Abstract
Preeclampsia is a major pregnancy-specific disorder affecting 5–7% of pregnancies worldwide. Although hypoxia caused by incomplete trophoblast invasion and impaired spiral arterial remodeling is thought to be a major cause of preeclampsia, how hypoxia affects placental development remains uncertain. GCM1 (glial cells missing homolog 1) is a transcription factor critical for placental development. In preeclampsia, GCM1 and its target genes syncytin 1 and placental growth factor, important for syncytiotrophoblast formation and placental vasculogenesis, are all decreased. Here we present evidence that GCM1 is a major target of hypoxia associated with preeclampsia. We show that hypoxia triggers GCM1 degradation by suppressing the phosphatidylinositol 3-kinase-Akt signaling pathway, leading to GSK-3β activation. Activated GSK-3β phosphorylates GCM1 on Ser322, which in turn recruits the F-box protein FBW2, leading to GCM1 ubiquitination and degradation. Importantly, the GSK-3β inhibitor LiCl prevented hypoxia-induced GCM1 degradation. Our study identifies a molecular basis for the disrupted GCM1 transcription network in preeclampsia and provides a potential avenue for therapeutic intervention.
Introduction
Trophoblast invasion, fetoplacental vascular development, and maternal vascular remodeling are key events for the formation of the hemochorial placenta in humans. Human placental trophoblasts make direct contact with maternal blood to mediate efficient gas and nutrient exchange between mother and fetus. It is evident that failure in the aforementioned key events will compromise placental function and have a significant impact on the health of both fetus and mother. Preeclampsia is a major pregnancy-specific disorder affecting 5–7% of pregnancies worldwide. The clinical features of preeclampsia include hypertension, proteinuria, and edema. Although the etiologic factors of preeclampsia are currently unknown, shallow trophoblast invasion and poor maternal vascular remodeling have been reported in preeclamptic placentas. It is thought that these defects impair the development of the fetal-maternal vasculature and result in placental ischemia and hypoxia, which contribute to the pathogenesis of preeclampsia (1–3). Consistent with this model, expression of the antiangiogenic protein soluble Flt-1 (Fms-like tyrosine kinase-1) (sFlt-1)3 is elevated, whereas expression of the proangiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PGF) is decreased in preeclampsia (4, 5). Systematic surveys of pregnant women have further revealed that the ratio between the circulating levels of sFlt-1 and PGF increases significantly prior to the onset of preeclampsia (6, 7). Therefore, an imbalance between pro- and antiangiogenic factors may be a contributing factor in preeclampsia pathogenesis.
GCM1 (also known as GCMa) is a key transcription factor in placental development. Genetic ablation of mouse GCM1 is embryonically lethal due to failure of the formation of the labyrinth layer and the fusion of trophoblasts to syncytiotrophoblasts (8, 9). Human GCM1 positively regulates syncytin 1 and PGF gene expression, which is critical for trophoblastic fusion and placental vasculogenesis (10, 11). Interestingly, decreased expression of GCM1 as well as its target genes, syncytin 1 and PGF, have been observed in preeclampsia and in placental cells under hypoxia (4, 11–15). Since hypoxia caused by incomplete trophoblast invasion and impaired spiral arterial remodeling is associated with preeclampsia (16, 17), we speculated that decreased GCM1 activity contributes to the pathogenesis of preeclampsia.
To better understand the role of GCM1 in the pathogenesis of preeclampsia, we have investigated the molecular mechanism by which hypoxia decreases GCM1 expression. We show here that the PI3K-Akt pathway is inactivated under hypoxia, which results in activation of GSK-3β in placental cells. Activated GSK-3β phosphorylates the Ser322 residue in GCM1 and promotes GCM1 ubiquitination and degradation via the SCFFBW2 E3 ligase. Importantly, hypoxia-induced GCM1 degradation was blocked by pharmacological inhibition of GSK-3β by LiCl. Moreover, we demonstrate that GCM1 positively autoregulates its own promoter activity, forming a positive feedback cascade that activates gene expression critical for placental development. Therefore, GCM1 degradation induced by hypoxia would result in profound decrease in expression of GCM1 target genes, contributing to preeclampsia. Our results delineate the signaling pathway responsible for hypoxia-induced GCM1 degradation, which could play a critical role in the pathogenesis of preeclampsia and offer potential novel therapeutic opportunity.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
The expression plasmids, pHA-GCM1, pGCM1-FLAG, pFBW2-Myc, and pHA-Ub, have been described previously (18). The expression plasmids for mutant HA-GCM1 and GCM1-FLAG with site-specific mutation were constructed by two-step PCR. The expression plasmids for Myc-tagged GSK-3β-wild type (WT), GSK-3β-constitutively active (CA), and GSK-3β-kinase-dead (KD) were kindly provided by Dr. Mien-Chie Hung (University of Texas M.D. Anderson Cancer Center). The expression plasmids, pMyc-p110*, pHA-myr-Akt, and pAkt-KA, have been described previously (19). The reporter plasmid, p(GBS)4E1BLuc, has been described previously (20).
Cell Culture and Transfection
293T, BeWo, and JAR cells were obtained from the American Type Culture Collection (Manassas, VA). Stable BeWo cells expressing HA-tagged GCM1 (BeWo31) were established as previously described (18). 293T cells were maintained at 37 °C in minimal essential medium α medium, with 10% fetal bovine serum, 100 mg/ml streptomycin, and 100 units/ml penicillin. JAR, BeWo, and BeWo31 were maintained at 37 °C in F-12K medium supplemented with 15% fetal bovine serum and the aforementioned antibiotics. Isolation and purification of villous cytotrophoblast (CTB) cells from term placentas was performed according to Kliman et al. (21). Purified CTB cells were maintained at 37 °C in Iscove's modified Dulbecco's medium containing 10% heat-inactivated fetal bovine serum and the aforementioned antibiotics. For transient expression, 293T cells were transfected with expression plasmid(s) using the calcium phosphate coprecipitation method. Transfection of siRNA into 293T cells was performed with the TransIT TKO reagent (Mirus, Madison, WI). Hypoxia was achieved by exposing cells to 1% O2, 5% CO2, and 94% N2 in a multigas incubator (Astec, Fukuoka, Japan) or to 250 μm CoCl2, an agent with effects that mimic hypoxic conditions, whereas normoxia was achieved with 21% O2, 5% CO2, and balanced N2.
Immunoprecipitation and Immunoblotting
For in vivo ubiquitination assays of GCM1, 293T cells were transfected with pGCM1-FLAG, pHA-Ub, and the indicated GSK-3β expression plasmid or siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), followed by treatment with MG132. Analysis of the ubiquitinated GCM1 was performed by immunoprecipitation using anti-FLAG monoclonal antibody (mAb; Sigma) and by immunoblotting using anti-HA mAb (Sigma) as previously described (18). Antibodies against Akt, Ser(P)308-Akt, Ser(P)473-Akt, GSK-3β, and Ser(P)9-GSK-3β were obtained from Cell Signaling (Danvers, MA). Antibody against Tyr(P)216-GSK-3β was obtained from Invitrogen. Phospho-specific antibodies to Ser322 and Ser326 were individually raised against chemically synthesized phosphopeptides, FPLTS(PO3)WPCSFSPS and FPLTSWPCS(PO3)FSPS in rabbits. The antisera were further affinity-purified against the respective phosphopeptides.
To study the effect of GSK-3β on GCM1 stability or compare the stability of wild-type GCM1 and its mutants harboring the S322A, S326A, or S322A/S326A mutation, 293T cells were transfected with the indicated expression plasmid and siRNA as described in the figure legends. At 24 h post-transfection, cells were treated with or without LiCl and cycloheximide for the indicated period of time and then harvested for immunoblotting with the indicated antibody. Band intensities were determined by densitometric analysis using an Eastman Kodak Co. DC290 zoom digital camera and Kodak 1D image analysis software. Student's t test was used to determine statistical significance for differences between means. A p value of less than 0.05 was considered significant.
Quantitative Real Time PCR
RNA was isolated using RNeasy reagents (Qiagen, Hilden, Germany) and then transcribed into cDNA using SuperScript III reagents (Invitrogen) with an oligo(dT)20 primer. Quantification of GCM1 mRNA was preformed in the LightCycler system (Roche Applied Science) using a commercial SYBR Green reaction reagent (Qiagen). The sequences of primer sets were 5′-CCTCTCATCCTCATCAGCAAT-3′ and 5′-TCGTCGTCCTTGTAATCTGGT-3′ for HA-GCM1, 5′-CTGACAAGGCTTTTTTCTTCACA-3′ and 5′-CCAGACGGGACAGGTTT-3′ for endogenous GCM1, 5′-GCCATCAATGACCCCTTCATT-3′ and 5′-TTGACGGTGCCATGGAATTT-3′ for glyceraldehyde-3-phosphate dehydrogenase, and 5′-AACTCCATCATGAAGTGTGACG-3′ and 5′-GATCCACATCTGCTGGAAGG-3′ for β-actin.
Immunohistochemistry
Placental tissue samples were collected and snap frozen in liquid nitrogen for cryosectioning. Indirect immunostaining was performed as previously described (13). In brief, the sections were blocked with protein block (Dako, High Wycombe, UK) for 20 min and then incubated with the antibody against Ser(P)9-GSK-3β (1:100 in phosphate-buffered saline) for 1 h at room temperature. After three washes in phosphate-buffered saline (5 min for each wash), sections were incubated with fluorescein isothiocyanate-conjugated secondary antibody (Dako) for 1 h at room temperature, followed by 4′,6′-diamidino-2-phenylindole staining. Immunofluorescence was examined under a Zeiss microscope (München-Hallbergmoos) equipped with a cooled charge-coupled device camera (Axiocam HRm; Zeiss) and imaging system Image-Pro Plus (Media Cybernetics, Bethesda, MD).
Pull-down Assay
For GST pull-down experiments, 293T cells were transfected with the indicated wild-type or mutant pGCM1-FLAG plasmid. At 48 h post-transfection, cells were harvested in the lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.2% Nonidet P-40, 1 mm EDTA, 0.5 mm NaF, 0.5 mm Na3VO5, 1 mm phenylmethylsulfonyl fluoride, and a protease inhibitor mixture (Sigma)). The cell lysate was incubated with GST or GST-FBW2 prebound to glutathione beads in the lysis buffer at 4 °C for 4 h before washing three times with the lysis buffer. The proteins pulled down were analyzed by immunoblotting with FLAG mAb. Nonphosphorylated and phosphorylated peptides corresponding to the amino acids 313–333 region of GCM1 were chemically synthesized and N-terminally conjugated with biotin. For peptide pull-down experiments, 293T cells were transfected with the indicated F-box protein expression plasmid. At 48 h post-transfection, cell lysates were prepared and incubated with the indicated biotinylated peptide prebound to streptavidin-conjugated magnetic beads (Polysciences, Warrington, PA) under similar conditions described above. After washing, the proteins pulled down were analyzed by immunoblotting with Myc mAb (9E10; Sigma).
RESULTS
Hypoxia Enhances GCM1 Degradation
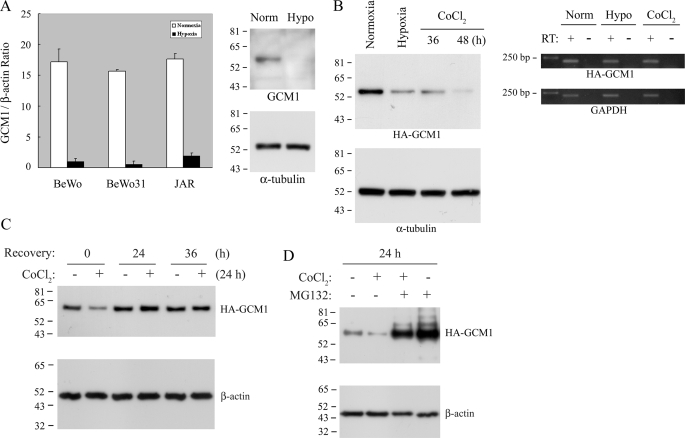
The development of preeclampsia is tightly associated with placental hypoxia and a disruption of the GCM1 transcription network. To investigate whether GCM1 is a critical target of placental hypoxia, we first investigated the effects of hypoxia on GCM1 expression in the placental cell lines, JAR and BeWo, and in a BeWo line (BeWo31) stably expressing HA-tagged GCM1 (HA-GCM1) under the cytomegalovirus promoter. The transcript levels of endogenous GCM1 in the three lines were significantly decreased under hypoxia (Fig. 1A, left), with a concomitant reduction in GCM1 protein levels (Fig. 1A, right). Interestingly, the protein level of ectopically expressed HA-GCM1 driven by the cytomegalovirus promoter in BeWo31 cells was also decreased under hypoxia or in the presence of the hypoxia mimic, CoCl2 (Fig. 1B). This observation was not due to a suppressive effect of hypoxia on the cytomegalovirus promoter, because comparable transcript levels of HA-GCM1 were detected in normoxic, hypoxic, and CoCl2-treated cells by RT-PCR analysis (Fig. 1B, right). Similar observations were also made in another HA-GCM1-expressing BeWo line, BeWo30 (data not shown). Furthermore, removal of CoCl2 restored the HA-GCM1 protein level in the CoCl2-pretreated BeWo31 cells, suggesting that the effect of hypoxia on GCM1 stability is reversible (Fig. 1C). Importantly, the hypoxia-induced loss of GCM1 could be counteracted by the proteasome inhibitor, MG132, indicating that GCM1 is degraded by the ubiquitin-proteasome system in response to hypoxia (Fig. 1D). In a parallel study of GCM1 gene expression, we demonstrated that GCM1 transactivates its own promoter activity (supplemental Fig. 1). Therefore, hypoxia would promote GCM1 degradation and further suppress GCM1 activity by impairing the positive feedback of GCM1 gene expression, leading to a reduction of GCM1 transcription (Fig. 1A).
FIGURE 1.
Hypoxia regulates GCM1 degradation. A, both GCM1 transcript and protein levels are decreased in placental cells under hypoxia. Total RNA was purified from BeWo, BeWo31, and JAR cells under normoxia (white bars) or hypoxia (black bars) for 48 h. One μg of RNA was converted into the first strand cDNA using oligo(dT)20 as primer, followed by quantitative real time PCR. Mean values and S.D. of the ratio of GCM1 to β-actin RNA copy number are shown (left). Cell protein extracts from BeWo cells under normoxia or hypoxia for 48 h were immunoblotted with GCM1 or α-tubulin antibody (right). B, hypoxia regulates GCM1 expression at the post-translational level. BeWo31 cells were cultured under hypoxia for 48 h or exposed to 250 μm CoCl2 for 36 or 48 h. Cells were then harvested for immunoblotting with HA or α-tubulin antibody (left) or for RT-PCR analysis of the transcript levels of HA-GCM1 and glyceraldehyde-3-phosphate dehydrogenase. C, the effect of hypoxia on GCM1 protein level is reversible. BeWo31 cells were mock-treated or pretreated with 250 μm CoCl2 for 24 h. The medium was then replaced with fresh medium without CoCl2, and culture was continued for the indicated period of time before harvesting the cells for immunoblotting with HA or β-actin antibody. D, proteasome is involved in the decreased GCM1 protein level induced by hypoxia. BeWo31 cells were mock-treated or treated with 250 μm CoCl2 in the presence or absence of 40 μm MG132 for 24 h. Cells were then harvested for immunoblotting with HA or β-actin antibody.
GSK-3β Promotes GCM1 Ubiquitination under Hypoxia
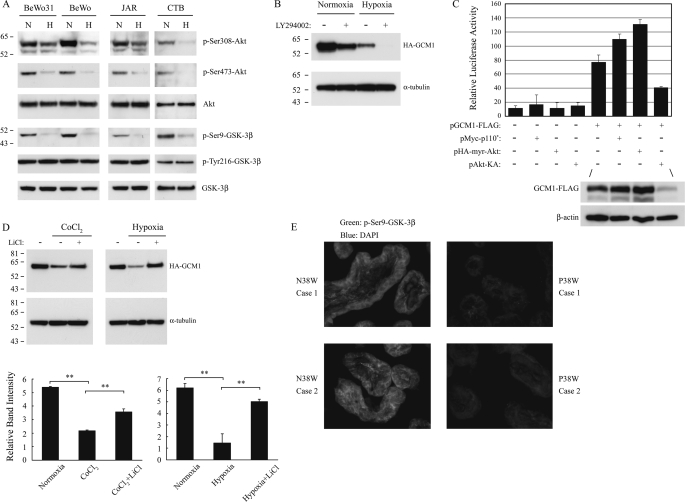
Recent studies have demonstrated that exposure to hypoxic conditions either activates or inactivates the PI3K-Akt signaling pathway, depending on the cell type (22, 23). To investigate whether PI3K-Akt signaling pathway plays a role in hypoxia associated with preeclampsia, we analyzed the effects of hypoxia on the villous CTB cells isolated from term placentas and in human placental cell lines. As shown in Fig. 2A, the levels of activated Akt (i.e. the Ser308-phosphorylated (Ser(P)308) Akt and Ser473-phosporylated (Ser(P)473) Akt) were significantly decreased in CTB cells and three cell lines under hypoxia for 48 h. This suggests that hypoxia may suppress the activity of the Akt upstream kinase, PI3K. Indeed, LY294002, an inhibitor of PI3K, significantly decreased the HA-GCM1 protein level in BeWo31 under normoxia conditions (Fig. 2B), whereas rapamycin, an mTOR inhibitor, did not affect the HA-GCM1 protein level under similar conditions (data not shown). In support of this finding, the expression of constitutively active mutants of p110 and Akt, Myc-p110* and HA-myr-Akt, were able to enhance GCM1-mediated transcriptional activation, whereas the dominant negative mutant of Akt, Akt-KA, inhibited GCM1-mediated transcriptional activation (Fig. 2C). These observations might be attributed to regulation of GCM1-FLAG stability by PI3K and Akt, because the protein level of GCM1-FLAG was increased in the presence of Myc-p110* and HA-myr-Akt but decreased in the presence of Akt-KA (Fig. 2C). Taken together, these results suggest that inactivation of the PI3K-Akt pathway by hypoxia promotes GCM1 degradation.
FIGURE 2.
Identification of the hypoxia-activated signaling pathway promoting GCM1 degradation. A, suppression of Akt and activation of GSK-3β by hypoxia in placental cells. BeWo331, BeWo, JAR, and villous CTB cells were incubated under normoxia (N) or hypoxia (H) for 48 h and then immunoblotted with the indicated antibody. B, inhibition of PI-3K promotes GCM1 degradation. BeWo31 cells were incubated in the presence or absence of 50 μm LY294002 under normoxia or hypoxia. After 24 h, cells were harvested and immunoblotted with HA or α-tubulin antibody. C, the transcriptional activity of GCM1 is regulated by PI3K-Akt pathway. 293T cells were co-transfected with 0.1 μg of p(GBS)4E1BLuc plus different combinations of 0.1 μg of expression plasmids. At 48 h post-transfection, cells were harvested for luciferase assay. The protein levels of GCM1-FLAG and β-actin in the indicated transfection groups were detected by FLAG and β-actin antibodies, respectively. D, inhibition of GSK-3β prevents GCM1 degradation under hypoxia. BeWo31 cells were incubated with or without 250 μm CoCl2 and 50 mm LiCl. In a separate experiment, cells were incubated under normoxia or hypoxia with or without 50 mm LiCl. After 48 h, cells were harvested for immunoblotting with HA or anti-α-tubulin antibody. Quantification of HA-GCM1 and α-tubulin band intensities was performed by densitometry as described under “Experimental Procedures.” The band intensity of HA-GCM1 was further normalized by that of α-tubulin. Mean values and the S.D. obtained from three independent experiments are presented. Asterisks, significant differences (**, p < 0.01). E, immunohistochemistry of Ser(P)9-GSK-3β in normal and preeclamptic (P) placentas. Third trimester (38 weeks) placental sections were stained with Ser(P)9-GSK-3β primary antibody and then fluorescein isothiocyanate-conjugated secondary antibody. Nuclei of the same sections were stained with 4′,6′-diamidino-2-phenylindole (DAPI). Stained sections were examined by fluorescence microscopy at ×400 magnification. Of note, the signals for Ser(P)9-GSK-3β were significantly lower in the preeclamptic trophoblast cells.
Because Akt phosphorylates the Ser9 residue of GSK-3β and hence inhibits GSK-3β activity (24), we studied whether GSK-3β regulates GCM1 stability under normoxia and hypoxia. Levels of the inhibitory Ser9-phosphorylated (Ser(P)9) GSK-3β in BeWo, BeWo31, JAR, and CTB cells fell significantly under hypoxia, whereas levels of the active Tyr216-phosporylated (Tyr(P)216) GSK-3β were not affected (Fig. 2A). Therefore, GSK-3β activity is elevated under hypoxic conditions and may be associated with GCM1 instability in placental cells. Indeed, treatment with LiCl, a GSK-3β inhibitor, was able to significantly stabilize HA-GCM1 proteins in cells treated with CoCl2 or exposed to hypoxia (Fig. 2D). Since placental hypoxia is associated with preeclampsia, the expression of GCM1 and Ser(P)9-GSK-3β in preeclamptic placentas was compared with that in gestation age-matched normal placentas by immunohistochemistry. In agreement with our previous study (13), expression of GCM1 was decreased in preeclamptic placentas (supplemental Fig. 2). Interestingly, the expression of Ser(P)9-GSK-3β in preeclamptic placentas was also decreased compared with that in normal placentas (Fig. 2E). Therefore, a relatively higher GSK-3β activity in preeclamptic placentas may be responsible for decreasing the GCM1 protein level in preeclampsia.
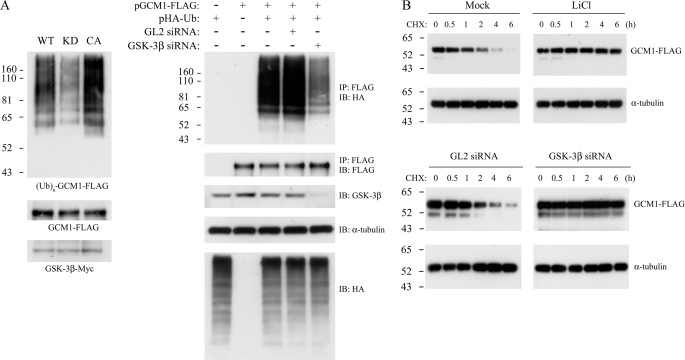
We next tested whether GSK-3β regulates GCM1 ubiquitination, which targets GCM1 to proteasomes for degradation (18). In vivo ubiquitination of GCM1 was analyzed in 293T cells cotransfected with pHA-Ub, pGCM1-FLAG, and an expression plasmid encoding the wild-type, kinase-dead (KD) or constitutively active (CA) GSK-3β. As shown on the left in Fig. 3A, the level of ubiquitinated GCM1-FLAG was decreased in the presence of the KD GSK-3β. In addition, knocking down the endogenous GSK-3β with a GSK-3β siRNA, but not an unrelated GL2 siRNA, also prevented GCM1 ubiquitination (Fig. 3A, right). Using cycloheximide to inhibit de novo protein synthesis, we further found that the half-life of GCM1-FLAG was prolonged to over 6 h in the LiCl-treated or GSK-3β siRNA-transfected cells but not in the mock-treated or GL2 siRNA-transfected cells (Fig. 3B). These results suggest that active GSK-3β promotes GCM1 ubiquitination and degradation.
FIGURE 3.
GSK-3β controls GCM1 ubiquitination and degradation. A, GCM1 ubiquitination is regulated by GSK-3β. 293T cells were transfected with 1 μg of pGCM1-FLAG, pHA-Ub, and the indicated GSK-3β expression plasmid. At 24 h post-transfection, cells were treated with 40 μm MG132 for an additional 4 h and then subject to ubiquitination analysis by immunoprecipitation (IP) with FLAG mAb and immunoblotting (IB) with HA mAb (left). In a separate experiment, 293T cells were transfected with 1 μg of pGCM1-FLAG and pHA-Ub plus 10 nm GL2 or GSK-3β siRNA and incubated as above for ubiquitination analysis. B, the half-life of GCM1 is prolonged by inhibition of GSK-3β. 293T cells were transfected with 0.35 μg of pGCM1-FLAG. At 24 h post-transfection, cells were pretreated with or without 50 mm LiCl for 2 h before incubation with 75 μm cycloheximide for the indicated period of time. In a separate experiment, 293T cells were transfected with 0.35 μg of pGCM1-FLAG plus 10 nm GL2 or GSK-3β siRNA. At 24 h post-transfection, cells were incubated with 75 μm cycloheximide for the indicated period of time. Cells were then harvested for immunoblotting with FLAG or α-tubulin antibody.
Phosphorylation of Serines 322 and 326 in GCM1 Is Required for FBW2 Recognition
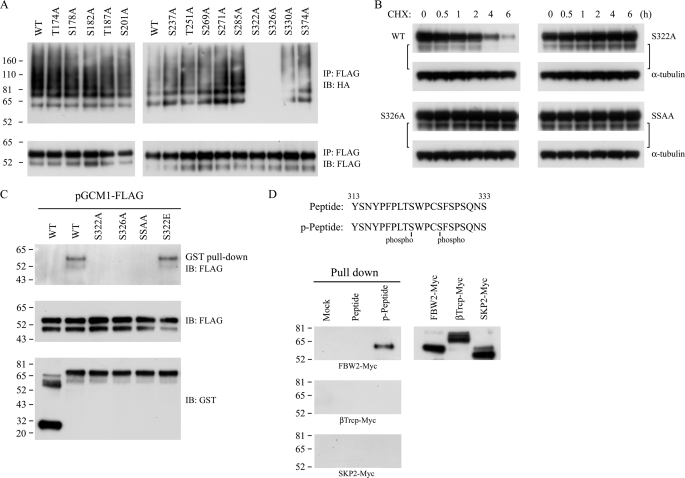
A series of GCM1 mutants on potential GSK-3β phosphorylation sites in GCM1 were generated and tested in in vivo ubiquitination assays in order to identify the functional GSK-3β sites in GCM1. As shown in Fig. 4A, changing Ser322 or Ser326 into alanine (i.e. mutants GCM1-FLAG-S322A and GCM1-FLAG-S326A) significantly impaired GCM1 ubiquitination. Moreover, the half-lives of both single mutants and the mutant harboring the double mutation of Ser322 and Ser326 (GCM1-FLAG-SSAA; SSAA) were significantly prolonged (Fig. 4B).
FIGURE 4.
Identification of GSK-3β phosphorylation sites and a C-terminal destruction motif in GCM1. A, Ser322 and Ser326 are required for GCM1 ubiquitination. 293T cells were transfected with 1 μg of pHA-Ub and wild-type or mutant pGCM1-FLAG, treated with MG132, and subject to ubiquitination analysis as described in the legend to Fig. 3A. B, Ser322 and Ser326 are involved in regulation of GCM1 stability. 293T cells were transfected with the indicated pGCM1-FLAG expression plasmid for protein stability analysis as described in the legend to Fig. 3B. C and D, FBW2 interacts with the C-terminal TpSWPCpS (where pS represents phosphoserine) destruction motif in GCM1. GST- or GST-FBW2-loaded glutathione beads were incubated with 100 μg of the cell lysate prepared from 293T cells transfected with wild-type or mutant pGCM1-FLAG expression plasmids for pull-down analysis, followed by immunoblotting with FLAG mAb. Biotinylated unmodified peptides or phosphopeptides covering amino acids 313–333 were incubated with 100 μg of cell lysate prepared from 293T cells transfected with pFBW2-Myc, pβTrcp-Myc, or pSKP2-Myc for pull-down analysis, followed by immunoblotting with Myc mAb. IP, immunoprecipitation; IB, immunoblot.
The F-box protein, FBW2, is the substrate recognition subunit of the SCFFBW2 E3 ligase responsible for GCM1 ubiquitination (18). We next investigated whether recognition of GCM1 by FBW2 depends on the phosphorylation status of Ser322 and Ser326. In pull-down experiments with GST-FBW2 and cell lysate from 293T cells expressing wild-type or mutant GCM1-FLAG harboring an individual or combined mutation in Ser322 and Ser326, interaction between FBW2 and the mutants S322A, S326A, and S322A/S326A was not detected (Fig. 4C). However, FBW2 did interact with the wild-type GCM1-FLAG and the mutant S322E, where Ser322 was changed into glutamic acid (Fig. 4C). As a complementary study, biotin-conjugated GCM1 peptides of amino acids 313–333 harboring unmodified or phosphorylated Ser322 and Ser326 were synthesized for pull-down experiments with different F-box proteins, including FBW2, βTrcp, and SKP2. As shown in Fig. 4D, neither mock beads nor unmodified peptide-loaded beads pulled down any of the three F-box proteins transiently expressed in 293T cells. However, when a peptide harboring both phospho-Ser322 and -Ser326 residues was tested, an interaction with FBW2 was observed (Fig. 4D). Therefore, phosphorylation of Ser322 and Ser326 forms a C-terminal destruction motif in GCM1 that permits interaction with FBW2.
GSK-3β Phosphorylates Serine 322 in GCM1 under Hypoxia
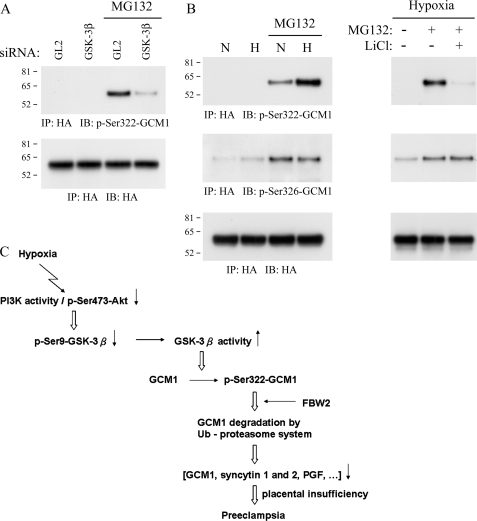
Phosphorylation site-specific antibodies were generated to investigate the physiological relevance of Ser322 and Ser326 phosphorylation sites (Ser(P)322 and Ser(P)326) in GCM1 (supplemental Fig. 3). With Ser(P)322-GCM1 antibody, we demonstrated that GSK-3β is involved in Ser322 phosphorylation in GCM1 in vivo, because the level of Ser(P)322-HA-GCM1 was significantly decreased in GSK-3β-knocked down and MG132-treated 293T cells (Fig. 5A). We further demonstrated that hypoxia stimulates Ser322 phosphorylation in BeWo31 placental cells (Fig. 5B, left), which could be counteracted by LiCl (Fig. 5B, right), supporting a requirement of GSK-3β for Ser322 phosphorylation under hypoxia. Similar results were also observed in JAR cells stably expressing HA-GCM1 (data not shown). Interestingly, the level of Ser(P)326-HA-GCM1 was not significantly different in BeWo31 cells under normoxia or hypoxia (Fig. 5B). Therefore, Ser326 is likely to be constitutively phosphorylated and to function as the priming phosphate for GSK-3β-mediated phosphorylation of Ser322 in GCM1 in placental cells. Overall, these results indicate that phosphorylation of Ser322 and Ser326 in GCM1 is critical for its degradation under hypoxia.
FIGURE 5.
Hypoxia induces Ser322 phosphorylation in GCM1 in vivo. A, GSK-3β phosphorylates Ser322 under hypoxia. 293T cells were transfected with pHA-GCM1 plus GL2 or GSK-3β siRNA. The transfected cells were subjected to hypoxic conditions for 24 h in the presence of MG132, followed by immunoprecipitation (IP) with HA mAb and immunoblotting (IB) with Ser(P)322-GCM1 Ab. B, Ser322 phosphorylation in placental cells under hypoxia. BeWo31 cells were cultured under normoxia or hypoxia for 48 h and mock-treated or treated with the indicated combination of MG132 and LiCl, followed by immunoprecipitation with HA mAb and immunoblotting with HA, Ser(P)322-GCM1, or Ser(P)326-GCM1 antibody. C, GCM1 degradation under placental hypoxia contributes to the pathogenesis of preeclampsia.
DISCUSSION
GCM1 is a critical transcription factor for placental development and function. Previous studies have demonstrated that GCM1 expression is decreased in preeclamptic placentas and placental cells under hypoxia (13, 25). Based on these observations, we wished to investigate in the present study how hypoxia regulates GCM1 expression in the placenta. One of the major cellular responses to hypoxia is expression of genes involved in glycolysis, glucose transport, erythropoiesis, and angiogenesis by hypoxia-inducible factors (HIFs). HIF is a heterodimeric transcription factor composed of HIFα and the arylhydrocarbon receptor nuclear translocator (ARNT; also known as HIF1β). Interestingly, transgenic mice studies have indicated that HIF1α, HIF2α, and ARNT are required for placental development (26, 27). Studies of trophoblast stem (TS) cells derived from wild-type, ARNT−/−, and HIF1α−/− HIF2α−/− placentas further suggested that HIF is involved in the differentiation of trophoblast giant cells. In addition, HIF-null TS cells predominantly differentiated into syncytiotrophoblasts (26–28). Accordingly, we had investigated the role of HIF in GCM1 gene expression when this study was initiated and did not detect any direct effect of HIF1 on GCM1 gene expression (data not shown). Instead, we found that GCM1 autoregulates its promoter activity (supplemental Fig. 1). Subsequently, we demonstrated that hypoxia down-regulates GCM1 activity at both transcriptional and post-translational levels by enhancing GCM1 degradation (Fig. 1). Recent studies have demonstrated that proteasome-mediated degradation of estrogen receptor α is induced by hypoxia via protein-protein interaction between estrogen receptor α and HIF1α (29, 30). We therefore tested the possibility of protein-protein interaction between GCM1 and HIF1α and did not detect any interaction between HIF1α and GCM1 (data not shown). Moreover, we have also tested whether hypoxia regulates GCM1 degradation through activation of FBW2 and UBE2D2 gene expression, which are the E3 and E2 components for the proteasome-dependent degradation of GCM1, respectively (31). Our results indicated that expression of the FBW2 and UBE2D2 genes was not affected by hypoxia (data not shown).
Because GCM1 autoregulates its own expression and hypoxia induces GCM1 degradation, the BeWo31 placental cells stably expressing HA-GCM1 provided a good system for further mechanistic study of hypoxia-induced GCM1 degradation at the post-translational level, because expression of HA-GCM1 is directed by the cytomegalovirus promoter, whose activity is not affected by hypoxia. We then came to investigate whether the hypoxia signaling pathway is involved in the regulation of GCM1 stability. Depending on the cell type, hypoxia either activates or inactivates the PI3K-Akt signaling pathway. For example, the pathway is activated in PC12, HT1080, and HeLa cells (22, 32, 33) and inactivated in HepG2 cells (23, 24) under hypoxia. Although the mechanism of activation or inhibition of the PI3K-Akt signaling pathway by hypoxia is not known, in the present study, we clearly demonstrated that this pathway is inhibited in BeWo, BeWo31, and JAR placental cells and primary cytotrophoblast cells under hypoxia (Fig. 2A). We further demonstrated that the level of the inhibitory form of GSK-3β, Ser(P)9-GSK-3β, is decreased when the PI3K-Akt signaling pathway is inhibited by hypoxia or in preeclamptic placentas. Consequently, the activated GSK-3β phosphorylates Ser322 in GCM1 and promotes GCM1 degradation. Our results are also in agreement with a recent study by Yung et al. (34), in which inactivation of Akt and activation of GSK-3β were found to be associated with protein synthesis inhibition and endoplasmic reticulum stress in placentas of intrauterine growth restriction and preeclampsia. Therefore, GSK-3β is a target of placental stress, and overactivated GSK-3β is very likely to cause dysregulation of protein translation, modification, and degradation in the pathogenesis of intrauterine growth restriction and preeclampsia.
Maternal obesity and insulin resistance before pregnancy, allelic polymorphism at the Thr235 residue of angiotensinogen, food shortage, induction of autoantibodies against angiotensin II type 1 receptor, and abnormal expression of angiogenic factors (sFlt-1, VEGF, and PGF) are all linked to preeclampsia (1, 35). In addition, recent studies have shown that both deficiency in 2-methoxyoestradiol production in catechol-O-methyltransferase knock-out mice and administration of purified autoantibodies against angiotensin II type 1 receptor into pregnant mice result in the development of preeclampsia (36, 37). Given that different factors are associated with preeclampsia, it occurs only during pregnancy, and its symptoms are resolved after delivery, it is very likely that the pathogenesis of this disease involves many regulatory systems, and dysfunction of any of these systems leads to placental insufficiency and development of the symptoms.
Expression of PGF and VEGF was found in villous trophoblasts and may regulate vessel formation in the chorionic villi (38, 39). PGF and VEGF expression was also detected in the invading CTBs, which invade and replace the endothelial cells in spiral arteries to achieve maternal vascular remodeling (40). Decreased circulating levels of VEGF and PGF have been demonstrated in preeclampsia and may account for endothelial dysfunction in preeclampsia (6, 7). Trophoblastic fusion is essential for the functional integrity of the syncytiotrophoblast layer, which very likely involves the fusogenic proteins syncytin 1 and 2. Syncytin 2 is a second placental fusogenic protein with an immunosuppressive activity (41). Expression of syncytin 2 is also regulated by GCM1 (data not shown) and is decreased in preeclampsia (42). Decreased expression of both fusogenic proteins in hypoxic conditions and preeclampsia may be responsible for the unstable villous structures and increased trophoblast deportation into the maternal circulation found in preeclampsia. Likewise, misexpression under placental hypoxia of the other genes regulated by GCM1, such as aromatase, may also contribute to placental insufficiency, leading to preeclampsia (43).
Based on the signaling pathway underlying hypoxia-induced GCM1 degradation, here we propose a model for the role of GCM1 in the pathogenesis of preeclampsia. GCM1 is increasingly subject to degradation under placental hypoxia. GCM1 gene expression is compromised due to impaired autoregulation of GCM1 gene expression. This feedback loop leads to a significant reduction of GCM1 under hypoxia. Reduced GCM1 levels caused a broad failure in the expression of GCM1 target genes, resulting in placental insufficiency (Fig. 5C). In particular, the misexpression of proangiogenic factors and fusogenic proteins is very likely to cause endothelial dysfunction and improper formation of the syncytiotrophoblast layer in the preeclamptic placentas.
Being a key placental transcription factor, the activity of GCM1 can be regulated by different post-translational modifications, which may fine tune placental development and function. For example, the cAMP-PKA pathway is able to enhance CBP-mediated acetylation of GCM1, which prevents GCM1 ubiquitination and thereby increases GCM1 activity (20). Here we have revealed a further new pathway responsible for decreased GCM1 activity in placental cells under hypoxia (i.e. activation of the hypoxia-PI3K-Akt-GSK-3β pathway leads to GCM1 phosphorylation, ubiquitination, and degradation). This pathway is very likely to play an important role in the pathogenesis of preeclampsia and provides an opportunity for therapeutic intervention in preeclampsia.
Supplementary Material
Acknowledgment
We thank Dr. Harry Wilson for manuscript editing.
This work was supported by National Science Council Grant 96-2311-B-001-034 (to H. C.) and a grant from Academia Sinica, Taiwan (to H. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- sFlt-1
- soluble Flt-1
- VEGF
- vascular endothelial growth factor
- PGF
- placental growth factor
- siRNA
- small interfering RNA
- mAb
- monoclonal antibody
- PMSF
- phenylmethylsulfonyl fluoride
- HA
- hemagglutinin
- CTB
- cytotrophoblast
- PI3K
- phosphatidylinositol 3-kinase
- WT
- wild type
- KD
- kinase-dead
- CA
- constitutively active
- HIF
- hypoxia-inducible factor
- ARNT
- arylhydrocarbon receptor nuclear translocator
- E2
- ubiquitin carrier protein
- E3
- ubiquitin-protein isopeptide ligase
- Ub
- ubiquitin.
REFERENCES
- 1.Maynard S., Epstein F. H., Karumanchi S. A. ( 2008) Annu. Rev. Med. 59, 61– 78 [DOI] [PubMed] [Google Scholar]
- 2.Wulff C., Weigand M., Kreienberg R., Fraser H. M. ( 2003) Reproduction 126, 569– 577 [DOI] [PubMed] [Google Scholar]
- 3.Soleymanlou N., Jurisica I., Nevo O., Ietta F., Zhang X., Zamudio S., Post M., Caniggia I. ( 2005) J. Clin. Endocrinol. Metab. 90, 4299– 4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torry D. S., Wang H. S., Wang T. H., Caudle M. R., Torry R. J. ( 1998) Am. J. Obstet. Gynecol. 179, 1539– 1544 [DOI] [PubMed] [Google Scholar]
- 5.Wikström A. K., Larsson A., Eriksson U. J., Nash P., Nordén-Lindeberg S., Olovsson M. ( 2007) Obstet. Gynecol. 109, 1368– 1374 [DOI] [PubMed] [Google Scholar]
- 6.Moore, Simas T. A., Crawford S. L., Solitro M. J., Frost S. C., Meyer B. A., Maynard S. E. ( 2007) Am. J. Obstet. Gynecol. 197, 244.e1– 8 [DOI] [PubMed] [Google Scholar]
- 7.Levine R. J., Lam C., Qian C., Yu K. F., Maynard S. E., Sachs B. P., Sibai B. M., Epstein F. H., Romero R., Thadhani R., Karumanchi S. A. ( 2006) N. Engl. J. Med. 355, 992– 1005 [DOI] [PubMed] [Google Scholar]
- 8.Anson-Cartwright L., Dawson K., Holmyard D., Fisher S. J., Lazzarini R. A., Cross J. C. ( 2000) Nat. Genet. 25, 311– 314 [DOI] [PubMed] [Google Scholar]
- 9.Schreiber J., Riethmacher-Sonnenberg E., Riethmacher D., Tuerk E. E., Enderich J., Bösl M. R., Wegner M. ( 2000) Mol. Cell. Biol. 20, 2466– 2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu C., Shen K., Lin M., Chen P., Lin C., Chang G. D., Chen H. ( 2002) J. Biol. Chem. 277, 50062– 50068 [DOI] [PubMed] [Google Scholar]
- 11.Chang M., Mukherjea D., Gobble R. M., Groesch K. A., Torry R. J., Torry D. S. ( 2008) Biol. Reprod. 78, 841– 851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munaut C., Lorquet S., Pequeux C., Blacher S., Berndt S., Frankenne F., Foidart J. M. ( 2008) Hum. Reprod. 23, 1407– 1415 [DOI] [PubMed] [Google Scholar]
- 13.Chen C. P., Chen C. Y., Yang Y. C., Su T. H., Chen H. ( 2004) Placenta 25, 413– 421 [DOI] [PubMed] [Google Scholar]
- 14.Chen C. P., Wang K. G., Chen C. Y., Yu C., Chuang H. C., Chen H. ( 2006) BJOG 113, 152– 158 [DOI] [PubMed] [Google Scholar]
- 15.Li H., Gu B., Zhang Y., Lewis D. F., Wang Y. ( 2005) Placenta 26, 210– 217 [DOI] [PubMed] [Google Scholar]
- 16.Redman C. W., Sargent I. L. ( 2005) Science 308, 1592– 1594 [DOI] [PubMed] [Google Scholar]
- 17.Brosens I. A., Robertson W. B., Dixon H. G. ( 1972) Obstet. Gynecol. Annu. 1, 177– 191 [PubMed] [Google Scholar]
- 18.Yang C. S., Yu C., Chuang H. C., Chang C. W., Chang G. D., Yao T. P., Chen H. ( 2005) J. Biol. Chem. 280, 10083– 10090 [DOI] [PubMed] [Google Scholar]
- 19.Huang W. C., Chen C. C. ( 2005) Mol. Cell. Biol. 25, 6592– 6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang C. W., Chuang H. C., Yu C., Yao T. P., Chen H. ( 2005) Mol. Cell. Biol. 25, 8401– 8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd ( 1986) Endocrinology 118, 1567– 1582 [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Tejado M., Alfranca A., Aragonés J., Vara A., Landázuri M. O., del Peso L. ( 2002) J. Biol. Chem. 277, 13508– 13517 [DOI] [PubMed] [Google Scholar]
- 23.Mottet D., Dumont V., Deccache Y., Demazy C., Ninane N., Raes M., Michiels C. ( 2003) J. Biol. Chem. 278, 31277– 31285 [DOI] [PubMed] [Google Scholar]
- 24.Flügel D., Görlach A., Michiels C., Kietzmann T. ( 2007) Mol. Cell. Biol. 27, 3253– 3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knerr I., Schubert S. W., Wich C., Amann K., Aigner T., Vogler T., Jung R., Dötsch J., Rascher W., Hashemolhosseini S. ( 2005) FEBS Lett. 579, 3991– 3998 [DOI] [PubMed] [Google Scholar]
- 26.Adelman D. M., Gertsenstein M., Nagy A., Simon M. C., Maltepe E. ( 2000) Genes Dev. 14, 3191– 3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowden Dahl K. D., Fryer B. H., Mack F. A., Compernolle V., Maltepe E., Adelman D. M., Carmeliet P., Simon M. C. ( 2005) Mol. Cell. Biol. 25, 10479– 10491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maltepe E., Krampitz G. W., Okazaki K. M., Red-Horse K., Mak W., Simon M. C., Fisher S. J. ( 2005) Development 132, 3393– 3403 [DOI] [PubMed] [Google Scholar]
- 29.Stoner M., Saville B., Wormke M., Dean D., Burghardt R., Safe S. ( 2002) Mol. Endocrinol. 16, 2231– 2242 [DOI] [PubMed] [Google Scholar]
- 30.Cho J., Kim D., Lee S., Lee Y. ( 2005) Mol. Endocrinol. 19, 1191– 1199 [DOI] [PubMed] [Google Scholar]
- 31.Chiang M. H., Chen L. F., Chen H. ( 2008) Biol. Reprod. 79, 914– 920 [DOI] [PubMed] [Google Scholar]
- 32.Chen E. Y., Mazure N. M., Cooper J. A., Giaccia A. J. ( 2001) Cancer Res. 61, 2429– 2433 [PubMed] [Google Scholar]
- 33.Beitner-Johnson D., Rust R. T., Hsieh T. C., Millhorn D. E. ( 2001) Cell. Signal. 13, 23– 27 [DOI] [PubMed] [Google Scholar]
- 34.Yung H. W., Calabrese S., Hynx D., Hemmings B. A., Cetin I., Charnock-Jones D. S., Burton G. J. ( 2008) Am. J. Pathol. 173, 451– 462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilekis J. V., Reddy U. M., Roberts J. M. ( 2007) Reprod. Sci. 14, 508– 523 [DOI] [PubMed] [Google Scholar]
- 36.Zhou C. C., Zhang Y., Irani R. A., Zhang H., Mi T., Popek E. J., Hicks M. J., Ramin S. M., Kellems R. E., Xia Y. ( 2008) Nat. Med. 14, 855– 862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanasaki K., Palmsten K., Sugimoto H., Ahmad S., Hamano Y., Xie L., Parry S., Augustin H. G., Gattone V. H., Folkman J., Strauss J. F., Kalluri R. ( 2008) Nature 453, 1117– 1121 [DOI] [PubMed] [Google Scholar]
- 38.Benirschke K., Kaufmann P. ( 2001) in Pathology of the Human Placenta, 4th Ed., pp. 134– 145, Springer-Verlag, New York, NY [Google Scholar]
- 39.Zhou Y., McMaster M., Woo K., Janatpour M., Perry J., Karpanen T., Alitalo K., Damsky C., Fisher S. J. ( 2002) Am. J. Pathol. 160, 1405– 1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y., Bellingard V., Feng K. T., McMaster M., Fisher S. J. ( 2003) Dev. Biol. 263, 114– 125 [DOI] [PubMed] [Google Scholar]
- 41.Mangeney M., Renard M., Schlecht-Louf G., Bouallaga I., Heidmann O., Letzelter C., Richaud A., Ducos B., Heidmann T. ( 2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20534– 20539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C. P., Chen L. F., Yang S. R., Chen C. Y., Ko C. C., Chang G. D., Chen H. ( 2008) Biol. Reprod. 79, 815– 823 [DOI] [PubMed] [Google Scholar]
- 43.Yamada K., Ogawa H., Honda S., Harada N., Okazaki T. ( 1999) J. Biol. Chem. 274, 32279– 32286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.