Abstract
Interleukin (IL)-32 is a recently described proinflammatory cytokine characterized by the induction of nuclear factor (NF)-κB activation. We studied IL-32 expression in human pancreatic tissue and pancreatic cancer cell lines. Tissue samples were obtained surgically. IL-32 expression was evaluated by standard immunohistochemical procedures. IL-32 mRNA expression was analyzed by Northern blotting and real time PCR analyses. IL-32 was weakly immunoexpressed by pancreatic duct cells. In the inflamed lesions of chronic pancreas, the ductal expression of IL-32 was markedly increased. A strong expression of IL-32α was detected in the pancreatic cancer cells. In pancreatic cancer cell lines (PANC-1, MIA PaCa-2, and BxPC-3 cells), the expression of IL-32 mRNA and protein was enhanced by IL-1β, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. An inhibitor of phosphatidylinositol 3-kinase (LY294002) significantly suppressed the IL-1β-, IFN-γ- and TNF-α-induced IL-32 mRNA expression. The blockade of NF-κB and activated protein-1 activation markedly suppressed the IL-1β-, IFN-γ-, and/or TNF-α-induced IL-32 mRNA expression. Furthermore, IL-32-specific small interfering RNA significantly decreased the uptake of [3H]thymidine and increased the annexin V-positive population (apoptotic cells) in PANC-1 cells. IL-32 knockdown also suppressed the mRNA expression of antiapoptotic proteins (Bcl-2, Bcl-xL, and Mcl-1). Pancreatic duct cells are the local source of IL-32, and IL-32 may play an important role in inflammatory responses and pancreatic cancer growth.
Introduction
Interleukin (IL)2-32 was first reported as a transcript in IL-2-activated NK and T cells (1–3) but has recently been recognized as a proinflammatory cytokine. The gene encoding IL-32 is located on human chromosome 16p13.3 and is organized into eight exons (4). There are four splice variants (IL-32α, IL-32β, IL-32δ, and IL-32γ), and IL-32α is the most abundant transcript (7). IL-32 is mainly expressed in natural killer cells, T cells, epithelial cells, and blood monocytes (5). It can induce the proinflammatory cytokines TNF-α and IL-1β in murine peritoneal macrophages as well as in phorbol ester-differentiated human THP-1 cells (2). Recently, a synergism between IL-32 and other well characterized players in innate immunity has been documented (6). Proteinase 3 has been identified as a specific IL-32α-binding protein and cleaves the cytokine to enhance its activity (7).
IL-32 has been implicated in inflammatory disorders, such as rheumatoid arthritis (5, 8–10), mycobacterium tuberculosis infections (6, 11), and inflammatory bowel disease (12). Furthermore, IL-32 expression by gastric and lung cancers has been reported (13). However, IL-32 expression in pancreatic tissues remains unclear. In this study, we investigated IL-32 expression in inflammatory lesions and malignant tissues from the human pancreas. Furthermore, we analyzed the molecular mechanisms controlling IL-32 expression in pancreatic cancer cell lines.
MATERIALS AND METHODS
Reagents
Recombinant human IL-1β, IL-17, and IFN-γ were purchased from R&D Systems (Minneapolis, MN), and the other cytokines were obtained from PeproTech (Rocky Hill, NJ). Anti-human IL-32α antibodies were purchased from R&D Systems. IL-32 isoforms share common amino acid sequences, and polyclonal anti-IL-32α antibodies react with other IL-32 isoforms (IL-32β, -γ, and -δ), as mentioned by Choi et al. (14). Therefore, we have used the term “IL-32” instead of “IL-32α.” All other reagents were purchased from Sigma.
Tissue Samples and Immunochemistry
Pancreatic cancer tissue was obtained from five patients who underwent pancreatectomies. Normal pancreatic tissue and chronic pancreatitis tissue were obtained from five patients who underwent total gastropancreatectomy due to gastric cancer. Diagnosis of chronic pancreatitis was made by histological analyses. The immunohistochemical analyses were performed according to the method described in our previous report (15). Briefly, goat polyclonal anti-human IL-32 antibodies (R&D Systems) were used as the primary antibodies. After incubation with the primary antibodies, the sections were treated with a biotin-conjugated goat anti-rabbit IgG (Vector, Burlingame, CA), and avidin-biotin-peroxidase complexes (ABC, Vector) were for visualization.
For double immunostaining procedures using the anti-IL-32 antibodies plus the anti-cytokeratin antibodies (DAKO, Kyoto, Japan), the mixture of anti-IL-32 antibodies (diluted 1:100) and anti-cytokeratin antibody was applied first and incubated overnight at 4 °C in a humidified chamber. Cy2-labeled anti-goat IgG (diluted 1:100 in phosphate-buffered saline containing 0.1% Tween 20; CHEMICON, Temecula, CA) plus the Cy3-labeled anti-cytokeratin antibodies (diluted 1:100) were then applied for 60 min at room temperature. The images were obtained with the digital confocal laser-scanning system MRC-600 (Bio-Rad).
Cells
The cell lines PANC-1 (16), MIA PaCa-2 (17), and BxPC-3 (16), which are derived from human pancreatic carcinomas, were obtained from the ATCC (Manassas, VA). The PANC-1 and MIA PaCa-2 cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Invitrogen), and the BxPC-3 cells were maintained in RPMI 1640 (Invitrogen) containing 10% fetal bovine serum, respectively. All culture media were supplemented with 50 units/ml penicillin and 50 μg/ml streptomycin.
Reverse Transcription (RT)-PCR
The expression of mRNA in the samples was assessed by RT-PCR and real time PCR analyses. RT-PCR was performed according to the methods described in our previous report (18). The oligonucleotide primers used in this study are shown in Table 1 (19–26). Real time PCR was performed using a LightCycler 2.0 system (Roche Applied Science). The PCR products were ligated into TA cloning vectors (Promega, Madison, WI) and sequenced. The PCR was conducted using a SYBR Green PCR Master Mix (Applied Biosystems, Foster city, CA). The data were normalized versus β-actin for human IL-32.
TABLE 1.
Oligonucleotides used in this study
| Gene name | Primers | Reference |
|---|---|---|
| IL-32 | ||
| Sense | 5′-AGCTGGAGGACGACTTCAAA-3′ | (17) |
| Antisense | 5′-AGAGCAGCAGAAACTCTGGA-3′ | |
| bcl-2 | ||
| Sense | 5′-GGTGCCACCTGTGGTCCAACCT-3′ | (18) |
| Antisense | 5′-CTTCACTTGTGGCCCAGATAGG-3′ | |
| bax | ||
| Sense | 5′-GACGAACTGGACAGTAACATG-3′ | (19) |
| Antisense | 5′-AGGAAGTCCAATGTCCAGCC-3′ | |
| bak | ||
| Sense | 5′-TGAAAAATGGCTTCGGGGGCAAGGC-3′ | (20) |
| Antisense | 5′-TCATGATTTGAAGAATCTTCGTACC-3′ | |
| bid | ||
| Sense | 5′-ATGGACTGTGAGGTCAACAACGG-3′ | (21) |
| Antisense | 5′-CACGTAGGTGCGTAGGTTCTGGTTA-3′ | |
| bad | ||
| Sense | 5′-GTTTGAGCCGAGTGAGCAGG-3′ | (22) |
| Antisense | 5′-ATAGCGCTGTGCTGCCCAGA-3′ | |
| bcl-x | ||
| Sense | 5′-TTGGACAATGGACTGGTTG-3′ | (23) |
| Antisense | 5′-GTAGAGTGGATGGTCAGTG-3′ | |
| mcl-1 | ||
| Sense | 5′-GAAAAGCAAGTGGCAAGAGG-3′ | (24) |
| Antisense | 5′-CATGGAAACCAAGCCAAAGT-3′ | |
| IL-32siRNA | ||
| Sense | 5′-GGGAGAGCUUUUGUGACAAGG-3′ | (25) |
| Antisense | 5′-UUGUCACAAAAGCUCUCCCCA-3′ | |
Northern Blot Analyses
Total cellular RNA was isolated by the acid guanidinium thiocyanate-phenol-chloroform method (27). Northern blotting was performed according to a previously described method (18). The used probe reacts with all IL-32 isoforms. The hybridizations were performed with 32P-labeled human probes generated by a random primed DNA labeling kit (Amersham Biosciences) and were evaluated by autoradiography.
Western Blot Analyses
For the analysis of IL-32 protein expression, the cells were exposed to cytokines for predetermined periods of time. The cells were then washed with phosphate-buffered saline and lysed in SDS sample buffer containing 100 μm orthovanadate. For Western blotting, 10 μg of protein from each sample was subjected to SDS-PAGE on a 4–20% gradient gel under reducing conditions (28). Biotinylated anti-human IL-32α antibodies were purchased from R&D Systems, and peroxidase-conjugated streptavidin was purchased from Dako Japan (Kyoto, Japan). Subsequently, detection was performed using the enhanced chemiluminescence Western blotting system (Amersham Biosciences).
For Akt phosphorylation analyses, the cells were exposed to cytokines for predetermined periods of time. Antibodies directed against phosphorylated and total Akt were purchased from Cell Signaling Technology (Beverly, MA), and the peroxidase-conjugated secondary antibodies were purchased from Amersham Biosciences.
Adenovirus-mediated Gene Transfers
We used a recombinant adenovirus expressing a stable mutant form of IκBα (Ad-IκBΔN) (29), a recombinant adenovirus expressing a dominant negative mutant of c-Jun (Ad-DN-c-Jun) (30), and a recombinant adenovirus containing bacterial β-galactosidase cDNA (Ad-LacZ). The stable mutant form of IκBα (IκBΔN) lacks the 54 NH2-terminal amino acids of wild type IκBα, and is neither phosphorylated nor proteolyzed in response to signal induction but fully inhibits NF-κB activation. The dominant negative mutant c-Jun (TAM67) lacks the transactivational domain of amino acids 3–122 of wild type c-Jun but retains the DNA-binding domain. In preliminary experiments, Ad-LacZ infections of colonic myofibroblasts with a multiplicity of infection of 10 showed a maximal expression (85% positive) of β-galactosidase (data not shown). The recombinant adenovirus was transferred into the cells, and the cells were made quiescent for 48 h before being assessed for the effects of the transferred gene.
IL-32 mRNA Interference Experiments
The siRNA for human IL-32 and a control siRNA were used. PANC-1 cells were cultured in complete medium that did not contain antibiotics for 4 days. The cells were seeded onto a 6-well plate 1 day prior to the transfection and cultured to 60–70% confluence on the following day. For the mRNA interference experiments, LipofectamineTM LTX and 2.5 μl of PLUSTM Reagent (Invitrogen) were used. IL-32 expression was determined by RT-PCR and Western blotting.
Nuclear Extracts and Electrophoretic Gel Mobility Shift Assays
Nuclear extracts were prepared from cells exposed to the cytokines for 1.5 h. The consensus oligonucleotides for NF-κB (5′-AGTTGAGGGGACTTTCCCAGCC) and AP-1 (5′-CGCTTGATGAGTCAGCCGGAA) were purchased from Promega (Madison, WI). The oligonucleotides were 5′-end-labeled with T4 polynucleotide kinase (Promega) and [γ-32P]ATP (Amersham Biosciences). The binding reactions were performed according to previously described methods (31).
[3H]Thymidine Incorporation
Subconfluent cultures (70–90%) of PANC-1 cells in 24-well plates were washed and then incubated in Dulbecco's modified Eagle's medium containing 0.2% fetal calf serum for 24 h to induce growth arrest. [3H]Thymidine (1 μCi/well) was added for another 12 h of incubation. At the end of this incubation, the cells were washed and fixed with methanol/glacial acetic acid (3:1) for 1 h. The cells were then lysed in 1 ml of 1 m NaOH. The supernatant was transferred into a scintillation vial along with 10 ml of scintillation mixture (Packard, Merden, CT) and counted in a LKB scintillation counter. The data were expressed as disintegrations/min and as -fold stimulation over the control value.
Apoptosis Detection
For apoptosis assay by fluorescence-activated cell sorting, PANC-1 cells were trypsinized, washed, and resuspended in 500 μl of binding buffer (Sigma). Annexin V conjugated with fluorescein isothiocyanate and propidium iodide solution were added to the cell preparations and incubated for 25 min in the dark. Apoptotic cells were identified by annexin-V-fluorescein isothiocyanate staining. The samples were analyzed by a FACSCalibur instrument equipped with CellQuest 3.3 software (BD Biosciences).
Statistical Analyses
The statistical significance of the differences was determined by the Mann-Whitney U test (Statview Version 4.5). Differences resulting in p values less than 0.05 were considered to be statistically significant.
RESULTS
Immunohistochemical Studies of IL-32 Expression in Human Pancreas
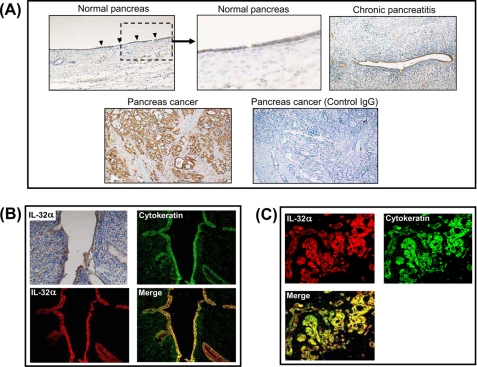
In normal human pancreatic tissue, there were weak IL-32 staining in duct cells (Fig. 1A). IL-32 expression by duct cells appeared increased in inflamed lesions of chronic pancreatitis (Fig. 1A) In these lesions, mesenchymal cells were also weakly stained. Furthermore, IL-32 was strongly positive in pancreatic cancer cells (Fig. 1A), whereas isotype control with goat IgG antibody shows negative staining.
FIGURE 1.
Representative immunohistochemical expression of IL-32 in the pancreas. A, IL-32 staining in normal pancreatic tissue (magnification ×100 and ×400), chronic pancreatitis (×100), pancreatic cancer (×100), and pancreatic cancer stained with control IgG. B, IL-32 staining in chronic pancreatitis. C, IL-32 staining in pancreatic cancer.
To further characterize the IL-32-expressing cells in the pancreas, double immunostaining for IL-32 and cytokeratin was performed in chronic pancreatitis. As shown in Fig. 1B, duct cells were strongly stained with cytokeratin. IL-32 staining was detected in duct cells, and surrounding mesenchymal cells were weakly stained. As shown in Fig. 1B, IL-32/cytokeratin double positive cells were detected as yellow, and cytokeratin-positive cells completely coincided with part of the IL-32-positive cells. Similarly, IL-32- and cytokeratin-positive cells completely coincided in pancreatic cancer cells (Fig. 1C).
Regulation of IL-32 Expression in Pancreatic Cancer Cell Lines
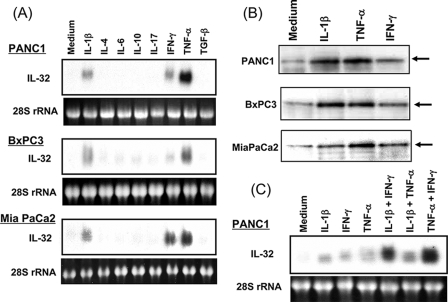
To investigate regulatory mechanisms underlying IL-32 induction in pancreatic cancer cell lines, cells were stimulated with various cytokines for 12 h, and IL-32 mRNA expression was detected by Northern blot analyses (Fig. 2A). In three pancreatic cancer cell lines (PANC-1, BxPC-3, and MIA PaCa-2), IL-32 mRNA was weakly expressed without any stimulus, and IL-1β, IFN-γ, and TNF-α markedly enhanced IL-32 mRNA expression. The effects of TNF-α were stronger than those induced by IL-1β and IFN-γ.
FIGURE 2.
IL-32 mRNA and protein expression in human pancreatic cancer cell lines. A, IL-32 mRNA expression. The cells were stimulated with cytokines (100 ng/ml) for 12 h. IL-32 mRNA expression was analyzed by Northern blotting. Ribosomal RNA, stained by ethidium bromide, is shown in the lower panel. B, intracellular IL-32 protein expression. The cells were stimulated with each cytokine (IL-1β (10 ng/ml), TNF-α (100 ng/ml), and IFN-γ (100 ng/ml)) for 24 h and then lysed with lysis buffer. IL-32 protein was analyzed by Western blotting. C, combined effects of cytokines on IL-32 mRNA expression. PANC-1 cells were stimulated with IL-1β (10 ng/ml), TNF-α (100 ng/ml), IFN-γ (100 ng/ml), and combinations of these cytokines for 12 h, and then IL-32 mRNA expression was determined by Northern blotting.
Similar results were observed at the protein level. The cells were stimulated for 24 h with IL-1β, IFN-γ, and TNF-α, and IL-32 protein expression was analyzed by Western blots. IL-32 was detected as a protein with a molecular mass of 25 kDa, which is comparable with a previous report (3). Stimulation with IL-1β, IFN-γ, and TNF-α enhanced intracellular accumulation of IL-32 protein (Fig. 2B). As observed at the mRNA level, TNF-α and IFN-γ effects were stronger than those induced by IL-1β. Next, we tested the effects of combinations of IL-1β, IFN-γ, and TNF-α in PANC-1 cells (Fig. 2C). Northern blot analysis showed that combinations of IL-1β plus IFN-γ and/or TNF-α plus IFN-γ synergistically enhanced IL-32 mRNA expression. Similar results were observed in BxPC-3 and MIA PaCa-2 cells (data not shown).
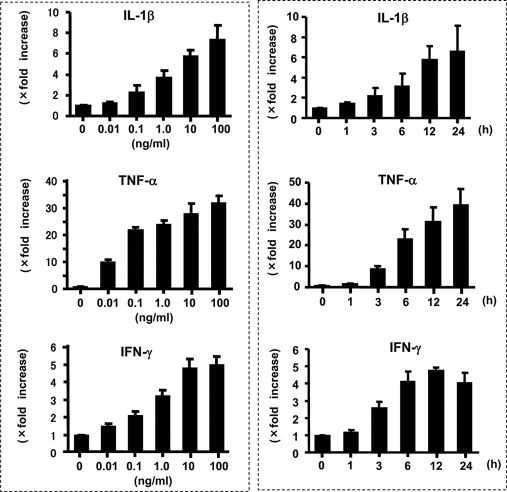
Dose- and Time-dependent Induction of IL-32 mRNA in Pancreatic Cancer Cell Lines
The effects of IL-1β, TNF-α, and IFN-γ on IL-32 mRNA expression were examined more precisely. PANC-1 cells were incubated for 12 h with increasing concentrations of IL-1β, TNF-α, and IFN-γ, and the IL-32 mRNA expression was analyzed by real time PCR. As shown in Fig. 3 (left), these cytokines dose-dependently up-regulated IL-32 mRNA expression. The kinetics of IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression was also evaluated. PANC-1 cells were stimulated with IL-1β (10 ng/ml), TNF-α (100 ng/ml), or IFN-γ (100 ng/ml), and sequential changes in IL-32 mRNA expression were determined by Northern blotting. As shown in Fig. 3 (right), time-dependent induction of IL-32 mRNA was detected. Similar results were observed in BxPC-3 and MIA PaCa-2 cells (data not shown).
FIGURE 3.
Dose- and time-dependent induction of IL-32 mRNA in PANC-1 cells. Left, cells were incubated with different doses of each cytokine, and IL-32 mRNA expression was determined by real time PCR. The data were expressed as IL-32 mRNA expression relative to β-actin mRNA expression (mean ± S.D. from four different experiments). Right, cells were stimulated with cytokines (IL-1β (10 ng/ml), TNF-α (100 ng/ml), and IFN-γ (100 ng/ml)) for predetermined times, and IL-32 mRNA expression was sequentially analyzed by real time PCR.
Role of Phosphatidylinositol 3-Kinase (PI3K) in IL-32 mRNA Induction
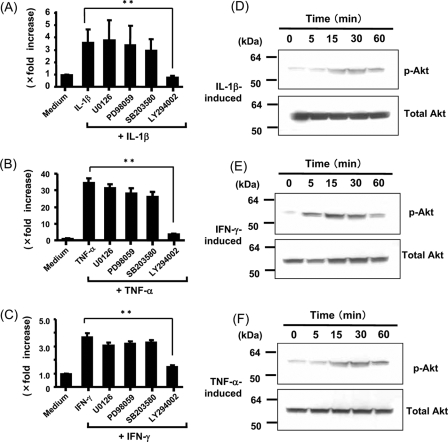
The MAPK and PI3K/Akt pathways are implicated in cytokine signaling in various cell types. To investigate molecular mechanisms underlying IL-32 induction in pancreatic cancer cells, we evaluated the effects of the following inhibitors: p42/44 MAPK inhibitors (PD98059 and U0216) (32, 33), a p38 MAPK inhibitor (SB203580) (34), and a PI3K inhibitor (LY294002) (35). Real time PCR demonstrated that treatment with MAPK inhibitors (PD98059 and U0216) or the p38 MAPK inhibitor (SB203580) had no effect on IL-1β-, TNF-α-, and/or IFN-γ-induced IL-32 mRNA in PANC-1 cells (Fig. 4, A–C). In contrast to these findings, the PI3K inhibitor, LY294002 (36), significantly blocked the effect of IL-1β-, TNF-α-, and/or IFN-γ on IL-32 mRNA expression (Fig. 4, A–C). Similar results were observed in BxPC-3 and MIA PaCa-2 cells (data not shown). These results suggest that PI3K activation is involved in IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression in pancreatic cancer cells.
FIGURE 4.
Effects of MAPK inhibitors and a PI3K inhibitor on IL-32 mRNA expression in PANC-1 cells. A–C, the cells were stimulated with each cytokine (IL-1β (10 ng/ml), TNF-α (100 ng/ml), and IFN-γ (100 ng/ml)) in the presence or absence of MEK inhibitors (PD98059 (20 μm) and U0216 (12.5 μm)), p38 inhibitor (SB203580 (25 μm)), and PI3K inhibitor (LY294002 (25 μm)) for 12 h, and then IL-32 mRNA expression was determined by real time PCR. The data were expressed as IL-32α mRNA expression relative to β-actin mRNA expression (mean ± S.D. from four different experiments). **, p < 0.01. D–F, kinetics of Akt activation in PANC-1 cells. The cells were stimulated with cytokines (IL-1β (10 ng/ml), TNF-α (100 ng/ml), and IFN-γ (100 ng/ml)), and phosphorylated (p-) and total Akt were sequentially detected by Western blotting.
In PANC-1 cells, the induction of Akt phosphorylation by IL-1β, TNF-α, and IFN-γ was evaluated by Western blotting. As shown in Fig. 4, D–F, IL-1β, TNF-α, and IFN-γ induced Akt phosphorylation. These data indicate that Akt, a protein kinase recruited by PI3K activation, is rapidly activated by IL-1β, TNF-α, and IFN-γ in PANC-1 cells.
NF-κB and AP-1 Activation Is Required for IL-32 mRNA Induction
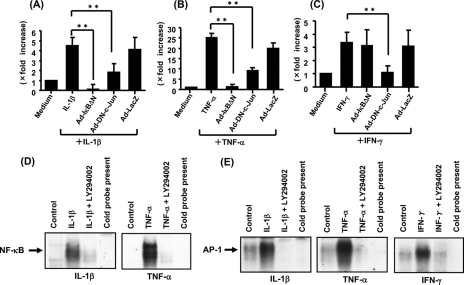
The promotor sequences analyzed by the UCSC Genome Browser created by the Genome Bioinformatics Group of the University of California (Santa Cruz, CA) showed consensus binding sites for NF-κB (at bp −638 to −649) and AP-1 (at bp −230 to −242) in promotor regions of the human IL-32 gene. To assess the role of transcription factors NF-κB and AP-1, we evaluated the effects of a recombinant adenovirus containing a stable mutant form of IκBα (Ad-IκBΔN) and a dominant negative mutant of c-Jun (Ad-DN-c-Jun) on cytokine-induced IL-32 mRNA expression. As shown in Fig. 5, A–C, PANC-1 cells were infected with recombinant adenovirus and were cultured for 48 h. The cells were stimulated for 12 h with IL-1β (10 ng/ml), TNF-α (100 ng/ml), and IFN-γ (100 ng/ml), and the expression of IL-32 mRNAs was determined by real time PCR. Ad-IκBΔN inhibited the effects of both IL-1β and TNF-α on IL-32 mRNA expression, and Ad-DN-c-Jun also suppressed the effects of IL-1β, TNF-α, and IFN-γ on IL-32 mRNA expression. Inhibitory effects were not induced by the Ad-LacZ gene, which was used as a negative control. These findings suggest that NF-κB and AP-1 play a role in IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression.
FIGURE 5.
Effects of NF-κB and/or AP-1 inhibition on IL-32 mRNA expression. A–C, PANC-1 cells were infected with an adenovirus expressing the IκBΔN or DN-c-Jun, and at 48 h after infection, cells were stimulated with IL-1β (10 ng/ml), TNF-α (100 ng/ml), or IFN-γ (100 ng/ml) for 12 h. IL-32 mRNA expression was determined by real time PCR. Adenovirus expressing LacZ was used as a negative control. The data were expressed by IL-32 mRNA expression relative to β-actin mRNA expression (mean ± S.D. from four different experiments). **, p < 0.01. D and E, electrophoretic gel mobility shift assays for NF-κB and/or AP-1 DNA binding activities. PANC-1 cells were incubated with medium alone, IL-1β (10 ng/ml), TNF-α (100 ng/ml), or IFN-γ (100 ng/ml) with or without LY294002 (25 μm) for 1.5 h, and then nuclear extracts were prepared.
To investigate the possibility that the PI3K/Akt pathway contributes to NF-κB/AP-1 activation, we investigated the effects of LY294002 on NF-κB and AP-1 activation in PANC-1 cells. As shown in Fig. 5D, electrophoretic gel mobility shift assays showed that PI3K inhibition by LY294002 suppressed both IL-1β- and TNF-α-induced NF-κB activation. Similarly, LY294002 blocked IL-1β-, TNF-α-, and IFN-γ-induced AP-1 activation (Fig. 5E). These findings suggest that IL-32 induction is mediated by PI3K/Akt pathway-dependent NF-κB/AP-1 activation.
Effects of IL-32 Knockdown on Cell Proliferation, Cell Death, and Apoptosis
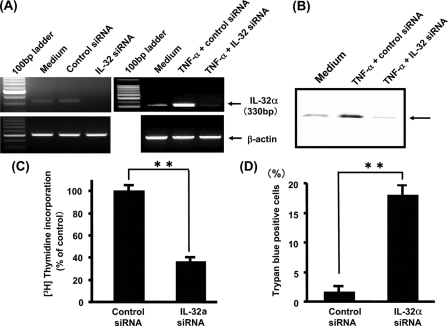
To investigate the role of IL-32 expression in pancreatic cancer cells, the mRNA interference technique was used. As shown in Fig. 6A, IL-32-specific siRNA effectively down-regulated the basal and TNF-α-induced expression of IL-32 mRNA in comparison with control siRNA. This was also confirmed by IL-32 protein expression (Fig. 6B). Similar effects were observed in BxPC-3 and MIA PaCa-2 cells (data not shown).
FIGURE 6.
IL-32 mRNA interference experiments. A, PANC-1 cells were transfected with IL-32 siRNA or control siRNA, and IL-32 mRNA expression was determined by RT-PCR. Similarly, PANC-1 cells were transfected with IL-32α siRNA or control siRNA and then stimulated by TNF-α for 12 h. IL-32 mRNA expression was determined by RT-PCR. B, PANC-1 cells were transfected with IL-32 siRNA or control siRNA and then stimulated by TNF-α for 24 h. IL-32 protein expression was determined by Western blotting. C, [3H]thymidine incorporation assay. PANC-1 cells were transfected with IL-32 siRNA or control siRNA and then cultured for 12 h in the presence of [3H]thymidine. The data were expressed as disintegrations/min and as -fold stimulation over the control value (mean ± S.D. from four different experiments). **, p < 0.01. D, trypan blue dye exclusion assay. PANC-1 cells were transfected with IL-32 siRNA or control siRNA and cultured for 24 h. The number of trypan blue-positive cells (dead cells) was counted under microscope (mean ± S.D. from four different experiments). **, p < 0.01.
Using this siRNA system, we evaluated how IL-32 knockdown modulates pancreatic cancer growth. A [3H]thymidine incorporation study was performed in PANC-1 cells with IL-32 or control siRNA. As shown in Fig. 6C, knockdown of IL-32 significantly decreased uptake of [3H]thymidine into the cells. Furthermore, IL-32 knockdown significantly increased the number of dead cells, which was determined by trypan blue-positive cells (Fig. 6D). These data suggest that constitutively expressed IL-32 may act as growth stimulator and inhibitor of cell death for pancreatic cancer cells.
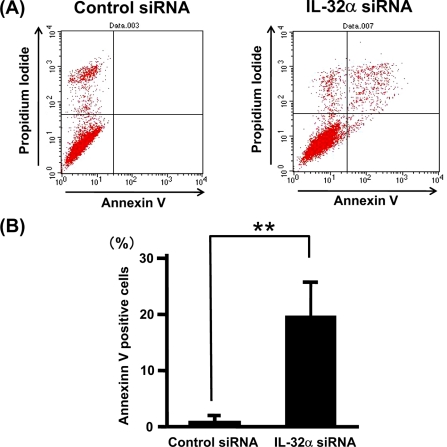
As a measure of apoptosis, we used annexin V-based flow cytometric analysis. This analysis indicated that knockdown of IL-32 significantly increased annexin V-positive (apoptotic) cells, indicating that IL-32 possesses antiapoptosis effects on pancreatic cancer cells (Fig. 7, A and B) and BxPC-3 and MIA PaCa-2 cells (data not shown).
FIGURE 7.
Knockdown of IL-32 gene stimulates apoptosis in PANC-1 cells. PANC-1 cells were transfected with IL-32α siRNA or control siRNA and cultured for 24 h. Apoptotic cells were analyzed by fluorescence-activated cell sorting using annexin V-fluorescein isothiocyanate. A, cytogram of fluorescence-activated cell sorting analyses. Annexin-V-positive cells are present in the upper and lower right. B, percentage of annexin V-positive cells (mean ± S.D. from four different experiments). **, p < 0.01.
Effects of IL-32 Knockdown on Pro- and Antiapoptotic Gene Expression
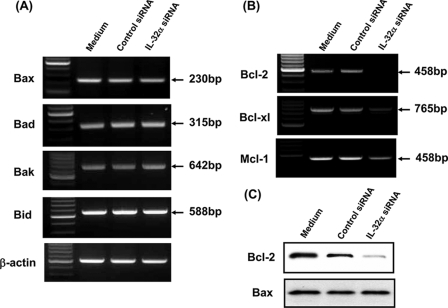
Whether a cell undergoes apoptosis in response to cellular stress is determined largely by interactions between proapoptotic proteins (e.g. Bax, Bak, Bid, and Bad) and prosurvival proteins (e.g. Bcl-2, Bcl-xL, and Mcl-1) (37). To clarify the role of IL-32 in apoptosis of pancreatic cancer cells, we analyzed the effects of IL-32 knockdown on the mRNA expression of proapoptotic and prosurvival proteins. As shown in Fig. 8, A and B, IL-32 knockdown down-regulated the mRNA expression of prosurvival proteins (Bcl-2, Bcl-xL, and Mcl-1), but it did not affect the mRNA expression of proapoptotic proteins (Bax, Bak, Bid, and Bad). This was also confirmed at the protein level (Fig. 8C). This suggests that constitutively expressed IL-32 may stimulate prosurvival protein expression in pancreatic cancer cells. Combined with the results of the apoptotic assay, IL-32 may play a role in growth and survival of pancreatic cancer cells.
FIGURE 8.
Effects of IL-32 knockdown on the mRNA expression of proapoptotic and prosurvival (antiapoptotic) proteins. A, PANC-1 cells were transfected with IL-32α siRNA or control siRNA, and the mRNA expression of proapoptotic proteins (Bax, Bad, Bak, and Bid) was determined by RT-PCR. B, PANC-1 cells were transfected with IL-32 siRNA or control siRNA, and the mRNA expression of prosurvival proteins (Bcl-2, Bcl-xl, and Mcl-1) was determined by RT-PCR. C, PANC-1 cells were transfected with IL-32 siRNA or control siRNA, and Bcl-2 and Bax protein expression was determined by Western blotting.
DISCUSSION
Recent studies have demonstrated that IL-32 is expressed by cells of epithelial origin, such as colon, gastric, and lung cancer cells (2, 3, 12, 13). However, IL-32 expression in the pancreas has not been precisely investigated. In the present study, we demonstrated several findings: (a) pancreatic duct cells are a source of IL-32; (b) IL-32 expression by duct cells is increased in chronic pancreatitis; (c) pancreatic cancer cells express IL-32 strongly; (d) IL-1β, TNF-α, and IFN-γ are potent stimulators for IL-32 induction; (e) PI3K/Akt activation and NF-κB/AP-1 activation participate in this IL-32 induction; and (f) IL-32 may play a role in the growth and survival of pancreatic cancer.
Pancreatic epithelial cells play an important role in the exocrine pancreas. The primary role of the pancreatic duct cells is to secrete water plus various electrolytes, but several lines of evidence have suggested that the pancreatic epithelial cells may also serve as active participants in various immunologic functions. For example, the expression of transforming growth factor-β1 has been detected in these cells in vivo (38, 39). In this study, we demonstrated that IL-32 is expressed by pancreatic duct cells and that it was increased in chronic pancreatitis. Furthermore, we detected IL-32 expression by pancreatic cancer cells and in the cancer cell lines. These observations indicate that human pancreatic epithelial (duct) cells are a local source of IL-32. Experiments using recombinant IL-32α also suggest that it is a proinflammatory cytokine and is characterized by the release of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and chemokines) through NF-κB and p38 MAPK activation pathways (2, 5, 14). Furthermore, IL-32 may play an important role in both inflammatory and immune responses in the pancreas.
IL-32 expression in ductal cells was enhanced in tissues from chronic pancreatitis and this suggests a regulatory mechanism for IL-32 expression. Previously, IL-1β, IL-12, IL-18, and IFN-γ have been identified as stimulators for IL-32 expression (2, 12). In the present study, we showed that in pancreatic cancer cells, IL-1β, TNF-α, and IFN-γ are potent inducers of IL-32 mRNA expression. To address the molecular mechanisms contributing to IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression, we evaluated the effects of MAPK inhibitors and a PI3K inhibitor on IL-32 induction. Although we have previously shown that MAPKs play a crucial role in inducing proinflammatory cytokines, such as IL-6 and IL-8 (28, 31, 40), p42/44 MAPK inhibitors (PD98059 and U0216) and the p38 MAPK inhibitor (SB203580) did not affect IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression. In contrast, a PI3K inhibitor (LY294002) effectively suppressed IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression. Furthermore, in these cells, IL-1β, TNF-α, and IFN-γ induced the phosphorylation of Akt, a protein kinase immediately recruited by PI3K activation (41). These observations indicated that the PI3K/Akt pathway contributes to proinflammatory cytokine-induced IL-32 mRNA expression in pancreatic cancer cells. Recent studies showed that the PI3K/Akt pathway plays an important role in pancreatic regenerative responses (42), acinar cell functions (43), and the endocrine pancreas (44). In addition, the PI3K/Akt pathway regulates trypsinogen activation during acute pancreatitis (45). Besides these functions, our findings suggested a novel role for the PI3K/Akt pathway in inflammatory immune responses in the pancreas.
The consensus binding sites for NF-κB and AP-1 are located in the promotor region of the human IL-32 gene (at bp −638 to −649 and bp −230 to −242, respectively), suggesting an involvement of NF-κB and AP-1 activation in IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression. To confirm this possibility, we used a recombinant adenovirus expressing a stable mutant form of IκBα (Ad-IκBΔN) (29) and a recombinant adenovirus expressing a dominant negative mutant of c-Jun (Ad-DN-c-Jun) (30). Successful infection of Ad-IκBΔN and/or Ad-DN-c-Jun fully inhibited NF-κB and AP-1 activation. As shown in Fig. 5, pretreatment with Ad-IκBΔN blocked IL-1β- and TNF-α-induced IL-32 mRNA expression, and treatment with Ad-DN-c-Jun also suppressed IL-1β-, TNF-α-, and IFN-γ-induced IL-32 mRNA expression. These findings indicate that NF-κB and AP-1 activation play a role in IL-32 mRNA induction in our system. In some cell types, NF-κB and AP-1 activation are regulated by the PI3K/Akt pathway (36, 46–49), and cross-talk between the PI3K/Akt pathway and NF-κB/AP-1 activation in cytokine-induced IL-32 mRNA expression can be hypothesized.
The significance of IL-32 expression in pancreatic cancer cells remains unclear. In this study, we showed that IL-32 knockdown significantly decreased [3H]thymidine incorporation and stimulated cell death and apoptosis in pancreatic cancer cells, suggesting that pancreatic cancer growth and apoptosis may be partially dependent on IL-32. Furthermore, IL-32 knockdown decreased the mRNA expression of prosurvival (antiapoptotic) proteins (Bcl-2, Bcl-xL, and Mcl-1) but did not affect the mRNA expression of proapoptotic proteins (Bax, Bak, Bid, and Bad). In the apoptosis cascade, the activation of proapoptotic proteins (Bax and Bak) is opposed by prosurvival Bcl-2 proteins, such as Bcl-2, Bcl-xL, and Mcl-1 (50). Bid has been shown to directly activate Bax-Bak, and Bcl-2 proteins (Bcl-2, Bcl-xL, and Mcl-1) sequester these molecules into a stable form and prevent the activation of Bax-Bak (50). In response to apoptotic stimuli, Bad is rapidly dephosphorylated and migrates to the mitochondria, where it induces cell death (50). Thus, IL-32 knockdown induced the suppression of prosurvival proteins, indicating that IL-32 stimulated the antiapoptotic activity of pancreatic cancer. Combined with the growth stimulation detected by [3H]thymidine incorporation, IL-32 may be one of the key factors contributing to the development of pancreatic cancer.
In this study, we could not clarify IL-32 isoforms, since IL-32 isoforms share common nucleotide and amino acid sequences (14). Our preliminary data of RT-PCR analyses with isoform-specific primers showed that all IL-32 isoforms are detectable in pancreatic cancer cell lines (data not shown). This suggests that our data include the behavior of all IL-32 isoforms. A recent report by Choi et al. (14) indicated that different IL-32 isoforms have similar biological effects, and isoform-specific experiments using isoform-specific probes and antibodies should be performed in the future.
In conclusion, we demonstrated that IL-32 is expressed in the human pancreas. IL-32 was expressed by pancreatic duct cells and may play an important role in inflammatory responses and pancreatic cancer growth. We still have limited data on IL-32 functions, but the findings in this study suggest the possibility that IL-32 can be a therapeutic target for pancreatitis and pancreatic cancer. The precise role of IL-32 in pancreatic disorders should be further investigated.
Footnotes
- IL
- interleukin
- PI3K
- phosphatidylinositol 3-kinase
- NF-κB
- nuclear factor-κB
- AP-1
- activated protein-1
- TNF
- tumor necrosis factor
- RT
- reverse transcription
- IFN
- interferon
- MAPK
- mitogen-activated protein kinase
- Ad
- adenovirus
- siRNA
- small interference RNA.
REFERENCES
- 1.Dahl C. A., Schall R. P., He H. L., Cairns J. S. ( 1992) J. Immunol. 148, 597– 603 [PubMed] [Google Scholar]
- 2.Kim S. H., Han S. Y., Azam T., Yoon D. Y., Dinarello C. A. ( 2005) Immunity 22, 131– 142 [DOI] [PubMed] [Google Scholar]
- 3.Netea M. G., Azam T., Ferwerda G., Girardin S. E., Walsh M., Park J. S., Abraham E., Kim J. M., Yoon D. Y., Dinarello C. A., Kim S. H. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 16309– 16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q., Carroll H. P., Gadina M. ( 2006) Vitam. Horm. 74, 207– 228 [DOI] [PubMed] [Google Scholar]
- 5.Dinarello C. A., Kim S. H. ( 2006) Ann. Rheum. Dis. 65, Suppl. 3, iii61– iii64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kundu M., Basu J. ( 2006) PLoS Med. 3, e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novick D., Rubinstein M., Azam T., Rabinkov A., Dinarello C. A., Kim S. H. ( 2006) Proc. Natl. Acad. Sci. U. S. A. 103, 3316– 3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cagnard N., Letourneur F., Essabbani A., Devauchelle V., Mistou S., Rapinat A., Decraene C., Fournier C., Chiocchia G. ( 2005) Eur. Cytokine Netw. 16, 289– 292 [PubMed] [Google Scholar]
- 9.Joosten L. A., Netea M. G., Kim S. H., Yoon D. Y., Oppers-Walgreen B., Radstake T. R., Barrera P., van de Loo F. A., Dinarello C. A., van den Berg W. B. ( 2006) Proc. Natl. Acad. Sci. U. S. A. 103, 3298– 3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoda H., Fujio K., Yamaguchi Y., Okamoto A., Sawada T., Kochi Y., Yamamoto K. ( 2006) Arthritis Res. Ther. 8, R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea M. G., Azam T., Lewis E. C., Joosten L. A., Wang M., Langenberg D., Meng X., Chan E. D., Yoon D. Y., Ottenhoff T., Kim S. H., Dinarello C. A. ( 2006) PLoS Med. 3, e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shioya M., Nishida A., Yagi Y., Ogawa A., Tsujikawa T., Kim-Mitsuyama S., Takayanagi A., Shimizu N., Fujiyama Y., Andoh A. ( 2007) Clin. Exp. Immunol. 149, 480– 486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K. H., Shim J. H., Seo E. H., Cho M. C., Kang J. W., Kim S. H., Yu D. Y., Song E. Y., Lee H. G., Sohn J. H., Kim J., Dinarello C. A., Yoon D. Y. ( 2008) J. Immunol. Methods 333, 38– 50 [DOI] [PubMed] [Google Scholar]
- 14.Choi J. D., Bae S. Y., Hong J. W., Azam T., Dinarello C. A., Her E., Choi W. S., Kim B. K., Lee C. K., Yoon D. Y., Kim S. J., Kim S. H. ( 2009) Immunology 126, 535– 542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. ( 2003) Gut 52, 65– 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieber M., Mazzetta J., Nelson-Rees W., Kaplan M., Todaro G. ( 1975) Int. J. Cancer 15, 741– 747 [DOI] [PubMed] [Google Scholar]
- 17.Yunis A. A., Arimura G. K., Russin D. J. ( 1977) Int. J. Cancer 19, 128– 135 [DOI] [PubMed] [Google Scholar]
- 18.Andoh A., Takaya H., Saotome T., Shimada M., Hata K., Araki Y., Nakamura F., Shintani Y., Fujiyama Y., Bamba T. ( 2000) Gastroenterology 119, 211– 219 [DOI] [PubMed] [Google Scholar]
- 19.Strausberg R. L., Feingold E. A., Grouse L. H., Derge J. G., Klausner R. D., Collins F. S., Wagner L., Shenmen C. M., Schuler G. D., Altschul S. F., Zeeberg B., Buetow K. H., Schaefer C. F., Bhat N. K., Hopkins R. F., Jordan H., Moore T., Max S. I., Wang J., Hsieh F., Diatchenko L., Marusina K., Farmer A. A., Rubin G. M., Hong L., Stapleton M., Soares M. B., Bonaldo M. F., Casavant T. L., Scheetz T. E., Brownstein M. J., Usdin T. B., Toshiyuki S., Carninci P., Prange C., Raha S. S., Loquellano N. A., Peters G. J., Abramson R. D., Mullahy S. J., Bosak S. A., McEwan P. J., McKernan K. J., Malek J. A., Gunaratne P. H., Richards S., Worley K. C., Hale S., Garcia A. M., Gay L. J., Hulyk S. W., Villalon D. K., Muzny D. M., Sodergren E. J., Lu X., Gibbs R. A., Fahey J., Helton E., Ketteman M., Madan A., Rodrigues S., Sanchez A., Whiting M., Madan A., Young A. C., Shevchenko Y., Bouffard G. G., Blakesley R. W., Touchman J. W., Green E. D., Dickson M. C., Rodriguez A. C., Grimwood J., Schmutz J., Myers R. M., Butterfield Y. S., Krzywinski M. I., Skalska U., Smailus D. E., Schnerch A., Schein J. E., Jones S. J., Marra M. A. ( 2002) Proc. Natl. Acad. Sci. U. S. A. 99, 16899– 16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleary M. L., Smith S. D., Sklar J. ( 1986) Cell 47, 19– 28 [DOI] [PubMed] [Google Scholar]
- 21.Apte S. S., Mattei M. G., Olsen B. R. ( 1995) Genomics 26, 592– 594 [DOI] [PubMed] [Google Scholar]
- 22.Chittenden T., Harrington E. A., O'Connor R., Flemington C., Lutz R. J., Evan G. I., Guild B. C. ( 1995) Nature 374, 733– 736 [DOI] [PubMed] [Google Scholar]
- 23.Wang K., Yin X. M., Chao D. T., Milliman C. L., Korsmeyer S. J. ( 1996) Genes Dev. 10, 2859– 2869 [DOI] [PubMed] [Google Scholar]
- 24.Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. ( 1995) Cell 80, 285– 291 [DOI] [PubMed] [Google Scholar]
- 25.Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. ( 1993) Cell 74, 597– 608 [DOI] [PubMed] [Google Scholar]
- 26.Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. ( 1993) Proc. Natl. Acad. Sci. U. S. A. 90, 3516– 3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chomczynski P., Sacchi N. ( 1987) Anal. Biochem. 162, 156– 159 [DOI] [PubMed] [Google Scholar]
- 28.Shimada M., Andoh A., Hata K., Tasaki K., Araki Y., Fujiyama Y., Bamba T. ( 2002) J. Immunol. 168, 861– 868 [DOI] [PubMed] [Google Scholar]
- 29.Obara H., Takayanagi A., Hirahashi J., Tanaka K., Wakabayashi G., Matsumoto K., Shimazu M., Shimizu N., Kitajima M. ( 2000) Arterioscler. Thromb. Vasc. Biol. 20, 2198– 2204 [DOI] [PubMed] [Google Scholar]
- 30.Yasumoto H., Kim S., Zhan Y., Miyazaki H., Hoshiga M., Kaneda Y., Morishita R., Iwao H. ( 2001) Gene Ther. 8, 1682– 1689 [DOI] [PubMed] [Google Scholar]
- 31.Hata K., Andoh A., Shimada M., Fujino S., Bamba S., Araki Y., Okuno T., Fujiyama Y., Bamba T. ( 2002) Am. J. Physiol. Gastrointest Liver Physiol. 282, G1035– 1044 [DOI] [PubMed] [Google Scholar]
- 32.Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F., Copeland R. A., Magolda R. L., Scherle P. A., Trzaskos J. M. ( 1998) J. Biol. Chem. 273, 18623– 18632 [DOI] [PubMed] [Google Scholar]
- 33.Alessi D. R., Cuenda A., Cohen P., Dudley D. T., Saltiel A. R. ( 1995) J. Biol. Chem. 270, 27489– 27494 [DOI] [PubMed] [Google Scholar]
- 34.Cuenda A., Rouse J., Doza Y. N., Meier R., Cohen P., Gallagher T. F., Young P. R., Lee J. C. ( 1995) FEBS Lett. 364, 229– 233 [DOI] [PubMed] [Google Scholar]
- 35.Fang J., Ding M., Yang L., Liu L. Z., Jiang B. H. ( 2007) Cell. Signal 19, 2487– 2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang C. H., Lu D. Y., Yang R. S., Tsai H. Y., Kao M. C., Fu W. M., Chen Y. F. ( 2007) J. Immunol. 179, 1292– 1302 [DOI] [PubMed] [Google Scholar]
- 37.Adams J. M., Cory S. ( 2007) Oncogene 26, 1324– 1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoh K., Shimosegawa T., Hirota M., Koizumi M., Toyota T. ( 1998) Pancreas 16, 468– 474 [DOI] [PubMed] [Google Scholar]
- 39.Detlefsen S., Sipos B., Zhao J., Drewes A. M., Klöppel G. ( 2008) Am. J. Surg. Pathol. 32, 986– 995 [DOI] [PubMed] [Google Scholar]
- 40.Andoh A., Shimada M., Bamba S., Okuno T., Araki Y., Fujiyama Y., Bamba T. (2002) Biochim. Biophys. Acta 1591, 69–74 [DOI] [PubMed] [Google Scholar]
- 41.Cantley L. C. ( 2002) Science 296, 1655– 1657 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H., Saito H., Rychahou P. G., Uchida T., Evers B. M. ( 2005) Gastroenterology 128, 1391– 1404 [DOI] [PubMed] [Google Scholar]
- 43.Gukovsky I., Cheng J. H., Nam K. J., Lee O. T., Lugea A., Fischer L., Penninger J. M., Pandol S. J., Gukovskaya A. S. ( 2004) Gastroenterology 126, 554– 566 [DOI] [PubMed] [Google Scholar]
- 44.Zawalich W. S., Tesz G. J., Zawalich K. C. ( 2002) J. Endocrinol. 174, 247– 258 [DOI] [PubMed] [Google Scholar]
- 45.Singh V. P., Saluja A. K., Bhagat L., van Acker G. J., Song A. M., Soltoff S. P., Cantley L. C., Steer M. L. ( 2001) J. Clin. Invest. 108, 1387– 1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Julien S., Puig I., Caretti E., Bonaventure J., Nelles L., van Roy F., Dargemont C., de Herreros A. G., Bellacosa A., Larue L. ( 2007) Oncogene 26, 7445– 7456 [DOI] [PubMed] [Google Scholar]
- 47.Rajaram M. V., Ganesan L. P., Parsa K. V., Butchar J. P., Gunn J. S., Tridandapani S. ( 2006) J. Immunol. 177, 6317– 6324 [DOI] [PubMed] [Google Scholar]
- 48.Peloponese J. M., Jr., Jeang K. T. ( 2006) J. Biol. Chem. 281, 8927– 8938 [DOI] [PubMed] [Google Scholar]
- 49.Li J., Chen H., Tang M. S., Shi X., Amin S., Desai D., Costa M., Huang C. ( 2004) J. Cell Biol. 165, 77– 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinicrope F. A., Rego R. L., Foster N. R., Thibodeau S. N., Alberts S. R., Windschitl H. E., Sargent D. J. ( 2008) Clin. Cancer Res. 14, 4128– 4133 [DOI] [PMC free article] [PubMed] [Google Scholar]