Abstract
Activation of the NADPH oxidase homolog dual oxidase 1 (DUOX1) within the airway epithelium represents a key mechanism of innate airway host defense, through enhanced production of H2O2, which mediates cellular signaling pathways that regulate the production of various inflammatory mediators. Production of the CXC chemokine interleukin (IL)-8/CXCL8 forms a common epithelial response to many diverse stimuli, including bacterial and viral triggers, environmental oxidants, and other biological mediators, suggesting the potential involvement of a common signaling pathway that may involve DUOX1-dependent H2O2 production. Following previous reports showing that DUOX1 is activated by extracellular ATP and purinergic receptor stimulation, this study demonstrates that airway epithelial IL-8 production in response to several bacterial stimuli involves ATP release and DUOX1 activation. ATP-mediated DUOX1 activation resulted in the activation of ERK1/2 and NF-κB pathways, which was associated with epidermal growth factor receptor (EGFR) ligand shedding by ADAM17 (a disintegrin and metalloproteinase-17). Although ATP-mediated ADAM17 activation and IL-8 release were not prevented by extracellular H2O2 scavenging by catalase, these responses were attenuated by intracellular scavengers of H2O2 or related oxidants, suggesting an intracellular redox signaling mechanism. Both ADAM17 activation and IL-8 release were suppressed by inhibitors of EGFR/ERK1/2 signaling, which can regulate ADAM17 activity by serine/threonine phosphorylation. Collectively, our results indicate that ATP-mediated DUOX1 activation represents a common response mechanism to several environmental stimuli, involving H2O2-dependent EGFR/ERK activation, ADAM17 activation, and EGFR ligand shedding, leading to amplified epithelial EGFR activation and IL-8 production.
Introduction
The respiratory tract is continuously subjected to inhaled microorganisms and environmental pathogens that can cause airway injury and impact on lung function, and the airway epithelium that lines the respiratory tract therefore provides a first-line defense, by constituting a physical barrier and as a source for various innate host defense mechanisms, including inflammatory cytokines/chemokines and mucus proteins (1, 2). Among the various components that include innate epithelial host defense, tracheobronchial and alveolar epithelial cells are capable of producing hydrogen peroxide (H2O2) in response to cell activation, which largely originates from the NADPH oxidase homolog dual oxidase 1 (DUOX1),2 located primarily at the apical surface (2–5). DUOX1-derived H2O2 forms a substrate for lactoperoxidase within airway secretions to facilitate oxidation of thiocyanate (SCN−) to the highly bactericidal hypothiocyanate (OSCN−) (3, 6). In addition to this direct host defense mechanism, recent studies have also linked airway epithelial DUOX1 to intracellular signaling pathways that are involved in the production of various mucins, matrix metalloproteinase-9, and other inflammatory mediators, in response to environmental or bacterial stimuli (7–10). Therefore, epithelial DUOX1 appears to participate in additional host defense actions, such as mucociliary clearance, and may contribute to inflammatory responses and epithelial repair processes during exogenous stress.
The respiratory epithelium is a major source of the CXC chemokine interleukin (IL)-8/CXCL8, which plays a pivotal role in controlling neutrophil and monocyte chemotaxis toward sites of infection, and it may also be involved in epithelial regeneration in response to injury (11). Epithelial production of IL-8/CXCL8 is enhanced in response to many diverse stimuli, including bacterial and viral triggers that activate Toll-like receptors (TLR), various environmental oxidants, and other biological mediators such as neutrophil elastase or lipid mediators (12–18). In many cases, IL-8 production by these diverse stimuli was found to involve cellular oxidant production (19–21), and extracellular oxidants such as H2O2 are capable of directly promoting IL-8 production (19, 22). A central role for cellular H2O2 in airway epithelial IL-8 production in response to various TLR ligands was recently linked to activation of DUOX1 (7, 12), although it is not yet understood how these various diverse stimuli activate DUOX1. Activation of DUOX1 is known to depend on intracellular Ca2+ mobilization, and one of the main biological mechanisms of epithelial Ca2+ mobilization and DUOX1 activation is the activation of P2Y purinergic receptor by ATP (6, 10). Many diverse mechanical or biological stimuli, including bacterial stimuli such as flagellin (14, 15), are known to be capable of provoking the release of cellular ATP and activating purinergic receptor-mediated intracellular Ca2+ signaling (2, 6, 10). This may suggest that cellular ATP release and activation of purinergic receptors present a central and common pathway leading to DUOX1 activation and downstream pro-inflammatory cell signaling cascades in response to various environmental and bacterial triggers.
Epithelial production of IL-8 production is regulated by both transcriptional and post-transcriptional mechanisms, and it involves the activation of mitogen-activated protein kinases and activation of the transcription factors, nuclear factor (NF)-κB, activator protein (AP)-1, and CCAAT/enhancer-binding protein (13, 23–26). A critical event in airway epithelial IL-8 production in response to many stimuli is the activation of ERK1/2, associated with increased activation of the epidermal growth factor receptor (EGFR) (12, 16, 27). In addition, EGFR stimulation can also promote the activation of NF-κB (28). Sustained EGFR activation by production of autocrine ligands such as transforming growth factor (TGF)-α, because of the activation of sheddases such as a disintegrin and metalloproteinase 17 (ADAM17, also known as TNF-α converting enzyme) (29), contributes to enhanced epithelial production of IL-8 and other mediators (30, 31). Indeed, recent studies have indicated that DUOX1 activation promotes epithelial EGFR signaling by activating ADAM17 (8, 9). It has been suggested that DUOX1-derived H2O2 directly stimulates a precursor form of ADAM17 by oxidative activation of its cysteine switch at the cell surface (8, 9), although this is inconsistent with known mechanisms of intracellular proteolytic ADAM17 processing and maturation (32), which may be independent of the cysteine switch (33, 34). Moreover, activation of mature ADAM17 at the cell surface is also subject to regulation by phosphorylation and may depend on initial EGFR/ERK activation (35–37).
In this study, we sought to determine whether ATP release and DUOX1 activation contribute to airway epithelial IL-8 production in response to several bacterial stimuli acting on diverse TLR receptors. Moreover, we wished to further clarify the mechanisms by which DUOX1 activation may be associated with ADAM17/EGFR activation in response to these diverse stimuli. Overall, our results indicate that various TLR ligands can promote ATP release to activate DUOX1, which contributes to IL-8 production by these stimuli. Moreover, DUOX1 participates in ATP-mediated activation of ERK1/2 and NF-κB signaling pathways, by intracellular activation of an ADAM17-EGFR amplification loop.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatments
Immortalized human bronchial epithelial (HBE1) cells were kindly provided by Drs. R. Wu and J. Yankaskas and cultured in Ham's F-12/Dulbecco's modified Eagle's medium (1:1) supplemented with insulin (5 μg/ml), transferrin (5 μg/ml), epidermal growth factor (10 ng/ml), dexamethasone (0.1 μm), cholera toxin (10 ng/ml), bovine hypothalamus extract (15 μg/ml), and bovine serum albumin (0.5 mg/ml) at 37 °C and 5% CO2 (38). Unless otherwise mentioned, cells were grown to confluence in 24-well plates and starved for 24 h by reducing the concentrations of supplement to 10% or by omitting epidermal growth factor from the growth media, prior to experimentation. Following starvation, medium was replaced with fresh starvation medium for 2 h, and various pharmacological inhibitors were added 30 min before cell stimulation with lipopolysaccharide (LPS from Escherichia coli; 10 μg/ml; Sigma), flagellin (from Salmonella typhimurium; 10 μg/ml; InvivoGen, San Diego), or an activating antibody against asialo-GM1 (α-ASGM1, 1:100; Wako Chemicals, Richmond, VA), the glycolipid receptor that mediates cell responses to flagellin (15). For comparison, cells were stimulated with exogenous ATP (100 μm). At various time points after stimulation, cells or conditioned media were collected for the various assays described below.
RNA Interference Silencing of DUOX1 Expression
After reaching ∼80% confluence, cells were transfected with pre-designed DUOX1 siRNA or nonspecific control siRNA, lacking any significant sequence similarity to mouse, rat, or human gene sequences (100 nm; Ambion, Austin, TX), using Lipofectamine 2000 reagent (Invitrogen) (10). After 48 h, medium was replaced by starvation medium for an additional 24 h prior to experimentation. Efficiency of DUOX1 silencing was confirmed by reverse transcription-PCR analysis of DUOX1 mRNA using the primer set 5′-GCA GGA CAT CAA CCC TGC ACT CTC-3′ and 5′-CTG CCA TCT ACC ACA CGG AGC TGC-3′.
Measurement of IL-8 Protein Levels and Gene Expression
Upon 24 h of stimulation, medium was collected to measure IL-8 protein levels, and cellular RNA was extracted for analysis of IL-8 mRNA expression. IL-8 protein levels were measured using an IL-8 ELISA kit (BD Biosciences) according to the manufacturer's manual. Total RNA was isolated using RNeasy extraction kit (Qiagen, Valencia, CA) and reverse-transcribed using superscript reverse transcriptase (Invitrogen). Subsequently, real time quantitative PCR was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) and primers specifically designed and validated for human IL-8 and the housekeeping gene GAPDH. The primer set used for IL-8 amplification was 5′-CGA TGT CAT GCA TAA AGA CA-3′ and 5′-TGA ATT CTC AGC CCT CTT CAA AAA-3′. The housekeeping gene GAPDH was amplified using the primer set 5′-TTC ATT GAC CTC AAC TAC AT-3′ and 5′-GAG GGG CCA TCC ACA GTC TT-3′. Using a Prism 7900HT sequence detection system (Applied Biosystems), 40 cycles of PCR were performed using the following cycling conditions: denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. The level of IL-8 expression was normalized to GAPDH levels, and relative IL-8 mRNA levels were determined according to the comparative threshold cycle (CT) method as described previously (39). In short, CT values were determined for all IL-8 and GAPDH samples, after which the ΔCT was calculated for each treatment by subtracting the CT of GAPDH from that of IL-8. Next, the ΔΔCT values were calculated by subtracting the ΔCT of the treated samples from the control. Finally, the ΔΔCT values were transformed into absolute values with the Equation 2−ΔΔCT.
Cellular H2O2 Production
Production of extracellular H2O2 was monitored as described previously (10). In short, cells were stimulated with ATP, LPS, or α-ASGM1 in Hanks' balanced salt solution for 1 h in the presence of l-tyrosine (1 mm) and lactoperoxidase (10 μg/ml), and formation of o,o′-dityrosine was determined using HPLC with fluorescence detection. Production of intracellular oxidants was monitored after cell loading with the oxidant-sensitive fluorescent probe DCFH2-DA (Invitrogen). Cells were seeded at confluence in 96-well plates and preincubated with DCFH2-DA for 15 min, after which media was replaced with Hanks' buffered saline solution, and cells were stimulated, and fluorescence was monitored in a Synergy HT fluorescence plate reader (BioTek, Winooski, VT) for up to 60 min.
Measurement of ATP Release
Cellular release of ATP release into the medium was examined using a luciferase/luciferin bioluminescence ATP determination kit (Molecular Probes, Eugene, OR). Upon addition of the bacterial stimuli, medium aliquots (100 μl) were taken at different time points and analyzed using luciferase/luciferin in a Lumat LB 9507 luminometer (Oak Ridge, TN). To confirm the presence of ATP, cells were stimulated in the presence of apyrase (10 units/ml). The amount of ATP released was calculated using external ATP standards (1–500 nm).
Western Blot Analysis
For analyses of protein tyrosine phosphorylation, or phosphorylation of ERK1/2 or IκBα, cells were washed twice with cold PBS after stimulation and collected in 100 μl of lysis buffer (50 mm Hepes, pH 7.4, 250 mm NaCl, 10% glycerol, 1% Triton, 1.5 mm MgCl2, 1 mm phenylmethylsulfonyl fluoride, 1 mm EGTA, 2 mm Na3VO4, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) per well. Cell lysates were kept on ice for 15 min and then collected by scraping and centrifuging (14,500 rpm, 5 min) to remove cell debris. Total protein content within cell lysates was measured using the BCA protein assay kit (Pierce), and samples containing equivalent amounts of protein (10–15 μg) were analyzed by SDS-PAGE and Western blotting using polyclonal antibodies (Cell Signaling, Beverly, MA) against α-phosphotyrosine (4G10; 1:1000), phosphorylated ERK (ppERK; 1:1000), total ERK1/2 (1:500), and pIκB-α (1:500). Primary antibody binding was visualized using horseradish-conjugated secondary antibody (Cell Signaling Technology) and enhanced chemiluminescence (Pierce).
Measurement of TGF-α
HBE1 cells were starved for 24 h and stimulated with either ATP or α-ASGM1 for 2 h, and conditioned media were collected for analysis of TGF-α protein levels using ELISA (BD Biosciences). To avoid TGF-α binding to EGFR, cells were in some cases pretreated with an α-EGFR mAb (225; 4 μg/ml; Calbiochem) for 30 min prior to stimulation.
Analysis of ADAM17 Activity
Upon starvation, cell monolayers were stimulated in the presence of 10 μm of fluorogenic ADAM17 Substrate II (Calbiochem) for 2 h, after which media were collected. ADAM17 activity was analyzed using a fluorescence plate reader (excitation, 310 nm; emission, 400 nm) and expressed relative to basal activity of untreated cells.
Immunohistochemical DUOX Analysis
HBE1 cells were seeded on glass chamber slides at subconfluence, fixed with 4% paraformaldehyde in PBS, and permeabilized with 0.2% Triton X-100 in 1% bovine serum albumin/PBS for 20 min. After blocking with 1% bovine serum albumin/PBS for 1 h, slides were incubated with α-DUOX antibody (1:100 dilution; kindly provided by Dr. Miot) for 2 h, and washed 4–5 times with PBS. Slides were subsequently incubated with AlexaFluor®568 goat anti-rabbit IgG (Invitrogen; 5 μg/ml in 1% bovine serum albumin/PBS) for 45 min and washed 4–5 times with PBS, and nuclei were stained with 4′,6-diamidino-2-phenylindole (10 μg/ml for 15 min), washed, and mounted for analysis by confocal microscopy.
Data Expression and Statistical Analysis
All experiments were performed at least in triplicate, and data are presented as means ± S.E. Statistical analysis to compare the different treatment and pro-treatment groups was performed using one-way analysis of variance, and differences were considered significant if p ≤ 0.05.
RESULTS
Bacterial Stimuli Induce Cellular ATP Release and Activate DUOX1
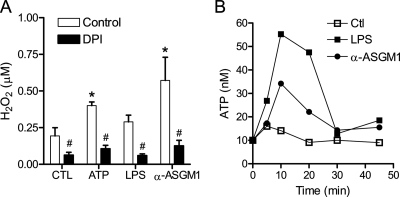
We first examined whether HBE1 cell stimulation with the TLR ligand LPS or ligation of the asialo-GM1 receptor, which is involved in TLR5-mediated responses to bacterial flagellin (15), resulted in enhanced extracellular H2O2 production, reflecting DUOX1 activation. As shown in Fig. 1A, HBE1 cell stimulation with the DUOX1 activator ATP (6, 10) or asialo-GM1 receptor ligation with α-ASGM1 (1:100) for 30 min induced extracellular H2O2 production. Cell stimulation with LPS (10 μg/ml) also slightly enhanced extracellular H2O2 production, as reported previously (40), although this did not reach statistical significance. In each case, H2O2 production was prevented by pretreatment with the NADPH oxidase inhibitor diphenylene iodonium (DPI; 1 μm), suggesting the involvement of DUOX1 activation. DPI pretreatment also significantly reduced basal H2O2 production, indicating NADPH oxidase-mediated H2O2 production under basal conditions. Because some bacterial stimuli are capable of inducing ATP release from cells (15), we hypothesized that ATP release and purinergic receptor activation might present a common mechanism of DUOX1 activation and cell responses to diverse stimuli such as LPS and α-ASGM1. Indeed, stimulation of the HBE1 cells with either LPS or α-ASGM1 resulted in a rapid and transient increase of extracellular ATP (Fig. 1B), which was abolished in the presence of the ATPase/ADPase apyrase (10 units/ml; not shown). Therefore, analogous to our recent studies demonstrating ATP release and DUOX1 activation in HBE1 cells in response to mechanical injury (10), these findings indicate that bacterial stimuli can also promote cellular ATP release, which subsequently may contribute to DUOX1 activation and cellular H2O2 production.
FIGURE 1.
Cellular ATP release and H2O2 production by TLR-activating stimuli. A, confluent HBE1 cells were stimulated with LPS (10 μg/ml), α-ASGM1 (1:100), or ATP (100 μm) in Hanks' buffered saline solution containing 1 mm l-tyrosine and 10 μg/ml lactoperoxidase. H2O2 production was quantitated by HPLC analysis of dityrosine, formed due to oxidative tyrosine cross-linking (10). CTL, control. Data (mean ± S.E., n = 3) used are as follows: *, p < 0.05 compared with control; #, p < 0.05 compared with the corresponding stimulation in the absence of DPI (n = 3). B, HBE1 cells were stimulated with LPS (10 μg/ml) or α-ASGM1 (1:100), and ATP release into the medium was measured at indicated time points using a luciferase/luciferin assay. Data are mean values of three separate experiments.
Bacterial Stimuli Induce IL-8 Expression by ATP-mediated DUOX1 Activation
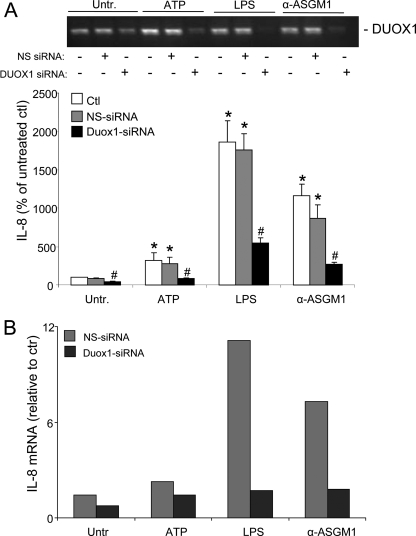
Confirming several previous reports (12, 16, 27, 41), we verified that HBE1 cell stimulation with either LPS or α-ASGM1 resulted in increased production of IL-8 (Fig. 2 and Table 1). Moreover, stimulation with exogenous ATP also induced IL-8 production, albeit to a lesser extent (Fig. 2A). The increase in IL-8 secretion was in each case accompanied by an increase in IL-8 mRNA (Fig. 2B), suggesting that these various stimuli transcriptionally activate IL-8 expression. IL-8 production by each of these stimuli was dramatically suppressed after preincubation with the P2Y receptor antagonist suramin (100 μm; Table 1), indicating the common involvement of ATP-dependent activation of P2Y receptors (16). Analysis of dose-dependent effects of ATP indicated that IL-8 production was enhanced by as little as 3 μm ATP, and responses were maximal at 30–100 μm ATP, consistent with the ability of ATP to stimulate these purinoceptors. The fact that IL-8 production by exogenous ATP was less pronounced compared with that by LPS or α-ASGM1 is not necessarily surprising, because IL-8 production by these latter stimuli most likely involves several concerted signaling mechanisms, some of which may be independent of ATP. However, the dramatic inhibitory effect of purinergic receptor blockade by suramin (Table 1) suggests that P2Y receptor stimulation is integral to the overall induction of IL-8 by these various stimuli.
FIGURE 2.
ATP-mediated IL-8 expression in HBE1 cells depends on DUOX1 activation. Starved confluent HBE1 cell monolayers were stimulated with either LPS (10 μg/ml), α-ASGM1 (1:100), or ATP (100 μm) for 24 h, and IL-8 protein secretion was measured in the medium (A), or RNA was extracted for analysis of IL-8 mRNA by reverse transcription-PCR (B). Cells were preincubated with DUOX1 siRNA or control nonspecific (NS) siRNA for 48 h prior to experimentation (10), and efficiency of DUOX1 silencing was evaluated by reverse transcription-PCR (A, top panel). Data are presented as a percentage of IL-8 protein production by unstimulated cells (A, mean ± S.E., n = 4; *, p < 0.05 compared with control incubation; #, p < 0.05 compared with the corresponding stimulation in the presence of NS siRNA) or relative to IL-8 mRNA expression in unstimulated cells (B, mean of two experiments). Ctl, untransfected control; Untr., untreated.
TABLE 1.
ATP-mediated IL-8 secretion by P2Y receptor stimulation, NADPH oxidase activation, and EGFR/ERK signaling
HBE1 cells were stimulated with ATP (100 μm), LPS (10 μg/ml), or α-ASGM1 (1:100), and secreted IL-8 protein was measured in the media after 24 h by ELISA. When indicated, cells were pretreated for 30 min with the NADPH oxidase inhibitor DPI, the P2Y receptor antagonist suramin, the MEK1 inhibitors U0126 and PD98058, or the EGFR tyrosine kinase inhibitor AG1478. Data are presented as a percentage of IL-8 production by unstimulated cells (mean ± S.E., n = 4).
| Untreated | ATP | LPS | α-ASGM1 | |
|---|---|---|---|---|
| Control | 100 ± 4.4 | 228 ± 64a | 238 ± 45a | 327 ± 87a |
| DPI (1 μm) | 36 ± 16b | 33 ± 12b | 49 ± 15b | 70 ± 18b |
| Suramin (100 μm) | 24 ± 12b | 34 ± 17b | 23 ± 10b | 37 ± 14b |
| U0126 (10 μm) | 36 ± 12b | 28 ± 8b | 32 ± 4b | 38 ± 9b |
| PD98058 (10 μm) | 28 ± 5b | 44 ± 14b | 46 ± 9b | 18 ± 7b |
| AG1478 (10 μm) | 16 ± 8b | 17 ± 8b | 16 ± 5b | 38 ± 8b |
ap < 0.05 as compared with unstimulated cells.
b p < 0.05 as compared with the corresponding controls.
To determine whether the increased IL-8 expression by these stimuli is associated with activation of DUOX1, we used siRNA to silence DUOX1 expression in HBE1 cells prior to cell stimulation. Consistent with our previous observations (10), transfection of HBE1 cells with DUOX1 siRNA significantly diminished DUOX1 expression (Fig. 2A, top). Moreover, in contrast to control siRNA transfection, DUOX1 siRNA-transfected cells showed significantly less IL-8 protein (Fig. 2A) and mRNA (Fig. 2B) in response to these stimuli. Similarly, pretreatment with the NADPH oxidase inhibitor DPI (1 μm) also markedly suppressed IL-8 production in each case (Table 1). Collectively, these data indicate the involvement of ATP release and DUOX1 activation in IL-8 expression induced by diverse bacterial stimuli.
DUOX1 Mediates IL-8 Production via Activation of EGFR, ERK1/2, and NF-κB
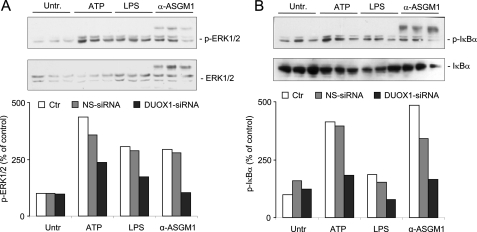
Previous studies have demonstrated the importance of the ERK1/2 in airway epithelial IL-8 production in response to various stimuli (12, 15, 42), and epithelial IL-8 expression is also under transcriptional control of nuclear factor (NF)-κB (43, 44). The importance of ERK1/2 in IL-8 production by ATP, LPS, or α-ASGM1 was verified by the marked inhibition by U0126 or PD98058, two pharmacological inhibitors of the upstream kinase MEK1 that activates ERK1/2 (Table 1). Following our recent studies showing that ATP-mediated DUOX1 stimulation activates ERK1/2 in HBE1 cells (10), we evaluated whether ATP as well as LPS or α-ASGM1 promote the activation of ERK1/2 and NF-κB, and whether this was the result of DUOX1 activation. As shown in Fig. 3A, Western blot analysis demonstrated significant activation of ERK1/2 by each stimulus, which was suppressed after cell transfection with DUOX1 siRNA but not by control NS siRNA (Fig. 3A). Similarly, each of these stimuli also enhanced the phosphorylation of the NF-κB inhibitor IκB-α, illustrating their ability to activate NF-κB, and this was again suppressed after DUOX1 siRNA silencing (Fig. 3B).
FIGURE 3.
DUOX1 mediates ATP-mediated phosphorylation of ERK1/2 and IκB. Confluent starved HBE1 cells were stimulated for 10 min with either ATP (100 μm), LPS (10 μg/ml), or α-ASGM1 (1:100), and harvested for Western blot analysis of phosphorylated and total ERK1/2 (A) or phospho-IκB (B). Where indicated, cells were preincubated for 48 h with pre-designed DUOX1 siRNA and control siRNA (NS-siRNA) (10). Representative blots and quantitative analysis by densitometry (n = 3) are shown. Ctr, untransfected control; Untr., untreated.
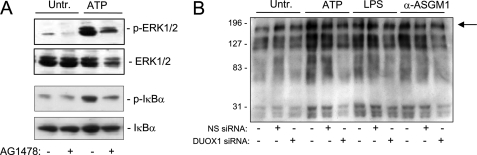
Activation of both ERK1/2 and NF-κB in response to several diverse stimuli has been linked to activation of EGFR (13, 45, 46), and recent studies have implicated DUOX1 in epithelial EGFR activation and IL-8 production (12, 21). Accordingly, inhibition of EGFR tyrosine kinase activity using AG1478 (1 μm) markedly decreased IL-8 production induced by ATP, LPS, or α-ASGM1 (Table 1). Similarly, addition of AG1478 also suppressed ATP-mediated phosphorylation of ERK1/2 and I-κBα (Fig. 4A), indicating the involvement of EGFR activation in these signaling events. Activation of EGFR or related tyrosine kinases by these stimuli was demonstrated more directly by Western blot analysis of tyrosine-phosphorylated proteins. As shown in Fig. 4B, HBE1 cell stimulation with either ATP, LPS, or α-ASGM1 resulted in increased phosphorylation of several proteins, including a protein of ±180 kDa, most likely representing EGFR autophosphorylation on tyrosine residues. These increases in tyrosine phosphorylation were in each case attenuated after DUOX1 siRNA silencing, but not by NS siRNA, indicating the involvement of DUOX1 in overall protein tyrosine phosphorylation and EGFR autophosphorylation and activation by these stimuli. Taken together, these results indicate that ATP-mediated DUOX1 activation promotes EGFR-dependent ERK and NF-κB activation, which subsequently enhances IL-8 expression and secretion.
FIGURE 4.
Role of EGFR in ATP-mediated signaling. A, HBE1 were stimulated for 10 min with ATP (100 μm) in the absence or presence of the EGFR inhibitor AG1478 (10 μm), and cell lysates were collected for Western blot analysis of phosphorylated and total ERK1/2 and IκB. B, HBE1 cells were preincubated with DUOX1 siRNA and NS siRNA and stimulated for 10 min with ATP, LPS, or α-ASGM1, as in Fig. 3, and cell lysates were analyzed for tyrosine-phosphorylated proteins, using α-PY antibody. A representative blot of three experiments is shown. Arrow indicates a major phosphorylated protein of ±180 kDa, presumably representing EGFR. Untr., untreated.
ATP/DUOX1 Activation Promotes TGF-α Production
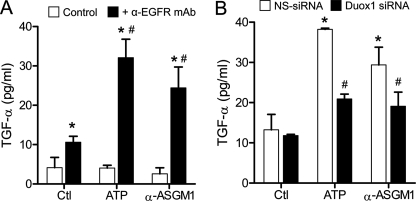
Upon demonstrating an important role for EGFR in the ATP/DUOX1-mediated IL-8 production, we assessed the potential involvement of an EGFR ligand. Based on previous studies implicating TGF-α in IL-8 production (12, 30), we considered that ATP stimulation might promote TGF-α activation. Indeed, as shown in Fig. 5A, HBE1 cell stimulation with either ATP or α-ASGM1 led to increased accumulation of TGF-α in the media, although this was observed only when cells were preincubated with a neutralizing EGFR antibody. This suggests that production of TGF-α by ATP or α-ASGM1 normally engages in EGFR binding and activation, thereby preventing its accumulation in the media to detectable levels. Silencing of DUOX1 attenuated the ATP- and α-ASGM1-induced TGF-α production observed in the presence of EGFR mAb (Fig. 5B), indicating the involvement of DUOX1 activation in ATP-induced TGF-α production and EGFR activation.
FIGURE 5.
ATP-mediated DUOX1 activation promotes TGF-α shedding in HBE1 cells. A, HBE1 cells were stimulated with either ATP or α-ASGM1 for 2 h, in the absence or presence of α-EGFR mAb (225; 4 μg/ml), and conditioned media were collected for analysis of TGF-α by ELISA. B, HBE1 cells were preincubated with NS siRNA (white bars) or DUOX1 siRNA (black bars) for 48 h and subsequently stimulated with ATP or α-ASGM1 in the presence of α-EGFR mAb for analysis of TGF-α. *, p < 0.05 compared with untreated control; #, p < 0.05 compared with the corresponding stimulation in the absence of α-EGFR mAb (A) or in the presence of NS siRNA (B, n = 4). Ctl, untreated control.
DUOX1 Mediates ATP-dependent Activation of ADAM17
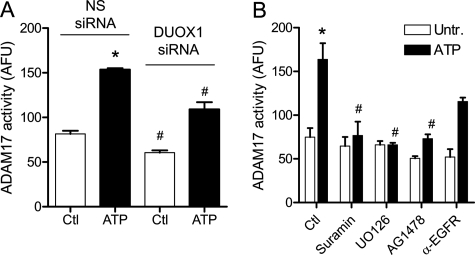
Activation of EGFR ligands such as TGF-α is commonly mediated by activation of sheddases such as ADAM17, which cleave membrane-bound forms of these ligands to promote EGFR activation (47). We therefore evaluated whether HBE1 cell stimulation with ATP or α-ASGM1 results in activation of ADAM17, and whether this is mediated by DUOX1 activation. As shown in Fig. 6A, both ATP and α-ASGM1 indeed resulted in enhanced ADAM17 activity, measured by increased cleavage of a fluorogenic ADAM17 substrate, and this was attenuated after siRNA silencing of DUOX1 (Fig. 6A) and in the presence of the NADPH oxidase inhibitor DPI (data not shown). Thus, our findings are consistent with previous studies indicating a role for DUOX1 in epithelial ADAM17 activation (12, 13).
FIGURE 6.
ATP-mediated activation of ADAM17 by activation of DUOX1-EGFR-ERK signaling. Confluent starved HBE1 cells were preincubated with either NS siRNA or DUOX1 siRNA (A) or with the P2Y antagonist suramin (100 μm), the MEK1 inhibitor U0126 (10 μm), or inhibitors EGFR (AG1478, 10 μm; α-EGFR mAb, 4 μg/ml) for 30 min each (B), and stimulated with ATP in the presence of a fluorogenic ADAM17 substrate (Substrate II, Calbiochem; 10 μm) for up to 2 h, and media were transferred to a fluorescence microplate for analysis of fluorescence (excitation, 310 nm; emission, 400 nm). *, p < 0.05 compared with control incubation; #, p < 0.05 compared with the corresponding incubation with NS siRNA or without inhibitor (n = 4–6). Ctr, control; Untr., untreated.
The importance of P2Y purinergic receptor stimulation in ADAM17 activation could be demonstrated by the ability of suramin to abolish the effects of both stimuli (Fig. 6B). Interestingly, ATP-mediated ADAM17 activation was also inhibited by blocking ERK signaling with U0126 (Fig. 6B), consistent with a role of ERK-mediated phosphorylation in ADAM17 activation (47, 48). Moreover, ATP-mediated ADAM17 activation was also suppressed by the EGFR kinase inhibitor AG1478 and, to a lesser extent, by preincubation with α-EGFR antibody (Fig. 6B), indicating a role for EGFR in ADAM17 activation.
Role of H2O2 in DUOX1-mediated ADAM17 Activation and IL-8 Production
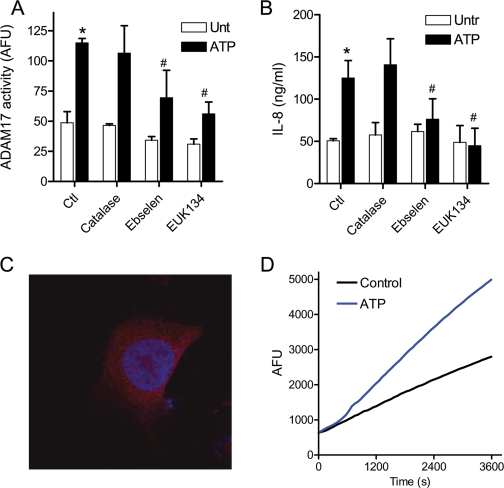
Based on the presence of a common “cysteine switch” consensus motif within the prodomain of matrix metalloproteinases/ADAM proteases, it was recently suggested that DUOX1 activation promotes ADAM17 activation by direct oxidation of the pro-ADAM17 cysteine at the cell surface (12, 13). However, the importance of the pro-domain cysteine switch in regulation of ADAM17 activation has recently been questioned (34), and ADAM17 activity is also regulated by intracellular signaling mechanisms such as EGFR/ERK-mediated pathways (as indicated above) (47), which could be regulated by H2O2-mediated signaling. To address the involvement of either extracellular or intracellular H2O2 in DUOX1-dependent activation of ADAM17, we performed experiments in the presence of catalase, which would rapidly detoxify extracellular H2O2. Alternatively, cells were preincubated with two structurally unrelated cell-permeable peroxidase/catalase mimetics, ebselen and EUK134, to quench intracellular H2O2. As shown in Fig. 7, addition of catalase (up to 2,000 units/ml) to HBE1 cells failed to prevent ATP-mediated activation of ADAM17 (Fig. 7A) and did not affect production of IL-8 (Fig. 7B). In contrast, addition of either ebselen or EUK134 significantly attenuated ATP-induced ADAM17 activation as well as IL-8 production (Fig. 7, A and B). Similar effects of catalase, ebselen, or EUK134 were observed on ADAM17 activation and/or IL-8 production in response to LPS or α-ASGM1 (data not shown). Hence, our results indicate that DUOX1-mediated activation of ADAM17 and EGFR/ERK signaling and production of IL-8 is primarily mediated by intracellular H2O2. Accordingly, analysis of DUOX protein within HBE1 cells by confocal imaging revealed that DUOX is largely present intracellularly (Fig. 7C), presumably in association with intracellular membrane structures of the endoplasmic reticulum or Golgi. Moreover, ATP stimulation also resulted in intracellular oxidant production, increase 2′,7′-dichlorofluorescein of HBE1 preloaded with DCFH2-DA, suggesting intracellular production of H2O2 and related oxidants (Fig. 7D). Thus, ATP-mediated activation of intracellular DUOX1 appears to participate in cellular signaling events that promote ADAM17 activation and production of IL-8.
FIGURE 7.
Effects of H2O2 scavengers on ATP-mediated signaling. A and B, confluent HBE1 cells were preincubated with catalase (2000 units/ml) or the peroxidase mimics ebselen (10 μm) or EUK134 (50 μm). B, for 30 min before stimulation with ATP for 2 h in the presence of fluorogenic ADAM17 Substrate II for analysis of ADAM17 activity (A) or 24 h for analysis of IL-8 production by ELISA (B). Ctl, control; Untr., untreated. *, p < 0.05 compared with control incubation; #, p < 0.05 compared with corresponding stimulation without antioxidant (n = 4). C, subconfluent HBE1 cells were fixed and stained with α-DUOX (red) and 4′,6-diamidino-2-phenylindole (blue) for confocal imaging. D, HBE1 cells in 24-well plates were preloaded with DCFH2-DA (10 μm; 15 min) and washed and stimulated with ATP, and fluorescence was monitored using a fluorescence plate reader. A typical result of three experiments is shown.
DISCUSSION
The results described in this study offer several new insights into the functional role of the NADPH oxidase DUOX1 in epithelial host defense responses. First, this study highlights a general role of ATP release and P2Y receptor activation in DUOX1-dependent H2O2 production in response to diverse bacterial triggers that act on various TLR receptors (41). As demonstrated, epithelial production of the neutrophil chemokine IL-8 in response to the TLR4 ligand LPS or activation of the glycolipid receptor for bacterial flagellin, ASGM1, which associates with TLR5 (16), involves the release of ATP and activation of DUOX1. Thus, in addition to recent studies indicating direct association of TLR4 with other NADPH oxidase homologs or MyD88-mediated phosphorylation of NOX activator proteins in other cell types (49, 50), our studies indicate that TLR-mediated activation of epithelial DUOX1 is related to release of cellular ATP and P2Y receptor activation, which stimulates DUOX1 by Ca2+-mediated signaling (6). However, the mechanisms by which these triggers provoke ATP release are still unknown (16, 51).
Second, our studies further address the mechanisms by which P2Y receptor stimulation may lead to activation of ADAM17-EGFR signaling cascades that mediate the activation of various host defense mechanisms, including IL-8 production (52, 53), and establish a role for DUOX1 in this signaling pathway. Thus, in addition to participating in innate host defense by providing H2O2 to produce antimicrobial oxidants (3), epithelial DUOX1 also appears to participate in additional host responses to infection, by mediating intracellular signaling pathways that result in production of neutrophil chemokines and other inflammatory mediators (7, 10).
Release of epithelial ATP in response to cell stimulation or injury can promote a variety of epithelial responses through activation of P2X and P2Y receptor types that are expressed on the epithelial surface (54, 55). Although we used extracellular ATP at up to 100 μm to mimic these responses, which considerably exceeds levels of released ATP in response to cell stimulation with either LPS or α-ASGM1 (Fig. 1B), it is important to note that such measurements in the bulk media typically underestimate local concentrations of released ATP at the cell surface that is often sufficient to activate purinoceptors (10). Moreover, even though these concentrations of exogenous ATP can activate both P2X and P2Y receptor subtypes (54), the inhibitory effects of the P2Y-selective antagonist suramin suggest that these responses are due to stimulation of this receptor type. Moreover, studies in mice deficient in P2Y1 and P2Y2 purinergic receptors have demonstrated the importance of these receptors in airway host defense against bacterial infection (56). Furthermore, our previous studies (10) and recent studies by others (3, 6) indicate that epithelial H2O2 production by exogenous ATP is primarily mediated by P2Y receptor stimulation of DUOX1, although we cannot fully rule out the potential contribution of other purinoceptors and alternative cellular sources of H2O2. The P2Y receptors belong to the family of G-protein-coupled receptors that mediate signaling through intracellular Ca2+ and protein kinase C signaling (57). Although it is not precisely understood how P2Y stimulation activates DUOX1, both Ca2+ mobilization and protein kinase C-activating stimuli have been shown to activate DUOX1-dependent H2O2 production in airway epithelial cells (6, 9). Recent studies indicated that basal DUOX1 activity is suppressed by interaction with NOXA1 and that Ca2+-mobilizing stimuli promote NOXA1 dissociation from DUOX1, and thereby enhance its NADPH oxidase activity (58).
In agreement with previous observations (9, 12, 13), our studies indicate that DUOX1 activation promotes the activation of EGFR and subsequent ERK1/2 and NF-κB signaling pathways, both critical in mediating IL-8 expression (43, 44). Moreover, our studies also demonstrate that DUOX1 activation contributes to the activation of ADAM17 and shedding of EGFR ligands such as TGF-α. Although the activation of ADAM17 and TGF-α shedding may contribute to EGFR activation and subsequent IL-8 production (12, 13), it is still unclear how DUOX1 activation leads to ADAM17 activation, and the overall regulation of ADAM17 activity is still incompletely understood (47). It was suggested that DUOX1 might directly mediate the activation of ADAM17 at the cell surface by oxidative disruption of the cysteine switch motif within the ADAM17 prodomain (13, 46), although direct evidence for such an oxidative activation mechanisms is lacking. Moreover, this hypothesis is also inconsistent with findings that ADAM17 maturation and proteolytic processing occur intracellularly and do not critically depend on the prodomain cysteine switch (34).
To address the role of oxidative mechanisms in ATP-mediated ADAM17 activation and subsequent IL-8 production, we used various extracellular or cellular antioxidant strategies. First, addition of the antioxidant enzyme catalase (up to 3,000 units/ml) completely failed to prevent DUOX1-mediated ADAM17 activation and subsequent IL-8 production, arguing against a role for extracellular H2O2. However, pretreatment with the cell-permeable peroxidase mimics ebselen, and EUK134 partially suppressed these responses, indicating the contribution of intracellular H2O2 or related oxidants in this signaling cascade.
In accordance with recent studies indicating the critical importance of subcellular compartmentalization of NOX-derived oxidant production, and its role in EGFR activation or NF-κB signaling (59, 60), our results suggest that DUOX1 activation not only generates extracellular H2O2 as a mechanism of epithelial antimicrobial defense (3, 6), but it also produces intracellular H2O2 or related oxidants to mediate intracellular signaling mechanisms involved in EGFR activation and expression of IL-8. In line with this notion, immunohistochemical analysis of HBE1 cells revealed that DUOX1 protein is largely detected intracellularly (Fig. 7C), consistent with studies of thyrocytes indicating that the bulk of DUOX1 protein is localized to intracellular compartments (61). Although intracellular DUOX proteins are usually considered immature and inactive (61), intracellular DUOX has been reported to possess some intrinsic oxidant-producing activity (62). Accordingly, ATP stimulation of HBE1 cells enhanced intracellular oxidant production, as determined by 2′,7′-dichlorofluorescein fluorescence (Fig. 7D), indicative of intracellular DUOX1 activation. Thus, although the innate host defense properties of airway epithelial DUOX1 are commonly believed to depend on maturated DUOX1 protein at the cell surface (3, 6), further studies will be required to determine the relative contribution of intracellular DUOX1 in this respect.
Whereas activation of ADAM17 and EGFR ligand shedding contribute importantly to autocrine EGFR activation and subsequent IL-8 production (12, 18), several reports suggest that ligand-independent EGFR activation by various stimuli promotes ADAM17-mediated shedding by ERK1/2-mediated Ser/Thr phosphorylation of ADAM17 (36, 48, 63). Accordingly, we observed that ATP-mediated ADAM17 activation was dramatically blocked by inhibitors of ERK1/2 or EGFR tyrosine kinase activity, but not by a blocking α-EGFR antibody, thus suggesting a role for ligand-independent EGFR/ERK1/2 activation in ADAM17 activation and EGFR ligand shedding. In this regard, various lines of evidence suggest that H2O2 can enhance EGFR activation by ligand-independent mechanisms, including oxidative inactivation of protein-tyrosine phosphatases to suppress EGFR dephosphorylation (59) and activation of the nonreceptor tyrosine kinase c-Src, which interacts with and phosphorylates EGFR (64–66). Indeed, c-Src is involved in ligand-independent activation of EGFR by several G-protein-coupled receptors, including P2Y2 purinergic receptors (67), and mediates epithelial IL-8 production in response to protease-activated receptor stimulation (24). Therefore, we propose that DUOX1-derived intracellular H2O2 mediates ATP-dependent EGFR activation by activating c-Src, analogous to its activation by other NADPH oxidases within endosomal compartments, a prominent site of EGFR activation (64, 68). In addition, observations that ADAM17 activity may also be directly regulated by the protein-tyrosine phosphatase PTPH1 (69) may suggest that intracellularly produced H2O2 could also more directly regulate ADAM17 activity and EGFR ligand shedding by inactivating PTPH1.
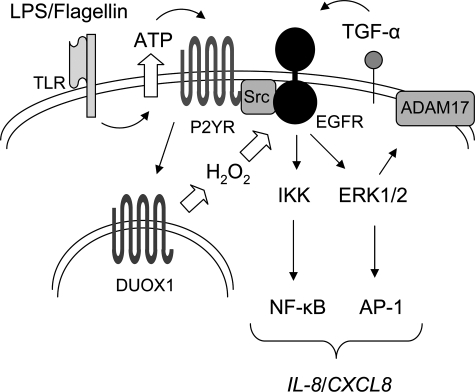
Our collective results indicate that EGFR activation in response to DUOX1 activation involves both ligand-dependent and -independent mechanisms, which may be required for amplified or prolonged EGFR activation to allow for appropriate activation of transcription factors and induction of IL-8 or other mediators (Fig. 8). Moreover, although some of these signaling events may be regulated by DUOX1-derived H2O2, it was recently reported that H2O2-mediated, ligand-independent activation of EGFR in airway epithelial cells may also depend on intracellular oxidant generation because of increased expression of NOX4 (70). This would tempt speculation that DUOX1-mediated activation of cell signaling pathways might also involve indirect oxidative signaling events because of induction and activation of NOX4. Future studies will be necessary to specifically address the importance of these various oxidative signaling events in DUOX1-mediated EGFR activation.
FIGURE 8.
Schematic representation of DUOX1 involvement in TLR-mediated IL-8 production in response to bacterial stimuli. Cell stimulation with LPS or α-ASGM1 mediates release of ATP, which activates EGFR-ERK1/2-NF-κB signaling cascades which is amplified by ADAM17-TGF-α activation cascade. Intracellular production of H2O2 by DUOX1 promotes this signaling pathway by ligand-independent EGFR activation or activation of upstream tyrosine kinases such as Src.
Because epithelial production of IL-8 forms an integral response in innate host defense as well as repair mechanisms in response to infection or injury, dysregulated production of IL-8 or other mediators by inappropriate DUOX activation may adversely affect these processes and contribute to lung disease pathology. For example, DUOX1 expression is decreased in large airways of smokers and patients suffering from mild or moderate chronic obstructive pulmonary disease (71), and it may be suppressed in lung cancers (72). Conversely, DUOX1 expression can be enhanced by Th2 cytokines such as IL-13 (10, 38), which can promote ADAM17 activation, TGF-α shedding, and subsequent epithelial cell proliferation (73), and may thereby contribute to epithelial hypertrophy and other features associated with airway remodeling in allergic asthma.
In summary, our present results demonstrate that ATP-mediated activation of epithelial DUOX1 represents a general response mechanism to diverse bacterial triggers that activate different TLR receptors, as illustrated by enhanced IL-8 production. Activation of epithelial DUOX1 augments pro-inflammatory ADAM17-TGF-α-ERK-EGFR cascades, which appears to be initiated by H2O2-mediated, ligand-independent EGFR activation. As such, epithelial DUOX1 is involved in innate immune responses in addition to direct antimicrobial extracellular oxidant production.
Acknowledgments
We thank Derek Sham for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL068865, HL074295, and HL089646 (to A. v. d. V.). This work was also supported by a Kootstra fellowship and a Niels Stensen Stipendium (to A. W. B.).
- DUOX1
- dual oxidase 1
- ASGM1
- asialo-GM1
- ADAM
- a disintegrin and metalloproteinase
- EGFR
- epidermal growth factor receptor
- ERK
- extracellular signal-regulated kinase
- LPS
- lipopolysaccharide
- TGF-α
- transforming growth factor-α
- IL
- interleukin
- HPLC
- high pressure liquid chromatography
- mAb
- monoclonal antibody
- PBS
- phosphate-buffered saline
- siRNA
- short interfering RNA
- DPI
- diphenylene iodonium
- ELISA
- enzyme-linked immunosorbent assay
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- NS
- nonspecific
- TLR
- Toll-like receptor
- DCFH2-DA
- 2′,7′-dichlorofluorescein diacetate.
REFERENCES
- 1.Knight D. A., Holgate S. T. ( 2003) Respirology 8, 432– 446 [DOI] [PubMed] [Google Scholar]
- 2.van der Vliet A. ( 2008) Free Radic. Biol. Med. 44, 938– 955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moskwa P., Lorentzen D., Excoffon K. J., Zabner J., McCray P. B., Jr., Nauseef W. M., Dupuy C., Bánfi B. ( 2007) Am. J. Respir. Crit. Care Med. 175, 174– 183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer H., Gonzales L. K., Kolla V., Schwarzer C., Miot F., Illek B., Ballard P. L. ( 2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1506– 1514 [DOI] [PubMed] [Google Scholar]
- 5.Geiszt M., Witta J., Baffi J., Lekstrom K., Leto T. L. ( 2003) FASEB J. 17, 1502– 1504 [DOI] [PubMed] [Google Scholar]
- 6.Forteza R., Salathe M., Miot F., Forteza R., Conner G. E. ( 2005) Am. J. Respir. Cell Mol. Biol. 32, 462– 469 [DOI] [PubMed] [Google Scholar]
- 7.Koff J. L., Shao M. X., Ueki I. F., Nadel J. A. ( 2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1068– 1075 [DOI] [PubMed] [Google Scholar]
- 8.Kuwahara I., Lillehoj E. P., Koga T., Isohama Y., Miyata T., Kim K. C. ( 2007) Am. J. Respir. Cell Mol. Biol. 37, 691– 698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao M. X., Nadel J. A. ( 2005) Proc. Natl. Acad. Sci. U.S.A. 102, 767– 772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wesley U. V., Bove P. F., Hristova M., McCarthy S., van der Vliet A. ( 2007) J. Biol. Chem. 282, 3213– 3220 [DOI] [PubMed] [Google Scholar]
- 11.Coraux C., Martinella-Catusse C., Nawrocki-Raby B., Hajj R., Burlet H., Escotte S., Laplace V., Birembaut P., Puchelle E. ( 2005) J. Pathol. 206, 160– 169 [DOI] [PubMed] [Google Scholar]
- 12.Nakanaga T., Nadel J. A., Ueki I. F., Koff J. L., Shao M. X. ( 2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1289– 1296 [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara I., Lillehoj E. P., Lu W., Singh I. S., Isohama Y., Miyata T., Kim K. C. ( 2006) Am. J. Physiol. Lung Cell Mol. Physiol. 291, L407– 416 [DOI] [PubMed] [Google Scholar]
- 14.Fu Z., Bettega K., Carroll S., Buchholz K. R., Machen T. E. ( 2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L353– 364 [DOI] [PubMed] [Google Scholar]
- 15.McNamara N., Khong A., McKemy D., Caterina M., Boyer J., Julius D., Basbaum C. ( 2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9086– 9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNamara N., Gallup M., Sucher A., Maltseva I., McKemy D., Basbaum C. ( 2006) Am. J. Respir. Cell Mol. Biol. 34, 653– 660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez M. I., Prince A. ( 2008) Pediatr. Pulmonol. 43, 11– 19 [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., He D., Saatian B., Watkins T., Spannhake E. W., Pyne N. J., Natarajan V. ( 2006) J. Biol. Chem. 281, 19501– 19511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biagioli M. C., Kaul P., Singh I., Turner R. B. ( 1999) Free Radic. Biol. Med. 26, 454– 462 [DOI] [PubMed] [Google Scholar]
- 20.Look D. C., Stoll L. L., Romig S. A., Humlicek A., Britigan B. E., Denning G. M. ( 2005) J. Immunol. 175, 4017– 4023 [DOI] [PubMed] [Google Scholar]
- 21.Koff J. L., Shao M. X., Ueki I. F., Nadel J. A. ( 2008) Am. J. Physiol. Lung. Cell Mol. Physiol. 294, L1068– 1075 [DOI] [PubMed] [Google Scholar]
- 22.Pelaia G., Cuda G., Vatrella A., Gallelli L., Fratto D., Gioffrè V., D'Agostino B., Caputi M., Maselli R., Rossi F., Costanzo F. S., Marsico S. A. ( 2004) J. Cell. Biochem. 93, 142– 152 [DOI] [PubMed] [Google Scholar]
- 23.Li J., Kartha S., Iasvovskaia S., Tan A., Bhat R. K., Manaligod J. M., Page K., Brasier A. R., Hershenson M. B. ( 2002) Am. J. Physiol. Lung Cell Mol. Physiol. 283, L690– 699 [DOI] [PubMed] [Google Scholar]
- 24.Lin C. H., Cheng H. W., Hsu M. J., Chen M. C., Lin C. C., Chen B. C. ( 2006) J. Immunol. 177, 3427– 3438 [DOI] [PubMed] [Google Scholar]
- 25.Strieter R. M. ( 2002) Am. J. Physiol. Lung Cell Mol. Physiol. 283, L688– 689 [DOI] [PubMed] [Google Scholar]
- 26.Yu Y., Nagai S., Wu H., Neish A. S., Koyasu S., Gewirtz A. T. ( 2006) J. Immunol. 176, 6194– 6201 [DOI] [PubMed] [Google Scholar]
- 27.Li J., Johnson X. D., Iazvovskaia S., Tan A., Lin A., Hershenson M. B. ( 2003) Am. J. Physiol. Lung Cell Mol. Physiol. 284, L307– 315 [DOI] [PubMed] [Google Scholar]
- 28.Sethi G., Ahn K. S., Chaturvedi M. M., Aggarwal B. B. ( 2007) Oncogene 26, 7324– 7332 [DOI] [PubMed] [Google Scholar]
- 29.Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C. P. ( 2004) J. Cell Biol. 164, 769– 779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter A., O'Donnell R. A., Powell R. M., Sanders M. W., Holgate S. T., Djukanović R., Davies D. E. ( 2002) Am. J. Respir. Cell Mol. Biol. 27, 85– 90 [DOI] [PubMed] [Google Scholar]
- 31.Hamilton L. M., Puddicombe S. M., Dearman R. J., Kimber I., Sandström T., Wallin A., Howarth P. H., Holgate S. T., Wilson S. J., Davies D. E. ( 2005) Eur. Respir. J. 25, 978– 985 [DOI] [PubMed] [Google Scholar]
- 32.Schlöndorff J., Becherer J. D., Blobel C. P. ( 2000) Biochem. J. 347, 131– 138 [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard J. D., Lin F., Milla M. E. ( 2005) Biochem. J. 387, 797– 805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milla M. E., Gonzales P. E., Leonard J. D. ( 2006) Cell Biochem. Biophys. 44, 342– 348 [DOI] [PubMed] [Google Scholar]
- 35.Fan H., Derynck R. ( 1999) EMBO J. 18, 6962– 6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gómez M. I., Seaghdha M. O., Prince A. S. ( 2007) EMBO J. 26, 701– 709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soond S. M., Everson B., Riches D. W., Murphy G. ( 2005) J. Cell Sci. 118, 2371– 2380 [DOI] [PubMed] [Google Scholar]
- 38.Harper R. W., Xu C., Eiserich J. P., Chen Y., Kao C. Y., Thai P., Setiadi H., Wu R. ( 2005) FEBS Lett. 579, 4911– 4917 [DOI] [PubMed] [Google Scholar]
- 39.Bevelander M., Mayette J., Whittaker L. A., Paveglio S. A., Jones C. C., Robbins J., Hemenway D., Akira S., Uematsu S., Poynter M. E. ( 2007) J. Immunol. 179, 3680– 3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koff J. L., Shao M. X., Kim S., Ueki I. F., Nadel J. A. ( 2006) J. Immunol. 177, 8693– 8700 [DOI] [PubMed] [Google Scholar]
- 41.Sha Q., Truong-Tran A. Q., Plitt J. R., Beck L. A., Schleimer R. P. ( 2004) Am. J. Respir. Cell Mol. Biol. 31, 358– 364 [DOI] [PubMed] [Google Scholar]
- 42.Tephly L. A., Carter A. B. ( 2007) Am. J. Physiol. Lung Cell Mol. Physiol. 293, L1143– 1155 [DOI] [PubMed] [Google Scholar]
- 43.Joseph T., Look D., Ferkol T. ( 2005) Am. J. Physiol. Lung Cell Mol. Physiol. 288, L471– 479 [DOI] [PubMed] [Google Scholar]
- 44.Roger T., Out T., Mukaida N., Matsushima K., Jansen H., Lutter R. ( 1998) Biochem. J. 330, 429– 435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K., Anderson G. P., Bozinovski S. ( 2008) Am. J. Respir. Cell Mol. Biol. 39, 305– 311 [DOI] [PubMed] [Google Scholar]
- 46.Shao M. X., Ueki I. F., Nadel J. A. ( 2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11618– 11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blobel C. P. ( 2005) Nat. Rev. Mol. Cell Biol. 6, 32– 43 [DOI] [PubMed] [Google Scholar]
- 48.Fan H., Turck C. W., Derynck R. ( 2003) J. Biol. Chem. 278, 18617– 18627 [DOI] [PubMed] [Google Scholar]
- 49.Park H. S., Jung H. Y., Park E. Y., Kim J., Lee W. J., Bae Y. S. ( 2004) J. Immunol. 173, 3589– 3593 [DOI] [PubMed] [Google Scholar]
- 50.Laroux F. S., Romero X., Wetzler L., Engel P., Terhorst C. ( 2005) J. Immunol. 175, 5596– 5600 [DOI] [PubMed] [Google Scholar]
- 51.Lazarowski E. R., Boucher R. C., Harden T. K. ( 2003) Mol. Pharmacol. 64, 785– 795 [DOI] [PubMed] [Google Scholar]
- 52.Buvinic S., Bravo-Zehnder M., Boyer J. L., Huidobro-Toro J. P., González A. ( 2007) J. Cell Sci. 120, 4289– 4301 [DOI] [PubMed] [Google Scholar]
- 53.Boucher I., Yang L., Mayo C., Klepeis V., Trinkaus-Randall V. ( 2007) Exp. Eye Res. 85, 130– 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwiebert E. M., Zsembery A. ( 2003) Biochim. Biophys. Acta 1615, 7– 32 [DOI] [PubMed] [Google Scholar]
- 55.Communi D., Paindavoine P., Place G. A., Parmentier M., Boeynaems J. M. ( 1999) Br. J. Pharmacol. 127, 562– 568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geary C., Akinbi H., Korfhagen T., Fabre J. E., Boucher R., Rice W. ( 2005) Am. J. Physiol. Lung Cell Mol. Physiol. 289, L890– 895 [DOI] [PubMed] [Google Scholar]
- 57.Ahmad S., Ahmad A., White C. W. ( 2006) Free Radic. Biol. Med. 41, 29– 40 [DOI] [PubMed] [Google Scholar]
- 58.Pacquelet S., Lehmann M., Luxen S., Regazzoni K., Frausto M., Noack D., Knaus U. G. ( 2008) J. Biol. Chem. 283, 24649– 24658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen K., Kirber M. T., Xiao H., Yang Y., Keaney J. F., Jr. ( 2008) J. Cell Biol. 181, 1129– 1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Q., Harraz M. M., Zhou W., Zhang L. N., Ding W., Zhang Y., Eggleston T., Yeaman C., Banfi B., Engelhardt J. F. ( 2006) Mol. Cell. Biol. 26, 140– 154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song Y., Driessens N., Costa M., De Deken X., Detours V., Corvilain B., Maenhaut C., Miot F., Van Sande J., Many M. C., Dumont J. E. ( 2007) J. Clin. Endocrinol. Metab. 92, 3764– 3773 [DOI] [PubMed] [Google Scholar]
- 62.Ameziane-El-Hassani R., Morand S., Boucher J. L., Frapart Y. M., Apostolou D., Agnandji D., Gnidehou S., Ohayon R., Noël-Hudson M. S., Francon J., Lalaoui K., Virion A., Dupuy C. ( 2005) J. Biol. Chem. 280, 30046– 30054 [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q., Thomas S. M., Lui V. W., Xi S., Siegfried J. M., Fan H., Smithgall T. E., Mills G. B., Grandis J. R. ( 2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6901– 6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donepudi M., Resh M. D. ( 2008) Cell. Signal. 20, 1359– 1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuang S., Schnellmann R. G., Zhougang S. ( 2004) Am. J. Physiol. Renal Physiol. 286, F858– 865 [DOI] [PubMed] [Google Scholar]
- 66.Khan E. M., Heidinger J. M., Levy M., Lisanti M. P., Ravid T., Goldkorn T. ( 2006) J. Biol. Chem. 281, 14486– 14493 [DOI] [PubMed] [Google Scholar]
- 67.Liu J., Liao Z., Camden J., Griffin K. D., Garrad R. C., Santiago-Pérez L. I., González F. A., Seye C. I., Weisman G. A., Erb L. ( 2004) J. Biol. Chem. 279, 8212– 8218 [DOI] [PubMed] [Google Scholar]
- 68.Li Q., Zhang Y., Marden J. J., Banfi B., Engelhardt J. F. ( 2008) Biochem. J. 411, 531– 541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y., Schlondorff J., Blobel C. P. ( 2002) J. Biol. Chem. 277, 42463– 42470 [DOI] [PubMed] [Google Scholar]
- 70.Kim H. J., Park Y. D., Moon U. Y., Kim J. H., Jeon J. H., Lee J. G., Bae Y. S., Yoon J. H. ( 2008) Am. J. Respir. Cell Mol. Biol. 39, 598– 609 [DOI] [PubMed] [Google Scholar]
- 71.Nagai K., Betsuyaku T., Suzuki M., Nasuhara Y., Kaga K., Kondo S., Nishimura M. ( 2008) Antioxid. Redox. Signal. 10, 705– 714 [DOI] [PubMed] [Google Scholar]
- 72.Luxen S., Belinsky S. A., Knaus U. G. ( 2008) Cancer Res. 68, 1037– 1045 [DOI] [PubMed] [Google Scholar]
- 73.Booth B. W., Sandifer T., Martin E. L., Martin L. D. ( 2007) Respir. Res. 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]