Abstract
Although there are numerous reports of carbohydrates enriched in cancer cells, very few studies have addressed the functions of carbohydrates present in normal cells that decrease in cancer cells. It has been reported that core3 O-glycans are synthesized in normal gastrointestinal cells but are down-regulated in cancer cells. To determine the roles of core3 O-glycans, we transfected PC3 and LNCaP prostate cancer cells with β3-N-acetylglucosaminyltransferase-6 (core3 synthase) required to synthesize core3 O-glycans. Both engineered cell lines exhibited reduced migration and invasion through extracellular matrix components compared with mock-transfected cells. Moreover we found that α2β1 integrin acquired core3 O-glycans in cells expressing core3 synthase with decreased maturation of β1 integrin, leading to decreased levels of the α2β1 integrin complex, decreased activation of focal adhesion kinase, and reduced lamellipodia formation. Upon inoculation into the prostate of nude mice, PC3 cells expressing core3 O-glycans produced much smaller tumors without metastasis to the surrounding lymph nodes in contrast to robust tumor formation and metastasis seen in mock-transfected PC3 cells. Similarly LNCaP cells expressing core3 O-glycans barely produced subcutaneous tumors in contrast to robust tumor formation by mock-transfected LNCaP cells. These findings indicate that addition of core3 O-glycans to β1 and α2 integrin subunits in prostate cancer cells suppresses tumor formation and tumor metastasis.
Cancer cells often express surface carbohydrates different from normal cells (1). One such change is expression of sialyl Lewis X and Lewis B blood group antigens in cancer cells (2, 3). These structural elements are seen as capping oligosaccharides attached to the underlying glycan backbone where they likely function as ligands for cell adhesion molecules.
The structure of underlying glycans also changes during malignant transformation and differentiation. In particular, there are several reports that an increase in the β1,6-N-acetylglucosaminyl branch in N-glycans synthesized by β1,6-N-acetylglucosaminyltransferase-V is associated with oncogenic transformation (4–7). Similar structural changes are seen in mucin-type O-glycans, which have N-acetylgalactosamine at the reducing end linked to polypeptide threonine or serine residues. Addition of different carbohydrate residues to N-acetylgalactosamine confers a variety of backbone structures on mucin-type O-glycans; the most abundant of those are classified as core1, core2, core3, and core4 O-glycans (8) (Fig. 1). Among these O-glycans, the synthesis of the core2 branch has been extensively studied particularly because conversion of core1 to core2 O-glycans was observed in T cell activation (9). Expression of core2 branch apparently represents an oncodifferentiation antigen because core2 branched O-glycans are synthesized in early stages of T cell differentiation, down-regulated in mature T cells, and reappear in T cell leukemia and immune deficiencies such as AIDS and Wiskott-Aldrich syndrome (for a review, see Ref. 10). In addition, overexpression of core2 O-glycans is seen in many cancers, including lung and breast carcinoma cells (11, 12).
FIGURE 1.
Biosynthetic pathways of mucin-type O-glycans. N-Acetylgalactosamine is transferred to a serine or threonine residue in a polypeptide. Resultant GalNAcα1→Ser/Thr is converted by core3 synthase (β3GnT-6) to GlcNAcβ1→3GalNAcα1→Ser/Thr (core3). Core3 is then converted to core4 by C2GnT-2 (C2GnT-M). GalNAcα1→Ser/Thr is also converted to core1, Galβ1→3GalNAcα1→Ser/Thr, by core1 synthase. Core1 is then converted to core2 by C2GnT-1, C2GnT-2, and C2GnT-3.
By contrast, core3 and core4 O-glycans are synthesized in normal cells but apparently down-regulated in gastric and colorectal carcinoma (13, 14). Core3 O-glycans are synthesized by core3 synthase (β3GnT-6),2 which adds β1,3-linked N-acetylglucosamine to N-acetylgalactosamine at the reducing terminus (15) (Fig. 1). Iwai et al. (16) showed that forced expression of core3 synthase in human fibrosarcoma HT1080 FP-10 cells resulted in significant reduction in the formation of lung tumor foci in mice after intravenous injection of tumor cells through a tail vein. However, the same study did not address whether the expression of core3 influences tumor metastasis because the cancer cells were intravenously injected and no primary tumor was formed to spread into the lung as metastasis in contrast to the other studies (17, 18). Core4 O-glycan is synthesized by addition of β1,6-linked N-acetylglucosamine to a core3 acceptor by core2 β1,6-N-acetylglucosamine M type (C2GnT-M) or C2GnT-2 (19, 20) (Fig. 1). Huang et al. (21) reported that C2GnT-M is down-regulated in colonic carcinoma cells and that forced expression of C2GnT-M in HCT116 colonic carcinoma cells significantly decreased cell invasion and subcutaneous tumor formation. How up-regulation of core3 and core4 O-glycans influences the pathophysiology of cells expressing core3 and core4 O-glycans has not been addressed.
Cell-extracellular matrix interaction plays an essential role during acquisition of migration and invasive behavior of cancer cells. For example, α2β1 integrin is the major receptor for collagen (22) and most abundantly expressed in prostate cancer cells (23). Glycosylation on integrin is one of the important modulators of integrin functions, and many glycan structures, mainly N-glycans, have been studied. An increase of bisecting GlcNAc structure on α5β1 integrin inhibits the cell spreading and migration (24), and induced β1,6-GlcNAc sugar chains on N-glycans of β1 integrin result in stimulation of cell migration (25). However, it has not been addressed whether changes in O-glycans affect integrin maturation and functions.
To determine the role of core3 O-glycans in tumor formation and metastasis, we analyzed PC3 and LNCaP human prostate cancer cells. We found that these cell lines express only small amounts of detectable core3 synthase; thus we transfected the cell lines with core3 synthase. Core3 synthase-transfected PC3 and LNCaP cells expressed increased amounts of core3 O-glycans in α2β1 integrin, showed the reduced maturation of β1 integrin and low levels of α2β1 integrin formation, migrated less efficiently through collagen and other extracellular matrix components, and were less invasive than mock-transfected cells. Moreover those cells exhibited decreased activation of focal adhesion kinase (FAK) compared with mock-transfected cells. Significantly PC3 cells expressing core3 O-glycans produced almost no primary tumors in the prostate and formed much fewer metastases in the draining lymph nodes than mock-transfected cells. Similarly LNCaP cells expressing core3 O-glycans produced much smaller subcutaneous tumors than mock-transfected LNCaP cells. These findings indicate that addition of core3 O-glycans to the α2β1 integrin leads to decreased cell migration and invasion, resulting in decreased prostate tumor formation and metastasis.
EXPERIMENTAL AND PROCEDURES
Cell Culture and Transfection
PC3 and LNCaP prostate cancer cell lines were obtained from American Type Culture Collection and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. cDNA encoding core3 synthase (β3GlcNAcT-6) (15) was amplified by reverse transcription (RT)-PCR and cloned into pcDNA 3.1(N) as described previously (26). pcDNA 3.1(N) was prepared by deleting the Zeocin resistance gene and f1 origin from pcDNA 3.1/Zeo as described previously (26). PC3 and LNCaP cells were transfected with pcDNA 3.1(N) harboring core3-synthase cDNA and pcDNA 3 harboring the neomycin resistance gene using Lipofectamine. Transfected cells were selected first in 200 μg/ml Geneticin® (Invitrogen) and maintained in 100 μg/ml Geneticin. Clonal transfected cells were obtained by dilution and tested for expression of carbohydrates reactive to peanut agglutinin (PNA) after neuraminidase treatment. PNA reacts with Galβ1→3GalNAcα1→R core1 structure (27). Core3 oligosaccharides do not react with PNA. For this assay, PC3 and LNCaP cells in cloning plates were dissociated into monodispersed cells using an enzyme-free dissociation solution (Hanks' balanced saline solution-based) purchased from Cell and Molecular Technologies. Dissociated cells were incubated with fluorescein isothiocyanate-conjugated PNA and subjected to fluorescence-activated cell sorting analysis using FACScan flow cytometry (BD Biosciences) as described previously (26). As controls, PC3 and LNCaP cells were transfected with empty pcDNA 3.1(N) and pcDNA 3 and selected in Geneticin. Mock-transfected and core3 synthase-expressing PC3 cells cultured on glass plates were stained with phalloidin to visualize F-actin as described previously (28).
Semiquantitative RT-PCR Analysis
Total RNA was isolated from PC3 and LNCaP cells using TRIzol (Invitrogen). RT-PCR of core3 synthase (β3GnT-6) (15), C2GnT-1 (29), C2GnT-2 (19), and C2GnT-3 (30) was undertaken. First strand cDNA was synthesized using Amplitag DNA polymerase (Applied Biosystems) and the following PCR primers: C2GnT-1, 5′-tcggtggacacctgacgactatat-3′ (5′-primer) and 5′-aggtcataccgcttcttccacctt-3′ (3′-primer); C2GnT-2, 5′-agtccagggaatctcaaagccagt-3′ (5′-primer) and 5′-tgagctctggagcaagtcttccat-3′ (3′-primer); C2GnT-3, 5′-gacatccagttctctagacctctg-3′ (5′-primer) and 5′-aaggcgaggtacttagggagtact-3′ (3′-primer); β3GnT-6, 5′-agcactgcagcagtggttc-3′ (5′-primer) and 5′-gaggaaggtgtccgcgaag-3′ (3′-primer); and glyceraldehyde-3-phosphate dehydrogenase, 5′-cctggccaaggtcatccatgaca-3′ (5′-primer) and 5′-atgaggtccaccaccctgttgct-3′ (3′-primer). The PCR was carried out at 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s and by a single incubation at 72 °C for 5 min. PCR products were separated by electrophoresis on 1% agarose gels.
Similarly the amounts of the transcripts for Cosmc (31) and core1 synthase were semiquantitatively estimated by PCR. Twenty-seven cycles of PCR was done using 5-cactgtgacaaagcaga-3 and 5-ggttggggtgataagtca-3 primers for Cosmc, and 29 cycles of PCR was done using 5-gtgggactgaaaaccaa-3 and 5-agatcagagcagcaacca-3 primers for core1 synthase. Expression levels were normalized by glyceraldehyde-3-phosphate dehydrogenase expression.
O-Glycan Structure Analysis
PC3-mock, LNCaP-mock, PC3-core3, and LNCaP-core3 cells were suspended in 0.1 m NH4HCO3, boiled for 10 min, and lyophilized. Dried samples were delipidated by chloroform-methanol (2:1 by volume) and then extracted by a standard 6 m guanidine chloride protocol followed by reduction and alkylation with dithiothreitol/iodoacetic acid. After dialysis, the samples were digested with trypsin/chymotrypsin (Sigma) and then with N-glycanase F (Roche Applied Science) and passed through a C18 Sep-Pak cartridge (Waters). De-N-glycosylated peptides were eluted stepwise from the C18 cartridge by 20–40% 1-propanol in 5% acetic acid and then treated with 0.05 m NaOH, 1 m NaBH4 at 37 °C for 3 days to release O-glycans. The samples were neutralized by acetic acid on ice until it stopped bubbling followed by passing through a Dowex 50-X8 column in 5% acetic acid and drying. Borates were then removed by repeated co-evaporation with 10% acetic acid in methanol under a stream of nitrogen. An aliquot of the released and desalted O-glycans was permethylated and analyzed by MALDI-MS and MS/MS on a 4700 Proteomics Analyzer (Applied Biosystem, Farmington, MA) as described previously (32, 33).
Immunoprecipitation and Western Blot Analysis
Cells were solubilized in lysis buffer composed of 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 1% (w/v) Nonidet P-40, 5 mm sodium pyrophosphate, 10 mm NaF, 1 mm sodium orthovanadate, 10 mm β-glycerophosphate, 1 mm phenylmethylsulfonyl fluoride, and a protease inhibitor mixture (Sigma). Equal amounts of cell lysates were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were incubated separately with polyclonal anti-β1 integrin antibody (Ab1952, Chemicon) (34), rabbit anti-α2 integrin antibody (Ab1936, Chemicon), rabbit anti-ERK antibody (Cell Signaling Technology), mouse anti-FAK antibody (BD Biosciences), and anti-FAK (Tyr(P)397) phosphospecific antibody (44–625G, BD Biosciences) and then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG or horseradish peroxidase-conjugated goat anti-rabbit IgG. In parallel, aliquots of the lysate were treated with N-glycanase (Calbiochem) as described previously (33) before separation by SDS-polyacrylamide gel electrophoresis. Alternatively cell lysates were incubated with polyclonal anti-β1 integrin antibody followed by protein A-agarose. Immunoprecipitates were dissociated from protein A-agarose by boiling for 5 min in sample buffer containing 1% SDS before electrophoresis. Solubilized proteins were separated on gels and blotted to a polyvinylidene difluoride membrane. The blot was incubated with anti-α2 integrin antibody followed by horseradish peroxidase-conjugated anti-rabbit IgG. ECL reagents (Amersham Biosciences) were used to detect signals. The membrane was then stripped by incubation with 1 n NaOH for 1–2 min followed by washing three times with 10 mm Tris-HCl buffer, pH7.4 containing 0.14 m NaCl and 0.05% Tween 20 (TBS-T) and blocking with TBS-T containing 5% skim milk again. This membrane was then incubated with anti-α2 integrin antibody. The membrane was also incubated with biotin-conjugated Griffonia simplifolia lectin II (GS-II) and visualized using a Vectastain ABC kit (Vector Laboratories Inc., Burlingame, CA) and, in parallel, with mouse monoclonal anti-β1 integrin antibody (610467, BD Biosciences) followed by horseradish peroxidase-conjugated anti-mouse IgG.
Flow Cytometry Analysis
Cells in semiconfluent conditions were detached from 10-cm culture dishes using enzyme-free cell dissociation solution (Chemicon) and resuspended in 50 μl of PBS. The suspended cells (5–10 × 106 cells) were incubated with and without a primary antibody (rabbit anti-α1 integrin polyclonal antibody (Chemicon), rabbit anti-human integrin α2 polyclonal (Chemicon), and rat anti-human monoclonal antibody for α6 integrin (BD Pharmingen) at a final concentration of 4 μg/ml for 1 h on ice. The cells were washed three times with PBS, then resuspended in PBS containing fluorescein isothiocyanate-conjugated secondary antibody, and further incubated for 1 h on ice. After washing three times with PBS, flow cytometry analyses were performed using a FACScan instrument (BD Biosciences) operated with CELLQuest software.
Migration and Invasion Assay
Cell migration was assayed using the 3-μm-pore size Transwell® Permeable Supports (Corning). The bottom part of the Transwell membrane was coated with human laminin mixture (Chemicon), rat laminin-5 (Chemicon), collagen I, or fibronectin (Sigma) at a concentration of 0.5 μg/ml in PBS at 4 °C overnight. 5 × 104 cells were added to the upper chamber, and 6 h later at 37 °C in a CO2 incubator, cells reaching the bottom layer were stained with 0.5% crystal violet and counted under a microscope. Cell invasion was assayed using an ECM Invasion Chamber (Chemicon) in which the upper layer of the transmembrane was coated with Matrigel. 2.8 × 105 cells for PC3 and 5 × 105 cells for LNCaP were loaded in the upper chamber. After 24 h, cells reaching the bottom layer were visualized by 0.5% crystal violet and counted. To determine the contribution of different integrins to invasion, cells were preincubated with 10 μg/ml mouse anti-human integrin α1 I domain monoclonal antibody (Chemicon), mouse anti-human α2 integrin monoclonal antibody (Chemicon), rat anti-human monoclonal antibody GoH3 for α6 blocking (BD Pharmingen), and mouse monoclonal 4B4 anti-β1 integrin-neutralizing antibody (34) (Beckman Coulter). In parallel, cells were incubated with control mouse IgG (10 μg/ml).
To exclude the possibility that clonal variation contributes to the difference in cell migration, the parent PC3 cells and LNCaP cells were transiently transfected with core3 synthase or empty vector. Three days after the transfection, cell migration was measured in the same way as described above.
Orthotopic Tumor Cell Inoculation
BALBc nude (nude/nude) mice (6–8-week-old males) obtained from Taconic were used for orthotopic tumor cell injection (35). Mice were anesthetized with Avertin®, and laparotomy was performed; 2 × 106 PC3-core3 and mock-transfected PC3 cells were suspended in 20 μl of serum-free RPMI 1640 medium and inoculated into the posterior lobe of the prostate. The wound was then closed with surgical clips. Eight weeks later, mice were sacrificed, prostates and surrounding lymph nodes were removed, and organs were weighted. Specimens were preserved by fixation in neutral buffered formalin.
RESULTS
Core3 Synthase-expressing Prostate Cancer Cell Lines Exhibit Abnormal Lamellipodia
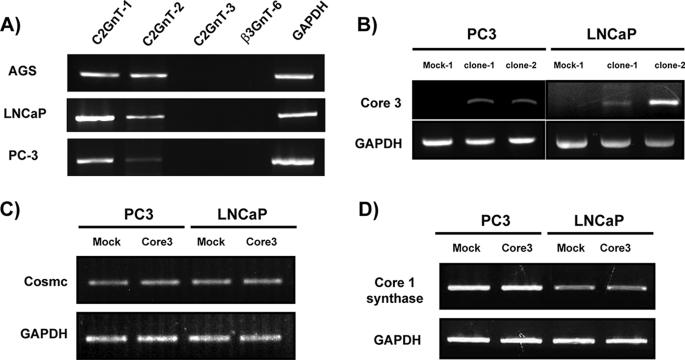
Previously it was shown that core3 synthase is down-regulated in colonic carcinoma cells relative to normal tissues (14). Expression of core3 synthase in a human fibrosarcoma cell line also resulted in decreased lung tumor foci formation compared with mock-transfected cells (16). However, these studies did not address the mechanisms regarding how core3 synthase expression results in decreased tumor formation and decreased tumor metastasis. To address these questions, we analyzed human PC3 and LNCaP prostate cancer cell lines because these cells metastasize to the lymph nodes. RT-PCR analysis of mRNAs encoding different glycosyltransferases showed that both PC3 and LNCaP cells express a significant amount of C2GnT-1 but only negligible or small amounts of core3 synthase (β3GnT-6). PC3 cells express only a small amount of C2GnT-2, which is much less than that expressed in gastric cancer AGS cells (Fig. 2A).
FIGURE 2.
Expression of enzymes forming mucin-type O-glycans. Total RNA was isolated from gastric cancer AGS cells, prostate cancer LNCaP, and PC3 cell lines and LNCaP-core3 and PC3-core3 cells. After reverse transcription, amounts of cDNAs for the enzymes were semiquantitatively estimated by PCR. β3GnT-6 and GAPDH refer to core3 synthase and glyceraldehyde-3-phosphate dehydrogenase, respectively. The parental cell lines (A) and transfected PC3 and LNCaP cells (B, C, and D) were subjected to the analysis. In C and D, PC3-core3 clone 2 and LNCaP-core3 clone 2 (see Fig. 3) were analyzed.
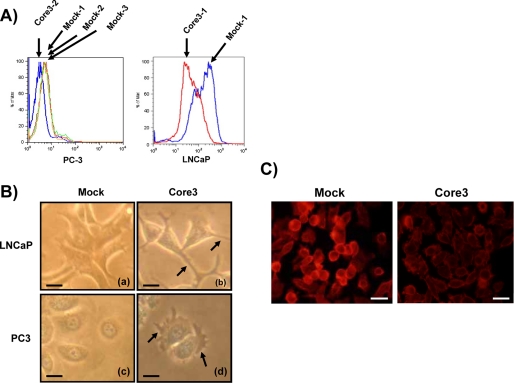
Individual vectors harboring core3 synthase (β3GnT-6) cDNA (15) and the neomycin (Geneticin) resistance gene were co-transfected into PC3 and LNCaP cells, and cells were selected in Geneticin. Transfected cells were subjected to Arthrobacter ureafaciens sialidase treatment and PNA staining followed by flow cytometry analysis. PNA binds to core1 O-glycan, Galβ1→3GalNAcα1→Thr/Ser, which can be formed by desialylation of sialylated core1 O-glycan. As shown in Fig. 3A, core3 synthase-transfected PC3 cell lines showed weaker PNA staining than did mock-transfected cells because some of the Galβ1→3GalNAc must be replaced by GlcNAcβ1→3GalNAc in the core3 synthase-transfected cells. Similarly LNCaP cells (clone 2) exhibited less PNA staining after transfecting with core3 synthase, although their level of PNA staining was much higher than those of PC3 cells for both mock-transfected and core3 synthase-transfected LNCaP cells. These cell lines transfected with core3 synthase were designated PC3-core3 and LNCaP-core3, respectively. Hereafter we studied clones 2 of PC3-core3 and LNCaP-core3 cells.
FIGURE 3.
Establishment of PC3 and LNCaP cells expressing core3 synthase. A, PC3 and LNCaP cells selected after transfection of core3 synthase and Geneticin resistance markers were subjected to fluorescence-activated cell sorting analysis after neuraminidase treatment and PNA staining. Mock-transfected clone 1 and clone 3-1 served as PC3 and LNCaP controls, respectively. B, micrographs of mock-transfected LNCaP (a), LNCaP-core3 (b), mock-transfected PC3 (c), and PC3-core3 (d) are shown. Lamellipodia formation is impaired as shown by arrows. C, F-actin was visualized by phalloidin. Scale bar, 0.025 mm for B and 0.05 mm for C.
RT-PCR analysis of PC3-core3 and LNCaP-core3 mRNAs showed that both cell lines expressed high levels of core3 synthase (Fig. 2B). By contrast, the expression level of core1 synthase and Cosmc (31) was not changed after core3 synthase transfection (Fig. 2, C and D). We also noted that transfected LNCaP and PC3 cells differed morphologically from controls in that they showed abnormal lamellipodia (Fig. 3B, arrows). Indeed F-actin visualized by phalloidin was decreased in core3 synthase-expressing PC3 cells compared with mock-transfected PC3 cells (Fig. 3C). By mass spectrometry analyses we confirmed the acquisition of core3 O-glycans after transfection with core3 synthase. As shown in Fig. 4A, core3-containing O-glycans (ions at m/z 779.3 and 1140.5) were seen only in core3-transfected PC3 cells and were absent in mock-transfected PC3 cells. The deduced structures for these molecular ion signals were further confirmed by MS/MS analyses (Fig. 4B). LNCaP-core3 cells displayed much more core3 O-glycans than mock-transfected LNCaP and much more than PC3-core3 cells (Fig. 4B). The latter finding is consistent with the difference in PNA labeling between PC3 and LNCaP cells as seen in Fig. 3A. The amount of core3 O-glycans in PC3-core3 cells is almost equivalent to that in colon cells of wild-type mice (36), whereas that of LNCaP-core3 cells apparently represents an overexpressed level of core3 O-glycans.
FIGURE 4.
Core3 O-glycans were revealed by mass spectrometric analyses in PC3-core3 and LNCaP-core3 cells but were barely detected in mock-transfected PC3 and LNCaP cells. MALDI-MS analysis of permethylated O-glycans in positive ion mode from PC3 (A) and LNCaP cells (B) is shown. The m/z values of the labeled peaks correspond to monoisotopic mass, and assignment of the molecular compositions is shown in the figure. Ions at m/z 895.4, 1256.5, 1344.6, and 1705.7 correspond to sialic acid→Gal→GalNAcitol, (sialic acid)2→Gal→GalNAcitol, sialic acid→[Gal→GlcNAc→(Gal→)GalNAcitol], and (sialic acid)2→[Gal→GlcNAc→(Gal→)GalNAcitol], respectively. Ions at m/z 779.3 and 1140.5 correspond to core3 O-glycans, Gal→GlcNAc→GalNAcitol, and sialic acid→Gal→GlcNAc→GalNAcitol, respectively. The ion at m/z 825.3 of PC3 cells corresponds to the in-source prompt fragment ion of sialic acid→Gal→GlcNAc. In B, the MS/MS spectrum of m/z 779 in the middle panel is shown at the bottom.
PC3 and LNCaP Cells Expressing Core3 O-Glycans Exhibit Reduced Migration and Invasion
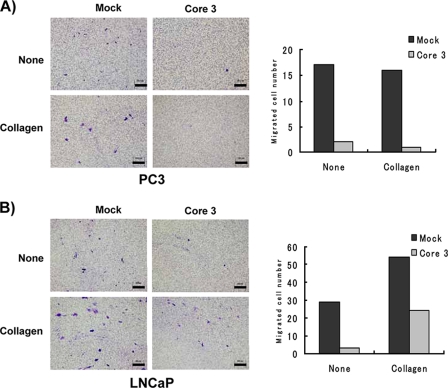
To determine how the expression of core3 synthase affects cell migration, we utilized a Transwell migration assay. For this assay, the bottom side of the Transwell membrane was coated with extracellular matrix components. Cells were then loaded on the upper side of the chamber, and migration to the bottom part of the membrane was determined 6 or 24 h later. Migration of PC3-core3 cells was much less efficient than that of mock-transfected PC3 cells (Fig. 5A). This reduction was observed on membranes coated with collagen, fibronectin, a mixture of laminin, or laminin-5 (Fig. 5A, lower panels). Similar results were obtained when LNCaP-core3 cells were tested (Fig. 5B). These results indicate that forced expression of core3 results in reduced migration on various components of the extracellular matrix.
FIGURE 5.
Core3 synthase inhibits cell migration of PC3 (A) and LNCaP (B) cells. Mock-transfected PC3 (a and c), PC3-core3 (b and d), mock-transfected LNCaP (a and c), and LNCaP-core3 (b and d) cells were seeded in a chamber containing different extracellular matrix components in the bottom layer of the Transwell membrane. Cells migrating to the bottom part of the Transwell membrane were fixed, stained with 0.5% crystal violet, and counted. A representative figure of three independent experiments is shown in the upper panels. Migration through type I collagen matrix (a and b are enlarged figures of c and d, respectively) is shown. Bar, 0.1 mm. The number of cells was counted in four different fields and tabulated in the bottom panel. Error bars represent S.D. of the mean. *, p < 0.05; **, p < 0.01 compared with mock transfectants.
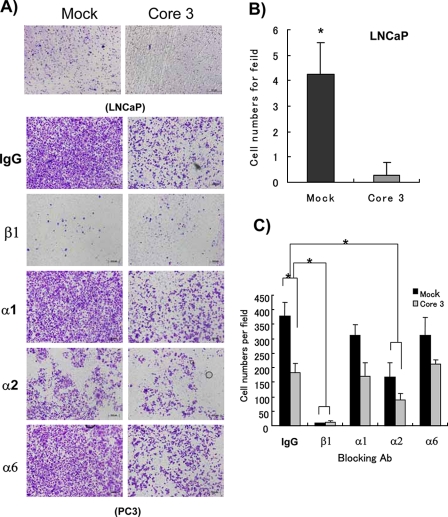
To determine how expression of core3 O-glycans influences invasion by prostate cancer cells, mock- and core3-transfected PC3 and LNCaP cells were seeded on the Transwell membrane coated with Matrigel in the upper chamber of a Boyden invasion chamber, and cells reaching the bottom layer were counted 24 h later. First we found that PC3- and LNCaP-core3 cells invade much less than mock-transfected cells (Fig. 6, A and B). Because the integrin family is a well known heteromeric receptor for extracellular matrix reported to have biological functions in protection against apoptosis (37) and malignant transformation (38, 39), we used functional blocking antibodies for several integrins to determine the major target integrin for core3 synthase. Invasiveness of PC3 cells was completely inhibited by treatment with anti-β1 integrin-blocking 4B4 antibody (34). Additionally a significant decrease of invasion was shown when cells were treated with α2-blocking antibody compared with α1- or α6-blocking antibody (Fig. 6C). These results show that decreased invasion by PC3-core3 cells is likely due to decreases in α2β1 integrin-mediated adhesion and migration.
FIGURE 6.
Core3 synthase inhibits PC3 and LNCaP cell invasion. A, mock-transfected and core3-expressing LNCaP cells (5 × 105 cells) and PC3 cells (2.8 × 105 cells) were seeded in the upper part of the transmembrane. The upper part of the transmembrane was precoated with Matrigel. Before seeding, PC3-mock and PC3-core3 transfectant cells were incubated with either control mouse IgG (10 μg/ml) or each functional blocking antibody (Ab) (10 μg/ml) against integrin for 10 min at 37 °C in a CO2 incubator. Cells reaching the bottom part of the filter were stained and counted. Invasive activity of mock and core3 transfectants was inhibited mostly by β1 integrin functional blocking antibody. B and C, results obtained by calculating four different fields are shown and repeated two times. Error bars represent S.D. of the mean. *, p < 0.01; scale bar = 0.2 mm.
To expand the above studies further, PC3 cells were transiently transfected with core3 synthase, and cell migration was measured. The results showed clearly that PC3 cells migrated much more slowly after transfection with core3 synthase compared with mock-transfected PC3 cells (Fig. 7A). Almost identical results were obtained with LNCaP cells (Fig. 7B). In addition, PC3-core3 and LNCaP-core3 cells shared all characteristics, although two cell lines were independently obtained. These results indicate that the results obtained after core3 synthase transfection are not due to clonal variation of the transfected cells and that core3 synthase is responsible for decreased migration and invasion and impaired tumor formation.
FIGURE 7.
Transient transfection of core3 synthase attenuates cell migration. PC3 (A) and LNCaP (B) cells were transiently transfected with core3 synthase or empty vector. Three days after the transfection, 5 × 104 PC3 or 2 × 105 LNCaP cells were seeded on the upper side of the membrane coated with or without collagen I in the same way as in Fig. 6. The number of cells at the bottom side of the Transwell membrane was counted after 6 h (for PC3) or 17 h (for LNCaP) of incubation. The results obtained by counting the migrated cell number are tabulated on the right side. Scale bar, 0.1 mm in PC3 and 0.2 mm in LNCaP.
Forced Expression of Core3 Synthase Decreases Prostate Cancer Formation and Lymph Node Metastasis
To determine how core3 expression influences tumor formation, PC3-mock and PC3-core3 cells were orthotopically inoculated into the prostate of nude mice as described previously (35). The prostate and the surrounding lymph nodes were isolated 8 weeks later and analyzed for tumor formation. Mice receiving mock-transfected cells showed larger prostate tumors, and the surrounding lymph nodes contained metastatic tumors (Fig. 8A). By contrast, mice inoculated with PC3-core3 cells showed much smaller prostate tumors, and the tumor metastasis to the surrounding lymph nodes was not noticed. These results indicate that core3 synthase expression results in reduction of both primary tumor and metastasis to the lymph node. Similar results were obtained on subcutaneously inoculated LNCaP-core3 cells. Compared with robust subcutaneous tumor formation by mock-transfected LNCaP cells, LNCaP-core3 cells barely formed subcutaneous tumor (Fig. 8B). These results demonstrate that expression of core3 O-glycans suppresses tumor formation and metastasis.
FIGURE 8.
Core3 synthase suppresses tumor formation and metastasis. Mock-transfected and core3-expressing PC3 cells (2 × 106 cells) and LNCaP cells (5 × 107 cells) were inoculated into nude mice. A, PC3-core3 and mock-transfected PC3 cells were orthotopically inoculated into the prostate of nude mice, and animals were sacrificed 8 weeks later. B, LNCaP-core3 and mock-transfected cells were subcutaneously injected with Matrigel (50:50, v/v), and the weight of tumors was measured after 3 months. Mock-transfected PC3 cells produced a large prostate tumor (arrow), whereas tumors produced by core3-expressing PC3 were not observed in the prostate (arrow). Seminal vesicles are also seen (arrowhead). Four representative prostate and lymph nodes with tumors are shown (left panel). Normal prostate represents prostates of the nude mice without any treatment. Wet weight of eight prostate and lymph nodes is shown (right panel). PC3 cells expressing core3 O-glycans formed almost no prostate tumors and no metastasis to the draining lymph node. In B, representative tumors are shown in the upper panel, and wet weight of six to seven tumors is shown in the lower panel. Core3 synthase-transfected LNCaP cells showed almost no tumor. Error bars represent S.D. of the mean. *, p < 0.05 compared with mock-transfectants using Mann-Whitney U test. Bar, 0.5 cm.
Maturation and Cell Surface Expression of β1 Integrin Is Attenuated in PC3-core3 Cells
Among different integrin subunits, we examined glycosylation status of β1 integrin because this protein has been shown to have mucin-type O-glycans (40). The N-acetylglucosaminyl terminus of core3 O-glycan, GlcNAcβ1→3GalNAcα1→Thr/Ser, can be detected by GS-II (41). Western blot analysis of β1 integrin immunoprecipitated from PC3- and LNCaP-core3 cells showed a strong band detected by GS-II. By contrast, β1 integrin from mock-transfected cells did not react with GS-II, although β1 integrin levels did not differ between the two cell types (Fig. 9A). α2 integrin of LNCaP cells but not PC3 cells apparently acquired core3 O-glycan, whereas α2 integrin from PC3 cells barely acquired it. In support of this finding, PNA binding to β1 integrin was slightly decreased in PC3-core3 cells compared with the mock-transfected PC3 cells. By contrast, PNA binding to β1 integrin and α2 integrin was significantly decreased in LNCaP-core3 cells (Fig. 9B). The results confirmed that β1 integrin from PC3-core3 cells and β1 integrin and α2 integrin from LNCaP-core3 cells express core3 O-glycans. The results are consistent with the conclusions obtained by mass spectrometric analysis (Fig. 4). It is possible that A. ureafaciens sialidase treatment did not efficiently remove sialic acid from core1 O-glycans of PC3 cells yielding weak signals by PNA staining (Fig. 3A), whereas the same treatment efficiently removed sialic acid from LNCaP cells.
FIGURE 9.
α2β1 integrin acquired β-N-acetylglucosaminyl residues and surface expression of α2β1 integrin is decreased in core3-expressing cells. A, β1 integrin was immunoprecipitated (IP) with rabbit anti-β1 integrin at 4 °C overnight and blotted to a polyvinylidene difluoride membrane. The membrane was incubated with GlcNAc-specific GS-II or the same anti-β1 integrin antibody. Similar experiments were carried out after immunoprecipitation of α2 integrin. B, binding of PNA to α2 and β1 integrins in PC3 and LNCaP cells. The experiment was carried out in the same way as in A except the PNA was used instead of GS-II. C, immunoblot analysis using rabbit anti-β1 integrin antibody shows decreased levels of mature, higher molecular weight β1 integrin indicated by an arrowhead. The amount of α2 integrin and control total ERK (t-ERK) protein is equivalent in both cell types. D, mock- and core3-transfected PC3 and LNCaP cells were incubated with anti-β1, anti-α2, and anti-α6 antibody and then stained with fluorescein isothiocyanate-conjugated secondary antibody for flow cytometry analysis. PNGase, peptide-N-glycosidase.
The immunoblotting using anti-β1 integrin antibody detected two forms of β1 integrin, and core3-expressing PC3 cells expressed lower levels of β1 integrin with the higher molecular weight (Fig. 9C, arrowhead) than did the mock-transfected cells, whereas the amount of α2 integrin was equivalent in both cell types (Fig. 9C). It was reported that the higher molecular weight form represents the mature form of β1 integrin (42). Two differently glycosylated forms of β1 integrin resolved to one lower molecular weight band after N-glycanase treatment (Fig. 9C).
We then examined the cell surface expression of several integrin subunits. Interestingly surface expression of β1 integrin was significantly reduced without changing that of α2 integrin in PC3-core3 cells (Fig. 9D). For LNCaP cells, reduced surface expression of α2 and α6 integrins as well as β1 integrin was detected. These results suggest that adding the core3 O-glycan could affect the maturation and surface expression for β1 integrin for PC3 cells and β1 integrin, α2 integrin, and possibly α6 integrin for LNCaP cells.
Association of α2 and β1 Integrin Is Attenuated in PC3-core3 and LNCaP-core3 Cells
Previously it was reported that PC3 cells express primarily α2β1 integrin (23), which is consistent with our data (Fig. 9B). The assays described in Fig. 6 indicate that invasion of PC3 cells is largely dependent on α2β1 integrin. Because the above results suggest that α2β1 integrin may not function well in PC3-core3 and LNCaP-core3 cells, we analyzed the amount of β1 integrin complexed with α2 integrin in those cells and compared them with mock-transfected cells. We thus immunoprecipitated β1 integrin from two cell types using rabbit anti-β1 integrin and incubated immunoprecipitates sequentially with anti-α2 integrin and anti-β1 integrin antibodies. As a complimentary experiment, α2 integrin was immunoprecipitated followed by blotting with anti-β1 antibody and -α2 antibody. Levels of α2 integrin co-immunoprecipitated with β1 integrin were significantly decreased in PC3 cells expressing core3 O-glycans compared with mock-transfected PC3 cells (Fig. 10A). Almost identical results were obtained for LNCaP cells (Fig. 10B). These results suggest that expression of core3 O-glycans in α2β1 integrin led to decreased heterodimerization. In this experiment, β1 integrin was immunoprecipitated by rabbit anti-β1 integrin antibody and then immunoblotted with the monoclonal anti-β1 integrin antibody or anti-α2 integrin antibody. Consistent with the previous report (42), the polyclonal anti-β1 antibody can detect two forms of β1 integrin by immunoblotting (Fig. 9C), whereas the same antibody and the monoclonal antibody immunoprecipitates mostly a major β1 integrin with lower molecular weight (Fig. 10) as shown previously (43).
FIGURE 10.
α2β1 integrin association in core3-expressing PC3 (A) and LNCaP (B) cells is impaired. α2 integrin was immunoprecipitated (IP) with rabbit anti-α2 integrin antibody, and the blot was incubated with mouse monoclonal anti-β1 integrin antibody. The membrane was then stripped by incubation with 1 n NaOH for 1–2 min and then incubated with anti-α2 integrin antibody. In parallel, β1 integrin was first immunoprecipitated by polyclonal anti-β1 integrin antibody, and the immunoprecipitates were sequentially incubated with anti-α2 antibody and anti-β1 antibody. The experiments were repeated three times, and a representative result is shown. Heterodimerization rate (α2/β1 or β1/α2) was estimated by scanning the gel and is tabulated in the lower panel.
Reduction in Integrin-mediated Activation of PC3-core3 Cells
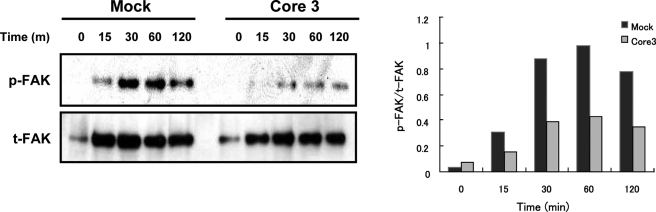
The above results suggested that PC3 and LNCaP cells expressing core3 synthase express lower levels of functional α2β1 integrin than do mock transfected PC3 cells. To support this conclusion, we examined the downstream signaling mediated by integrin. Integrin-mediated cell adhesion activates various protein tyrosine kinases, such as FAK, which is directly activated by integrin signaling and leads to association of integrins with the cytoskeleton (38). In PC3-core3 cells, the levels of phosphorylated FAK on collagen-coated plates were significantly decreased relative to mock-transfected cells, although both cell types expressed similar levels of FAK (Fig. 11). These results suggest that expression of core3 O-glycan is associated with decreased levels of functional α2 and β1 integrin complexes, leading to decreased levels of activation of downstream signaling as indicated by FAK phosphorylation.
FIGURE 11.
FAK phosphorylation is impaired in PC3-core3 cells. Left panel, cells were seeded on collagen I (1 μg/ml)-coated plates and harvested at various times as indicated (m, minutes). The blot was incubated with mouse anti-FAK (Tyr(P)397) phosphospecific antibody (p-FAK). This membrane was stripped by incubation with 1 n NaOH for 1–2 min at room temperature and washed three times with TBS-T and then used for detection of total FAK (t-FAK). Total FAK amounts were detected using mouse anti-FAK antibody. Right panel, activation (phosphorylation) rate of FAK (p-FAK/t-FAK) was estimated using the Image J program.
DISCUSSION
This study shows that forced expression of core3 oligosaccharides in the PC3 prostate cancer cell line significantly reduces both primary prostate tumor formation and tumor metastasis to the draining lymph nodes. In the previous work on core3 O-glycans, tumors were directly formed in the lung without forming a primary tumor (16). This is different from metastasis where metastatic tumor is formed by cells that migrated from the primary tumor (17, 44). By contrast, it has been shown that lung tumor formation after intravenous injection is equivalent to the lung tumor colonization seen in many studies (for example, see Refs. 45 and 46). In our present study, we inoculated prostate cancer cells in the prostate and assayed the formation of tumor in the prostate (primary tumor) and in the draining lymph node (metastasis). As a variation of this, we also measured the tumor formation of LNCaP cells after subcutaneous injection. In this case, the formed tumor is mostly primary tumor, but some of the cells invaded into surrounding tissues. Our assay therefore measured the tumor formation in the primary site and metastatic sites. Thus, we can definitely say that the increased core3 structure can attenuate the prostate tumor formation as well as metastasis. We also show that PC3 and LNCaP cells expressing increased amounts of core3 oligosaccharides display incomplete maturation of β1 integrin, which contains mucin-type O-glycans, resulting in formation of fewer α2β1 integrin complexes and possibly α6β1 integrin complexes. Reduced amounts of α2β1 integrin complex lead to impaired cell migration by PC3 and LNCaP cells expressing core3 O-glycans. Reduced cell migration then results in reduced tumor cell invasion, tumor formation, and tumor metastasis. Our work is the first report that modulation of mucin-type O-glycans in α2β1 integrin decreases tumorigenicity and tumor metastasis.
Recently it was reported that mouse embryonic fibroblasts from β1,6-N-acetylglucosaminyltransferase-V null mice exhibit increased α5β1 integrin activation due to elevated protein kinase C signaling, which increases cell surface expression of α5β1 integrins. These results suggest that decreases in N-acetylglucosamine in N-glycans increase cell surface expression of α5β1 integrin (25). The 1,6-GlcNAc-branched N-glycans on the α3 subunit of integrin was reduced by β1,4-N-acetylglucosaminyltransferase-III, and this leads to reduced migration (47). Moreover it was discovered that N-glycans of the β-propeller domain of the integrin α5 subunit is essential for α5β1 heterodimerization. At the same time, loss of the same N-glycan resulted in the decreased cell surface expression of α5β1 (48). These reports are consistent with our findings that incomplete N-glycosylation leads to the reduced cell surface expression of α2β1.
In our study, the α2, β1 integrin of LNCaP and β1 integrin of PC3 cells increased core3 structure with decreased core1 structure, and these cells showed decreased cell surface expression of α2β1 integrin (Fig. 9, A, B, and D). Although it is not clear how these different glycosylations modulate the cell surface expression, there is a possibility that increased core3 structure hindered Ν-glycosylation of α2β1 integrin resulting in reduced cell surface expression.
We also showed that core3 synthase expression affects β1 integrin glycosylation and that a greater amount of β1 integrin remains incompletely N-glycosylated in PC3-core3 and LNCaP-core3 cells compared with the mock-transfected cells (Fig. 9C). Thus it is likely that addition of core3 O-glycan may hinder glycosyltransferases that form complex-type N-glycans due to the hydrophobic nature of neighboring O-glycans. Incompletely N-glycosylated β1 integrin apparently associates less efficiently with α2 integrin than does mature β1 integrin. Although it is not formally proven yet, it is likely that the mature form of β1 integrin contains more N-glycan chains due to an increased number of N-glycans per molecule because N-glycanase treatment converted two forms to one form with a lower molecular weight. Alternatively N-glycans are not fully matured when core3 O-glycans are attached. It is tempting to speculate that β1 integrin containing immature N-glycans may have a different conformation than the completely N-glycosylated β1 integrin, resulting in the decreased α2β1 complex conformation. The decrease in α2β1 integrin complex formation led to decreased FAK phosphorylation, resulting in reduced lamellipodia formation. Conversely forced expression of C2GnT-M resulted in decreases in paxillin but not FAK phosphorylation (21). Because paxillin phosphorylation likely occurs downstream of FAK phosphorylation in integrin signaling (38), C2GnT-M may affect other molecules in addition to integrin, thereby modulating paxillin phosphorylation.
Core3 synthase is expressed in normal intestine and colon, but its activity is down-regulated in cancer cells (13, 14). It was also shown that forced expression of core3 synthase in fibrosarcoma cells reduces lung tumor focus formation when cells are injected intravenously (16). These results are consistent with our findings that core3 synthase expression suppresses tumor formation. Our studies extend previous studies by showing that PC3 and LNCaP cells expressing core3 O-glycans form much fewer metastases and subcutaneous tumors, respectively, than mock-transfected cells. Moreover our studies show that PC3 and LNCaP cells expressing core3 synthase exhibit impaired β1 integrin maturation, resulting in impaired cytoskeletal organization as assayed by stress fiber formation, decreased FAK phosphorylation, and decreased cell migration, leading to decreased tumor metastasis spread from primary tumors. These results are striking because only a small increase of core3 O-glycans in PC3 cells resulted in the dramatic change in these phenotypes. The difference in the phenotypes of these cells was as dramatic as LNCaP cells that significantly increased core3 O-glycans.
More recently, formation of colonic carcinoma after treatment with azoxymethane and dextran sodium sulfate was shown to be much greater in core3 synthase-deficient mice than in wild-type mice (36). This was associated with reduction in MUC2 protein and increased permeability of the intestinal barrier. Our findings suggest that one of the tumor-suppressing functions of core3 O-glycan is to attenuate the integrin-extracellular matrix interaction, making cells prone to growth control and rendering them less motile and invasive. These findings suggest that forced expression of core3 synthase suppresses tumor formation. Further studies should address development of therapies via ectopic expression of core3 synthase.
Acknowledgments
We thank Dr. Kiyohiko Angata and the rest of the staff members of the laboratory of Drs. Minoru and Michiko Fukuda for useful discussion, Dr. Elise Lamar for critical reading of the manuscript, and Aleli Morse for organizing the manuscript.
This work was supported by National Institutes of Health Grants CA33000 and in part by CA48737 (to M. F.) and PO1CA71932 (to M. F. and M. N. F.) and by grants from Taiwan National Science Council (to K. H. K.).
- core3 synthase or β3GnT-6
- β3-N-acetylglucosaminyltransferase-6
- C2GnT
- core2β1,6-N-acetylglucosaminyltransferase
- FAK
- focal adhesion kinase
- MS
- mass spectrometry
- RT
- reverse transcription
- PNA
- peanut agglutinin
- MALDI
- matrix-assisted laser desorption ionization
- ERK
- extracellular signal-regulated kinase
- GS-II
- Griffonia simplifolia lectin II
- PBS
- phosphate-buffered saline
- GalNAcitol
- N-acetylgalactosaminitol.
REFERENCES
- 1.Hakomori S. ( 2002) Proc. Natl. Acad. Sci. U. S. A. 99, 10231– 10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukushima K., Hirota M., Terasaki P. I., Wakisaka A., Togashi H., Chia D., Suyama N., Fukushi Y., Nudelman E., Hakomori S. ( 1984) Cancer Res. 44, 5279– 5285 [PubMed] [Google Scholar]
- 3.Nakamori S., Kameyama M., Imaoka S., Furukawa H., Ishikawa O., Sasaki Y., Kabuto T., Iwanaga T., Matsushita Y., Irimura T. ( 1993) Cancer Res. 53, 3632– 3637 [PubMed] [Google Scholar]
- 4.Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. ( 1984) J. Biol. Chem. 259, 10834– 10840 [PubMed] [Google Scholar]
- 5.Pierce M., Arango J. ( 1986) J. Biol. Chem. 261, 10772– 10777 [PubMed] [Google Scholar]
- 6.Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. ( 1987) Science 236, 582– 585 [DOI] [PubMed] [Google Scholar]
- 7.Hubbard S. C. ( 1987) J. Biol. Chem. 262, 16403– 16411 [PubMed] [Google Scholar]
- 8.Schachter H., Brockhausen I. ( 1992) The Biosynthesis of Serine (Threonine)-N-acetylgalactosamine-linked Carbohydrate Moieties, pp. 263– 332, Marcel Dekker, New York [Google Scholar]
- 9.Piller F., Piller V., Fox R. I., Fukuda M. ( 1988) J. Biol. Chem. 263, 15146– 15150 [PubMed] [Google Scholar]
- 10.Fukuda M. ( 1996) Cancer Res. 56, 2237– 2244 [PubMed] [Google Scholar]
- 11.Machida E., Nakayama J., Amano J., Fukuda M. ( 2001) Cancer Res. 61, 2226– 2231 [PubMed] [Google Scholar]
- 12.Dalziel M., Whitehouse C., McFarlane I., Brockhausen I., Gschmeissner S., Schwientek T., Clausen H., Burchell J. M., Taylor-Papadimitriou J. ( 2001) J. Biol. Chem. 276, 11007– 11015 [DOI] [PubMed] [Google Scholar]
- 13.Vavasseur F., Dole K., Yang J., Matta K. L., Myerscough N., Corfield A., Paraskeva C., Brockhausen I. ( 1994) Eur. J. Biochem. 222, 415– 424 [DOI] [PubMed] [Google Scholar]
- 14.Vavasseur F., Yang J. M., Dole K., Paulsen H., Brockhausen I. ( 1995) Glycobiology 5, 351– 357 [DOI] [PubMed] [Google Scholar]
- 15.Iwai T., Inaba N., Naundorf A., Zhang Y., Gotoh M., Iwasaki H., Kudo T., Togayachi A., Ishizuka Y., Nakanishi H., Narimatsu H. ( 2002) J. Biol. Chem. 277, 12802– 12809 [DOI] [PubMed] [Google Scholar]
- 16.Iwai T., Kudo T., Kawamoto R., Kubota T., Togayachi A., Hiruma T., Okada T., Kawamoto T., Morozumi K., Narimatsu H. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 4572– 4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsson L., Forchhammer J. ( 1984) Proc. Natl. Acad. Sci. U. S. A. 81, 3389– 3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta G. P., Perk J., Acharyya S., de Candia P., Mittal V., Todorova-Manova K., Gerald W. L., Brogi E., Benezra R., Massagué J. ( 2007) Proc. Natl. Acad. Sci. U. S. A. 104, 19506– 19511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh J. C., Ong E., Fukuda M. ( 1999) J. Biol. Chem. 274, 3215– 3221 [DOI] [PubMed] [Google Scholar]
- 20.Schwientek T., Nomoto M., Levery S. B., Merkx G., van Kessel A. G., Bennett E. P., Hollingsworth M. A., Clausen H. ( 1999) J. Biol. Chem. 274, 4504– 4512 [DOI] [PubMed] [Google Scholar]
- 21.Huang M. C., Chen H. Y., Huang H. C., Huang J., Liang J. T., Shen T. L., Lin N. Y., Ho C. C., Cho I. M., Hsu S. M. ( 2006) Oncogene 25, 3267– 3276 [DOI] [PubMed] [Google Scholar]
- 22.Emsley J., Knight C. G., Farndale R. W., Barnes M. J., Liddington R. C. ( 2000) Cell 101, 47– 56 [DOI] [PubMed] [Google Scholar]
- 23.Bonaccorsi L., Carloni V., Muratori M., Salvadori A., Giannini A., Carini M., Serio M., Forti G., Baldi E. ( 2000) Endocrinology 141, 3172– 3182 [DOI] [PubMed] [Google Scholar]
- 24.Isaji T., Gu J., Nishiuchi R., Zhao Y., Takahashi M., Miyoshi E., Honke K., Sekiguchi K., Taniguchi N. ( 2004) J. Biol. Chem. 279, 19747– 19754 [DOI] [PubMed] [Google Scholar]
- 25.Guo H. B., Lee I., Bryan B. T., Pierce M. ( 2005) J. Biol. Chem. 280, 8332– 8342 [DOI] [PubMed] [Google Scholar]
- 26.Mitoma J., Petryniak B., Hiraoka N., Yeh J. C., Lowe J. B., Fukuda M. ( 2003) J. Biol. Chem. 278, 9953– 9961 [DOI] [PubMed] [Google Scholar]
- 27.Lotan R., Skutelsky E., Danon D., Sharon N. ( 1975) J. Biol. Chem. 250, 8518– 8523 [PubMed] [Google Scholar]
- 28.Sugihara K., Sugiyama D., Byrne J., Wolf D. P., Lowitz K. P., Kobayashi Y., Kabir-Salmani M., Nadano D., Aoki D., Nozawa S., Nakayama J., Mustelin T., Ruoslahti E., Yamaguchi N., Fukuda M. N. ( 2007) Proc. Natl. Acad. Sci. U. S. A. 104, 3799– 3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierhuizen M. F., Fukuda M. ( 1992) Proc. Natl. Acad. Sci. U. S. A. 89, 9326– 9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwientek T., Yeh J. C., Levery S. B., Keck B., Merkx G., van Kessel A. G., Fukuda M., Clausen H. ( 2000) J. Biol. Chem. 275, 11106– 11113 [DOI] [PubMed] [Google Scholar]
- 31.Ju T., Cummings R. D. ( 2002) Proc. Natl. Acad. Sci. U. S. A. 99, 16613– 16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S. Y., Wu S. W., Khoo K. H. ( 2006) Glycoconj. J. 23, 355– 369 [DOI] [PubMed] [Google Scholar]
- 33.Mitoma J., Bao X., Petryanik B., Schaerli P., Gauguet J. M., Yu S. Y., Kawashima H., Saito H., Ohtsubo K., Marth J. D., Khoo K. H., von Andrian U. H., Lowe J. B., Fukuda M. ( 2007) Nat. Immunol. 8, 409– 418 [DOI] [PubMed] [Google Scholar]
- 34.Takada Y., Puzon W. ( 1993) J. Biol. Chem. 268, 17597– 17601 [PubMed] [Google Scholar]
- 35.Inaba Y., Ohyama C., Kato T., Satoh M., Saito H., Hagisawa S., Takahashi T., Endoh M., Fukuda M. N., Arai Y., Fukuda M. ( 2003) Int. J. Cancer 107, 949– 957 [DOI] [PubMed] [Google Scholar]
- 36.An G., Wei B., Xia B., McDaniel J. M., Ju T., Cummings R. D., Braun J., Xia L. ( 2007) J. Exp. Med. 204, 1417– 1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardone M. H., Salvesen G. S., Widmann C., Johnson G., Frisch S. M. ( 1997) Cell 90, 315– 323 [DOI] [PubMed] [Google Scholar]
- 38.Giancotti F. G., Ruoslahti E. ( 1999) Science 285, 1028– 1032 [DOI] [PubMed] [Google Scholar]
- 39.Hynes R. O. ( 2002) Cell 110, 673– 687 [DOI] [PubMed] [Google Scholar]
- 40.Clément M., Rocher J., Loirand G., Le Pendu J. ( 2004) J. Cell Sci. 117, 5059– 5069 [DOI] [PubMed] [Google Scholar]
- 41.Lyer P. N., Wilkinson K. D., Goldstein L. J. ( 1976) Arch. Biochem. Biophys. 177, 330– 333 [DOI] [PubMed] [Google Scholar]
- 42.Bellis S. L., Newman E., Friedman E. A. ( 1999) J. Cell. Physiol. 181, 33– 44 [DOI] [PubMed] [Google Scholar]
- 43.Dulabon L., Olson E. C., Taglienti M. G., Eisenhuth S., McGrath B., Walsh C. A., Kreidberg J. A., Anton E. S. ( 2000) Neuron 27, 33– 44 [DOI] [PubMed] [Google Scholar]
- 44.Chen S., Kawashima H., Lowe J. B., Lanier L. L., Fukuda M. ( 2005) J. Exp. Med. 202, 1679– 1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meromsky L., Lotan R., Raz A. ( 1986) Cancer Res. 46, 5270– 5275 [PubMed] [Google Scholar]
- 46.Hatakeyama S., Sugihara K., Nakayama J., Akama T. O., Wong S. M., Kawashima H., Zhang J., Smith D. F., Ohyama C., Fukuda M., Fukuda M. N. ( 2009) Proc. Natl. Acad. Sci. U. S. A. 106, 3095– 3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y., Nakagawa T., Itoh S., Inamori K., Isaji T., Kariya Y., Kondo A., Miyoshi E., Miyazaki K., Kawasaki N., Taniguchi N., Gu J. ( 2006) J. Biol. Chem. 281, 32122– 32130 [DOI] [PubMed] [Google Scholar]
- 48.Isaji T., Sato Y., Zhao Y., Miyoshi E., Wada Y., Taniguchi N., Gu J. ( 2006) J. Biol. Chem. 281, 33258– 33267 [DOI] [PubMed] [Google Scholar]