Abstract
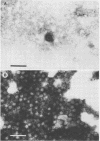
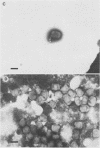
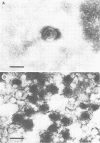
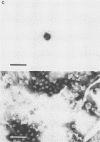
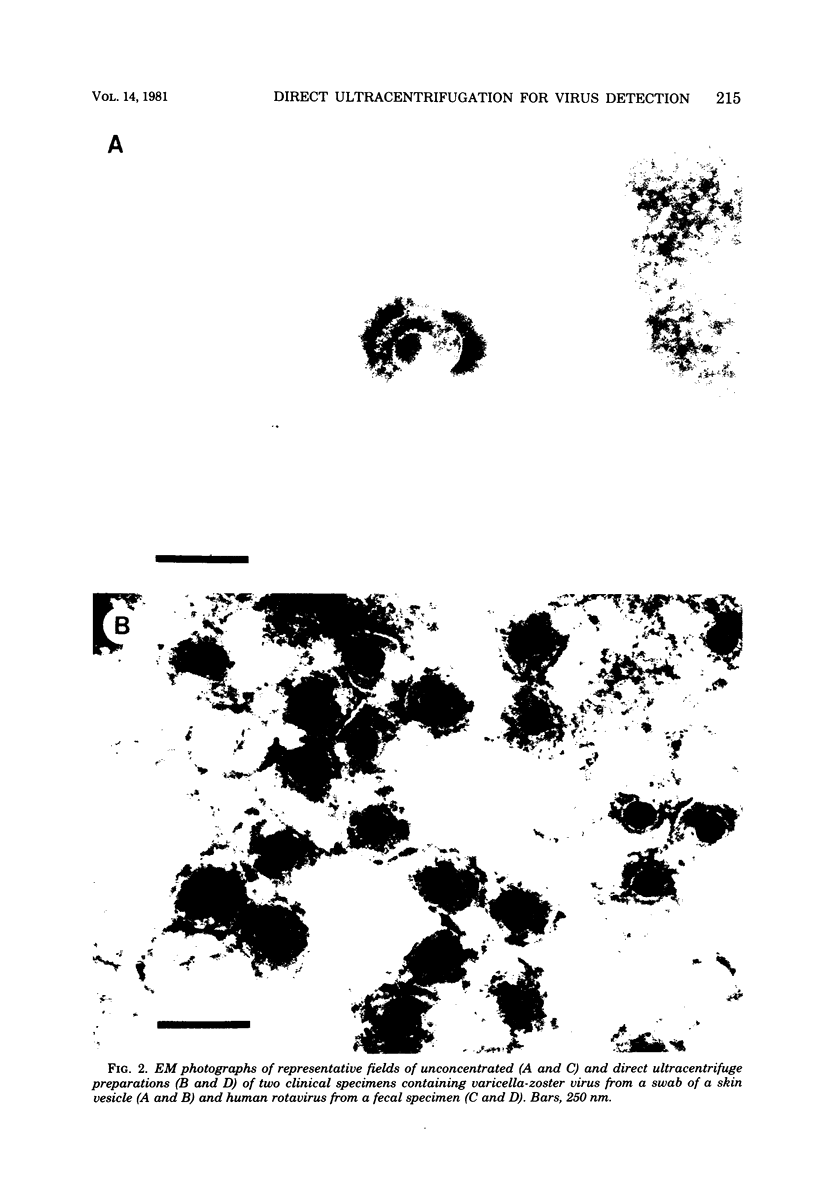
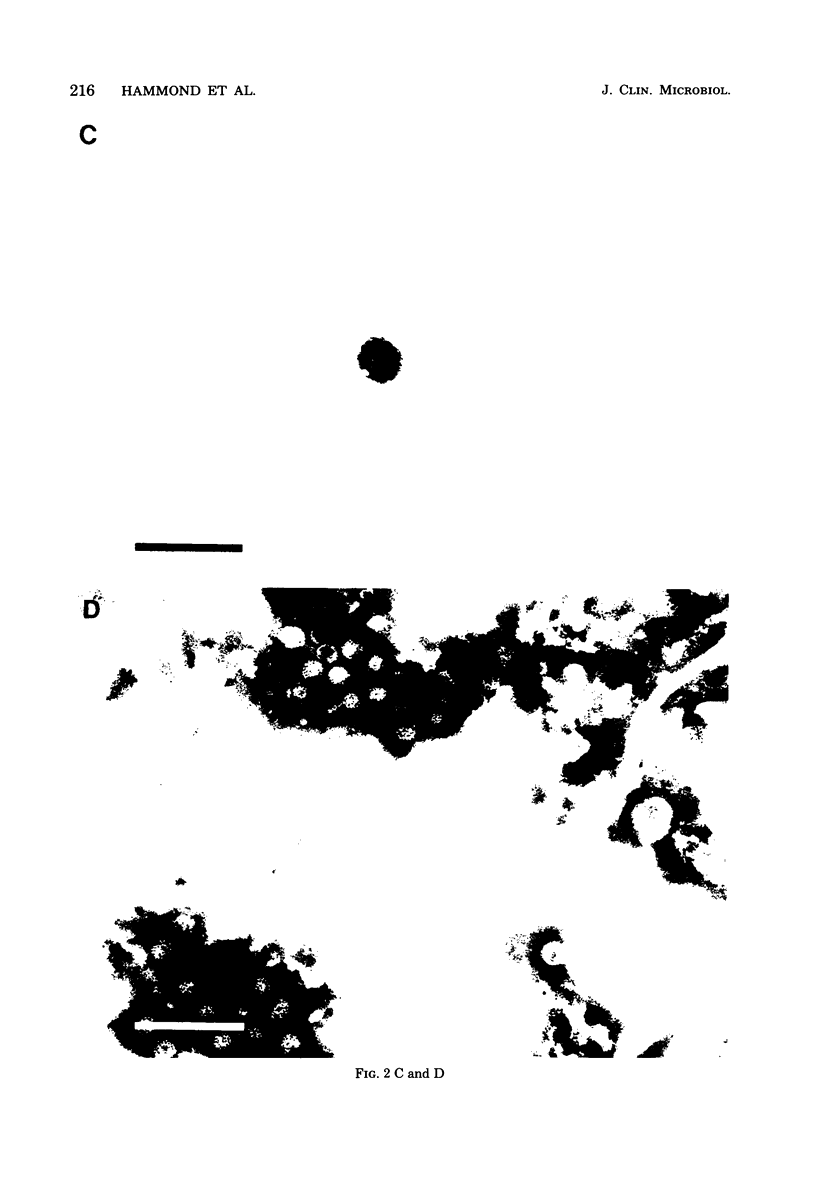
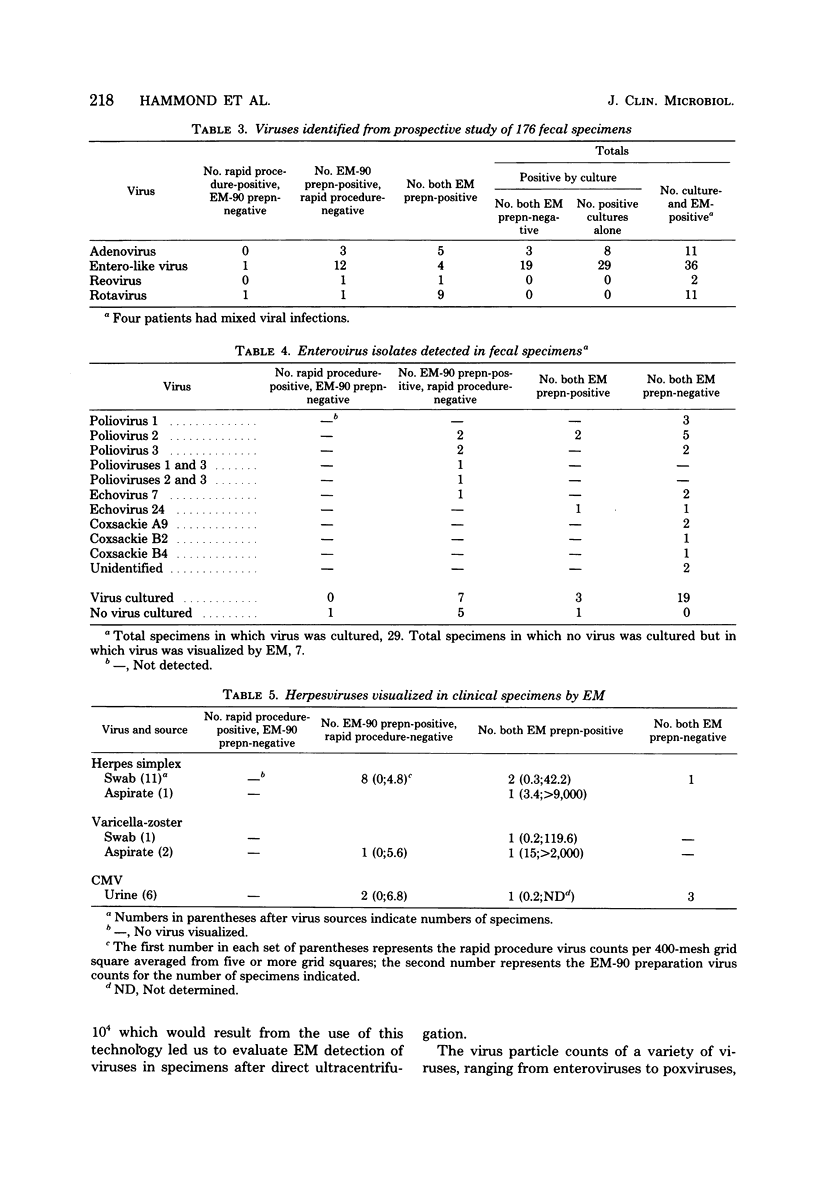
We have adapted the Beckman Airfuge air turbine ultracentrifuge and the new EM-90 particle-counting rotor to improve detection by electron microscopy of viruses in clinical specimens. Samples were clarified by centrifugation, pelleted in the EM-90 rotor directly to Formvar-coated copper grids, and strained with 1.5% sodium phosphotungstate. Virus counts and endpoint titrations of serial dilutions of partially purified preparations of poliovirus, SA11 rotavirus, herpes simplex virus, and vaccinia virus showed an increase of ca. 1.5 log10 to 3.0 log10 over the virus titers of unconcentrated preparations of the same material. An increased yield of 14% more positive specimens for rotavirus was obtained after preparation of clinical samples by direct ultracentrifugation versus a method without virus concentration (82 versus 72). A prospective study showed that detection of adenoviruses, herpesviruses, and enteroviruses increased when specimens were prepared by direct ultracentrifugation. Direct ultracentrifugation with the EM-90 rotor in the Airfuge ultracentrifuge is a rapid concentration method which enhances the rate and yield of virus detection from clinical specimens by electron microscopy and is easily adaptable to a diagnostic virology laboratory.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D. Editorial: Visualization of fecal viruses. N Engl J Med. 1975 Jun 26;292(26):1403–1405. doi: 10.1056/NEJM197506262922614. [DOI] [PubMed] [Google Scholar]
- Almeida J. D. Practical aspects of diagnostic electron microscopy. Yale J Biol Med. 1980 Jan-Feb;53(1):5–18. [PMC free article] [PubMed] [Google Scholar]
- Blaskovic P. J., Kuderewko O., McLaughlin B., Yong D. C., Ball F. R. Rapid diagnosis by electron microscopy of nonbacterial gastroenteritis in children. Can Med Assoc J. 1980 Jan 26;122(2):150–152. [PMC free article] [PubMed] [Google Scholar]
- Doane F. W. Virus morphology as an aid for rapid diagnosis. Yale J Biol Med. 1980 Jan-Feb;53(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Bryden A. S., Davies H. Diagnostic electron microscopy of faeces. I. The viral flora of the faeces as seen by electron microscopy. J Clin Pathol. 1974 Aug;27(8):603–608. doi: 10.1136/jcp.27.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne L., Marsolais G., Marois P., Assaf R. Diagnosis of rotavirus, adenovirus, and herpesvirus infections by immune electron microscopy using a serum-in-agar diffusion method. Can J Microbiol. 1980 Feb;26(2):261–264. doi: 10.1139/m80-041. [DOI] [PubMed] [Google Scholar]
- Lee F. K., Nahmias A. J., Stagno S. Rapid diagnosis of cytomegalovirus infection in infants by electron microscopy. N Engl J Med. 1978 Dec 7;299(23):1266–1270. doi: 10.1056/NEJM197812072992302. [DOI] [PubMed] [Google Scholar]
- Narang H. K., Codd A. A. Preponderance of rotavirus in clumped form in patients with acute gastroenteritis. Lancet. 1980 May 31;1(8179):1192–1193. doi: 10.1016/s0140-6736(80)91650-5. [DOI] [PubMed] [Google Scholar]
- Pavilanis V., Joncas J. H., Berthiaume L., Williams R., Beaudry P. Diagnosis of viral respiratory infections by electron microscopy. Lancet. 1969 May 10;1(7602):956–959. doi: 10.1016/s0140-6736(69)91858-3. [DOI] [PubMed] [Google Scholar]
- Rice S. J., Phillips A. D. Rapid preparation of faecal specimens for detection of viral particles by electron microscopy. Med Lab Sci. 1980 Oct;37(4):371–372. [PubMed] [Google Scholar]