Abstract
Background: Locomotor training (LT) to improve walking ability in people poststroke can be accomplished with therapist assistance as needed to promote continuous stepping. Various robotic devices also have been developed that can guide the lower limbs through a kinematically consistent gait pattern. It is unclear whether LT with either therapist or robotic assistance could improve kinematic coordination patterns during walking.
Objective: The purpose of this study was to determine whether LT with physical assistance as needed was superior to guided, symmetrical, robotic-assisted LT for improving kinematic coordination during walking poststroke.
Design: This study was a randomized clinical trial.
Methods: Nineteen people with chronic stroke (>6 months’ duration) participating in a larger randomized control trial comparing therapist- versus robotic-assisted LT were recruited. Prior to and following 4 weeks of LT, gait analysis was performed at each participant's self-selected speed during overground walking. Kinematic coordination was defined as the consistency of intralimb hip and knee angular trajectories over repeated gait cycles and was compared before and after treatment for each group.
Results: Locomotor training with therapist assistance resulted in significant improvements in the consistency of intralimb movements of the impaired limb. Providing consistent kinematic assistance during robotic-assisted LT did not result in improvements in intralimb consistency. Only minimal changes in discrete kinematics were observed in either group.
Limitations: The limitations included a relatively small sample size and a lack of quantification regarding the extent of movement consistency during training sessions for both groups.
Conclusions: Coordination of intralimb kinematics appears to improve in response to LT with therapist assistance as needed. Fixed assistance, as provided by this form of robotic guidance during LT, however, did not alter intralimb coordination.
Locomotor training (LT) using a treadmill with body-weight support (BWS) has been advocated for improving walking in individuals with hemiparesis poststroke.1 Specific afferent inputs provided during LT, including maximizing lower-limb weight bearing during stance,2 training at gait speeds approximating normal walking speeds,3 and generating reciprocal lower-limb kinematics associated with locomotion,4,5 are thought to facilitate locomotor recovery in individuals with spinal cord injury (SCI) and stroke. In individuals with substantial gait impairment, successfully completing each step during LT often requires physical assistance, which can be achieved in 1 of 2 ways. Commonly, therapists assist patients manually to approximate the lower-limb kinematic trajectories associated with human gait. Providing such assistance is labor intensive, however, and may result in variable, inconsistent kinematic trajectories during training. Increased variability of intralimb kinematics may represent diminished coordination,6 and deficits in consistency or stability from stride to stride are thought to predict gait instability and fall risk.7
In contrast, the use of clinical robotic locomotor devices can relieve therapists of the physical effort often required during LT by providing consistent, repetitive guidance to the lower extremities.8–10 The ability of robotic devices to provide stable, repetitive LT is thought to supply many of the sensory-specific cues related to walking, which may strengthen neural pathways associated with the production of coordinated locomotion.10,11
Contrary to the notion that consistent sensory information during LT is critical to enhancing stepping, a long-standing body of research has indicated the importance of practice variability when learning a motor task.12 Recent data in experimental models of SCI indicate that variable, assist-as-needed step training improves the consistency of stepping compared with constrained guidance through a fixed trajectory.13 Furthermore, such fixed training paradigms are thought to reduce voluntary participation14 and the central nervous system's ability to fully explore various movement options.13 Thus, training with robotic devices that provide strict guidance of limb kinematics may limit improvements in the recovery of motor coordination by reducing movement variability, particularly compared with variable, compliant, assist-as-needed LT paradigms.13,15
Previous work investigating the effects of robotic- versus therapist-assisted training on recovery of walking function in subjects with hemiparesis poststroke focused on alterations in gait speed and symmetry and functional outcomes following training.16 In the present study, we sought to determine whether LT with therapist assistance as needed was superior to guided, symmetrical robotic-assisted LT at improving kinematic coordination during walking. An estimate of intralimb coordination has been quantified by other investigators6,17 as the repeatability or consistency of the coupling of hip and knee kinematics during multiple gait cycles. In the present study, gait kinematics were assessed in a subpopulation of individuals with chronic (>6 months’ duration) stroke from a larger randomized controlled study prior to and following 4 weeks of LT performed on a treadmill. Specific analyses were performed to determine alterations in intralimb coordination during overground walking and their relationship to functional improvements. Secondary analysis was performed to determine whether absolute joint angles and excursions were altered following LT with therapist or robotic assistance. Based on previous work,6,17 we hypothesized that therapist-assisted LT using variable assistance as needed would elicit greater improvements in intralimb coordination than robotic-assisted LT using fixed movement patterns.
Method
Participants
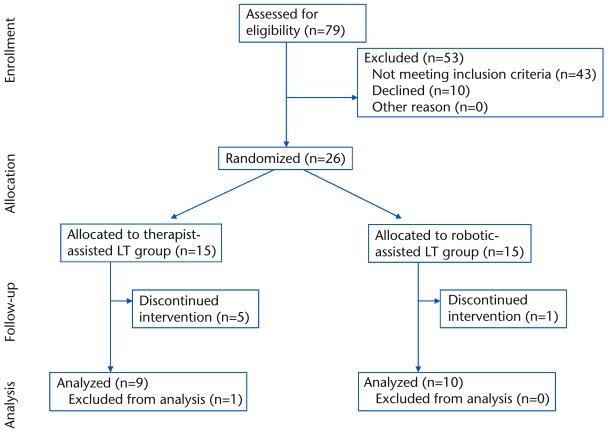
Participants recruited for the current investigation represent an intentional subgroup analysis of the final 26 of 62 individuals recruited for a larger randomized clinical trial, comparing functional outcomes following LT with either robotic or therapist assistance.16 Data collection for the project presented here began midway through the larger randomized clinical trial16 because our motion capture equipment was unavailable at the beginning of the larger trial. Individuals with chronic (>6 months’ duration) hemiparesis following unilateral ischemic or hemorrhagic stroke were recruited for participation (Fig. 1). Lesion location was confirmed by imaging (ie, magnetic resonance imaging, computed tomography, positron emission tomography), with no evidence of brain-stem, cerebellar, or bilateral lesions. Participants were included only if they could walk at least 10 m overground without physical assistance and at a self-selected gait speed of <0.8 m/s, indicative of limited community ambulation.18 Exclusion criteria consisted of significant cardiorespiratory or metabolic disease that limited exercise participation, history of previous orthopedic or neurological conditions that may limit walking ability, and size limitations of the harness-counterweight system and robotic orthosis.16 No participant had received botulinum toxin therapy in the lower limbs in the past 6 months, and all participants were prohibited from receiving physical therapy training outside of the study. Participants with scores of <22 on the Mini Mental Status Exam were excluded. All participants required medical clearance to participate. Participants were randomly assigned to receive either robotic-assisted LT (n=11) or therapist-assisted LT (n=15) using sealed envelopes concealed from view. All participants were informed of the purpose of and procedures for the study and signed an informed consent form approved by the Institutional Review Board of Northwestern University prior to participation.
Figure 1.
CONSORT flow diagram representing participant enrollment, allocation, and analysis throughout the study. LT=locomotor training.
Training
All individuals participated in 12 sessions of LT (3 sessions per week for 4 weeks), with up to 30 minutes of stepping during a 1-hour session.16 Speed was gradually increased during the first session and remained at 3.0 kmph (0.83 m/s) for the remainder of LT. Body-weight support was provided by a harness-counterweight system, with up to 40% BWS at the first session and reduced as tolerated.16 Treadmill training speeds and the amount of unloading were similar between groups.16
Participants randomly assigned to the robotic-assisted LT group used the Lokomat,* a robotic gait trainer, to assist the lower extremities in a consistent, symmetrical walking pattern during treadmill stepping. The design and control of this device have been described previously.11 Participants were fixed to the device with adjustable cloth straps placed around the trunk, pelvis, and lower extremities, with hip and knee joints aligned with computer-controlled actuators. Spring-loaded cloth straps were attached around the participants’ forefoot to ensure toe clearance during swing on the paretic side. The robotic device provided continuous assistance in kinematic trajectories approximating “normal” gait. Participants were given continuous visual feedback of bilateral hip and knee torques during walking and were encouraged to generate maximal effort in the paretic limb.
Participants randomly assigned to the therapist-assisted LT group received manual assistance from a single therapist for limb advancement or pelvic control. An ankle-foot orthosis (AFO) was used only if an individual was unable to step safely without it. Manual facilitation was provided only if a participant could not step continuously at the required treadmill speed (ie, an assist-as-needed paradigm) and was not provided to normalize kinematics between limbs.
Gait Analysis
Gait analysis was performed for all participants less than 1 week prior to the initiation of LT and was repeated less than 1 week following the last LT session. Eight participants (4 in each group) required some form of ankle bracing (7 AFOs, 1 ankle stirrup brace), and use of orthoses and assistive devices was consistent during testing sessions. Participants ambulated at least 5 times across a 10-m walkway at their comfortable, self-selected speed, while an 8-camera motion capture system† recorded the 3-dimensional (3D) movement of 25.4-mm (1-in) retroreflective markers affixed to the pelvis, thighs, shanks, and feet. Specifically, markers were placed on the posterior sacrum, bilateral anterior-superior iliac spine, medial and lateral femoral condyles, medial and lateral malleoli, and posterior heel counter of the shoe and dorsally over the second metatarsal head to identify segment ends. The motion of the thighs and shanks was tracked by 3 markers rigidly affixed to thermoplastic shells, which, in turn, were wrapped securely around each limb segment.
Data Management
The marker trajectories were identified and low-pass filtered (6 Hz) to track the 3D motion of the pelvis and lower-limb segments using EvaRT software.† Relative positions and intersegmental joint angles (eg, hip, knee, and ankle angles) were calculated using a rigid body analysis19 and normalized to a stride cycle using OrthoTrak 6.2.4.†
The consistency of intralimb coordination between the hip and knee joints was quantified by calculating the average coefficient of correspondence (ACC) as described by Field-Fote and Tepavac.17 The ankle joint was not assessed because some participants used AFOs to restrict ankle kinematics. The ACC uses a vector coding technique to analyze the sagittal-plane hip and knee angles on an angle-angle plot. The difference between successive frames (one frame equals 1% of the gait cycle) on the phase plane is represented by a vector whose length is calculated by:
| (1) |
where, xi and yi are the change in hip angle and knee angle from frame i to frame i+1, respectively. Consequently, there are 100 l values for each stride. Determination of the direction of the vector at each frame is calculated from the sine and cosine of the angle between l and the x-axis for each frame:
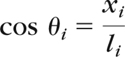 |
(2) |
and
| (3) |
Then the mean cos(θ) and sin(θ) for a given frame over multiple steps was calculated. The mean vector angle for each frame can then be evaluated by:
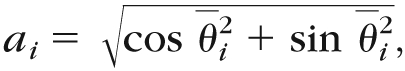 |
(4) |
where cos θ̄i and sin θ̄i are the mean cos(θ) and sin(θ) for each i frame. The ai values are a measure of dispersion across strides of the hip and knee angle pair at each percent of the gait cycle. Values equal to 1 would indicate that there is perfect consistency across all steps for that given frame. To represent all the ai values as a single variable, the mean of the a values for all i frames was calculated to represent the hip and knee ACC. Individuals who are unimpaired walking at their self-selected speeds exhibit a hip and knee ACC of .9417 to .97.6 A representative example of the hip and knee angle-angle plot and the steps involved in the calculation of hip and knee ACC in an individual poststroke is shown in Figure 2. Overall, an average of 15 strides (SD=8) on the involved side and an average of 16 strides (SD=9) on the uninvolved side per participant were analyzed.
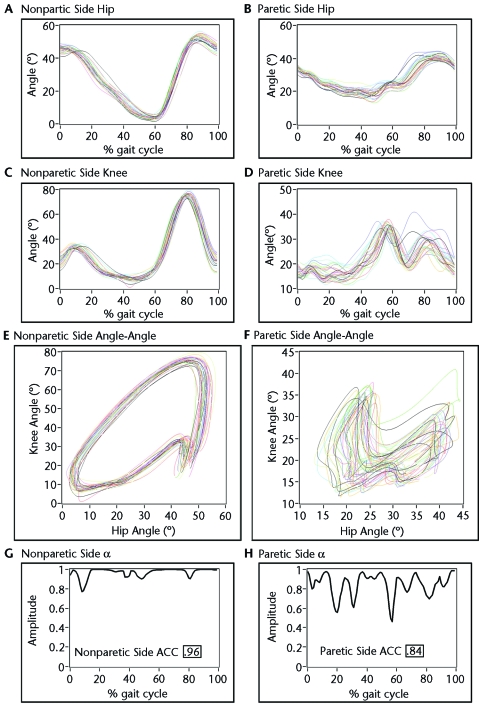
Figure 2.
Representative example of data obtained before locomotor training (LT) for a participant enrolled in the robotic-assisted LT group. The left column represents the nonparetic-side hip (A) and knee (C) angles plotted against percentage of gait cycle for all steps, with an angle-angle plot for the hip and knee (E). The bottom graph represents the a values calculated for each percentage of the stride cycle, with the hip and knee average coefficient of correspondence (ACC) value (G). Similarly, the right column shows the more-variable paretic-side hip (B) and knee (D) angles with the respective angle-angle plot (F) and a values (H) used to calculate hip and knee ACC.
In addition to hip and knee ACC, secondary outcome measures included alterations in spatiotemporal gait parameters (gait speed, cadence, and stride length) and changes in discrete joint kinematics, as well as the extent of limb circumduction during walking. Gait speed was calculated during the gait analysis session as the speed of the sacral marker in the direction of forward progress. Cadence and stride length were determined from the OrthoTrak software. Hip, knee, and ankle joint angles were calculated for both the stance phase (ie, peak hip and knee extension and ankle plantar flexion) and the swing phase (ie, peak hip and knee flexion and ankle dorsiflexion), in addition to maximum flexion to extension excursion. Finally, we calculated limb circumduction as the maximum lateral deviation of the heel during swing with respect to the position of the ipsilateral heel during consecutive stance phases (immediately prior to and following the measured swing phase).20
Data Analysis
All statistical analyses were performed with SPSS, version 15.0.‡ The effect of LT on the consistency of intralimb coordination was evaluated with a 2-group (robotic-assisted LT and therapist-assisted LT) × 2-session (pretraining and posttraining) analysis of variance (ANOVA) for repeated measures for session. Post hoc testing of within-group differences for comparison of pretraining and posttraining values was performed using paired t tests. Group data are reported as mean (SD), with α=.05. The relationship between gait speed and intralimb coordination was assessed with Pearson correlation coefficients. Secondary measures of peak joint angle and excursions are presented using descriptive statistics (ie, mean and SD), but pretraining and posttraining values were not compared, as these were not primary outcomes. In addition, a stepwise linear regression analysis was used to determine the relative contributions of cadence and stride length to changes in walking speed. Cadence and stride length for each group were compared before and after training with paired t tests.
Results
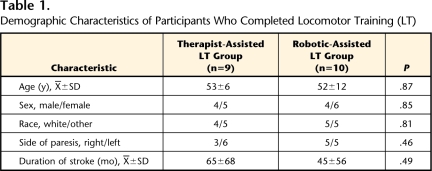
A total of 26 participants were enrolled in the study. During the training period, 6 participants could not complete the study (5 in the therapist-assisted LT group and 1 in the robotic-assisted LT group). Dropouts were secondary to an orthopedic injury related (n=1) or unrelated (n=1) to training, difficulty with transportation (n=1), fear of falling (n=1), or self-reported exercise intolerance (n=2). There was no difference in dropout rates between groups (χ2=2.10, P=.147). In addition, data collection procedures on 1 participant in the therapist-assisted LT group resulted in unusable kinematic data, yielding a total of 10 participants in the robotic-assisted LT group and 9 participants in the therapist-assisted LT group for data analysis. Demographic characteristics of these 19 participants are provided in Table 1.
Table 1.
Demographic Characteristics of Participants Who Completed Locomotor Training (LT)
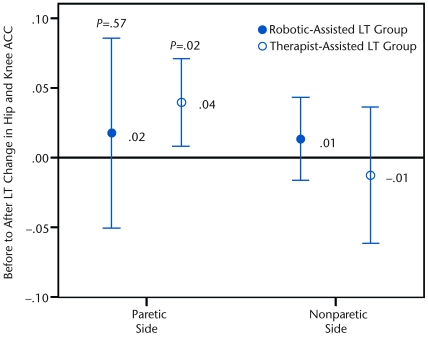
The mean and standard deviation of pretraining and posttraining intralimb consistency of movement (hip and knee ACC) are shown in Table 2 for each group. Prior to training, both groups had significantly less consistency of the relative hip and knee movement on the paretic side compared with the nonparetic side (paretic side: .79 [.11]; nonparetic side: .88 [.10]; ANOVA: P<.001). Furthermore, higher gait speeds were significantly associated with larger hip and knee ACC values (R=.785, P<.001). Changes in paretic- and nonparetic-side hip and knee ACC following LT are shown in Figure 3. Following training, between-group analysis demonstrated that the therapist-assisted LT group did not exhibit a significantly greater increase in paretic-side hip and knee ACC than the robotic-assisted LT group (P=.53, effect size=0.30). However, a within-group analysis showed that the therapist-assisted LT group demonstrated an increase in consistency of relative paretic-side hip and knee movement (P=.02), whereas the robotic-assisted LT group did not (P=.57). Neither the therapist-assisted LT group (P=.58) nor the robotic-assisted LT group (P=.33) demonstrated a significant change in nonparetic hip and knee ACC as a result of training.
Table 2.
Mean (SD) Values for Hip and Knee Average Coefficient of Correspondence Before and After Locomotor Training (LT) for the Robotic- and Therapist-Assisted LT Groups
Figure 3.
Graph depicts the mean change in paretic- and nonparetic-side hip and knee average coefficient of correspondence (ACC) from before to after locomotor training (LT) for the robotic-assisted LT group (filled circle) and the therapist-assisted LT group (open circle). The error bars represent the 95% confidence interval. Although there was no between-group difference for the paretic side (P=.53), a significant within-group difference was observed for the therapist-assisted LT group only (P=.02).
Overall, the participants walked at 0.43 (0.22) m/s prior to training and 0.47 (0.24) m/s following training. The participants in the robotic-assisted LT group did not demonstrate a significant increase in self-selected walking speed following training (0.01-m/s increase, P=.45; Tab. 3). In contrast, the participants in the therapist-assisted LT group significantly increased their self-selected walking speed after training (0.06-m/s increase, P=.05). A moderate effect size (0.58) was observed for walking speed. For cadence and stride length, there were no significant changes in either variable within groups (all P>.24). To determine the contribution of each variable (eg, stride length, cadence) to increases in gait speed in the therapist-assisted LT group, a stepwise, linear regression revealed a significant relationship (R2=.896, P<.001) between the change in stride length (β=.946) and improved gait speed. After including the change in stride length into the analysis, the change in cadence did not meet criteria for inclusion (ie, P<.05) in the model. Additionally, there was no association between changes in gait speed and change in hip to knee ACC from before to after training (R=.146, P=.55).
Table 3.
Spatiotemporal Patterns and Joint Kinematics Before and After Locomotor Training (LT) for the Robotic- and Therapist-Assisted LT Groupsa
Values represent mean (SD). Hip extension, knee extension (ie, hyperextension), and ankle plantar-flexion joint angles are negative; hip flexion, knee flexion, and ankle dorsiflexion joint angles are positive.
Maximum joint angles and excursions of the hip, knee, and ankle joints also are shown in Table 3. Although training altered the variability of intralimb kinematics of the paretic limb, these data generally indicate only small mean increases (<3°) in joint angles and excursions throughout the gait cycle following LT with either therapist or robotic assistance. Likewise, neither group substantially altered limb circumduction, with only small changes (<0.2 cm) observed in either group following LT.
Discussion
This study indicates that improvements in intralimb coordination (hip and knee ACC) can occur following 4 weeks of LT with therapist assistance but not robotic assistance. Consistent with the results from the larger study,16 improvements in walking speed also were evident, although only in the therapist-assisted LT group. In contrast, clinically meaningful changes in peak joint kinematics or excursions did not appear to be present.
Investigations of improvements in acquisition and retention of novel motor tasks following variable and consistent practice have been performed in subjects who were neurologically intact. In general, variable practice conditions appear to decrease motor performance during learning compared with constant task practice, although better retention or transfer is observed with more-variable practice.21,22 In contrast, few data are available regarding the effects of variable or consistent practice conditions for improving locomotor function in individuals poststroke.
Significant between-group differences in robotic- versus therapist-assisted LT were not revealed, further indicating the general efficacy of locomotor training. Nevertheless, robotic-assisted LT did not elicit improvements in hip and knee ACC. Instead, intralimb coordination as quantified using the hip and knee ACC, was improved with therapist-assisted training and might suggest that mechanically imposed practice of movement consistency during training does not, by itself, improve intralimb coordination (ie, consistency of hip and knee trajectories). The extent of error allowed by the patient during training may influence changes in intralimb coordination. During robotic-assisted LT, hip and knee joints were rigidly “guided” through a given movement trajectory, thereby minimizing movement errors. In contrast, although therapists can encourage consistent kinematic movements associated with locomotion, they cannot minimize error to the same extent. The resultant variability in lower-limb trajectories with therapist assistance may allow the patient to explore various solutions to accomplish the locomotor task and adapt to a more consistent locomotor trajectory.13
Although the paretic-side hip and knee ACC of the therapist-assisted LT group increased significantly, there was no significant change in hip and knee ACC for the nonparetic limb of either group. The nonparetic limb exhibited somewhat less-consistent movements than what has been reported for individuals who were unimpaired (eg, ACC values=.94–.97).6,17 Nevertheless, the hip and knee ACC for the nonparetic limb was similar to values reported by Daly and colleagues6 for the nonparetic limb of their participants. Unfortunately, they did not report training-induced changes to the nonparetic limb, although it appears from our data that LT with either robotic or therapist assistance is unable to significantly alter the nonparetic limb's hip and knee ACC.
A limitation to the present study is that we did not quantify the extent of movement consistency during training sessions for either group, and we are unsure of the extent of error allowance or consistency achieved with therapist assistance. However, the lower-limb trajectories during robotic-assisted treadmill stepping are extremely consistent, with very little error in kinematic trajectories.23 It is unlikely that therapist-assisted LT provides consistency of movement similar to that of robotic-assisted treadmill stepping, where joint kinematic trajectories are so tightly controlled.11
Although the allowance of errors during training may facilitate improvements in locomotor consistency in animal models of SCI13 and in the current data in individuals with hemiparesis poststroke, the type of feedback that facilitates improvement in coordination still is uncertain. Specifically, participants who received robotic-assisted LT were provided visual feedback of their relative hip and knee torques during walking (eg, kinetics) to increase volitional effort during stepping,14,24 but they did not improve their hip and knee ACC. A previous study in individuals without neurological injury indicated that continuous physical guidance with visual feedback during learning of a novel task may limit retention and transfer,25 particularly compared with subjects who received no guidance and a reduced schedule of feedback. More-recent data obtained during learning of a novel 3D upper-limb task indicate that mechanical guidance through the required trajectory that combined proprioceptive and visual input was slightly inferior to a simple visual demonstration that involved no limb movement.26 Comparative data of the effect of visual, kinematic, or kinetic feedback and error in improving locomotor function in individuals poststroke are lacking, however, and further investigation is needed.27
As a secondary measure, gait speed also improved significantly following LT, but only in the therapist-assisted LT group by an average of 0.06 m/s. Results from the regression analysis indicated that gait speed increases were determined by changes in stride length, although stride length did not increase significantly after training. The magnitudes of changes in gait speed in both groups were lower than those observed in the larger randomized training trial, although relative differences in gait speed improvements between groups were consistent between studies.16 Although this change in gait speed in the therapist-assisted LT group may be relatively small, such an improvement is thought to constitute a “small meaningful change”28 according to recently established estimates obtained, in part, in subjects with subacute (1–5 months’ duration) stroke (see Fulk and Echternach,29 however, for changes in acute stroke). The relatively smaller sample size may have contributed to nonsignificant differences between LT groups, and studies with larger sample sizes are needed to reveal differences in walking speed16 as well as kinematic coordination between groups.
Additional secondary measures of joint kinematic data indicate very little changes in joint angle kinematics on the paretic extremity. Importantly, kinematic parameters of interest were peak angles during specific gait phases and net joint excursions and did not take into account alterations in angular velocities, which may be present with changes in gait speed, particularly in the therapist-assisted LT group. Accordingly, although increased hip and knee ACC values were observed in the therapist-assisted LT group, it is unclear whether this response to training is beneficial or detrimental. Increasing movement consistency has traditionally been viewed as a favorable outcome, as excessive movement variability has been purported to indicate inappropriate motor control and decreased movement efficiency.30–32 In the therapist-assisted LT group, however, the relative lack of changes in discrete kinematic data points suggests that although the movements became more similar after training, individuals were not repeating “correct” patterns. Therefore, the therapist-assisted training may reinforce impaired gait patterns that have been ingrained in the chronic stages poststroke.
Possible reasons for the relative lack of changes in absolute joint angular excursions in both groups may be multifactorial. To begin, it is possible that training intensities were not high enough to elicit alterations in kinematic variables. This is particularly relevant for the robotic-assisted LT group, which may have allowed participants to walk more “passively” than those in the therapist-assisted LT group due to the continuous physical guidance from the Lokomat. Walking with robotic assistance has been shown in ambulatory subjects with incomplete SCI to reduce metabolic costs of stepping compared with therapist-assisted stepping.14 Reduced volitional effort during training may limit the possibility of enhanced learning of skilled movements.33 Providing feedback during training, as performed in our study, however, mitigates these effects.14 Nevertheless, as we did not measure “effort” during LT, it is possible that the capacity for passive walking in the robotic-assisted LT group contributed to a lack of improvements in hip and knee ACC and gait speed. Furthermore, the protocol used for the present study required the treadmill speed during LT to be limited by the device used, with maximal speeds of 3.0 km/h used in the present study. Although training speeds were greater than the self-selected speed of all participants and similar to those of previous studies of LT in individuals poststroke,34 our inability to further increase stepping speed during training may have reduced walking intensity, even during therapist-assisted LT, when passive assistance was limited. Stepping at higher speeds may have required greater joint excursions35 and has been shown to elicit greater changes in locomotor ability (ie, walking speed, spatiotemporal parameters) than walking at slower speeds.34,36 There is, however, little evidence to support improvements in kinematic patterns as a result of increased training speeds, although we cannot exclude this possibility.
Alternatively, our data may suggest that the ambulatory individuals with chronic stroke recruited for this training program had well-established abnormal gait patterns that could not be modified in 4 weeks of treadmill training with therapist or robotic assistance. Altering these persistent, learned gait deviations may require training programs that begin more acutely,37 consist of longer, more-frequent sessions, continue for a longer period of time,38 or offer a different training method. Other researchers, however, have used LT for a 12-week period (eg, control group described by Daly et al39) and also did not show a change in gait kinematics. These data, therefore, may indicate that much-longer training is necessary to elicit changes poststroke or that simply providing appropriate repetitions for task practice may be an insufficient stimulus for evoking substantial changes in kinematic trajectories during walking. Perhaps additional sensorimotor stimuli may be necessary to elicit changes during walking in individuals with chronic stroke. An enhanced physical stimulus, such as the addition of neuromuscular or reflex electrical stimulation during locomotor training, for instance, may evoke substantial changes to joint kinematics in individuals poststroke39 and those with incomplete SCI.17
Another potential limitation of this study is the relatively small sample size tested. Although we were able to document changes in intralimb coordination following therapist-assisted LT, we did not find differences between groups. Additionally, it appeared that kinematic changes were not appreciably altered. Nevertheless, other researchers34,39,40 have used equivalent or even smaller sample sizes to demonstrate that LT with specific modalities or protocols has the ability to elicit changes following stroke. Recruitment of substantially larger sample sizes may reveal significant differences in relative hip-knee coordination between groups, although the differences in the observed magnitude of altered gait kinematics may not be clinically meaningful.
Conclusion
A 4-week LT program with variable assistance as needed (therapist-assisted) LT significantly increased intralimb coordination and walking speed. In contrast, robotic-assisted LT did not facilitate such improvements. Although traditional therapies have advocated the assistance and facilitation of movement of the impaired extremities of people with neurological injury in an effort to ensure the quality of movement,41 our results contribute to a body of evidence that suggests mechanically or passively minimizing errors may not be the optimal strategy to improve motor coordination or function in individuals with chronic hemiparesis. Thus, attempts to provide consistent, precise kinematic trajectories of the lower limbs during assisted stepping, whether applied with therapist assistance or through automated technology, may not be optimal. Rather, providing assistance only as needed and allowing some variability during task practice,15,42 or even augmenting error,15 may facilitate greater improvements in motor coordination and function for ambulatory individuals with chronic stroke.
It is unclear whether there is a positive effect on functional outcomes from improving intralimb coordination without eliciting substantial alterations in discrete joint kinematics. Further research is needed to determine more-optimal training parameters, including duration, intensity, and timing, following stroke to optimize both outcomes related to impairments and functional limitations. In addition, the efficacy of additional physical (eg, electrical stimulation)6 or other therapeutic interventions to “normalize” kinematic patterns needs to be more thoroughly investigated.
Dr Lewek, Ms Moore, Dr Dhaher, and Dr Hornby provided concept/idea/research design. Dr Lewek, Ms Cruz, and Dr Hornby provided writing. Dr Lewek, Ms Cruz, Ms Moore, Ms Roth, and Dr Hornby provided data collection. Dr Lewek, Ms Cruz, Ms Moore, and Dr Hornby provided data analysis. Dr Lewek, Ms Roth, and Dr Dhaher provided project management. Dr Dhaher and Dr Hornby provided fund procurement and facilities/equipment. Ms Roth and Dr Hornby provided participants. Ms Cruz, Ms Moore, and Ms Roth provided consultation (including review of manuscript before submission).
This study was approved by the Northwestern University Institutional Review Board.
Portions of these results were presented at the Combined Sections Meeting of the American Physical Therapy Association; February 14–18, 2007; Boston, Massachusetts.
Funding for this project was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (grant F32AR053447), and the National Institute on Disability and Rehabilitation Research (grants H133G040065 and H133B031127).
Hocoma AG, Industriestrasse 4, CH-8604 Volketswil, Switzerland.
Motion Analysis Corp, 3617 Westwind Blvd, Santa Rosa, CA 95403.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References
- 1.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29:1122–1128. [DOI] [PubMed] [Google Scholar]
- 2.Harkema SJ, Hurley SL, Patel UK, et al. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. [DOI] [PubMed] [Google Scholar]
- 3.Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127(pt 10):2232–2246. [DOI] [PubMed] [Google Scholar]
- 4.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. [DOI] [PubMed] [Google Scholar]
- 5.Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol (Lond). 2000;528:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly JJ, Sng K, Roenigk K, et al. Intra-limb coordination deficit in stroke survivors and response to treatment. Gait Posture. 2007;25:412–418. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesse S, Werner C, Uhlenbrock D, et al. An electromechanical gait trainer for restoration of gait in hemiparetic stroke patients: preliminary results. Neurorehabil Neural Repair. 2001;15:39–50. [DOI] [PubMed] [Google Scholar]
- 9.Colombo G, Wirz M, Dietz V. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord. 2001;39:252–255. [DOI] [PubMed] [Google Scholar]
- 10.Hesse S, Uhlenbrock D, Sarkodie-Gyan T. Gait pattern of severely disabled hemiparetic subjects on a new controlled gait trainer as compared to assisted treadmill walking with partial body weight support. Clin Rehabil. 1999;13:401–410. [DOI] [PubMed] [Google Scholar]
- 11.Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev. 2000;37:693–700. [PubMed] [Google Scholar]
- 12.Schmidt RA, Lee T. Motor Control and Learning: A Behavioral Emphasis. 4th ed. Champaign, IL: Human Kinetics Inc; 2005.
- 13.Cai LL, Fong AJ, Otoshi CK, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006;26:10564–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther. 2006;86:1466–1478. [DOI] [PubMed] [Google Scholar]
- 15.Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res. 2006;168:368–383. [DOI] [PubMed] [Google Scholar]
- 16.Hornby TG, Campbell DD, Kahn JH, et al. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke. a randomized controlled study. Stroke. 2008;39:1786–1792. [DOI] [PubMed] [Google Scholar]
- 17.Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82:707–715. [PubMed] [Google Scholar]
- 18.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. [DOI] [PubMed] [Google Scholar]
- 19.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22:51–56. [DOI] [PubMed] [Google Scholar]
- 21.Shea CH, Kohl RM. Specificity and variability of practice. Res Q Exerc Sport. 1990;61:169–177. [DOI] [PubMed] [Google Scholar]
- 22.Heitman RJ, Pugh SF, Kovaleski JE, et al. Effects of specific versus variable practice on the retention and transfer of a continuous motor skill. Percept Mot Skills. 2005;100(3 pt 2):1107–1113. [DOI] [PubMed] [Google Scholar]
- 23.Hidler J, Wisman W, Neckel N. Kinematic trajectories while walking within the Lokomat robotic gait-orthosis. Clin Biomech (Bristol, Avon). 2008;23:1251–1259. [DOI] [PubMed] [Google Scholar]
- 24.Banz R, Bolliger M, Colombo G, et al. Computerized visual feedback: an adjunct to robotic-assisted gait training. Phys Ther. 2008;88:1135–1145. [DOI] [PubMed] [Google Scholar]
- 25.Winstein CJ, Pohl PS, Lewthwaite R. Effects of physical guidance and knowledge of results on motor learning: support for the guidance hypothesis. Res Q Exerc Sport. 1994;65:316–323. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Cramer SC, Reinkensmeyer DJ. Learning to perform a new movement with robotic assistance: comparison of haptic guidance and visual demonstration. J Neuroeng Rehabil. 2006;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vliet PM, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence? Disabil Rehabil. 2006;28:831–840. [DOI] [PubMed] [Google Scholar]
- 28.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 29.Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther. 2008;32:8–13. [DOI] [PubMed] [Google Scholar]
- 30.Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86:1501–1510. [DOI] [PubMed] [Google Scholar]
- 31.Candau R, Belli A, Millet GY, et al. Energy cost and running mechanics during a treadmill run to voluntary exhaustion in humans. Eur J Appl Physiol Occup Physiol. 1998;77:479–485. [DOI] [PubMed] [Google Scholar]
- 32.Furuya S, Kinoshita H. Expertise-dependent modulation of muscular and non-muscular torques in multi-joint arm movements during piano keystroke. Neuroscience. 2008;156:390–402. [DOI] [PubMed] [Google Scholar]
- 33.Lotze M, Braun C, Birbaumer N, et al. Motor learning elicited by voluntary drive. Brain. 2003;126(pt 4):866–872. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–691. [DOI] [PubMed] [Google Scholar]
- 35.Lamontagne A, Fung J. Faster is better: implications for speed-intensive gait training after stroke. Stroke. 2004;35:2543–2548. [DOI] [PubMed] [Google Scholar]
- 36.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33:553–558. [DOI] [PubMed] [Google Scholar]
- 37.Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J Neurophysiol. 2005;94:255–264. [DOI] [PubMed] [Google Scholar]
- 38.Smith JL, Smith LA, Zernicke RF, Hoy M. Locomotion in exercised and nonexercised cats cordotomized at two or twelve weeks of age. Exp Neurol. 1982;76:393–413. [DOI] [PubMed] [Google Scholar]
- 39.Daly JJ, Roenigk KL, Butler KM, et al. Response of sagittal plane gait kinematics to weight-supported treadmill training and functional neuromuscular stimulation following stroke. J Rehabil Res Dev. 2004;41:807–820. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira-Salmela LF, Nadeau S, McBride I, Olney SJ. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. J Rehabil Med. 2001;33:53–60. [DOI] [PubMed] [Google Scholar]
- 41.Bobath B. Adult Hemiplegia: Evaluation and Treatment. 3rd ed. Newton, MA: Butterworth-Heinemann; 1990.
- 42.Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: a randomized controlled pilot study. J Neuroengineering Rehabil. 2006;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]