Abstract
The mechanisms that ensure an equal inheritance of cellular organelles during mitosis are an important area of study in cell biology. For the mitochondria fragment during mitosis, however, the cellular links that signal these changes are largely unknown. We recently identified a SUMO protease, SenP5, that deSUMOylates a number of mitochondrial targets, including the dynamin-related fission GTPase, DRP1. In interphase, SenP5 resides primarily within the nucleoli, in addition to a cytosolic pool. Here we report the relocalization of SenP5 from the nucleoli to the mitochondrial surface at G2/M transition prior to nuclear envelope breakdown. The recruitment of SenP5 results in a significant loss in mitochondrial SUMOylation, and a concomitant increase in the labile pool of DRP1 that drives mitochondrial fragmentation. Importantly, silencing of SenP5 leads to an arrest in the cell cycle precisely at the time when the protease is translocated to the mitochondria. These data indicate that transition of SenP5 to the mitochondria plays an important role in mitochondrial fragmentation during mitosis. The altered intracellular localization of SenP5 represents the first example of the mitochondrial recruitment of a SUMO protease and provides new insights into the mechanisms of interorganellar communication during the cell cycle.
Introduction
The regulation of the cell cycle is based upon a number of critical checkpoints that ensure the cell is healthy, the DNA is correctly replicated, there is sufficient metabolic energy, and that the organelles are properly partitioned during mitosis. Each of the cell cycle checkpoints are maintained through precise signaling cascades, whose activities determine whether the cycle proceeds, remains quiescent, or whether the cell may enter into apoptotic death. A complete understanding of all cell cycle checkpoints is critical for the identification of new therapeutic targets for both cancer and for the development of regenerative technologies. Recently, genetic models in Drosophila melanogaster have identified at least two novel retrograde signaling pathways that ensure sufficient metabolic capacity and health at the G1/S checkpoint (1, 2). Mutations in a component of electron transport chain complex IV led to a 60% decrease in cellular ATP, thereby activating AMP-activated protein kinase and p53-dependent degradation of cyclin E (1). In a parallel pathway, the increased production of cellular ROS through mutations in a component of complex I led to the activation of the c-Jun NH2-terminal kinase (JNK)-FOXO cascade that up-regulates the cyclin E inhibitor Dacapo, causing cell cycle arrest at G1/S (2). These two pathways highlight the emerging importance of the mitochondria as an essential component of intracellular signaling cascades and cell cycle regulation.
The mitochondria cannot be formed de novo, therefore the proliferation and segregation of these organelles during mitosis may be just as important to cellular survival as their role in signaling the health of the cell during G1. Mitochondrial segregation during mitosis has been investigated in yeast model organisms where the active delivery of the organelles into the growing bud is essential (3, 4). However, the regulation of mitochondrial segregation in mammalian cell division has attracted less attention because it is generally considered to be a primarily stochastic event. This idea has been challenged recently by studies investigating mitochondrial morphology during each cell cycle phase. Notably, the fragmentation of mitochondria requires a member of the dynamin family of GTPases, the dynamin-related protein 1 (DRP1),3 which was recently found to be phosphorylated by the cell cycle-specific kinase Cdk1/cyclin B1 during mitosis (5). The phosphorylation of DRP1 increased fission activity of the protein specifically during anaphase, further hinting that morphology and distribution of the mitochondria may be tightly controlled to ensure equal segregation into the daughter cells. On a molecular level, these findings support the idea that activity of the mitochondrial shaping proteins is modulated by cellular signaling cascades.
In addition to the phosphorylation of DRP1, we have previously documented that this fission GTPase is also subject to covalent conjugation by the small ubiquitin-like modifier, SUMO1 (6–8). SUMOylation requires the sequential action of the ATP-dependent E1 enzyme heterodimer Aos1/SAE1 that forms a thioester bond with the terminal diglycine motif in SUMO, followed by its transfer to Ubc9, the E2 ligating enzyme (reviewed in Refs. 9 and 10). After this, a tightly localized SUMO E3 ligase is required to provide substrate specificity and facilitate the transfer of SUMO to the final target protein. The SUMOylated targets are thought to acquire a new conformation, leading to alternate protein interactions, cellular locations, or protein stability (9). In the case of DRP1, SUMOylation appears to stabilize the protein from degradation, leading to an increase in mitochondrial fragmentation (6–8). Relative to the abundance of documented ubiquitin E3 ligases, there are only a few identified SUMO E3 ligases that are primarily localized within the nucleus. Those identified to date include the Siz1/PIAS (protein inhibitor of activated STAT (signal transducer and activator of transcription)) family that contains the SP-RING motif (11), the nuclear pore component RanBP2 (12), the polycomb2 protein (13), the dual ubiquitin and SUMO E3 ligase TOPORS (14–16), and the transcriptional corepressor KAP1/TIF1β, which contains a RING-related PHD domain (17). These ligases do not function enzymatically, and instead provide a scaffold for the SUMO E2 ligase, Ubc9, to conjugate SUMO to the substrate (18). Importantly, SUMOylation is a transient, substoichiometric modification that is removed by the action of SUMO (or Sentrin) proteases. The yeast Saccharomyces cerevisiae has two ubiquitin like proteases, Ulp1 and Ulp2, whereas the mammalian genome encodes 6, named Sentrin protease SenP1–3 and SenP5–7. SUMO proteases bind directly to the SUMO protein, and not the substrate, which allows their broad specificity. These proteases are differentially localized and thought to have specific cellular functions, including regulation of cell cycle progression (19–22). To date, no SUMO E3 ligases or proteases function directly on the mitochondrial membranes, although many mitochondrial SUMO targets, including DRP1, have been reported.
In an effort to understand the function of mitochondrial SUMOylation, we recently identified a specific SUMO protease, SenP5, which is responsible for the deSUMOylation of DRP1 in steady state (8). SenP5 is localized primarily to the nucleoli, but there is also a substantial amount of the endogenous protein found within the cytosol, where we proposed that it functions to deSUMOylate DRP1 (8). SenP1, SenP5, and SenP3 were the first SUMO proteases to demonstrate a preference to deSUMOylate SUMO2 and SUMO3 from substrates relative to SUMO1 (23, 24). However, recent data has shown that the conformation of SUMO within the substrate can lead to differential deSUMOylation. For example, SenP5 could remove SUMO1 from Lys65 of promyelocytic leukemia, but not Lys160 or Lys490 of the same substrate (24). Interestingly, SenP5 could remove the two SUMO1 paralogues, SUMO2 and SUMO3, from their conjugation at Lys160 or Lys490. This, combined with evidence that mixed chains containing all three paralogues are found on native substrates (25), strongly suggests that there is a much higher level of complexity and specificity in the SUMOylation pathways than previously suspected. Indeed, SUMO2/3 were shown to specifically conjugate to a microtubule motor protein CENP-E, which was required to target it to kinetochores during mitosis (26). In contrast, SUMO1 was shown to conjugate proteins that bind directly to the spindles, indicating very distinct functional roles for the SUMO proteins. Finally, SUMO proteins have also been shown to be themselves subject to regulated phosphorylation (27), although the extent and functional consequences of this are still under investigation.
One of the most common general functions for SUMOylation appears to be in the regulation of the cell cycle. The loss of the SUMO proteases in yeast (called Ulp1 and Ulp2) led to a cell cycle arrest, suggesting that deSUMOylation is important for cell cycle progression (28). Similar studies in mammalian cells have shown that global SUMOylation tends to favor cellular senescence rather than proliferation (29). Because SUMOylation plays a global role in cell cycle-dependent regulation of many different proteins (19, 20, 29, 30), and that SenP5 was specifically shown to be essential for cell cycle progression (21), we have examined the localization of SenP5 throughout the cell cycle. We here report its relocation from the nucleoli to the mitochondria specifically at the G2/M transition. This movement leads to an increase in the labile pool of DRP1 that drives mitochondrial fragmentation and significantly decreases total mitochondrial SUMOylation. Our data provide the first insights into the role of SenP5 in facilitating a change in global mitochondrial SUMOylation during cell cycle progression.
EXPERIMENTAL PROCEDURES
Materials
COS-7 and sHeLa cells were purchased from American Type Culture Collection (Manassas, VA). Polyclonal anti-SenP5 serum was obtained from rabbit immunization with recombinant SenP5-His6 (pQE31:SenP5) as described (8). Polyclonal anti-SenP5 directed against the first 124 amino acids (mitochondrial targeting signal) of the total sequence of 756 of the SenP5 protein was obtained from immunizing rabbits with recombinant MTS-His6 (pQE30:MTS), at 21st Century Biochemicals (Marlboro, MA). Polyclonal anti-TOM20 was a generous gift from Dr. Gordon Shore (McGill University, Canada). Rabbit monoclonal anti-ERK1 was obtained from Cell Signaling (Danvers, MA). Monoclonal anti-cyclin B1, anti-DRP1, and anti-cytochrome c were obtained from BD Biosciences. Monoclonal anti-cyclin A was obtained from Transduction Laboratories. Monoclonal anti-bromodeoxyuridine (BrdUrd)-Alexa Fluor 594 conjugated was obtained from Molecular Probes (Eugene, OR). Monoclonal anti-SUMO1 (anti GMP1) was obtained from Zymed Laboratories Inc., polyclonal anti-SUMO1 was obtained from Cell Signaling. Monoclonal, anti-KI-67 was obtained from DAKO (Denmark). Monoclonal anti-HSP60 was obtained from Sigma and monoclonal anti-HSP70 from Stressgen (Victoria, Canada). Anti-MFN2 serum was obtained by immunizing rabbits with recombinant MFN2-glutathione S-transferase as described (31). Anti-rabbit Alexa Fluor 514 and anti-mouse Alexa Fluor 647 secondary antibodies were obtained from Invitrogen. Thymidine, nocodazole, puromycin, ATP, BrdUrd, and N-methylmaleimide were obtained from Sigma. Dulbecco's modified Eagle's medium, suspension minimum essential medium, Hoechst 33258, Lipofectamine 2000, and DNase I were obtained from Invitrogen. Arrest-In transfection lipid was obtained from Open Biosystems (Huntsville, AL). Protease inhibitor mixture was obtained from Roche (Switzerland). Trypsin was obtained from Fluka Biochemika (Buchs, Switzerland), and Triton X-100 was obtained from Bio-Rad. Fetal bovine serum was obtained from HyClone.
DNA Constructs
pOCT:CFP, SenP5:YFP, and pQE31:SenP5 were described previously (6, 8). To obtain pQE30:MTS (for recombinant protein expression and antibody production) MTS:FLAG was digested with BamHI and HindIII. The fragment was ligated to pQE30, cut with BamHI and HindIII.
Cell Culture and Synchronization
COS-7 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% heat-inactivated fetal bovine serum (HyClone), 2 mm l-glutamine, penicillin, and streptomycin. Transfections with OCT:CFP and SenP5:YFP were performed overnight with COS-7 cells at 80–90% confluence, using Lipofectamine 2000 (Invitrogen). Synchronization experiments were performed as described (32). Briefly, cells were incubated with 2 mm thymidine for 12 h to block DNA replication, followed by a release in thymidine-free medium for 9 h. A second incubation with 2 mm thymidine for 12 h was performed to bring most cells to synchrony. For synchronization of cells in mitosis (prophase) a double thymidine block followed by 100 ng/ml nocodazole incubation for 14 h was performed. For sHeLa cells, suspension minimum essential medium was used, and cells were incubated in Fernbach flasks (Fisher Scientific) for at least 5 days, until reaching a density of 100,000/ml. Then thymidine (2 mm) or Nocodazole (100 ng/ml) was added to the flasks and cells were incubated for 20 h, to become synchronized, and finally harvested and subjected to fractionation.
Cell Proliferation Assay
Approximately 400,000 COS-7 stable cells of either pSHAG-MAGIC2 vector alone or pSHAG-MAGIC2-SenP5 shRNA, were seeded on 6-well plates, in triplicate, in Dulbecco's modified Eagle's medium + 10% fetal calf serum containing 20 μg/ml puromycin. At days 3, 5, and 7 cells were counted and reseeded. Vector stable cells were reseeded 1:4 at day 3, and 1:3 at day 5, to avoid confluence, to keep the cells growing in logarithmic phase (for the final cell numbers dilution factors were considered in the calculations).
Flow Cytometry
HeLa cells stably silenced for vector-shRNA, SenP5-shRNA I or SenP5-shRNA II were grown in complete culture medium + 2 μg/ml puromycin as a selective agent. To optimize the silencing of SenP5 for cell cycle studies (DNA content), the cells were grown in 20 μg/ml puromycin and samples (100,000 cells aliquots) were taken at day 3, then fixed with cold ethanol for 20 min and stained with 50 μg/ml propidium iodide (Sigma) + 100 μg/ml RNase A for 20 min at room temperature. Cells were then analyzed for DNA content by FACS using the 488 nm laser (FACSAria, BD Biosciences).
Immunofluorescence and Microscopy Studies
For immunofluorescence, cells previously seeded onto coverslips were washed 3 times with PBS and fixed in 4% paraformaldehyde/PBS for 15 min at 37 °C. Cells were then quenched with 50 mm ammonium chloride in PBS for 10 min at room temperature, and permeabilized with 0.1% Triton X-100/PBS for 10 min. Cells were then blocked with 5% FBS in PBS for 1 h at room temperature, then incubated with primary antibody for 2 h followed by 3 washes in blocking solution. Cells were next incubated with goat anti-mouse or goat anti-rabbit conjugated (515 or 647 nm) Alexa Fluor secondary antibodies (Invitrogen) for 45 min at room temperature followed by 3 washes with PBS. A final incubation with 2.5 μg/ml Hoechst in PBS (to observe DNA) was performed, followed by 3 PBS washes and finally mounting onto slides. For anti-BrdUrd staining, cells grown on coverslips and previously transfected with OCT-CFP + SenP5-YFP were incubated with 20 μm BrdUrd for 30 min at 37 °C. Cells were then fixed, washed, and permeabilized, followed by incubation with a mixture containing 0.15 m NaCl, 4.2 mm MgCl2, 10 μm HCl, 5000 units/ml DNase I (Invitrogen), for 30 min at 37 °C. Cell were then washed and incubated with 1 n HCl for 10 min at room temperature, followed by PBS washes. The 2 latest incubation steps are to make the chromatin accessible to the antibody. Cells were then blocked and stained with monoclonal anti-BrdUrd conjugated to Alexa Fluor 594, washed with PBS, and mounted for microscopy. Fixed cells transfected with fluorescence constructs or stained with antibodies were imaged on Olympus IX70 microscope with a ×100 objective U Plan Apochromat, NA 1.35–0.50 objective, excited at 514 (YFP), 434 (CFP), and 350 nm (Hoechst), with the Polychrome IV monochrometer (Till Photonics, Grafelfing, Germany). The emitted light was filtered through either Till double CFP/YFP or Fura filters. Confocal images were obtained with a ×100 objective NA1.4 on an Olympus IX81 inverted microscope with appropriate lasers (440 nm for CFP 434, 515 nm Argon for YFP 515, and 633 nm for Alexa Fluor 647), using Olympus FV1000 confocal scanning microscope. Images acquired were saved as tiff images and overlaid in Adobe Photoshop for image assembly.
To quantify the recruitment of DRP1 to mitochondrial membranes, synchronized cells were fixed and stained with monoclonal anti-DRP1 and polyclonal anti-TOM20. The cytosolic pool of DRP1 is visible by the antibody and appears as small, granular staining. Therefore, in each cell 10 short line scans were plotted across the cytosolic DRP1 signal and an additional 10 scans were taken crossing DRP1 foci that were aligned along the TOM20-labeled mitochondria. This was done for 7 cells in the G1/S- and G2/M-synchronized cells, with care taken to maintain the confocal scanning conditions below saturation and constant for each coverslip (including the laser power, PMT voltage, and scanning pixel resolution). The maximum pixel intensity across the line scans on mitochondrial DRP1 foci were averaged per cell and divided by the maximum pixel intensities across the scans taken from the cytosolic DRP1 signals within the same cell. This provides a ratio defining the relative increase in endogenous DRP1 recruitment to the mitochondrial membranes compared with the cytosolic signal. The standard deviation of this ratio was then calculated and graphed accordingly.
Fluorescence recovery after photobleaching was done as described (7). Briefly, HeLa cells grown on coverslips were transfected with DRP1-YFP, and the following day, were synchronized with either a double thymidine block or with an additional 12-h incubation with nocodazole. Cells were loaded into a live imaging chamber and incubated with Mitofluor Red 633 for 15 min to label the mitochondria. Using the Olympus FV1000, the DRP1-YFP signal within a region of interest was bleached with 3 scans using the 514-nm laser line at 50% power. 50 images of the entire cell were then taken every 6 s, and the percentage of DRP-YFP fluorescence within the region of interest relative to the entire cell was calculated as described (7), and the average of the recovery plotted with standard deviations indicated. The initial slopes were calculated from the data obtained in the first 40 s following the bleach, and S.E. obtained with their associated p values. Given the very rounded shape of cells following nocodazole incubation, we released the cells for 1 h in complete medium before imaging. In this way the fluorescence recovery after photobleaching analysis from nocodazole-treated cells were limited to prophase.
Cell Fractionation and Trypsin Digestion
Unsynchronized and synchronized cells were washed in PBS and harvested by trypsinization and centrifugation (HeLa) or centrifugation (sHeLa), followed by 2 washes in cold PBS and centrifugation. The cell pellets were resuspended in 1 ml of homogenization buffer (220 mm mannitol, 68 mm sucrose, 20 mm Hepes, pH 7.4, 80 mm KCl, 0.5 mm EGTA, 2 mm MgOAc2, protease inhibitors). Cells were broken in a cell cracker (10 passages, 8.002 ball) and after taking an aliquot of the total fraction, the nuclear fraction was separated by centrifugation at 800 × g for 10 min at 4 °C. The post-nuclear supernatant was centrifuged at 9,300 × g for 20 min at 4 °C to pellet mitochondria. Post-mitochondrial supernatant was centrifuged at 200,000 × g to clear the cytosolic fraction of light membranes. Total, mitochondrial, and cytosolic fractions were normalized for protein content, and around 50 μg were loaded for electrophoresis and Western blotting. For the trypsin digestion experiment, mitochondria were isolated from synchronized sHeLa suspension cultured cells and 20-μg aliquots were incubated on ice for 30 min with 100 μg/ml trypsin (Fluka Biochemika), or buffer containing trypsin plus 1% Triton X-100 (Bio-Rad), then processed for electrophoresis and Western blotting.
shRNA Studies
shRNA was performed using the prepared sh-activated mRNA silencing system (Open Biosystems). Two different shRNAs inserted into pSHAG-MAGIC2 vectors were as follows: (shRNAI) 5′-TGCTGTTGACAGTGAGCGACCAGTTTACTTGGAATAGACAGTAGTGAAGCCACAGATGTA-3′, and (shRNAII) 5′-TGCTGTTGACAGTGAGCGCGCGCAGATGGTTTGTTACTTGAATAGTGAAGCCACAGATGTAT-3′. COS-7 cells were transfected using Arrest-In transfection reagent (Open Biosystems) and incubated for 48 h. Transfectants were selected in 3 μg/ml puromycin (Sigma) for 12 days. Control cells were transfected with pSHAG-MAGIC2 vector.
In Vitro SUMO Conjugation Assay
The SUMO conjugation assay was performed in 20-μl volumes containing 100 nm SUMO activating enzyme (Boston Biochem), 250 nm UBC9 (Biomol), 10 μm His6-SUMO1 (Boston Biochem), buffer A (50 mm Tris-HCl, pH 8.0, 100 mm NaCl, 5 mm MgCl2), 1 mm dithiothreitol, and either an ATP regenerating system (2.5 units creatine kinase, 125 nm creatine phosphate, 5 mm ATP) or 0.1 unit of apyrase in the ATP minus control; and finally, 100 μg (per reaction) of synchronized and purified sHeLa mitochondria. Reactions were incubated for 90 min at 30 °C. Streptavidin-coupled magnetic beads (Dynal) were washed in buffer B (buffer A with 2 mg/ml bovine serum albumin and 1% Triton X-100) using a magnetic particle concentrator (Dynal). For each reaction, 20 μl of packed beads were suspended in 180 μl of buffer B and incubated with the SUMO1 conjugation reaction for 35 min, rocking at 25 °C. The beads were then subjected to several washing cycles with buffer C (buffer A with 500 mm NaCl and 1% Triton X-100). The mixture was finally subjected to SDS-polyacrylamide gel electrophoresis on 4–20% gels, followed by standard immunoblotting procedures using SUMO1 antibody.
Cross-linking and Immunoprecipitation
For cross-linking studies, HeLa cells synchronized either for the G1/S or G2/M borders were harvested, washed, and a fractionation was performed in mitochondrial extraction buffer containing 20 mm N-methylmaleimide and protease inhibitors. After normalization, post-nuclear supernatants (containing both cytosolic and mitochondrial fractions) were then treated or not (controls) with 0.2, 1, 2, or 10 mm of the BMH (bis(maleimido)hexane) cross-linker, in mitochondrial buffer, for 2 h at 4 °C, to freeze protein-protein interactions. The reaction was then stopped by incubation with 10 mm dithiothreitol for 15 min at 4 °C, followed by SDS-PAGE running and inmunoblotting for DRP1, as well as controls for loading and synchronization efficiency.
For immunoprecipitation experiments, HeLa cells were synchronized for either the G1/S or G2/M border and fractionated for total, cytosolic, and mitochondrial fractions, in mitochondrial extraction buffer containing 20 mm N-methylmaleimide and protease inhibitors, as previously indicated. Each fraction (totals, cytosol, and mitochondria), from each condition, was then solubilized by adding Triton X-100 to a final concentration of 1%, for 30 min, at 4 °C, followed by ultracentrifugation at 200,000 × g for 40 min, at 4 °C. Supernatants were normalized for protein content and incubated with either a nonspecific mouse IgG or with monoclonal anti-DRP1 (2.5 μg for each mg of protein per IP point), conjugated to protein G-Sepharose beads, for 12 h, at 4 °C. After centrifugation at 10,000 × g to spin down the protein G-Sepharose-immune complexes, 3 bead washes were made in mitochondrial buffer containing 1% Triton X-100. Beads were then run by SDS-PAGE electrophoresis followed by immunoblots with either monoclonal SUMO1 or monoclonal DRP1 antibodies.
Quantification and Statistical Analysis
Quantification of blots was performed using the Scion Image program (Scion Image Corporation). Graphs with data from blots quantification or microscopy counts and statistical analysis (p values) using unpaired t test were obtained using Sigma Plot.
RESULTS
SenP5 Translocates to Mitochondria during Mitosis
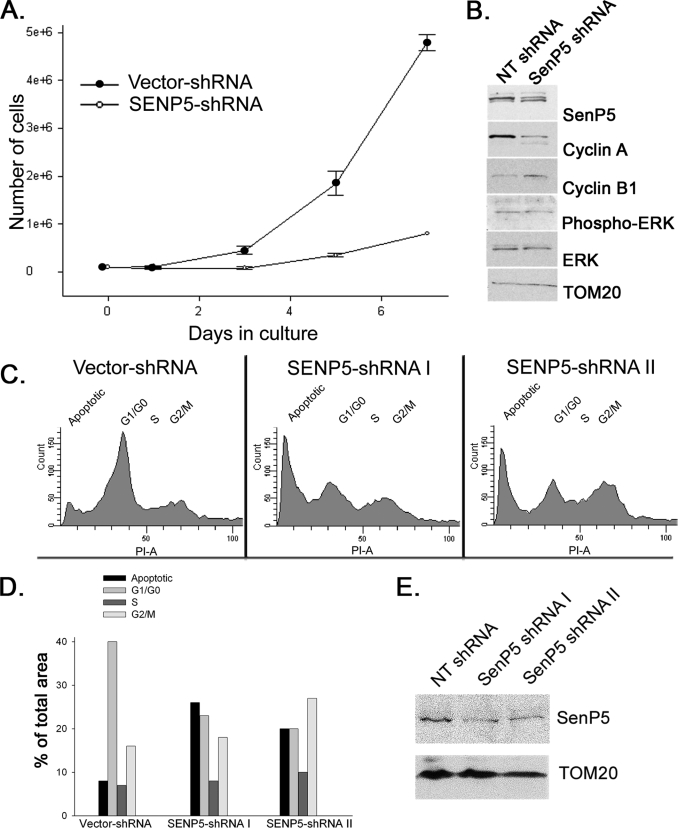
Previously it was reported that silencing the expression of SenP5 induces cell cycle arrest in cytokinesis (21). We confirmed this in COS7 cells stably transfected with SenP5-shRNA. As previously shown (8), the maximal loss of SenP5 in the targeted shRNA containing cells was 48%. The experiment shown in Fig. 1 demonstrates that a reduction in SenP5 levels led to cell cycle arrest relative to the control cells (Fig. 1A). To determine at which stage of the cycle the cells were arrested, we probed the lysates for two cell cycle markers, cyclin B1 (accumulates during mitosis) and cyclin A (accumulates in S and G2). The loading control Tom20 confirmed equal protein amounts, however, SenP5-shRNA cells showed a higher relative level of cyclin B1 (Fig. 1B) and a lower level of cyclin A than control cells, indicating cell cycle arrest occurred at the G2/M transition. Western blots of cell extracts also show no change in phospho-ERK, indicating that the G1 growth phase of cells silenced for SenP5 was not affected relative to controls (33)(Fig. 1B). We confirmed this result using FACS sorting of HeLa cells expressing either the vector shRNA, or two different SenP5 shRNA stable lines targeting unique regions of the SenP5 message. The reduction in SenP5 in the HeLa cells was similar to the COS7 cell line (Fig. 1E). Cells expressing the vector control shRNA showed a majority of cells containing 1 n DNA (40%), and 16% containing 2 n DNA, which reflects cells at G2/M (Fig. 1, C and D). In contrast, upon silencing of SenP5 with two independent shRNA, the cells showed an increase in 2 n DNA content after 3 days, consistent with biochemical data indicating a cell cycle arrest at G2/M (Fig. 1, C and D). In addition, there was a significant increase in apoptotic fragments within the cells silenced for SenP5 (from 8% in control to 20–26% in shRNA cells), which is a common event when the cell cycle is delayed (34). Therefore, consistent with previous results (21), the reduction of SenP5 is sufficient to significantly affect cell proliferation and trigger cell death. The further loss of SenP5 is likely lethal and explains why we could not obtain lower expression levels in our stable shRNA cell lines.
FIGURE 1.
Silencing of SenP5 leads to a cell cycle arrest at G2/M. A, COS-7 cells stably expressing non-targeted shRNA or SenP5 shRNA were cultured and cell numbers quantified. The graph plots the cell numbers obtained at 3, 5, and 7 days of culture. Results express the mean ± S.D. of two independent experiments. B, aliquots obtained at 7 days of the experiment in A were processed for electrophoresis and Western blots with the indicated antibodies. C, HeLa cells expressing vector-shRNA, SenP5-shRNA I, or SenP5-shRNA II were grown in 20 μg/ml puromycin for 3 days, harvested, stained with propidium iodide, and analyzed for DNA content by FACS. D, the peaks were quantified using Summit version 4.3 software and plotted, as indicated. E, an aliquot of the HeLa cells used for the FACS analysis were analyzed by Western blot to confirm the extent of SenP5 silencing using each hairpin.
Given that we have previously demonstrated a role for SenP5 in the regulation of mitochondrial dynamics and morphology (8), we examined whether cell cycle arrest induced in the absence of SenP5 may involve its mitochondrial function. Although over 90% of cells examined showed primarily nucleolar localization of SenP5 (Fig. 2A, top panels), we noticed a small percentage of cells that exhibited a different pattern of localization (Fig. 2A). We examined the mitochondria using the matrix marker pOCT-CFP (6) and followed SenP5-YFP localization in multiple cells. Interestingly, some cells showed a dual location for SenP5, both within the nucleoli and the mitochondria (Fig. 2A, middle panels), where in other cells SenP5 was recruited almost exclusively to the mitochondria (Fig. 2A, bottom panels). Finally, in the mitotic cells identified by the condensed chromosomes we could observe that SenP5-YFP was completely targeted to the mitochondria (Fig. 2B). To better characterize whether the shift of SenP5 to the mitochondria occurred in a cell cycle-dependent manner, we synchronized COS-7 cells either at the G1/S boundary, or at G2/M. We fractionated the cytosol and mitochondria and probed the fractions with antibodies against endogenous SenP5. The data revealed a shift of endogenous SenP5 to the mitochondria during mitosis (Fig. 2C), consistent with the SenP5-YFP translocation observed by microscopy. Although the total cellular levels of DRP1 did not change during the cell cycle, we observed a significant reduction in the stable association of DRP1 with mitochondrial membranes during mitosis (Fig. 2C). Interestingly, DRP1 appears as a doublet on the 6% SDS-PAGE, and in addition to an overall reduction in mitochondrial associated DRP1, the top band of the doublet is significantly reduced within the mitochondrial fraction at G2/M, consistent with a shift in post-translational modifications at this time. The success of the synchronization was revealed by cell cycle markers cyclin B1, whose levels are maximal at G2/M, and cyclin A, maximal at G1/S. Western blot analysis of the mitochondrial SenP5 protein present at G2/M revealed that it was completely degraded by the addition of trypsin, whereas the intermembrane space protein, cytochrome c, was protected (Fig. 2D). This demonstrates that SenP5 is not imported, but is recruited to the surface of the mitochondria. This localization would suggest that the majority of SenP5 substrates are either outer membrane or peripherally associated proteins such as DRP1.
FIGURE 2.
SenP5 translocates to the mitochondrial membrane during the G2/M phase. A, COS-7 cells were transfected with OCT-CFP, SenP5-YFP, fixed, and stained with Hoechst, prior to analysis by fluorescence microscopy. B, mitotic cells at prophase, anaphase, and telophase, showing SenP5-YFP on the mitochondria (red). Chromatin distribution was revealed by Hoechst (green). Scale is 5 μm. C, COS-7 cells were synchronized with either a double thymidine block alone (G1/S), or the thymidine block followed by a 12-h release in 100 ng/ml nocodazole (G2/M). These cells were harvested, and total extracts, cytosolic and mitochondrial fractions were obtained. Then, 25 μg of protein from each fraction was loaded on the gel and processed for electrophoresis and Western analysis with antibodies against endogenous SenP5, DRP1, HSP60, TOM20, and HSP70, as shown. Cyclin A (G1/S) and cyclin B1 (G2/M) were probed as synchronization controls. Asterisks indicate the higher migrating form of DRP1 that is lost upon the delivery of SenP5 to the mitochondria in nocodazole-treated cells. D, COS-7 cells were synchronized with double thymidine block, followed by a 6-h release to enrich endogenous SenP5 on the mitochondria. Cells were harvested, mitochondria were isolated, and 20-μg aliquots were either left untreated (lane 1), or treated with 0.1 mg/ml trypsin in the presence or absence of 1% Triton X-100 (second and third lanes 2). Western blots were probed for endogenous SenP5, and cytochrome c as an internal control.
To precisely map the stage within the cell cycle when SenP5 transits outside of the nucleus to the mitochondria we stained COS-7 cells transfected with SenP5-YFP with a monoclonal antibody against the cell cycle marker KI-67, a nuclear protein present in cycling cells that exhibits unique subnuclear localizations in each stage of the cell cycle (35). Based on these properties, we imaged the transiently transfected pOCT-CFP and SenP5-YFP, and observed that SenP5-YFP mostly colocalizes with nucleolar KI-67 in G1 and S phases (Fig. 3A, top two panels). During G2, when KI-67 is found in irregular nucleoli and nucleoplasm, SenP5 exhibits faint mitochondrial localization, additional to its colocalization with KI-67 in irregular nucleoli (Fig. 3A, third panels). At prophase, KI-67 associates with condensing chromosomes, whereas SenP5 migrates to the mitochondria (bottom panels). These results show that SenP5 starts to exit the nucleus at G2, whereas the nuclear envelope is still intact. Upon disassembly of the nucleoli and nuclear envelope, SenP5 associates with mitochondria.
FIGURE 3.
Quantification of SenP5 recruitment to the mitochondria using multiple cell cycle markers. A, unsynchronized COS-7 cells were transfected with OCT-CFP and SenP5-YFP overnight. The cells were then fixed and stained with an antibody against the cell cycle marker KI-67. Representative fluorescence microscopy pictures at different stages of the cell cycle are shown. Insets show colocalization of SenP5-YFP and the mitochondrial marker OCT-CFP. Scale bar, 5 μm. B, quantification of SenP5-YFP distribution along the cell cycle. COS-7 cells were transfected with OCT-CFP and SenP5-YFP overnight, then stained with monoclonal antibodies against different markers of the cell cycle: anti-cyclin D1 and -2 for G1 phase, anti-BrdUrd for S phase, anti-cyclin A for S and G2 phases, anti-cyclin B1 (cytosolic) for the G2 phase, and nuclear cyclin B1 staining reflected the M phase. The distribution of SenP5-YFP was scored as nuclear, nuclear and mitochondrial, or mitochondrial, and determined as a percentage of total cells positive for both SenP5-YFP transfection and for the different cell cycle markers. Results express the mean ± S.D. of three independent experiments, with at least 50 cells examined in each case.
We expanded this approach by quantifying the localization of SenP5-YFP in cells stained with a number of established cell cycle markers, including cyclin D1 antibodies for G1 phase, BrdUrd incorporation and anti-cyclin A staining for S phase, and cyclin B1 staining for G2 (cytosolic) and prophase (nuclear). In each condition, cells were cotransfected with SenP5-YFP and the mitochondrial marker pOCT-CFP. Cells that showed bright cyclin D1 fluorescence had SenP5-YFP mainly within the nucleus (at the nucleoli) (Fig. 3B), cells that incorporated anti-BrdUrd fluorescence at the nucleus (S) had SenP5-YFP mainly at the nucleoli and a few cells showed faint mitochondrial staining. Cells with nuclear cyclin A fluorescence (S/G2) had SenP5 in the nucleus and on the mitochondria. Cells that showed bright anti-cyclin B1 fluorescence in the cytosol (G2) had SenP5-YFP mostly in the nucleus and recruited to the mitochondria and some of them only on the mitochondria, and cells showing bright cyclin B1 fluorescence in the nucleus (prophase) had SenP5-YFP mostly on the mitochondria. These results confirmed that SenP5 starts to actively move out of the nucleus between S and G2, before being recruited to the mitochondria during mitosis.
SenP5 deSUMOylates Mitochondrial Targets and Alters Morphology
The presence of SenP5 on the mitochondria was accompanied by a reduction in the stable association of DRP1 with the mitochondrial membrane (Fig. 2C). Therefore we tested whether this was also accompanied by a change in mitochondrial morphology. COS7 cells were synchronized at G1/S, or in mitosis, and morphology of the mitochondria in these cells was quantified. Consistent with previously published data (5), the quantification revealed a significant increase in cells containing fragmented mitochondria at G2/M (Fig. 4, A and B). To quantify the recruitment of endogenous DRP1 to the mitochondrial membrane, we stained cells with anti-DRP1 antibodies (Fig. 4C). In both thymidine- and nocodazole-treated cells, confocal imaging reveals DRP1 fluorescence within small foci localized in the cytosol, some of which may likely localize to microtubules, as previously described (36, 37). In addition, there are also DRP1 foci that colocalize with the mitochondria. To score for an enrichment of DRP1 recruitment, we performed line scans across the cytosolic and mitochondrial DRP1 foci and generated a ratio of fluorescence (Fig. 4, C and D). In thymidine-treated cells, the intensity of DRP1 fluorescence on the mitochondria is similar to those within the cytosol. However, in nocodazole-treated cells, the mitochondrial association is enriched by 2-fold relative to cytosolic DRP1 (Fig. 4D). Therefore, although biochemical fractionation demonstrated a reduction in the stable membrane-associated DRP1 (Fig. 2C), immunofluorescence staining of DRP1 indicated that within intact cells there is an increased amount of DRP1 localizing along the mitochondrial tubules during G2/M (Fig. 4, C and D). This suggests that the association of DRP1 on the mitochondria is more labile during mitosis than at the G1/S transition, and is more readily washed away from the mitochondria during biochemical fractionation, as seen in Fig. 2.
FIGURE 4.
Mitochondria fragment in mitosis and a more labile pool of DRP1 is enriched on the membranes. A, panels showing cells synchronized as indicated, fixed, and stained with anti-Tom20 antibodies. Scale bar, 10 μm. B, graph showing the mitochondrial phenotype in G1/S (thymidine) versus G2/M (nocodazole)-synchronized cells (results are mean ± S.D. of 3 coverslips examined in each case, with 50 cells examined per coverslip). C, COS-7 cells were synchronized as indicated, then fixed and stained with monoclonal anti-DRP1 (red) and polyclonal anti-Tom20 (green), for analysis by confocal fluorescence microscopy. Line scans were drawn across DRP1 puncta that localized with mitochondria, and those that were found within the cytosol. Examples of these foci are shown for each condition (inset) and the line scans are indicated. Scale bars, 5 μm. D, the relative intensity of endogenous DRP1 foci along the mitochondrial tubules relative to the background cytosolic signal were quantified using line scans in cells synchronized in G1/S (thymidine) and G2/M (nocodazole). The results are the mean ± S.D. of 10 cytosolic DRP1 line scans versus 10 mitochondrial DRP1 line scans per cell with n = 7 cells examined for both G1/S and G2/M phases.
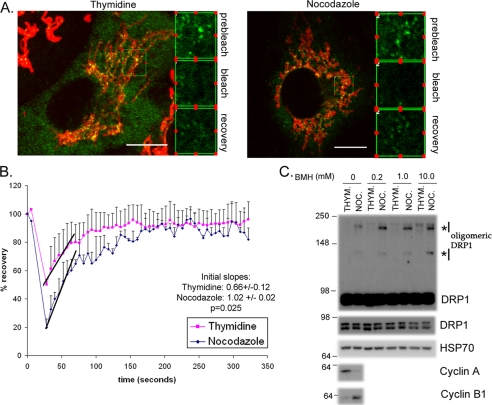
To confirm whether cycling of DRP1 recruitment to mitochondria is more labile during mitosis, we employed fluorescence recovery after photobleaching experiments (Fig. 5, A and B). HeLa cells were transfected with DRP1-YFP, and synchronized either in G1/S or G2/M. The data show that the recovery of DRP1-YFP onto mitochondria following photobleaching occurs in both conditions; however, the rates of recovery are increased nearly 2-fold in prophase (Fig. 5B). This is consistent with an increase in endogenous DRP1 recruitment (Fig. 4, C and D), but an increase in recycling rates is also consistent with the biochemical evidence that mitochondrial associated DRP1 is less stably associated with the membranes (Fig. 2C). To determine whether increased rates of recycling may correlate to an increase in functional fission activity, we performed cross- linking studies on post-nuclear supernatants to examine oligomerization of DRP1 (Fig. 5C). It has previously been shown that an increase in dynamin oligomerization reflects an increase in the fission-competent rings formed during constriction (38–41). We observe a cell cycle-specific increase in DRP1 oligomers during mitosis (Fig. 5C), which is consistent with an increase in mitochondrial fission observed at this time (Fig. 4, A and B). Together these data show an increase in the lability of DRP1, which leads to an increased formation of functional oligomers during mitosis.
FIGURE 5.
DRP1 association with mitochondria is more labile, leading to an increase in functional oligomers during mitosis. A, HeLa cells transfected with DRP1-YFP were synchronized with thymidine or nocodazole. The nocodazole was washed out for 60 min to facilitate a more complete attachment of the cells to the coverslips during imaging. Cells were labeled with Mitofluor Red 633 and the extent of DRP1-YFP recovery after photobleaching is equivalent in both thymidine- and nocodazole-synchronized cells. B, the quantification of fluorescence recovery after the bleach shows that the rates of recovery are distinct. The average curves are shown from (n = 3) thymidine- and (n = 2) nocodazole-treated cells. The lines indicate the initial slopes of DRP1-YFP recovery following the bleach. Quantification of the slopes are indicated along with the statistical significance (inset). C, HeLa cells synchronized with thymidine or nocodazole were harvested, and the post-nuclear supernatants were incubated with increasing concentrations of bis(maleimido)hexane (BMH) cross-linker, as indicated. Western blots with anti-DRP1 antibodies reveal the enrichment of oligomeric forms of DRP1, which is specific to the nocodazole-treated cells (asterisks). HSP70 antibodies control for equal loading in each lane, where cyclins A and B indicate the efficiency of synchronization.
To test whether SenP5 delivery to the mitochondria directly affects mitochondrial targets of SUMOylation, including DRP1, we examined isolated mitochondria probed for endogenous SUMO conjugates. Western blots probing for endogenous SUMO1 conjugates revealed an overall reduction of mitochondrial SUMO targets during mitosis (Fig. 6A, top panel). As expected, we also saw a reduction in SUMO2/3 conjugates on the mitochondria (data not shown). These results demonstrate that the recruitment of SenP5 to the mitochondria during mitosis (Fig. 6A, middle panel) leads to a general reduction of mitochondrial SUMO1 targets. In addition, the data reveal that the global pattern of mitochondrial SUMOylated targets were reduced, consistent with the biochemical evidence that SUMO proteases do not exhibit substrate specificity, and instead bind to the SUMO1 protein within the conjugates (42, 43).
FIGURE 6.
Mitochondrial SUMOylation is decreased during mitosis. A, sHeLa cells were grown in suspension and synchronized for 20 h with either 2 mm thymidine or 100 ng/ml nocodazole, then harvested and processed for mitochondria purification. SUMO1-conjugated targets in G1/S (thymidine) versus mitotic (nocodazole) mitochondria. Mitochondria isolated from the synchronized cells show a decrease in endogenous SUMO1 conjugates, concomitant with the recruitment of SenP5 during mitosis (HSP60 shown as internal loading control). B, aliquots of the total cell fractions were probed for cyclin A and cyclin B1 to monitor the synchronization efficiency (HSP70 was used as loading control). C, mitochondria were incubated in the indicated conditions (“Experimental Procedures”) and probed for the association of conjugated His6-SUMO1. The conjugation of SUMO1 to the mitochondrial targets is ATP dependent. HSP60 was probed to monitor mitochondrial loading. D, the data from three independent conjugation experiments, using mitochondria from two independent fractionations were quantified and, after subtraction of background signal (−ATP conditions), the percentage of total SUMO1 conjugates intensity are plotted in G1/S and G2/M. E, sHeLa cells synchronized as indicated were fractionated into cytosol or mitochondrial fractions, solubilized, and anti-DRP1 antibodies used to immunoprecipitate endogenous DRP1. Extracts were loaded on two independent gels, with one probed with anti-SUMO1 antibodies, and the other with DRP1 antibodies. The data shows that DRP1 immunoprecipitates are immunoreactive for SUMO1, with a higher migrating species being lost specifically when SenP5 is delivered to the mitochondria in nocodazole-synchronized cells (asterisk).
To further confirm whether active mitochondrial SUMOylation is reduced during G2/M, we performed in vitro SUMOylation reactions on mitochondria isolated from synchronized cells. Mitochondria were isolated from suspension HeLa cells synchronized at G1/S, or at G2/M, then assayed for the energy-dependent conjugation of recombinant His6-SUMO in the presence of E1 and E2 sumoylating enzymes. The efficiency of synchronization was confirmed using the cell cycle-specific markers cyclin A and cyclin B1 (Fig. 6B). Mitochondria isolated from G1/S-synchronized cells were able to form SUMOylated conjugates in an energy-dependent manner (Fig. 6C, first and second lanes). This indicates that the isolated mitochondria must contain an endogenous SUMO E3 ligase sufficient to conjugate exogenously added His6-SUMO to native substrates. In contrast, mitochondria isolated from cells synchronized at G2/M are not able to efficiently SUMOylate their targets relative to interphase cells (Fig. 6C, third lane versus first lane). Quantification of these results revealed a 60% reduction in active SUMOylation targets on mitotic mitochondria (Fig. 6D), consistent with the recruitment of the protease SenP5.
This data suggested that the mitochondrial associated DRP1 would also be efficiently deSUMOylated by SenP5 along with all other targets. To test this directly, we immunoisolated endogenous DRP1 from total cell extracts, fractionated cytosol and mitochondria synchronized in G1/S or G2/M. The data reveal that the doublet form of DRP1 is immunoreactive for SUMO1 at G1/S, however, only the lower molecular weight band is SUMO positive at G2/M, both from total extracts and cytosol (Fig. 6E). This is consistent with the release of SenP5 from the nucleoli, where it would deSUMOylate DRP1. Within the mitochondrial fraction, we again observe a reduction in the biochemical association of DRP1 with the membranes, but we also observe the selective loss of the upper, SUMOylated band of DRP1 at G2/M. Interestingly, the cytosolic pool of DRP1 is found in doublet form in both conditions, yet only the lower band is SUMO positive. This may indicate a replacement of SUMOylated DRP1 with a phosphorylated or ubiquitinated form that could retain the same mobility (5, 44). Nevertheless, our data demonstrate the loss of a SUMOylated form of DRP1 during mitosis, which is consistent with the selective delivery of SenP5 to the mitochondrial membranes. That the lower molecular weight band of DRP1 remains SUMOylated indicates that the cycle of SUMOylation is retained during mitosis, consistent with our previous observation that the SUMOylation of DRP1 enhances its recruitment and fission activity (6–8). From this data, we consider that the delivery of SenP5 stimulates the cycle of DRP1 SUMOylation and deSUMOylation, which increases the rate of oligomerization, mitochondrial fission, and DRP1 recycling (Fig. 7).
FIGURE 7.
Schematic of SenP5 transition and the DRP1 SUMOylation cycle. A, illustrates the translocation of SenP5 from the nucleoli to the mitochondria at G2/M, which coincides with the global deSUMOylation of mitochondrial targets and increase in mitochondrial fission. B, our data indicates that the SUMOylation cycle of DRP1 is enhanced upon delivery of SenP5 to the membrane. The deSUMOylation of DRP1 helps to drive the oligomerization of DRP1, which promotes mitochondrial fission during mitosis.
DISCUSSION
Recent data has highlighted the importance of metabolic stability and mitochondrial function before cells commit to DNA synthesis in S phase (1, 2). This illustrates the high degree of cross-talk between the mitochondrial and cell signaling pathways (45). During mitosis, it is equally important that the intracellular organelles, including the mitochondria, be equally and efficiently segregated into the daughter cells. Work presented here provides new insights into how events within the nucleus can be communicated directly to the mitochondrial surface to initiate the morphological changes that accompany cell division. The use of SUMOylation as a means to translate these changes is consistent with its role in multiple aspects of cell cycle regulation. For example, it has been shown in yeast that translocation of the SUMO E3 ligase Siz1p from the nuclear envelope to the cleavage furrow facilitates septin SUMOylation and oligomerization during cytokinesis (46). Following this, the carefully timed release of the SUMO protease, Ulp1, from the nuclear envelope into the cytosol ensures the efficient deSUMOylation of the septin ring following cytokinesis (46). Therefore, cellular localization of the SUMOylation enzymes is emerging as a critical factor that controls cell cycle progression. Given this precedent, it is significant that the silencing of SenP5 leads to a cell cycle arrest at G2/M, precisely at the point where the protein should be recruited to the mitochondria. This suggests that the gain of SenP5-mediated deSUMOylation activity on mitochondrial targets plays an important role in the G2/M transition. Just as the mitochondria exert a cell cycle checkpoint at the G1/S transition (1, 2), these data hint toward a second requirement for mitochondrial transitions at the G2/M checkpoint.
The translocation of SenP5 to the mitochondria supports growing evidence that the changes in SUMOylation can be mediated, at least in part, through the regulated translocation of SUMO proteases and/or ligases between intracellular compartments. In addition, the specific recruitment of SenP5 to the mitochondrial surface highlights the emerging importance of this modification as a highly regulated tool to modulate mitochondrial behavior. At least one of the functional requirements for SenP5 recruitment to the mitochondria is to modulate the activity of its known target, DRP1 (8). Our previous studies have shown that conditions where SUMOylation becomes stabilized, through the silencing of SenP5 (8), overexpression of SUMO1 (6), or during apoptosis (7), lead to increased mitochondrial fragmentation. The data presented here show that movement of SenP5 to the mitochondria at the onset of mitosis appears to accelerate the binding and release cycles of DRP1, which also resulted in increased mitochondrial fragmentation. The ongoing presence of a SUMOylated form of DRP1 in both G1/S and G2/M (Fig. 6E) indicates that SUMOylation is not blocked during mitosis, rather that the timing of deSUMOylation is increased. Given the data presented here along with our previous studies, we consider that SUMOylation may play a chaperone-like function to drive DRP1 recruitment to the mitochondrial membrane (see model, Fig. 7). During apoptosis, DRP1 SUMOylation is stabilized in a Bax/Bak-dependent manner, and this stabilizes the protein on the membrane. The data here show that stimulating deSUMOylation of DRP1 on the mitochondrial membrane during mitosis enhances its incorporation into functional oligomers, and leads to an increase in overall recycling rates, a process that is required to facilitate membrane scission. In this way the deSUMOylation appears essential to complete the cycle and release DRP1 from the membrane (Ref. 7 and this study). Finally, because DRP1 also undergoes regulated ubiquitination, phosphorylation, and dephosphorylation, it is likely that the combinatorial use of post-translational modifications contributes to its recruitment, assembly, and constriction to drive mitochondrial fission (5, 44, 47–50). We observe a change in DRP1 SUMOylation during mitosis, however, the cytosolic pool of DRP1 continues to migrate as a doublet (Fig. 6E), suggesting the involvement of another modification at this time. Therefore, cell cycle-dependent changes in other post-translational modifications may also contribute to the labile properties of DRP1 during G2/M (5). These data highlight the integration of mitochondrial morphology with cellular cues, and provide insight into the role of SUMOylation enzymes as a switch to modulate mitochondrial shape.
Importantly, our previous data (6, 8), along with this study (Fig. 5), reveal additional mitochondrial targets that likely also play important roles in the G2/M transition. We are currently pursuing the mitochondrial SUMO proteome in an effort to understand the contribution of mitochondrial deSUMOylation to cell cycle transition. Because SenP5 also deSUMOylates SUMO2/3 (21, 24), it will be important to follow the potential function of these conjugates and the substrates of mixed chains between SUMO1, -2, and -3. For now, these data are the first to provide a direct molecular link between the breakdown of the nucleoli and the preparation of the mitochondria to complete the cell cycle. Future work will uncover the mechanisms that mediate SenP5 movement from the nucleoli to the mitochondria and provide further insights into how these two organelles are functionally integrated during progression of the cell cycle.
This work was supported in part by Canadian Institutes of Health Research Grant 68833.
- DRP1
- dynamin-related protein 1
- ERK
- extracellular signal-regulated kinase
- BrdUrd
- bromodeoxyuridine
- YFP
- yellow fluorescent protein
- CYP
- cyan fluorescent protein
- shRNA
- short hairpin RNA
- FACS
- fluorescence-activated cell sorter
- PBS
- phosphate-buffered saline
- E1
- ubiquitin-activating enzyme
- E2
- ubiquitin carrier protein.
REFERENCES
- 1.Mandal S., Guptan P., Owusu-Ansah E., Banerjee U. ( 2005) Dev. Cell 9, 843– 854 [DOI] [PubMed] [Google Scholar]
- 2.Owusu-Ansah E., Yavari A., Mandal S., Banerjee U. ( 2008) Nat. Genet. 40, 356– 361 [DOI] [PubMed] [Google Scholar]
- 3.Yaffe M. P. ( 1999) Science 283, 1493– 1497 [DOI] [PubMed] [Google Scholar]
- 4.Boldogh I. R., Fehrenbacher K. L., Yang H. C., Pon L. A. ( 2005) Gene 354, 28– 36 [DOI] [PubMed] [Google Scholar]
- 5.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. ( 2007) J. Biol. Chem. 282, 11521– 11529 [DOI] [PubMed] [Google Scholar]
- 6.Harder Z., Zunino R., McBride H. ( 2004) Curr. Biol. 14, 340– 345 [DOI] [PubMed] [Google Scholar]
- 7.Wasiak S., Zunino R., McBride H. M. ( 2007) J. Cell Biol. 177, 439– 450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zunino R., Schauss A., Rippstein P., Andrade-Navarro M., McBride H. M. ( 2007) J. Cell Sci. 120, 1178– 1188 [DOI] [PubMed] [Google Scholar]
- 9.Geiss-Friedlander R., Melchior F. ( 2007) Nat. Rev. Mol. Cell Biol. 8, 947– 956 [DOI] [PubMed] [Google Scholar]
- 10.Martin S., Wilkinson K. A., Nishimune A., Henley J. M. ( 2007) Nat. Rev. Neurosci. 8, 948– 959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palvimo J. J. ( 2007) Biochem. Soc. Trans. 35, 1405– 1408 [DOI] [PubMed] [Google Scholar]
- 12.Pichler A., Gast A., Seeler J. S., Dejean A., Melchior F. ( 2002) Cell 108, 109– 120 [DOI] [PubMed] [Google Scholar]
- 13.Kagey M. H., Melhuish T. A., Wotton D. ( 2003) Cell 113, 127– 137 [DOI] [PubMed] [Google Scholar]
- 14.Pungaliya P., Kulkarni D., Park H. J., Marshall H., Zheng H., Lackland H., Saleem A., Rubin E. H. ( 2007) J. Proteome Res. 6, 3918– 3923 [DOI] [PubMed] [Google Scholar]
- 15.Hammer E., Heilbronn R., Weger S. ( 2007) FEBS Lett. 581, 5418– 5424 [DOI] [PubMed] [Google Scholar]
- 16.Rajendra R., Malegaonkar D., Pungaliya P., Marshall H., Rasheed Z., Brownell J., Liu L. F., Lutzker S., Saleem A., Rubin E. H. ( 2004) J. Biol. Chem. 279, 36440– 36444 [DOI] [PubMed] [Google Scholar]
- 17.Ivanov A. V., Peng H., Yurchenko V., Yap K. L., Negorev D. G., Schultz D. C., Psulkowski E., Fredericks W. J., White D. E., Maul G. G., Sadofsky M. J., Zhou M. M., Rauscher F. J., 3rd ( 2007) Mol. Cell 28, 823– 837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson E. S. ( 2004) Annu. Rev. Biochem. 73, 355– 382 [DOI] [PubMed] [Google Scholar]
- 19.Li S. J., Hochstrasser M. ( 1999) Nature 398, 246– 251 [DOI] [PubMed] [Google Scholar]
- 20.Taylor D. L., Ho J. C., Oliver A., Watts F. Z. ( 2002) J. Cell Sci. 115, 1113– 1122 [DOI] [PubMed] [Google Scholar]
- 21.Di Bacco A., Ouyang J., Lee H. Y., Catic A., Ploegh H., Gill G. ( 2006) Mol. Cell. Biol. 26, 4489– 4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay D., Dasso M. ( 2007) Trends Biochem. Sci. 32, 286– 295 [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay D., Ayaydin F., Kolli N., Tan S. H., Anan T., Kametaka A., Azuma Y., Wilkinson K. D., Dasso M. ( 2006) J. Cell Biol. 174, 939– 949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong L., Yeh E. T. ( 2006) J. Biol. Chem. 281, 15869– 15877 [DOI] [PubMed] [Google Scholar]
- 25.Matic I., van Hagen M., Schimmel J., Macek B., Ogg S. C., Tatham M. H., Hay R. T., Lamond A. I., Mann M., Vertegaal A. C. ( 2008) Mol. Cell. Proteomics 7, 132– 144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X. D., Goeres J., Zhang H., Yen T. J., Porter A. C., Matunis M. J. ( 2008) Mol. Cell 29, 729– 741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matic I., Macek B., Hilger M., Walther T. C., Mann M. ( 2008) J. Proteome Res. 7, 4050– 4057 [DOI] [PubMed] [Google Scholar]
- 28.Felberbaum R., Hochstrasser M. ( 2008) Cell Cycle 7, 52– 56 [DOI] [PubMed] [Google Scholar]
- 29.Bischof O., Schwamborn K., Martin N., Werner A., Sustmann C., Grosschedl R., Dejean A. ( 2006) Mol. Cell 22, 783– 794 [DOI] [PubMed] [Google Scholar]
- 30.Li S. J., Hochstrasser M. ( 2003) J. Cell Biol. 160, 1069– 1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuspiel M., Zunino R., Gangaraju S., Rippstein P., McBride H. M. ( 2005) J. Biol. Chem. 280, 25060– 25070 [DOI] [PubMed] [Google Scholar]
- 32.Stein G. S., Borun T. W. ( 1972) J. Cell Biol. 52, 292– 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meloche S., Pouysségur J. ( 2007) Oncogene 26, 3227– 3239 [DOI] [PubMed] [Google Scholar]
- 34.Blagosklonny M. V. ( 2007) Cell Cycle 6, 70– 74 [DOI] [PubMed] [Google Scholar]
- 35.Scholzen T., Gerdes J. ( 2000) J. Cell. Physiol. 182, 311– 322 [DOI] [PubMed] [Google Scholar]
- 36.Varadi A., Johnson-Cadwell L. I., Cirulli V., Yoon Y., Allan V. J., Rutter G. A. ( 2004) J. Cell Sci. 117, 4389– 4400 [DOI] [PubMed] [Google Scholar]
- 37.Cereghetti G. M., Stangherlin A., Martins de Brito O., Chang C. R., Blackstone C., Bernardi P., Scorrano L. ( 2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15803– 15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin H. W., Takatsu H., Mukai H., Munekata E., Murakami K., Nakayama K. ( 1999) J. Biol. Chem. 274, 2780– 2785 [DOI] [PubMed] [Google Scholar]
- 39.Fukushima N. H., Brisch E., Keegan B. R., Bleazard W., Shaw J. M. ( 2001) Mol. Biol. Cell 12, 2756– 2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhar D., Karren M. A., Babst M., Shaw J. M. ( 2006) J. Biol. Chem. 281, 17312– 17320 [DOI] [PubMed] [Google Scholar]
- 41.Ingerman E., Perkins E. M., Marino M., Mears J. A., McCaffery J. M., Hinshaw J. E., Nunnari J. ( 2005) J. Cell Biol. 170, 1021– 1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z., Chau S. F., Lam K. H., Chan H. Y., Ng T. B., Au S. W. ( 2006) Biochem. J. 398, 345– 352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ihara M., Koyama H., Uchimura Y., Saitoh H., Kikuchi A. ( 2007) J. Biol. Chem. 282, 16465– 16475 [DOI] [PubMed] [Google Scholar]
- 44.Karbowski M., Neutzner A., Youle R. J. ( 2007) J. Cell Biol. 178, 71– 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McBride H. M., Neuspiel M., Wasiak S. ( 2006) Curr. Biol. 16, R551– 560 [DOI] [PubMed] [Google Scholar]
- 46.Makhnevych T., Ptak C., Lusk C. P., Aitchison J. D., Wozniak R. W. ( 2007) J. Cell Biol. 177, 39– 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yonashiro R., Ishido S., Kyo S., Fukuda T., Goto E., Matsuki Y., Ohmura-Hoshino M., Sada K., Hotta H., Yamamura H., Inatome R., Yanagi S. ( 2006) EMBO J. 25, 3618– 3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura N., Kimura Y., Tokuda M., Honda S., Hirose S. ( 2006) EMBO Rep. 7, 1019– 1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang C. R., Blackstone C. ( 2007) J. Biol. Chem. 282, 21583– 21587 [DOI] [PubMed] [Google Scholar]
- 50.Cribbs J. T., Strack S. ( 2007) EMBO Rep 8, 939– 944 [DOI] [PMC free article] [PubMed] [Google Scholar]