia alone is capable of causing cell cycle arrest in tissue culture cells (5, 17), and this arrest was generally observed in late G1 or early S phases (18). In addition, cells in G2/M phases have been reported to be insensitive to hypoxia (19), progressing through mitosis to G1, where arrest may occur. In contrast, recent in vivo studies have shown that although the greatest number of hypoxic cells reside in G1/G0 phases (20), the phase of the cell cycle with the highest proportion of hypoxia was G2/M (20).
p53 is a sequence-specific transcription factor known to be activated in response to hypoxia as a result of the phosphorylation of multiple serine residues (15, 21, 22). It has been suggested that phosphorylation of p53 on Ser-15 and/or Ser-20 residues facilitates the detachment and subsequent degradation of MDM2, resulting in enhancement of p53 stability and its function as a transcription factor (23, 24). p53 is phosphorylated on Ser-15/Ser-20 via an ataxia telangiectasia-mutated (ATM) or ATM-Rad3-related (ATR)-dependent pathway (25). ATM or ATR has been shown to phosphorylate checkpoint kinase 1 (Chk1) or Chk2 in response to hypoxia (22, 26). Chk1 or Chk2 is activated by phosphorylation and phosphorylates p53 on Ser-20 residue, which activates transcription factor function of p53. Genomic approaches have shown that p53 induces or inhibits the expression of more than 150 genes, including CDKN1A (p21, waf1 and cip1), gadd45, mdm2, and bax (23), all of which mediate arrest of mammalian cells at G1 or G2 cell cycle checkpoints (23). The biochemical links between p53, G1 arrest, senescence, and apoptosis are cell type- and stress type-dependent.
Cellular oxidation-reduction (redox) equilibrium is an important modulator of various cellular functions. Essentially, aerobic cells are in redox equilibrium; perturbation of such equilibrium would be expected to induce adaptive mechanisms favorable to the cell. Because the partial pressure of oxygen is a major variable in cellular redox equilibrium, hypoxic cells would be expected to be in an altered redox state, compared with normoxic cells. Thioredoxin (Trx) is an important protein that maintains the cellular redox status, and the oxidation state of Trx can influence the overall redox equilibrium of a cell. Trx is a low molecular mass protein (12 kDa) with cytoplasmic, membrane, extracellular, and mitochondrial distribution (27, 28). It was originally identified in Escherichia coli as a hydrogen donor for ribonucleotide reductase (RNR), the essential enzyme providing deoxyribonucleotide for DNA replication (27, 28). The Trx system includes Trx, Trx reductase (TrxR), and peroxiredoxins, and uses NADPH as a source of reducing equivalents; Trx is an efficient protein disulfide reductase. Besides being an antioxidant itself, Trx also is an important regulator of the expression of other antioxidant genes, such as manganese superoxide dismutase (MnSOD) (29), and is known to modulate the activation of redox-responsive transcription factors, such as nuclear factor κB (30).
Proliferating cells require large amounts of deoxyribonucleotide only during the S phase of the cell cycle, when duplication of the genome occurs. The RNR level in mammalian cells is therefore closely linked to the phase of the cell cycle. In addition, because the reduction of ribonucleotides is the first metabolic function committed to DNA synthesis, RNR is an important target for regulation of cell growth, and several RNR inhibitors are being used or have been proposed as chemotherapy agents for cancer (31). Mammalian RNR is a complex of a large (R1) and a small (R2) subunit (31). Catalysis involves binding of the substrate to the reduced form of R1 and the subsequent transfer of electron from R2 to R1. The electron needed for this reduction is provided via a redox chain that involves electron transfer from NADPH-dependent TrxR, followed by Trx, and finally a cysteine pair on the surface of R1 (31). Thus, NADPH ultimately provides the power to reduce RNR (31). Because of the role of oxygen in RNR action, the premise that reduced oxygen concentrations affect DNA replication through RNR is plausible (32). However, results published by Giaccia and co-workers (15, 19, 33) indicate that replenishing the ribonucleotide pool during exposure to hypoxia does not rescue cells from S phase arrest. In addition, whereas aphidicolin or hydroxyurea induce DNA damage, comet assays (single-cell gel electrophoresis assay for induced DNA damage) show that hypoxia does not do so (19).
In this study we show that cellular increase of Trx expression profoundly affects cell cycle progression through various phases in hypoxia. Using overexpression of mutant Trx in cells, we further show that redox-active Trx overexpression abrogates hypoxia-mediated p53 expression and its DNA binding activity demonstrating a crucial role of cellular redox state in control of cell cycle in hypoxia. We further show that cellular Trx becomes oxidized in hypoxia, and RNR is inactivated in hypoxia. These studies establish a critical role of cellular Trx redox status in control of cell cycle via inactivation of RNR.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
6-Aminonicotinamide, guanidine hydrochloride, DTT, iodoacetic acid, aprotinin, and antipain were purchased from Sigma. Poly(dI-dC) was purchased from GE Healthcare. All other chemicals were of purest available grade. The antibodies used in our study were obtained from the following vendors: Trx, p21, and Chk1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); RNR, MnSOD, and Chk2 antibodies were obtained from Millipore (Billerica, MA); poly(ADP-ribose) polymerase (PARP), p53, phospho-p53 (Ser-15), and pChk1 antibodies were obtained from Cell Signaling Technologies (Beverly, MA). Secondary horseradish peroxidase-conjugated antibodies for various IgGs were obtained from Santa Cruz Biotechnology or Cell Signaling Technologies; mouse monoclonal PAb421-p53 antibody was obtained from Oncogene (Cambridge, MA).
Cell Culture Treatments, Transfections, and Adenovirus Infection
MCF-7, HEK293T, or HCT116 cells were obtained from ATCC (Manassas, VA) and grown in Dulbecco's modified Eagle's medium or McCoy's 5A medium supplemented with 10% fetal bovine serum and 100 units each of penicillin and streptomycin. Stable cell lines of MCF-7 were generated using pCMV-Trx or pCMV-dnTrx constructs described previously (30, 34). Stable clones of HCT116 cells expressing Trx or dnTrx were generated by transfection of Trx or dnTrx constructs into HCT116 cells using FuGENE 6 transfection reagent (Roche Applied Science). Stable transfectants were selected using G418 (500 μg/ml). The activity of Trx was determined by Trx activity assay. Cells, in 60- or 100-mm2 tissue culture dishes containing 10–12 ml of medium, were exposed to hypoxia (0.2 or 1% oxygen + 5% CO2 + N2) at a flow rate of 10 liters/min for 10 min in humidified modular exposure chambers (Billups-Rothenburg, Del Mar, CA) following which the chamber was sealed and incubated in a 37 °C incubator or to room air containing 5% CO2 (normoxia, 21% oxygen) in a CO2 incubator (Stericult, Thermo Scientific, Waltham, MA) for 16–24 h. At the end of the incubation, cells were washed with nitrogen-equilibrated PBS and were either processed for total cell lysates for Western blotting or other assays. Exposure of cells to hypoxia was performed in the log phase of cell growth, and cells were seeded at low density to prevent any contact inhibition at the end of the exposure period. pcDNA3-MnSOD construct was obtained from Dr. Larry Oberley (University of Iowa, Iowa City, IA) and was transfected into HEK293T cells using FuGENE 6 transfection reagent (Roche Applied Science). This construct contains a 24-amino acid leader sequence in the N-terminal region of coding sequence that direct its expression to the mitochondria (35, 36).
AdenoX system was obtained from Stratagene Corp. (La Jolla, CA), and Trx or mutant Trx cDNA (30) was cloned into pAdenoX vector as described previously (34). Recombinant virus was allowed to infect HEK293 cells for generation of viral particles. For transfection, MCF-7 cells were infected with ∼1 × 108 infectious units (per million cells), and after 48 h Trx protein expression was determined using enzyme-linked immunosorbent assay (37).
RNA Interference
Small interfering RNAs were obtained for Trx, RNR, or MnSOD from Dharmacon Inc. (Arvada, CO). Nontargeting siRNA control (siCONTROL number 2, catalog number D-001210-02-20), which is a mixture of four 21-mer duplex siRNA sequences tested to be used as nontargeting sequence, was obtained from Dharmacon. RNR siRNA was purchased from Dharmacon, which is a mixture of four duplex sequences targeted to RNR. siRNA sequences for MnSOD and Trx are as follows: MnSOD, 3′-GGA GCA CGC UUA CUA CCU UUU dTdT-5′; Trx, 3′-UGA AGC AGA UCG AGA GCA AUU dTdT-5′.
For transfection of Trx, RNR, or nontargeting siRNA into cells (MCF-7, HCT116, or HEK293T), cells were seeded in 60-mm dishes to obtain 50% confluency at the time of transfection. Xtreme siRNA transfection reagent (Roche Applied Science) was used to transfect siRNA to a final concentration of 100 nm. The down-regulation of these proteins was confirmed by Western analysis using respective antibodies.
Microarray Analysis of Cell Cycle Regulatory Genes
MCF-7 clones were exposed to normoxia or hypoxia for 24 h, and RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA). We used a GEArray human cell cycle checkpoint assay kit (Super Array Inc., Bethesda) for analysis of cell cycle checkpoint genes. Briefly, 10 μg of total RNA was reverse-transcribed with GEAprimer mix using 5 μl (10 mCi/ml) of [α-32P]dCTP and 2 μl (50 units/μl) of Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) at 42 °C for 20 min. The reaction was stopped, and the probe was denatured at 68 °C with the appropriate buffers. The labeled cDNA probe was then added to the GEArray hybridization solution and incubated for 16–18 h at 68 °C with continuous agitation. The washed, wet membrane was sealed in a hybridization bag and exposed to x-ray film at −70 °C until sufficient exposure was achieved. The developed spots were identified with the grid card provided in the kit.
Trx Activity Assay
Thioredoxin activity assay was performed as described by Holmgren and Bjornstedt (28). Briefly, the reaction mixture contained NADPH (200 μm), porcine insulin (80 μm), and bovine thioredoxin reductase (0.1 μm) in 0.05 m potassium phosphate buffer, pH 7.0, containing EDTA (1 mm) in a total volume of 0.5 ml. The reaction was started by addition of bovine TrxR (0.1 μm). Trx activity was calculated as micromoles of NADPH oxidized per min per mg of protein at 25 °C.
Redox Assay of Trx
MCF-7 or HCT16 cells stably expressing redox-inactive mutant Trx (dnTrx) or Trx were exposed to hypoxia or normoxia for 24 h in modular exposure chambers; carboxymethylation of cell lysates was followed as described previously (29, 38). Briefly, cells were homogenized in 0.5 ml of carboxymethylation buffer (0.1 m Tris-HCl, pH 8.8, 6 m guanidine hydrochloride, and iodoacetic acid, 10 mg/ml). After homogenization of cells, 5 μl of 10% Triton X-100 was added to the tubes, and the tubes were incubated for 1 h at 37 °C in the dark. At the end of incubation, samples were centrifuged in a tabletop refrigerated centrifuge (Eppendorf, Westbury, NY) for 10 min at 14,000 rpm. Supernatant (0.5 ml) was transferred to a desalting column to remove excess guanidine hydrochloride. Protein content was determined by the Bradford method (Bio-Rad). Twenty μg of carboxymethylated cell homogenate was fractionated on a 15% native polyacrylamide gel. The protein was transferred to polyvinylidene difluoride membrane (Bio-Rad) using a Miniprotean transblot apparatus (Bio-Rad). Polyvinylidene difluoride membrane was washed and incubated with anti-Trx (Santa Cruz Biotechnology, Santa Cruz, CA). After washing, the blot was incubated with rabbit IgG-horseradish peroxidase conjugate (Santa Cruz Biotechnology) for 1 h at room temperature. Binding of secondary antibody was detected using ECL detection system (GE Healthcare).
Trx Reductase Assay
Mammalian thioredoxin reductase reduces 5,5′-dithiobis(nitrobenzoic acid) using reducing equivalents from NADPH. The product in the reaction, 5′-thionitrobenzoic acid, is yellow and has an absorbance maximum at 412 nm. We used this reduction of 5,5′-dithiobis(nitrobenzoic acid) to determine the TR activity in an assay as described by Holmgren and Bjornstedt (28). Briefly, the reaction mixture contained NADPH (200 μm), 5,5′-dithiobis(nitrobenzoic acid) (5 mm), 1% bovine serum albumin, and 1% ethanol in potassium phosphate buffer, pH 7.0, containing EDTA (10 mm) in a total volume of 1 ml. The reaction was started by the addition of cell lysates. TrxR activity is expressed as A412 units as described by Holmgren and Bjornstedt (28) using millimolar extinction coefficient of 13.6 for 5′-thionitrobenzoic acid.
Nuclear Extract and EMSA
Nuclear extract was prepared as described previously (30). Briefly, cells were washed in ice-cold PBS and harvested in 2 ml of ice-cold PBS by centrifugation. Cell pellets were resuspended in 400 μl of Buffer A (10 mm HEPES, pH 7.8, 10 mm KCl, 0.1 mm EDTA, 1 mm DTT, 1 mm phenylmethylsulfonyl fluoride, and 50 μg/ml of leupeptin and antipain) by gentle pipetting. Cells were allowed to swell on ice for 15 min followed by addition of 25 μl of 10% Nonidet P-40 and vortexed at full speed for 10 s. The homogenate was centrifuged for 30 s at 14,000 rpm. The nuclear pellet was resuspended in Buffer C (20 mm HEPES, pH 7.8, 0.42 m NaCl, 5 mm EDTA, 1 mm DTT, 1 mm phenylmethylsulfonyl fluoride in 10% v/v glycerol), and tubes were rocked gently at 4 °C for 30 min on a shaking platform. The extracts were then centrifuged at 14,000 rpm for 25 min, and the supernatant was saved as nuclear extract at −70 °C for further experiments. Protein was quantified using Bradford protein assay (Bio-Rad).
Electrophoretic Mobility Shift Assay (EMSA)
For EMSA, the p53 consensus oligonucleotide was obtained from Sigma Genosys (5′-GGCATGTCCGGGCATGTCC-3′) and was end-labeled using T4 polynucleotide kinase (New England Biolabs, Beverly, MA) and [γ-32P]ATP (PerkinElmer Life Sciences) in 10× kinase buffer supplied with the enzyme. Ten micrograms of nuclear protein was preincubated in 5 μl of 5× binding buffer (20% glycerol, 5 mm MgCl2, 5 mm EDTA, 5 mm DTT, 500 mm NaCl, 50 mm Tris-HCl, 0.4 mg/ml calf thymus DNA), 200 ng of anti-p53 polyclonal 421 antibody, and 2 μg of poly(dIdC) for 15 min followed by binding with labeled oligonucleotide for 30 min. The nuclear protein was separated by electrophoresis using 4% native polyacrylamide gel and 0.25× of TBE (Tris borate/EDTA) as running buffer. Gels were dried and exposed to Kodak Biomax x-ray film overnight.
Flow Cytometry
HCT116 clones (vector, Trx, or dnTrx) were grown in McCoy's 5A medium with 10% fetal calf serum and 300 μg/ml G418 to 80% confluency in 35-mm tissue culture dishes. After 24 h, cells were cultured in conditions of normoxia (21% O2 and 5% CO2) or hypoxia (0.2% O2 + 5% CO2 + balance N2) for 24 h. Cells were trypsinized and were washed twice in phosphate-buffered saline and fixed in cold 70% ethanol until staining and analysis. For the DNA content analysis, cells were suspended in freshly prepared propidium iodide staining solution (0.1% bovine serum albumin containing phosphate-buffered saline, 0.1% RNase A, and 50 μg/ml propidium iodide) for 30 min in the dark. Cell cycle analysis was carried out with the help of the flow cytometry core facility at the University of Arkansas for Medical Sciences. Data analysis was performed with ModFit LT (Verity Software House Inc., Topsham, ME).
Western Analysis
Protein lysates were prepared using radioimmunoprecipitation assay buffer containing 5% sodium deoxycholate, 1% SDS, 1% Igepal (Sigma) in PBS with protease inhibitors, and protein concentration was determined using Bio-Rad protein assay reagent (Bio-Rad). Equal amounts of protein were resolved by 10% SDS-PAGE and transferred onto nitrocellulose membrane (Hybond-ECL, GE Healthcare). The blot was treated with appropriate dilutions of primary antibody and visualized using either LumiGLO (Cell Signaling Technology, Beverly, MA) or ECL plus system (GE Healthcare) with appropriate horseradish peroxidase-conjugated secondary antibody.
RNR Assay
After exposure to normoxia or hypoxia, the cell pellets were resuspended in buffer containing 50 mm HEPES, pH 8.0, 50 mm KCl, 2 mm MgCl2, and 1 mm phenylmethylsulfonyl fluoride. The cell suspensions were sonicated and spun at 15,000 rpm for 10 min, and then the supernatants were collected for measuring RNR activity. RNR activity was determined by measuring conversion of [3H]CDP to [3H]dCDP, as described previously (39) with modifications. Assays were done in the absence of DTT and NADPH. Each extract was assayed in duplicate, and the data represent averages of these determinations. To determine whether dnTrx by itself decreases RNR activity, some assays were performed in the presence or absence of NADPH, and with or without DTT.
RESULTS
Effect of Trx or dnTrx on Cell Cycle Progression in Normoxia and Hypoxia
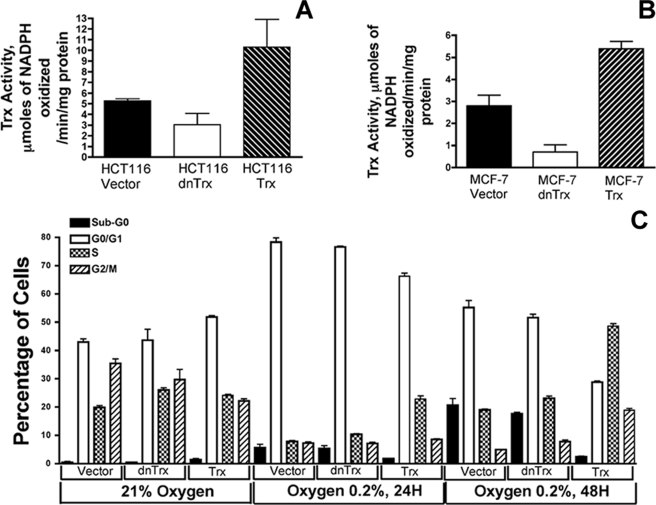
To determine how Trx redox state affects the cell cycle progression in normoxia or hypoxia, we generated stable clones of HCT116 cells expressing Trx or dnTrx. dnTrx cDNA was generated by site-directed mutagenesis of the human Trx cDNA by replacing redox-active Cys-32 and Cys-35 with alanine residues as we described previously (30). dnTrx has been shown to be a competitive inhibitor of reduction of Trx by TrxR with a Ki of 1.8 μm (40). When the dnTrx is overexpressed in the cell, the activity of endogenous Trx is decreased because of failure of TrxR to regenerate Trx. Hence, overexpression of dnTrx in cells functions as dominant-negative because it suppresses the activity of endogenous Trx (30, 34, 40, 41). As shown in Fig. 1A, HCT116-Trx cells showed about 2-fold increase in Trx activity compared with HCT116-vec cells. In contrast, HCT116-dnTrx cells showed about 60% less activity compared with vector only cells. Similar results were also obtained for MCF-7 clones (Fig. 1B). MCF-7 clones were generated and extensively described in our previous publication (34).
FIGURE 1.
A, activity of Trx in HCT116 clones. Trx activity was analyzed in HCT116-vector1, HCT116-Trx13, and HCT116-dnTrx4 cells as described under “Experimental Procedures.” The experiment was performed in triplicate. B, activity of Trx in MCF-7 clones. Trx activity was analyzed in MCF-7-vector1, MCF-7-Trx9, and dnTrx4 cells as described under “Experimental Procedures.” The experiment was performed in triplicate. C, cell cycle analysis in hypoxia. For HCT116 stable cell lines overexpressing Trx, dnTrx cells were exposed to normoxia (21% O2 and 5% CO2) or hypoxia (0.2% O2 and 5% CO2) for 24 or 48 h. Following exposure, cells were trypsinized and washed twice in phosphate-buffered saline and fixed in cold 70% ethanol until staining and analysis. For the DNA content analysis, cells were suspended in freshly prepared propidium iodide staining solution (0.1% bovine serum albumin containing phosphate-buffered saline, 0.1% RNase A, and 50 μg/ml propidium iodide) for 30 min in the dark. Cell cycle analysis was carried out with help of the flow cytometry core facility, University of Arkansas for Medical Sciences. Data analysis was performed with ModFit LT (Verity Software House Inc., Topsham, ME). The experiment was performed in triplicate.
HCT116-vector1, Trx13, or dnTrx4 clones were exposed to normoxia or hypoxia for 24 or 48 h, and the cell cycle distribution was analyzed by flow cytometry. As demonstrated in Fig. 1C, there was no difference between percentage of vector only or dnTrx cells in sub-G0 phase in normoxia. However, the percentage of Trx cells in sub-G0 phase was significantly higher in normoxia. There was no significant difference in the G1 phase distribution of vector or dnTrx cells in normoxia. However, a significantly higher percentage of Trx cells were in G1 phase in normoxia as compared with vector or dnTrx cells in normoxia (Fig. 1C). The S phase population in Trx cells exposed to hypoxic conditions for 24 h was comparable with those experiencing normoxia. In contrast, vector or dnTrx cells showed a very high percentage of cells in G1 phase as evidenced by a significantly smaller percentage of cells in S phase. Although the distribution of S phase in vector or dnTrx cells in 48 h of hypoxia was increased, there was no significant difference between G1 phase distributions between normoxia or 48 h of hypoxia. In contrast, we observed a significant increase in the S phase distribution of Trx cells exposed for 48 h. Furthermore, there was no significant difference between percentages of Trx cells in G2/M phase (Fig. 1C) in normoxia compared with G2/M phase cells in 48 h of hypoxia.
Because cells were exposed to prolonged hypoxia, we tested the viability of these cells using ViCell cell counter that determines the percentage of viable cells (ViCell, Beckman Co, CA). As demonstrated in Table 1, there was no significant change between 21 or 0.2% oxygen-exposed cells for 24 h in MCF-7 or HCT116 clones. However, when the cells were exposed to 48 h of hypoxia, the viability was decreased by about 15 or 20% compared with MCF-7 or HCT116 cells in normoxia. The viable cells that adhered to dishes were trypsinized for cell cycle analysis in flow cytometry.
TABLE 1.
Percentage of viability
Viability of MCF-7 or HCT116 clones exposed to 24 or 48 h of hypoxia is shown. MCF-7 or HCT116 clones were exposed either to normoxia or hypoxia (0.2% O2), and cells were counted using ViCell cell counter (Beckman Coulter). Viability is expressed as percentage of cells that are viable using total cell number counted as 100%. The experiment was performed in triplicate.
| Cell type | 21% oxygen |
0.2% oxygen, 24 h |
0.2% oxygen, 48 h |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Vector | dnTrx | Trx | Vector | dnTrx | Trx | Vector | dnTrx | Trx | |
| MCF-7 | 94 ± 1 | 94 ± 1 | 93 ± 1 | 83 ± 2 | 88 ± 1 | 89 ± 1 | 75 ± 2 | 75 ± 2 | 83 ± 2 |
| HCT116 | 70 ± 3 | 78 ± 2 | 82 ± 2 | 67 ± 2 | 70 ± 6 | 67 ± 2 | 67 ± 2 | 61 ± 3 | 64 ± 5 |
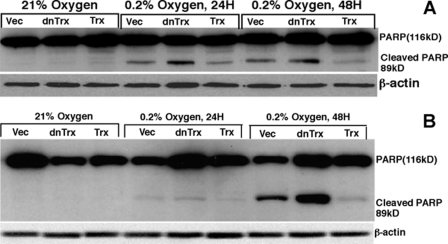
Hypoxia Induces Cleavage of PARP in Vector or dnTrx Cells but Not in Trx Cells
Our cell cycle data show that vector or dnTrx cells undergo enhanced apoptosis in 24 or 48 h of hypoxia, but Trx cells did not. To further confirm the apoptosis induced by hypoxia, we used cleavage of PARP (89-kDa product) as a marker of apoptosis in these cells. PARP is cleaved by caspases that have been used as a hallmark of apoptosis (42, 43). As demonstrated in Fig. 2, exposure of HCT116 (Fig. 2A) or MCF-7 cells (Fig. 2B) to 24 or 48 h of hypoxia induced the appearance of 89-kDa PARP cleavage product in vector or dnTrx cells but not in Trx cells. These data confirm our sub-G0 data (Fig. 1C) and show that increased expression of Trx prevent hypoxia-induced apoptosis in cancer cells.
FIGURE 2.
Effect of hypoxia on PARP cleavage in HCT116 (A) or MCF-7 (B) clones. HCT116 or MCF-7 clones were exposed to normoxia or hypoxia (24 or 48 h). Cell lysates were prepared and PARP Western analysis was performed using PARP and β-actin antibodies. Vec, vector.
p21 Is Increased in dnTrx Cells but Not in Trx Cells
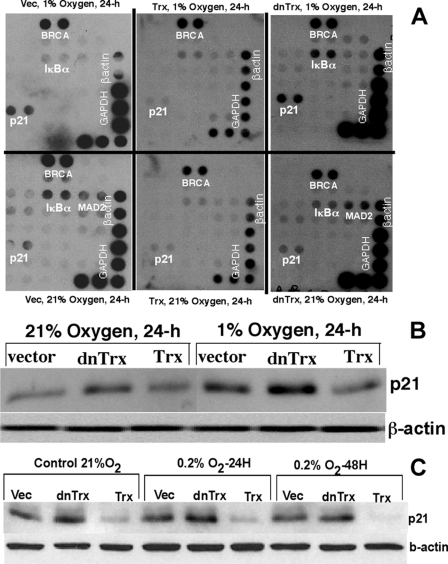
To identify the subset of cell cycle regulatory genes affected by hypoxia in Trx redox-altered MCF-7 cells, we used a simple microarray analysis of 24 cell cycle checkpoint genes, employing methods we used previously (44). The genes spotted onto the array membrane were as follows: atm, brca1, c-abl, chk1, chk2, gadd45, hus1, ikba, mad2L1, mcm4, mcm6, mcm7, mre11a, mre11b, nbs1, p21waf1, tp53, rad17, rad50, rad51, rad9, rpa, and timp3. Expression levels of these genes did not change in Trx-overexpressing cells, whether exposed to hypoxia or normoxia (Fig. 3A). However, in MCF-7 cells expressing dnTrx, the levels of p21 and inhibitor of nuclear factor κB kinase α subunit (IκBα) were increased under hypoxia compared with normoxia (Fig. 3A). These results are important because a previous study indicated that, although p53 is up-regulated during hypoxia, it is transcriptionally inactive (15). Our microarray data suggest that during hypoxia p21, which is a p53-regulated gene, is transcriptionally up-regulated in MCF-7 cells that are deficient in redox-active Trx but not in cells that express a higher level of redox-active Trx (Fig. 3A). The expression of p21 protein in MCF-7 clones was increased in vector or dnTrx cells but not in Trx cells (Fig. 3B). These data show that p21 up-regulation in hypoxia in MCF-7 cells is transcriptional as well as translational. HCT116 vector cells exposed to hypoxia did not show significant increase in p21 expression in Western analysis (Fig. 3C). In contrast, dnTrx cells demonstrated a slight increase in p21 expression. However, the base-line level of p21 expression was abrogated in Trx-overexpressing cells in hypoxia. Thus although p21 transcriptional up-regulation occurs in dnTrx cells, Trx-overexpressed cells do not show any increase in p21 expression in hypoxia. We next determined whether binding of p53 to DNA, which is a measure of p53 activation as a transcription factor, increases during hypoxia.
FIGURE 3.
A, effect of hypoxia on cell cycle regulatory genes. For MCF-7 clones (vector (Vec), Trx, or dnTrx), cells were exposed to normoxia or hypoxia (1% O2 + 5% CO2 + 94% N2) for 24 h. Cells were harvested, and micro-array analysis was performed as described previously (44). Top panel, vector, Trx, or dnTrx cells exposed to hypoxia; bottom panel, vector, Trx, or dnTrx cells exposed to normoxia. B, effect of hypoxia on p21 expression in MCF-7 cells. MCF-7-vector, MCF-7-Trx, or MCF-7 dnTrx was exposed to 1% oxygen for 24 h, followed by Western analysis of p21 in the cellular lysates as described under “Experimental Procedures.” C, HCT16-vector, HCT116-Trx, or HCT116-dnTrx cells were exposed to 0.2% oxygen for 24 or 48 h, and p21 Western analysis was performed on cellular lysates.
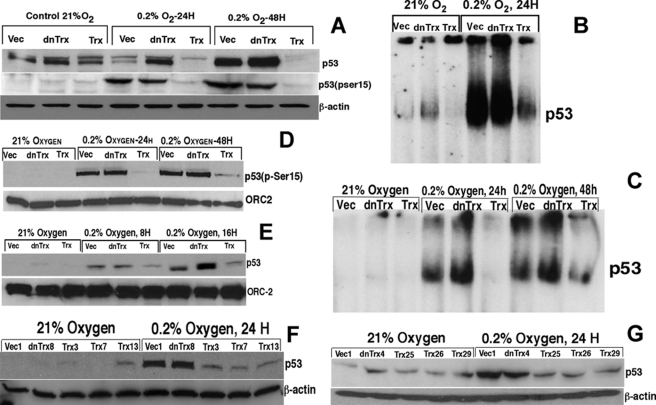
Hypoxia Induces p53 Expression and DNA Binding in Vector or dnTrx Cells but Not in Trx Cells
p53 is a key transcription factor that regulates cell cycle progression, apoptosis, and other cellular processes in response to stress. Because we observed significant apoptosis and G1 arrest in vector or dnTrx cells, but not in Trx cells, we hypothesized that p53 is activated in vector or dnTrx cells but not in Trx cells. Therefore, we sought to determine whether p53 expression and DNA binding is compromised in hypoxia in Trx cells. As demonstrated in Fig. 4A, HCT116 cells showed increased p53 protein levels and p53 (Ser-15) phosphorylation in vector- or dnTrx-transfected cells. In contrast, cells overexpressing Trx showed no increase in expression of p53 or phosphorylation of p53 on Ser-15 residues (Fig. 4A). Additionally, Trx cells showed significantly lower levels of p53 DNA binding compared with vector or dnTrx cells in response to hypoxia (Fig. 4B). As shown in Fig. 4C, exposure of MCF-7 vector or dnTrx cells to hypoxia induced increased p53 DNA binding. However, cells expressing a higher level of Trx did not show a significant level of p53 binding to DNA. These findings are in agreement with our microarray data demonstrating that p21 is not induced in cells overexpressing Trx in response to hypoxia (Fig. 3A), because p21 expression is regulated by p53. Additionally, the level of p53 (Ser-15) phosphorylation was significantly increased in vector or dnTrx cells when MCF-7 stable clones were assayed (Fig. 4D). In contrast, there was almost no increase in p53 (Ser-15) phosphorylation levels in MCF-7 cells expressing Trx (Fig. 4D). These observations indicate that Trx overexpression overrides p53 activation as a transcription factor in hypoxia. The experiment was done using 24 or 48 h of exposure of cells to hypoxia. To test whether similar effects are seen at a lower time point, we exposed MCF-7 clones to 8 or 16 h of hypoxia and evaluated p53 expression. As demonstrated in Fig. 4E, p53 expression was increased in 8 or 16 h in vector or dnTrx cells but did not increase in Trx cells, confirming our observations with 24 or 48 h of exposure. Furthermore, we tested whether other clones of MCF-7 or HCT116 cells would show similar response to hypoxia in their expression of p53. As shown in Fig. 4, F and G, HCT116 or MCF-7 Trx clones did not show increased p53 expression in hypoxia. In contrast, vector or dnTrx showed increased p53 expression.
FIGURE 4.
Effect of Trx on p53 expression and DNA binding in hypoxia. A, HCT116 cells (vector (Vec), Trx, or dnTrx) were exposed to normoxia or hypoxia (0. 2% O2) for 24 or 48 h. Cell lysates were analyzed for p53 expression and p53 (Ser-15) phosphorylation by Western analysis as detailed under “Experimental Procedures.” Top panel, expression of total p53; middle panel, expression of phospho-p53 (Ser-15); bottom panel, expression of β-actin. B, HCT116 cells (vector, dnTrx, or Trx) were exposed to 21% oxygen or 0.2% O2 + 5% CO2 + 94% N2 for 24 h. Cells were harvested; nuclear extract was prepared, and p53 EMSA was performed as described under “Experimental Procedures.” C, MCF-7 cells (vector, Trx, of dnTrx cells) were exposed to normoxia or hypoxia as mentioned for A, and p53 EMSA was performed as detailed under “Experimental Procedures.” D, MCF-7 clones were treated and processed as described in A. Origin recognition complex-2 (ORC-2) in the nuclear lysates was detected as a loading control. E, MCF-7 clones were exposed to 21% oxygen or 8 or 16 h of hypoxia, and p53 expression was analyzed using Western analysis as mentioned in B. F, various clones of HCT116 cells were exposed to normoxia or hypoxia (0.2% 24 h), and p53 Western analysis was performed as described under “Experimental Procedures.” G, various clones of MCF-7 cells were exposed to normoxia or hypoxia (0.2% 24 h), and p53 Western analysis was performed as described under “Experimental Procedures.”
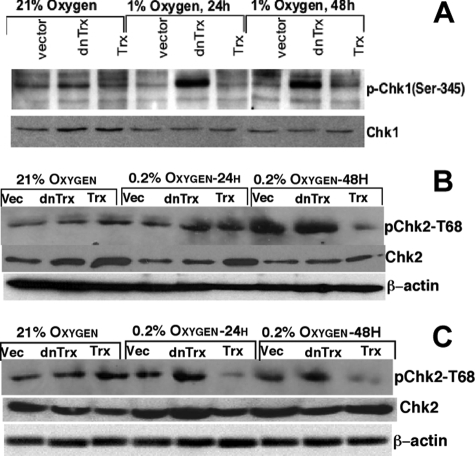
Phosphorylation of Chk1/Chk2 Was Increased in dnTrx Cells but Not in Trx Cells in Hypoxia
P53 is known to be phosphorylated on Ser-15/Ser-20 by Chk1 or Chk2 in response to stress, including hypoxia (22, 26). This phosphorylation stabilizes p53 and activates its transactivation potential to increase or decrease gene expression (25). We have observed that whereas vector or dnTrx cells show increased phosphorylation of Ser-15 residue on p53 in hypoxia, Trx cells did not show increased p53 phosphorylation. Therefore, we reasoned that the activation of kinases such as Chk1 or Chk2 that are upstream of p53 might be affected by Trx redox state modulation in hypoxia. We determined the phosphorylation of Chk1 (Ser-345) or Chk2 (Thr-68) using phospho-specific antibodies in an immunoblotting assay because these kinases are activated by phosphorylation. As shown in Fig. 5A, in MCF-7 cells Chk1 was strongly phosphorylated in dnTrx cells after 24 or 48 h of hypoxia, but not in vector or Trx cells. Chk2 was also strongly phosphorylated in MCF-7-vector and dnTrx cells but not in Trx cells (Fig. 5B). HCT116 stable cell lines showed activation of Chk2 in vector or dnTrx cells but not in Trx cells (Fig. 5C).
FIGURE 5.
Effect of Trx on Chk1 or Chk2 phosphorylation in hypoxia. A, MCF-7 cells expressing vector (Vec), dnTrx, or Trx were exposed to normoxia or hypoxia (1 or 0.2% O2 + 5% CO2 + 94% N2) for 24 or 48 h in modular exposure chambers as described under “Experimental Procedures.” Phospho-Chk1 and Chk1 were detected with phospho-specific (Ser-345) antibody and Chk1 antibody as described under “Experimental Procedures.” B, MCF-7 cells were exposed to normoxia or hypoxia, and phospho-Chk2 or Chk2 was detected as described under “Experimental Procedures.” C, HCT116 cells (vector, dnTrx, or Trx) were exposed to hypoxia and phospho-Chk2 or Chk2 was detected as mentioned under “Experimental Procedures.”
Hypoxia Modulates Trx Redox State
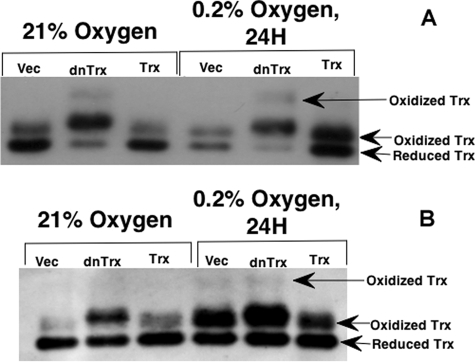
Although exposure of cells to hypoxia increased p53 and Chk1/Chk2 activation in vector or dnTrx cells, Trx cells did not show increased p53 or Chk1/Chk2 phosphorylation. Therefore, we reasoned that Trx could act as an antioxidant in hypoxia-induced oxidative stress, because hypoxia has been shown to generate ROS (45, 46), and ROS-induced DNA damage could invoke a p53 response. Therefore, we sought to determine whether hypoxia would oxidize Trx because of the generation of ROS. We exposed MCF-7 or HCT116 cells to 24 h of hypoxia (0.2% O2) followed by determination of levels of oxidized or reduced Trx in MCF-7 or HCT116 cells using a previously described method (38). Fully reduced Trx is carboxymethylated on two of its sulfhydryl groups and thus migrates as a dicarboxymethylated band, and fully oxidized Trx remains noncarboxymethylated and is the slowest moving form (29, 38). As demonstrated in Fig. 6A (MCF-7 cells) and Fig. 6B (HCT116 cells), the level of oxidized Trx increased in response to hypoxia in vector or dnTrx cells, compared with that in normoxic cells. Cells overexpressing Trx, however, showed a significantly lower level of oxidized Trx in hypoxia. The results indicate that exposure of cells to hypoxia induces oxidation of Trx, but cells overexpressing Trx are able to overcome increased oxidation of Trx in hypoxia. Interestingly, dnTrx cells showed a higher Trx oxidation band in normoxia as well as hypoxia in MCF-7 cells. However, HCT116 cells showed a higher oxidation band in vector or dnTrx cells exposed to hypoxia but not in Trx cells.
FIGURE 6.
Hypoxia modulates the redox state of Trx. A, MCF-7 cells (vector (Vec), dnTrx, or Trx) were exposed to hypoxia (0.2% O2) or normoxia for 24 h in modular exposure chambers; carboxymethylation of cell lysates was prepared, and oxidized or reduced Trx was detected as described under “Experimental Procedures.” B, HCT116 cells were exposed to hypoxia, and Trx redox state was determined as described under “Experimental Procedures.”
Increased Expression of p53 in Hypoxia Does Not Depend on ROS
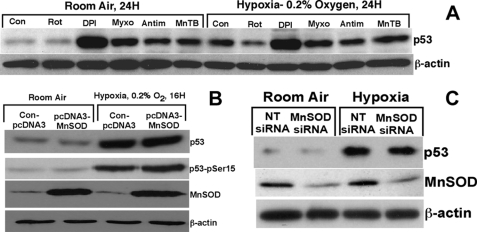
Although controversial, increasing evidence suggests that mitochondrial ROS is generated in hypoxia (45–48). Therefore, we reasoned that in our hypoxia model (0.2% oxygen) increased production of ROS could be involved in the oxidation of Trx and enhanced expression of p53. We pretreated cells with diphenyl iodonium, a complex I inhibitor (49), myxothiazole, a complex III inhibitor (45), or rotenone, a complex I inhibitor (47), to evaluate whether hypoxia-induced p53 expression involves ROS. In addition, we also used antimycin A, which has been shown to increase the ROS generation in complex III (45, 50) to demonstrate whether use of this compound would increase p53 expression. Furthermore, we used MnTBAP, a ROS scavenger (51, 52), that would decrease p53 expression in hypoxia if ROS were to induce p53 expression. As shown in Fig. 7A, none of these compounds decreased the expression of p53 in response to hypoxia. To our surprise, all of these compounds induced p53 expression in normoxia, indicating that inhibition of mitochondrial electron transport chain might be inducing a metabolic stress that resulted in the induction of p53. Our results do not support a role for the mitochondrial ROS in the expression of p53 in hypoxia (0.2% O2).
FIGURE 7.
Role of ROS in p53 expression in hypoxia. A, effect of mitochondrial respiratory chain inhibitors on p53 expression in hypoxia. HCT116 cells were pretreated with rotenone (Rot) (3 μg/ml), diphenyl iodonium (DPI) (3 μm), myxothiazole (Myxo) (100 ng/ml), antimycin A (Antim) (5 μg/ml), or MnTBAP (MnTB) (20 μm) for 2 h followed by exposure of cells to room air or hypoxia (0.2% O2) for 16–24 h as described under “Experimental Procedures.” Con, control. Cells were harvested, and total cell lysate was prepared using radioimmunoprecipitation assay buffer, and p53 Western analysis was performed as described under “Experimental Procedures.” B, effect of MnSOD overexpression on p53 expression in hypoxia. HEK293T cells were transfected with either pcDNA3 vector or pcDNA3-MnSOD construct as described under “Experimental Procedures.” Transfected cells were exposed to room air or hypoxia, and Western analysis for p53, p53 (Ser-15), MnSOD, or β-actin was performed as described under “Experimental Procedures.” C, effect of down-regulation of MnSOD expression on p53 expression in hypoxia. MCF-7 cells were transfected with MnSOD siRNA as mentioned under “Experimental Procedures,” and transfected cells were exposed either to room air or hypoxia (0.2% O2) for 16 h. Western analysis of p53, MnSOD, or β-actin was performed as described under “Experimental Procedures.” NT, nontargeting.
If mitochondrial ROS is required for the expression of p53 in hypoxia, then overexpression of MnSOD, a mitochondrial superoxide dismutase, should prevent the increase in p53 expression in hypoxia. Conversely, down-regulation of MnSOD by RNA interference should increase the expression of p53, as ROS level would be increased in the absence of MnSOD in the mitochondria. Hence, we employed a genetic system to conclusively delineate the role of ROS in p53 expression in hypoxia. We increased the level of MnSOD in the mitochondria by using an MnSOD overexpression construct containing a 24-amino acid leader sequence as the mitochondrial localization signal that transports the protein to the mitochondria (the construct was kindly provided by Dr. Larry Oberley (University of Iowa, Iowa City) and has been described) (35, 36). As demonstrated in Fig. 7B, the expression of MnSOD was significantly increased in HEK293T cells transfected with the pcDNA3-MnSOD construct. However, when these cells were exposed to hypoxia, there was no difference in the expression or phosphorylation of p53 in MnSOD up-regulated cells or cells expressing a normal level of MnSOD (Fig. 7B). In a converse experiment, we down-regulated the MnSOD expression by RNA interference in HEK293T cells and exposed these cells to hypoxia. As shown in Fig. 7C, the expression and phosphorylation of p53 remain unchanged. Thus, modulation of the expression of MnSOD, a potent mitochondrial superoxide dismutase, does not affect p53 expression when cells are exposed to hypoxia. Therefore, this genetic experiment suggests that mitochondrial ROS is not involved in p53 expression in hypoxia.
p53 Expression and Phosphorylation Is Increased in Normoxia and Is Potentiated in Hypoxia in 6-AN-treated Cells
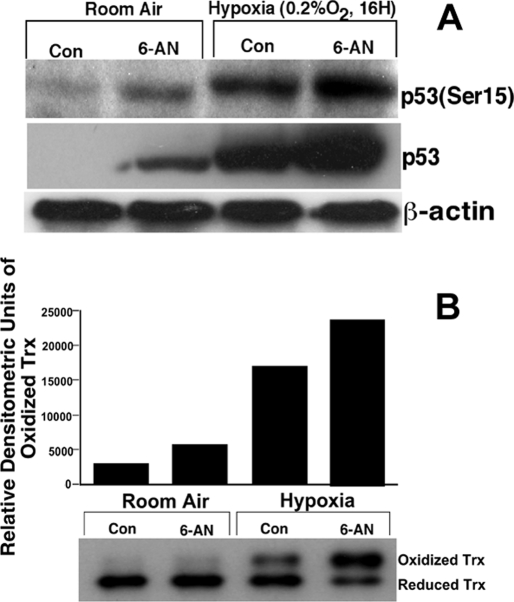
The predominant pathway that generates the reducing power of cells is the pentose phosphate pathway (PPP). The PPP generates NADPH via glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase (53). TrxR maintains the redox state of Trx by utilizing NADPH. Therefore, if NADPH is rate-limiting for the reduction of Trx in hypoxia, then inhibition of the PPP should show similar effects to that of hypoxia in terms of Trx oxidation and p53 activation. Therefore, we used a pharmacologic inhibitor of the PPP, 6-AN, to inhibit the PPP and determine its effect on p53 expression and p53 phosphorylation. As shown in Fig. 8A, MCF-7 cells treated with 6-AN indeed induced increased level of p53 expression in normoxia. Furthermore, cells treated with 6-AN and exposed to hypoxia demonstrated a potentiation of p53 expression and p53 (Ser-15) phosphorylation compared with either 6-AN-treated cells alone or cells exposed only to hypoxia.
FIGURE 8.
p53 expression and phosphorylation are increased in normoxia and potentiated in hypoxia in 6-AN-treated cells. A, HEK293T cells were pretreated with 6-AN (100 μm) for 2 h followed by exposure to 16 h in hypoxia (0.2% oxygen). Cell lysates were prepared, and p53 Western analysis was performed as described under “Experimental Procedures.” Con, control. B, oxidation of Trx increased in 6-AN-treated cells in normoxia and is potentiated in 6-AN-treated cells exposed to hypoxia. MCF-7 cells were treated with 6-AN (50 μm), and following exposure oxidized and reduced Trx was analyzed as described under “Experimental Procedures.” Upper panel, densitometry of oxidized band; lower panel, Western analysis of Trx oxidation state.
Loss of reducing power in the form of NADPH should create a more oxidizing condition in the absence of ROS. The level of NADPH has been shown to be decreased in hypoxia (54). Because Trx gets its reducing power from NADPH via TrxR cells treated with 6-AN, an inhibitor PPP should show higher levels of Trx oxidation in normoxia. Additionally, cells treated with 6-AN and exposed to hypoxia should show potentiation of Trx oxidation compared with each of 6-AN- or hypoxia-treated cells alone. To determine whether the loss of NADPH in hypoxia oxidizes Trx, we treated cells with 6-AN, exposed them to hypoxia, and determined the redox state of Trx. As shown in Fig. 8B, cells treated with 6-AN alone showed a 2-fold increase in Trx oxidation compared with room air-exposed cells. Additionally, cell exposed to hypoxia showed a 5-fold increase in Trx oxidation. However, when 6-AN-treated cells were exposed to hypoxia, we observed about a 7-fold increase in Trx oxidation.
Effect of Hypoxia on TrxR Activity
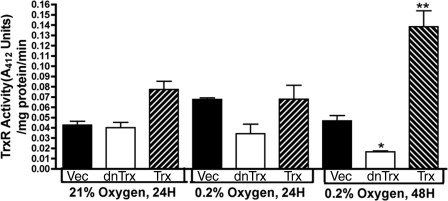
TrxR reduces Trx, and therefore, inactivation of TrxR in hypoxia could result in failure of Trx reduction resulting in accumulation of oxidized Trx. To test whether TrxR is inactivated in hypoxia, we determined the activity of TrxR in MCF-7 clones exposed to 24 or 48 h of hypoxia. As demonstrated in Fig. 9, the activity of TrxR decreased in dnTrx cells exposed to 48 h of hypoxia. In contrast, TrxR activity increased in Trx cells in 48 h of hypoxia. Therefore, cells with a decreased level of Trx showed a decreased level of TrxR activity in hypoxia. This observation agrees with our Trx oxidation data, which suggest that a decrease activity of TrxR will contribute to accumulation of oxidized Trx in hypoxia because of a decreased level of regeneration of oxidized Trx.
FIGURE 9.
Effect of hypoxia on TrxR activity. HCT116 cells were exposed to normoxia or hypoxia (0. 2% O2; 24 or 48 h) and TrxR activity was analyzed as described under “Experimental Procedures.” The experiment was performed in triplicate. Vec, vector. *, significantly lower than the vector cells exposed to 48 h of hypoxia; **, significantly higher than the vector cells exposed to 48 h of hypoxia.
RNR Activity Is Decreased in dnTrx Cells but Not in Trx Cells in Hypoxia
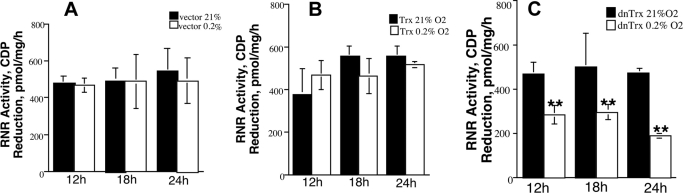
Trx is a major electron donor for the catalytic cycle of RNR and is the first committed step in DNA replication. Therefore, oxidation of Trx in hypoxia is expected to compromise the activity of RNR because of decreased supply of reducing equivalents. Therefore, we investigated the effect of either Trx depletion or mutant Trx expression on RNR activity. Cells expressing a high level of Trx or a normal level of Trx (vector control) did not show a significant decrease in RNR activity when exposed to 0.2% oxygen for 12, 18, or 24 h (Fig. 10, A and B). Cells expressing mutant Trx, however, showed a significant decrease in RNR activity at all time points in response to hypoxia (Fig. 10C). Therefore, when redox-compromised cells are further exposed to hypoxia, RNR activity is significantly decreased. We verified this phenomenon by transiently overexpressing Trx or mutant Trx by adenovirus infection of MCF-7 cells with expression vectors. Adenovirus infection increased the level of Trx about 2-fold, and the level of dnTrx was increased about 3-fold as determined by enzyme-linked immunosorbent assay (Table 2, 2nd column). As demonstrated in Table 2, cells infected with mutant Trx construct had a significantly lower level of RNR activity in response to 24 h of hypoxia. We depleted the cellular Trx level by RNA interference, as performed previously (34), and observed a significant decrease in RNR activity after the cells experienced hypoxia for 24 h (Table 2). Because dnTrx cells demonstrated decreased RNR activity, there is a reason to believe that dnTrx protein itself may decrease RNR activity. Therefore, we performed the RNR assay with or without NADPH or DTT for dnTrx cell lysates exposed to normoxia or hypoxia. As shown in Table 3, the activity of RNR was not significantly affected by the presence or absence of NADPH or DTT in normoxia. However, dnTrx cells show decreased activity of RNR with or without NADPH or DTT in hypoxia, which demonstrates that dnTrx protein per se does not affect RNR activity either in normoxia or hypoxia. Thus, Trx oxidation in hypoxia could further impair the DNA replication process in hypoxia because of inactivation of RNR.
FIGURE 10.
Activity of RNR decreases in hypoxia in dnTrx cells. MCF-7 cells (vector, Trx or dnTrx) cells were exposed to normoxia or hypoxia (0. 2%O2), and RNR activity was assayed as mentioned under “Experimental Procedures.” The activity was expressed as picomoles of CDP reduction/mg/h. A, vector only cells exposed to normoxia or hypoxia. B, Trx cells exposed to normoxia or hypoxia. C, dnTrx cells exposed to normoxia or hypoxia. The experiment was performed in triplicate. **, significantly lower than dnTrx cells exposed to 21% oxygen.
TABLE 2.
Effect of transient overexpression of Trx or dnTrx and down-regulation of Trx on RNR activity in hypoxia
MCF-7 cells were transfected with AdenoX constructs containing Trx or dnTrx cDNA as mentioned under “Experimental Procedures,” and after 48 h Trx enzyme-linked immunosorbent assay was performed on some samples. The transfected cells were exposed to normoxia or hypoxia for 24 h, and RNR activity assay was performed as described under “Experimental Procedures.” In some experiments nontargeting siRNA or Trx RNA was transfected into MCF-7 cells, and RNR activity was determined as described under “Experimental Procedures.” The experiment was performed in triplicate.
| Trx | CDP reduction |
p value | ||
|---|---|---|---|---|
| 21% O2 | 0.2% O2 | |||
| pg/mg protein | pmol/mg/h | |||
| AdenoX LacZ | 742 ± 44 | 287 ± 52 | 251 ± 91 | 0.29 |
| AdenoX Trx | 1643 ± 85 | 327 ± 43 | 290 ± 76 | 0.25 |
| AdenoX dnTrx | 2375 ± 96 | 242 ± 84 | 109 ± 33 | 0.03 |
| MCF (NT siRNA) | 479 ± 58 | 521 ± 27 | 0.84 | |
| MCF Trx siRNA | 496 ± 72 | 322 ± 65 | 0.01 | |
TABLE 3.
Effect of NADPH and DTT on RNR activity in hypoxia in dnTrx cells
MCF7-dnTrx cells were exposed to normoxia or hypoxia, and RNR activity was determined as mentioned under “Experimental Procedures” with or without NADPH in reactions containing DTT or without DTT. The experiment was performed in triplicate.
| Samples | CDP reduction |
||
|---|---|---|---|
| Without NADPH, with DTT | With NADPH, with DTT | Without NADPH, without DTT | |
| pmol/mg/h | pmol/mg/h | pmol/mg/h | |
| dnTrx, 21% O2 | 406 ± 131 | 503 ± 116 | 468 ± 49 |
| dnTrx, 0.2% O2 | 314 ± 132 | 260 ± 41 | 282 ± 39 |
DISCUSSION
The mechanism of cell cycle arrest in hypoxia remains unclear. This study provides several pieces of new information into the mechanism of cell cycle arrest in hypoxia. Our novel findings include the following. 1) Hypoxia induces oxidation of Trx that is independent of ROS. 2) inhibition of NADPH-generating enzyme glucose-6-phosphate dehydrogenase by 6-AN induces p53 expression and potentiates its expression in hypoxia. 3) Hypoxia decreases the activity of TrxR and RNR in dnTrx cells. 4) Overexpression of Trx inhibits the expression and DNA binding of p53 and the phosphorylation of Chk1/Chk2. 5) Overexpression of Trx prevents hypoxia-induced apoptosis.
Our cell cycle analysis showed that in hypoxia vector and dnTrx cells underwent massive apoptosis in 24 or 48 h compared with Trx-overexpressing cells, which did not show a significant level of apoptosis. This finding is consistent with our data demonstrating that vector or dnTrx cells show increased p53 expression and DNA binding that is required for induction of apoptosis or cell cycle arrest. On the contrary, cells overexpressing Trx did not undergo apoptosis nor did they show enhanced p53 expression or DNA binding. Furthermore, Trx cells did not show G1 arrest, but a large number of cells were in S phase after 48 h of hypoxia indicating an S phase delay of these cells. However, there was no significant change in the G2/M phase population between normoxia and 48 h of hypoxia. This fact suggested that although Trx-overexpressed cells were transiting from S to G2/M phase, they were delayed in S phase. The exact reason for this phenomenon is currently unknown. Thus, our data show that although cell cycle checkpoint is activated in hypoxia in vector or dnTrx cells, Trx cells were able to bypass checkpoint activation.
Activation of p53 induces cell cycle arrest, apoptosis, or DNA repair that depends on the specific stress type. It is well established that p53 is induced in hypoxia. However, the mechanism of p53 activation in hypoxia, which is considered a nongenotoxic stress, remains unclear. Abrogation of p53 expression and DNA binding in hypoxia in Trx-overexpressed cells suggests that these cells continue to proliferate in hypoxia without the activation of cell cycle checkpoint. Therefore, overexpression of Trx must have attenuated a specific biological response in hypoxia that is required for p53 up-regulation. Trx is a disulfide reductase, an antioxidant protein, and an electron donor for RNR, a crucial enzyme for the synthesis of deoxyribonucleotide, a first committed step in DNA replication. Therefore, it is possible that increased oxidative stress or inactivation of RNR in hypoxia could result in activation of cell cycle checkpoint and that the checkpoint activation is inhibited in cells overexpressing Trx.
We evaluated the role of oxidative stress in the activation of p53 in hypoxia, because ROS has been shown to be generated in hypoxia. Although there are indications that ROS are generated by the mitochondrial electron transport chain in response to hypoxia (45–48), several findings lead us to question this intriguing possibility. In circumstances where oxygen acts as an acceptor of electrons for formation of ROS, their generation during anoxia or severe hypoxia is not only counter-intuitive but brings into question the methodology used to measure ROS in this condition (48, 55). For example, ρ0 cells, mitochondrial complex inhibitors, and dicarboxyfluorescein diacetate oxidation have been employed as tools to document ROS generation during hypoxia (48). Although these measurements are meticulously performed and rigorously tested, other researchers have questioned the validity of these approaches (55–57); for example, ρ0 cells have been shown to have other responses (58, 59). In contrast to some of the published studies (36), we have observed that the expression of p53 is increased in hypoxia in a ROS-independent manner (Fig. 7). MnSOD is a critical antioxidant in the mitochondria that neutralizes the superoxide anion generated because of oxidative phosphorylation in the electron transport chain. Therefore, an increased level of MnSOD is expected to protect against higher level of superoxide anion generated in hypoxia. However, in our experiment using MnSOD overexpression or MnSOD depletion, we did not find any change in p53 expression. This finding suggests that a p53 increase in hypoxia is independent of ROS (Fig. 7). On the contrary, when we inhibited the PPP by 6-AN, we observed an increase in p53 expression that was potentiated in hypoxia. These data demonstrate that the failure of generation of reducing equivalents in hypoxia could account for the increase in p53. In addition, we have observed that the activity of TrxR is decreased in dnTrx cells exposed to hypoxia. Reduced levels of TrxR activity could impair the regeneration of reduced Trx, and hence, oxidized Trx is expected to accumulate. A recent study has demonstrated that the level of NADPH decreases in endothelial cells in hypoxia (54), suggesting that NADPH supply could be a limiting factor in hypoxia, which may result in the oxidation of Trx in the absence of ROS. Therefore, if p53 expression were linked to the oxidation of Trx in hypoxia, then we should observe increased Trx oxidation in 6-AN-treated cells in normoxia, and the oxidation of Trx should be potentiated in hypoxia. Indeed, we observed that Trx oxidation is potentiated in 6-AN-treated cells exposed to hypoxia. Thus our data demonstrate that oxidation of Trx is independent of ROS but is dependent on the loss of reducing power of the cell. This is an appropriate response to hypoxia, because in the absence of a high level of cellular energy that is required for cell division, the cell must stop cycling to conserve energy.
Therefore, the intriguing question is as follows: “How does the oxidation of Trx in hypoxia translate into a cellular signal for p53 activation, which triggers a cell cycle arrest?” One key signaling molecule could be ribonucleotide reductase whose activation depends on the supply of reducing equivalents shuttled by the Trx-TrxR system. We have shown that whereas normal or Trx-overexpressed cells retain the activity of RNR, cells overexpressing mutant Trx show decreased levels of RNR activity in hypoxia (Fig. 10, A–C). The activity of RNR was comparable among all treatments in normoxia demonstrating that the amount of cellular Trx/TrxR present in the lysates is adequate for driving the mammalian RNR assay system, because DTT and NADPH were omitted in our assay system. However, the activity of RNR was decreased in dnTrx cells exposed to hypoxia, but not in vector or Trx cells. These data indicate that redox-active Trx could be a limiting factor for the activity of RNR in hypoxia because of severe oxidation of Trx in dnTrx cells. To this effect a recent publication (60) has shown that the activity of mammalian RNR can proceed without DTT or NADPH because of the presence of Trx/TrxR in the cell extract, which is a dominant electron donor for RNR. Therefore, our data show that reduced Trx is a limiting factor for RNR activity in hypoxia in dnTrx cells. Therefore oxidation of Trx in hypoxia because of loss of reducing power of the cell would inhibit the RNR activity resulting in the failure of deoxyribonucleotide synthesis, a rate-limiting step in DNA replication. This process could inhibit DNA replication triggering a checkpoint response that could activate p53.
Thus, our study suggests that decreased activity of RNR in hypoxia could be a link between metabolic depletion of reducing power of the cells to that of cell cycle arrest. This process can occur without the involvement of ROS in hypoxia. Our results also show that oxidation of Trx is a critical event that links metabolic depletion of the reducing power of the cells to that of regulation of cell proliferation in hypoxia.
Acknowledgment
We gratefully acknowledge the generous gift of MnSOD plasmid by Dr. Larry Oberley (University of Iowa, Iowa City).
This work was supported, in whole or in part, by National Institutes of Health Grant R01 HL071558. This work was also supported by the University of Arkansas for Medical Science Research Council (to K. C. D.).
- ROS
- reactive oxygen species
- Trx
- thioredoxin
- TrxR
- thioredoxin reductase
- Chk1
- checkpoint kinase-1
- Chk2
- checkpoint kinase-2
- 6-AN
- 6-aminonicotinamide
- ATM
- ataxia telangiectasia mutated
- ATR
- ATM-Rad-3-related kinase
- RNR
- ribonucleotide reductase; replication protein A
- MnSOD
- manganese superoxide dismutase
- dnTrx
- dominant-negative Trx
- siRNA
- small interfering RNA
- PBS
- phosphate-buffered saline
- DTT
- dithiothreitol
- PARP
- poly(ADP-ribose) polymerase
- EMSA
- electrophoretic mobility shift assay
- PPP
- pentose phosphate pathway.
REFERENCES
- 1.Brown J. M. ( 1999) Cancer Res. 59, 5863– 5870 [PubMed] [Google Scholar]
- 2.Brown J. M., Giaccia A. J. ( 1998) Cancer Res. 58, 1408– 1416 [PubMed] [Google Scholar]
- 3.Brown J. M. ( 2000) Mol. Med. Today 6, 157– 162 [DOI] [PubMed] [Google Scholar]
- 4.Blancher C., Harris A. L. ( 1998) Cancer Metastasis Rev. 17, 187– 194 [DOI] [PubMed] [Google Scholar]
- 5.Green S. L., Giaccia A. J. ( 1998) Cancer J. Sci. Am. 4, 218– 223 [PubMed] [Google Scholar]
- 6.Höckel M., Vaupel P. ( 2001) Semin. Oncol. 28, 36– 41 [PubMed] [Google Scholar]
- 7.Vaupel P., Schlenger K., Knoop C., Höckel M. ( 1991) Cancer Res. 51, 3316– 3322 [PubMed] [Google Scholar]
- 8.Teicher B. A. ( 1994) Cancer Metastasis Rev. 13, 139– 168 [DOI] [PubMed] [Google Scholar]
- 9.Tomida A., Tsuruo T. ( 1999) Anticancer Drug. Des. 14, 169– 177 [PubMed] [Google Scholar]
- 10.Chi J. T., Wang Z., Nuyten D. S., Rodriguez E. H., Schaner M. E., Salim A., Wang Y., Kristensen G. B., Helland A., B⊘rresen-Dale A. L., Giaccia A., Longaker M. T., Hastie T., Yang G. P., van de Vijver M. J., Brown P. O. ( 2006) PLoS Med. 3, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito S., Goodarzi A. A., Higashimoto Y., Noda Y., Lees-Miller S. P., Appella E., Anderson C. W. ( 2002) J. Biol. Chem. 277, 12491– 12494 [DOI] [PubMed] [Google Scholar]
- 12.Rundle N. T., Xu L., Andersen R. J., Roberge M. ( 2001) J. Biol. Chem. 276, 48231– 48236 [DOI] [PubMed] [Google Scholar]
- 13.Rotman G., Shiloh Y. ( 1997) BioEssays 19, 911– 917 [DOI] [PubMed] [Google Scholar]
- 14.Danielsen T., Hvidsten M., Stokke T., Solberg K., Rofstad E. K. ( 1998) Br. J. Cancer 78, 1547– 1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond E. M., Giaccia A. J. ( 2005) Biochem. Biophys. Res. Commun. 331, 718– 725 [DOI] [PubMed] [Google Scholar]
- 16.Krtolica A., Krucher N. A., Ludlow J. W. ( 1999) Br. J. Cancer 80, 1875– 1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilodeau J. F., Faure R., Piedboeuf B., Mirault M. E. ( 2000) Exp. Cell Res. 256, 347– 357 [DOI] [PubMed] [Google Scholar]
- 18.Giaccia A. J., Simon M. C., Johnson R. ( 2004) Genes Dev. 18, 2183– 2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond E. M., Green S. L., Giaccia A. J. ( 2003) Mutat. Res. 532, 205– 213 [DOI] [PubMed] [Google Scholar]
- 20.Webster L., Hodgkiss R. J., Wilson G. D. ( 1998) Br. J. Cancer 77, 227– 234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond E. M., Dorie M. J., Giaccia A. J. ( 2003) J. Biol. Chem. 278, 12207– 12213 [DOI] [PubMed] [Google Scholar]
- 22.Hammond E. M., Denko N. C., Dorie M. J., Abraham R. T., Giaccia A. J. ( 2002) Mol. Cell. Biol. 22, 1834– 1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appella E., Anderson C. W. ( 2001) Eur. J. Biochem. 268, 2764– 2772 [DOI] [PubMed] [Google Scholar]
- 24.Shieh S. Y., Taya Y., Prives C. ( 1999) EMBO J. 18, 1815– 1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham R. T. ( 2001) Genes Dev. 15, 2177– 2196 [DOI] [PubMed] [Google Scholar]
- 26.Gibson S. L., Bindra R. S., Glazer P. M. ( 2005) Cancer Res. 65, 10734– 10741 [DOI] [PubMed] [Google Scholar]
- 27.Holmgren A. ( 1985) Annu. Rev. Biochem. 54, 237– 271 [DOI] [PubMed] [Google Scholar]
- 28.Holmgren A., Björnstedt M. ( 1995) Methods Enzymol. 252, 199– 208 [DOI] [PubMed] [Google Scholar]
- 29.Das K. C., Lewis-Molock Y., White C. W. ( 1997) Am. J. Respir. Cell Mol. Biol. 17, 713– 726 [DOI] [PubMed] [Google Scholar]
- 30.Das K. C. ( 2001) J. Biol. Chem. 276, 4662– 4670 [DOI] [PubMed] [Google Scholar]
- 31.Kolberg M., Strand K. R., Graff P., Andersson K. K. ( 2004) Biochim. Biophys. Acta 1699, 1– 34 [DOI] [PubMed] [Google Scholar]
- 32.Xue L., Zhou B., Liu X., Wang T., Shih J., Qi C., Heung Y., Yen Y. ( 2006) Cancer Res. 66, 1900– 1905 [DOI] [PubMed] [Google Scholar]
- 33.Hammond E. M., Giaccia A. J. ( 2004) DNA Repair. 3, 1117– 1122 [DOI] [PubMed] [Google Scholar]
- 34.Ravi D., Muniyappa H., Das K. C. ( 2005) J. Biol. Chem. 280, 40084– 40096 [DOI] [PubMed] [Google Scholar]
- 35.Li J. J., Colburn N. H., Oberley L. W. ( 1998) Carcinogenesis 19, 833– 839 [DOI] [PubMed] [Google Scholar]
- 36.St Clair D. K., Holland J. C. ( 1991) Cancer Res. 51, 939– 943 [PubMed] [Google Scholar]
- 37.Das K. C., White C. W. ( 1998) J. Immunol. Methods 211, 9– 20 [DOI] [PubMed] [Google Scholar]
- 38.Das K. C., Guo X. L., White C. W. ( 1999) Am. J. Physiol. 276, L530– L539 [DOI] [PubMed] [Google Scholar]
- 39.Slabaugh M. B., Johnson T. L., Mathews C. K. ( 1984) J. Virol. 52, 507– 514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oblong J. E., Berggren M., Gasdaska P. Y., Powis G. ( 1994) J. Biol. Chem. 269, 11714– 11720 [PubMed] [Google Scholar]
- 41.Gallegos A., Gasdaska J. R., Taylor C. W., Paine-Murrieta G. D., Goodman D., Gasdaska P. Y., Berggren M., Briehl M. M., Powis G. ( 1996) Cancer Res. 56, 5765– 5770 [PubMed] [Google Scholar]
- 42.Plummer E. R. ( 2006) Curr. Opin. Pharmacol. 6, 364– 368 [DOI] [PubMed] [Google Scholar]
- 43.Soldani C., Scovassi A. I. ( 2002) Apoptosis 7, 321– 328 [DOI] [PubMed] [Google Scholar]
- 44.Das K. C., Dashnamoorthy R. ( 2004) Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L87– 97 [DOI] [PubMed] [Google Scholar]
- 45.Chandel N. S., Maltepe E., Goldwasser E., Mathieu C. E., Simon M. C., Schumacker P. T. ( 1998) Proc. Natl. Acad. Sci. U. S. A. 95, 11715– 11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandel N. S., McClintock D. S., Feliciano C. E., Wood T. M., Melendez J. A., Rodriguez A. M., Schumacker P. T. ( 2000) J. Biol. Chem. 275, 25130– 25138 [DOI] [PubMed] [Google Scholar]
- 47.Chandel N. S., Vander Heiden M. G., Thompson C. B., Schumacker P. T. ( 2000) Oncogene 19, 3840– 3848 [DOI] [PubMed] [Google Scholar]
- 48.Cash T. P., Pan Y., Simon M. C. ( 2007) Free Radic. Biol. Med. 43, 1219– 1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambert A. J., Buckingham J. A., Boysen H. M., Brand M. D. ( 2008) Biochim. Biophys. Acta 1777, 397– 403 [DOI] [PubMed] [Google Scholar]
- 50.Park W. H., Han Y. W., Kim S. H., Kim S. Z. ( 2007) J. Cell. Biochem. 102, 98– 109 [DOI] [PubMed] [Google Scholar]
- 51.Faulkner K. M., Liochev S. I., Fridovich I. ( 1994) J. Biol. Chem. 269, 23471– 23476 [PubMed] [Google Scholar]
- 52.Day B. J., Shawen S., Liochev S. I., Crapo J. D. ( 1995) J. Pharmacol. Exp. Ther. 275, 1227– 1232 [PubMed] [Google Scholar]
- 53.Wolin M. S., Ahmad M., Gupte S. A. ( 2005) Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L159– 173 [DOI] [PubMed] [Google Scholar]
- 54.Gupte S. A., Wolin M. S. ( 2006) Am. J. Physiol. Heart Circ. Physiol. 290, H2228– 2238 [DOI] [PubMed] [Google Scholar]
- 55.White C. W. ( 2006) Free Radic. Biol. Med. 40, 923– 927 [DOI] [PubMed] [Google Scholar]
- 56.Srinivas V., Leshchinsky I., Sang N., King M. P., Minchenko A., Caro J. ( 2001) J. Biol. Chem. 276, 21995– 21998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doege K., Heine S., Jensen I., Jelkmann W., Metzen E. ( 2005) Blood 106, 2311– 2317 [DOI] [PubMed] [Google Scholar]
- 58.Hagen T., Taylor C. T., Lam F., Moncada S. ( 2003) Science 302, 1975– 1978 [DOI] [PubMed] [Google Scholar]
- 59.Metzen E., Zhou J., Jelkmann W., Fandrey J., Brüne B. ( 2003) Mol. Biol. Cell 14, 3470– 3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avval F. Z., Holmgren A. ( 2009) J. Biol. Chem. 284, 8233– 8240 [DOI] [PMC free article] [PubMed] [Google Scholar]