Abstract
Objective: Accurate and prospective assessments of treatment-emergent suicidal thoughts and behaviors are essential to both clinical care and randomized clinical trials. The Sheehan Suicidality Tracking Scale is a prospective, patient self-report or clinician-administered rating scale that tracks both treatment-emergent suicidal ideation and behaviors. The Sheehan Suicidality Tracking Scale was incorporated into a multicenter, randomized, double-blind, placebo-controlled, and active comparator study examining the efficacy of an experimental corticotropin-releasing factor antagonist (BMS-562086) for the treatment of generalized anxiety disorder.
Method: The Sheehan Suicidality Tracking Scale was administered to subjects at baseline, Week 2, Week 4, and Week 8 or early termination. Subjects completed theSheehan Suicidality Tracking Scale by self report. The Sheehan Suicidality Tracking Scale was designated as an exploratory outcome measure in the study protocol, and post-hoc analyses were performed to examine the performance of the Sheehan Suicidality Tracking Scale.
Results: A total of 82 subjects completed the Sheehan Suicidality Tracking Scale during the course of the study. Altogether, these subjects provided 297 completed Sheehan Suicidality Tracking Scale ratings across the study time points. Sixty-one subjects (n=25 placebo, n=24 BMS-562086, and n=12 escitalopram) had a baseline and at least one post-baseline Sheehan Suicidality Tracking Scale measurement. The mean change from baseline at Week 8 in the Sheehan Suicidality Tracking Scale total score was -0.10, -0.02, and -0.06 for escitalopram, placebo, and BMS-562086 groups, respectively. The sensitivity of the Sheehan Suicidality Tracking Scale and HAM-D Item #3 (suicide) for identifying subjects with suicidal thoughts or behaviors was 100 percent and 63 percent, respectively.
Conclusions: The Sheehan Suicidality Tracking Scale may be a sensitive psychometric tool to prospectively assess for treatment-emergent suicidal thoughts and behaviors. Despite the small sample size and low occurrence of suicidal ideation during the course of this clinical trial, the self-reported Sheehan Suicidality Tracking Scale demonstrated increased sensitivity over the rater administered HAM-D Item #3 in identifying suicide related ideations and behaviors. Further research in larger study samples as well as in other psychiatric disorders are needed.
Keywords: treatment emergent suicidality, suicide, suicide rating scale, assessment of suicidal behaviors
Introduction
Suicide remains the 11th leading cause of death in adults and the third leading cause of death in adolescents in the United States.1 Clinical evaluation of suicidal thoughts and behaviors is a central component of the psychiatric interview.2 Assessment of suicidal thoughts and behaviors is commonly used to make treatment determinations, such as the need for hospitalization, frequency of outpatient visits, and psychopharmacological interventions to treat underlying psychiatric symptoms. Although standards for assessment of suicidal behaviors and thoughts have been established in the clinic,3 appropriate tools to monitor potential treatment-emergent suicidal ideations or behaviors during the course of clinical research trials have yet to be clearly established.
Suicidality is a broad and somewhat controversial term that has received increased attention since concerns arose about potentially increased rates of suicidality in adolescents treated with serotonin reuptake inhibitor (SRI) medications. The term suicidality includes passive suicidal ideation, active suicidal ideation, interrupted or aborted suicide attempts, preparatory behaviors toward suicide, actual suicide attempts, and completed suicide.4 Experts disagree whether research regarding treatment-emergent suicidality should encompass such broad categories of suicide-related terms given the lack of empirical evidence demonstrating an etiological connection between those terms and completed suicide. Although suicidal ideation is certainly a clinical risk factor for suicide, the majority of individuals who experience suicidal ideation do not go on to engage in suicidal behaviors or commit suicide. Thus, an increase in suicidality may or may not be associated with actual increases in suicide or suicide-related behaviors.
The possible association between suicidality and certain classes of medications has appropriately led to increased interest in improved methodologies to monitor for treatment-emergent suicidal ideation and behaviors during randomized, clinical trials (RCTs). Concerns regarding antidepressant medications in adolescents stemmed from a retrospective review of several clinical trials involving SRI medications.5 The regulatory warnings from this retrospective analysis led to reduced antidepressant prescribing for children and adolescents despite the absence of suicides during the studies included in the retrospective analysis.6 With exception of the HAM-D suicide Item 3 and Item 10 on the Childhood Depression Rating Scale (which showed no association between SRI treatment and suicide), there was no systematically or prospectively gathered suicide assessment data during these RCTs to more accurately assess potential treatment-emergent drug effects versus placebo. However, recently published data suggest that, for the first time in decades, there has been an increase in suicides in the adolescent population following the black box warning.6,7 Regardless of one’s own belief whether or not retrospectively gathered suicidality data accurately predict treatment-emergent risks for suicide, the attention that this issue has garnered highlights the importance of incorporating accurate and prospective suicidality assessments in future RCTs.
Although a number of psychometric tools are available to potentially monitor suicide thoughts and behaviors (Table 1), many have not yet been widely used or validated in RCTs. Systematic prospective monitoring for treatment-emergent suicidality during RCTs is increasing rapidly in drug development programs and is likely to be mandated in the near future. While the field determines the best practices to follow regarding prospective assessment of suicidality during clinical trials, currently available suicide assessment tools need to be evaluated to determine the reliability and validity of these tools for suicidality assessment. An essential property in a suicidality assessment scale is that not only should it map to a suicidality classification system acceptable to regulatory authorities, like the Columbia Classification Algorithm of Suicide Assessment (C-CASA), but it should also be very sensitive to treatment effects (both improvement and worsening).
TABLE 1.
Psychometric rating scales measuring suicidality
|
The Sheehan Suicidality Tracking Scale (Sheehan-STS) is a prospective rating scale that tracks both treatment-emergent suicidal ideation and behaviors. The Sheehan-STS is an eight-item scale that can be administered either by a clinician or patient through self report (Figure 1). Each item in the Sheehan-STS is scored on a 5-point Likert scale (0=not at all, 1=a little, 2=moderately, 3=very, and 4=extremely). Data from the Sheehan-STS can be analyzed as individual item scores, suicidal ideation subscale score (sum of scores from items 2, 3, and 4, plus score from item 5 if ≤1), suicidal behavior subscale score (sum of scores from items 6, 7a, and 8, plus score from item 5 if >1), and total score. The Sheehan-STS was adapted from the Suicidality Module of the Mini International Neuropsychiatric Interview (MINI) Structured Diagnostic Interview for DSM-IV.8 The MINI is one of the most cited diagnostic tools with extensive reliability and validity testing; however, there has been no independent evaluation of the Sheehan-STS. We present pilot data from the use of the Sheehan-STS in a subset of patients who were enrolled in a large, multicenter, clinical drug study for generalized anxiety disorder (GAD).
FIGURE 1.

Suicidality Tracking Scale (STS)
Methods
The Sheehan-STS was incorporated into a multicenter, randomized, double-blind, placebo-controlled, and active comparator (escitalopram) study examining the efficacy of an experimental corticotropin-releasing factor (CRF) antagonist (BMS-562086) for the treatment of GAD. The study was conducted at approximately 50 centers in the United States between July 2007 and January 2008. The study was approved by the institutional review board at each study site or a central review board (Western Institutional Review Board). All study subjects provided written informed consent. The trial was registered at www.clinicaltrials.gov (NCT00481325) prior to enrollment.
Female outpatients ages 18 to 65 who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for the diagnosis of GAD were eligible for study participation.9 Diagnosis was confirmed using the MINI, and a psychiatric evaluation was performed by a board-eligible or -certified psychiatrist. Additionally, subjects met the following criteria on site-administered rating scales: 1) Hamilton Anxiety scale (HAM-A) total score of 18 or greater at screening and baseline;10 2) baseline HAM-A total score not more than 30 percent below the score at screening; 3) HAM-A anxiety and tension item scores of two or greater at both screening and baseline; and 4) Clinical Global Impression of Severity score of four or greater (moderately or more severely ill) at both screening and baseline. We excluded patients with any of the following: other current Axis I or Axis II psychiatric diagnoses; serious medical problems that would interfere with safety or efficacy assessments; a 17-item Hamilton Depression (HAM-D-17) scale score greater than 19 or a HAM-D-17 depression item score greater than 1; any significant alcohol or illicit drug abuse or dependence within the past 12 months; significant suicide risk based on the clinical judgment of the principal investigator; and nonresponse to escitalopram or nonresponse to three or more adequate trials of any selective SRIs.
Subjects were randomly assigned to double-blind treatment with either BMS-562086, matching placebo, or escitalopram 20mg in a 2:2:1 fashion. After a protocol amendment approving the use of the Sheehan-STS, the Sheehan-STS was administered to subjects at baseline, Week 2, Week 4, and Week 8 (or early termination). Subjects completed the Sheehan-STS by self report, and a clinician was required to review the Sheehan-STS results prior to the subject leaving the site. The Sheehan-STS was designated an exploratory outcome measure in the study protocol and post-hoc analyses were performed to examine the performance of the Sheehan-STS.
Results
With regard to the HAM-A primary outcome measure, escitalopram demonstrated statistically significant efficacy compared with placebo on the primary efficacy criterion, the mean change from baseline HAM-A total score, while BMS-562086 failed to separate from placebo.11 The mean HAM-D-17 total score at baseline for escitalopram, placebo, and BMS-562086 groups was 13.76, 13.31, and 13.56, respectively. Escitalopram also demonstrated statistically significant improvement on the mean change from baseline HAM-D-17 total score, while BMS-562086 failed to separate from placebo.
A total of 82 subjects completed the Sheehan-STS during the course of the study. Altogether, these subjects provided 297 completed Sheehan-STS ratings across the study time points. Sixty one subjects (n=25 placebo, n=24 BMS-562086, and n=12 escitalopram) had a baseline and at least one post-baseline Sheehan-STS measurement.
The sensitivity of both the Sheehan-STS and HAM-D Item #3 (suicide) for identifying subjects with suicidal thoughts or behaviors is shown in Table 2. The true positive rate for the sensitivity calculations was defined as any evidence of suicidal thoughts or behaviors from review of AEs, SAEs, HAM-D Item #3 >0, or Sheehan-STS score >0. Table 3 shows data from those subjects who were rated as having a HAM-D Item #3 (suicide) score of zero but self-reported suicidality items on the Sheehan-STS. For those patients with both a baseline and post-baseline Sheehan-STS measurement, the mean change in the Sheehan-STS total score over time is shown in Figure 2. The mean change from baseline in the Sheehan STS at week 8 was -0.10 for escitalopram and -0.04 for the BMS-562086 and placebo groups combined. This difference between groups was not statistically significant. The mean change from baseline at Week 8 in the Sheehan-STS total score was -0.10, -0.02, and -0.06 for escitalopram, placebo, and BMS-562086 groups, respectively.
TABLE 2.
Sensitivity of Sheehan STS and HAM-D Item #3 in identifying suicidal thoughts or behaviors, n=297 timepoints.
| *True positive (TP) defined as any record of suicidal thoughts/behaviors from review of AEs, SAEs, HAM-D Item #3 ›0 or STS Score ›0 | |||
| Sensitivity of HAM-D Item #3 | # of TP | 12 | 63% |
| # TP+ #FN | 12+7 | ||
| Sensitivity of Sheehan-STS | # of TP | 12 | 100% |
| # TP+ #FN | 12+0 | ||
TABLE 3.
Instances with HAM-D Item #3 (suicide) score=0 and STS score ≥1, n=297 timepoints.
| TIMEPOINT* | HAM-D ITEM #3 SCORE | STS TOTAL SCORE | DESCRIPTION OF STS ITEM(S) WITH SCORE ›0 |
|---|---|---|---|
| * Each entry represents a different patient. | |||
| Baseline | 0 | 3 | Endorsed “moderately” on STS Item #2 (“think that you would be better off dead or wish you were dead”) Endorsed “a little” on STS Item #4 (“think about suicide”) |
| Baseline | 0 | 1 | Endorsed “a little” on STS Item #2 (“think that you would be better off dead or wish you were dead”) |
| Baseline | 0 | † | †Endorsed suffering an accident in the last week without intent to harm self |
| Baseline | 0 | 2 | Endorsed “a little” on STS Item #2 (“think that you would be better off dead or wish you were dead”) Endorsed “a little” on STS Item #4 (“think about suicide”) |
| Baseline | 0 | 1 | Endorsed “a little” on STS Item #2 (“think that you would be better off dead or wish you were dead”) |
| Baseline | 0 | 6 |
Endorsed “very” on STS Item #2(“think that you would be better off dead or wish you were dead”) Endorsed “very” on STS Item #4 (“think about suicide”) |
| Week 8 | 0 | 2 |
Endorsed “a little” on STS Item #2 (“think that you would be better off dead or wish you were dead”) Endorsed “a little” on STS Item #4 (“think about suicide”) |
| Week 8 | 0 | 2 | Endorsed “moderately” on STS Item #2 (“think that you would be better off dead or wish you were dead”) |
FIGURE 2.
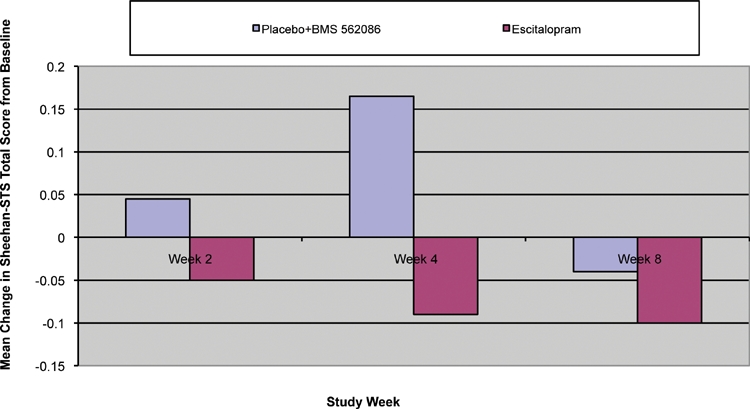
Mean change from baseline Sheehan-STS total score by week across study groups (LOCF data set, Efficacy Sample). Sheehan STS Total Score is from 0 to 8. A negative score signifies improvement. Changes were not statistically significant.
Conclusions
The Sheehan-STS may be a sensitive psychometric tool to prospectively assess for treatment-emergent suicidal thoughts and behaviors. Despite the small sample size and low occurrence of suicidal ideation during the course of this clinical trial, the self-reported Sheehan-STS demonstrated increased sensitivity over the rater administered HAM-D Item #3 in identifying suicide-related ideations and behaviors. Moreover, the Sheehan-STS did not fail to capture any occurrences of suicidal thoughts or behaviors as confirmed by review of AEs, SAEs, and HAM-D ratings. By contrast, the rater administered HAM-D Item #3 failed to capture seven out of a total of 12 instances of suicidality identified during the study. Our findings appear consistent with other studies, suggesting self report of current suicide-related ideations and behaviors may be more sensitive than rater-administered assessments.12–14
Effective treatment with escitalopram showed numerical improvement over placebo and the study medication with regard to the mean change in the Sheehan-STS total score over time. Although improvements in the mean Sheehan-STS total score over time were not statistically significant in this underpowered study, these preliminary data suggest that the Sheehan-STS could be used to detect a statistically significant change in suicidality in larger studies or over the course of a drug development program. Based on the greatest effect size of 0.36 observed in the mean change from baseline Sheehan-STS total score in the escitalopram and placebo/BMS-562086 groups, approximately 123 patients per treatment group would be needed to observe a statistically significant difference in suicidality using this scale. Such sample sizes are typically within the scope of most Phase 2 and Phase 3 RCTs. To our knowledge, no suicidality tracking instrument has been shown to be sensitive to treatment effects in this way.
Accurate and prospective assessments for treatment-emergent suicidal thoughts and behaviors are essential to both clinical care and neuroscience research trials. The Sheehan-STS demonstrated significant utility during this study and its advantages include the following: 1) it can be administered either via self report or by clinicians; 2) it is completed in only 1 to 2 minutes; 3) it does not require rater training; 4) it assesses multiple domains of suicidality, including passive suicidal ideation, active suicidal ideation, suicidal behaviors, self-injurious behaviors, and accidents; and 5) it can be analyzed with regard to individual item scores, total score, suicidal ideation subscale score, or suicidal behaviors subscale score. The Sheehan-STS also has the potential to detect changes in the frequency and intensity of suicidal thoughts or behaviors over time. The Sheehan-STS items maps directly to the C-CASA (Table 4), the suicidality classification coding system used by the FDA. This permits comparisons to studies that use a retrospective analysis of suicidality. Further research in larger study samples as well as other diagnostic categories are needed. The generalizability of our study results are limited by the small sample size, female only subjects, GAD study population and screening out subjects with significant risk for suicide.
TABLE 4.
Using the Sheehan-STS in clinical trials and mapping to the FDA coding system (C-CASA)
| CODE NUMBER | FDA CODING CATEGORY | STS |
|---|---|---|
| 1 | Completed suicide | Obtain from SAE |
| 2 | Suicide attempt (Self-injurious behavior associated with some intent to die. Intent can be stated or inferred by rater.) | Any positive response to STS Question 8; any positive response to STS Question 1b |
| 3 | Preparatory acts toward imminent suicide behavior (Person takes steps to injure self but is stopped by self or other. Intent to die is either stated or inferred.) | Any positive response to STS Question 5 and/or 6 with negative response to Question 8 |
| 4 | Suicidal ideation (Passive thoughts about wanting to be dead or active thoughts about killing oneself, not accompanied by preparatory behavior.) | Any positive response to STS Question 2, 3, or 4 with negative response to Questions 5, 6, or 8. Any positive response to STS |
| 5 | Self-injurious behavior, intent unknown (Self-injurious behavior where associated intent to die is unknown and cannot be inferred.) | Question 7 without unambiguous response to Question 7a and Question 8; Any positive response to STS Question 1a without an unambiguous response to Question 1b or 8. |
| 6 | Not enough information (fatal) | Obtain from SAE |
| 7 | Self-injurious behavior, no suicide intent | Any positive response to STS Question 7 or 7a with negative response to Question 8; any positive response to STS Question 1a with a negative response to Question 1b. |
| 8 | Other (accidental, psychiatric, medical), no deliberate self harm | Positive response to STS Question 1 with negative responses to Questions 1a and 1b. |
| 9 | Not enough information (nonfatal) | Obtain from AE/SAE |
Acknowledgments
Our deepest appreciation to the patients who participated in this clinical trial. We also thank the Pexacerfont Development Team for their outstanding implementation of this study protocol, including Kimberly A. Gentile, BS, Olive Watson-Coleman, RN, Cynthia McHugh, Sarah Bevivino, Tamara Bratt, Jennifer Mack, Kathleen Monaco, Lorenzo Biscotti, Caroline Clairmont, Xiaoling Wu, PhD, and Dan Oren, MD.
We are grateful for the hard work and efforts of following principal investigators and their clinical staff: Bernadette B. D’Souza, MD; Daniel L. Zimbroff, MD; Ronald Brenner, MD; Valerie K. Arnold, MD; Donald Garcia, MD; Mahmoud S. Okasha, MD; Andrew Goddard, MD; Jason D. Baron, MD; Michael Downing, MD; Tanya Vapnik, PhD; Nick Vatakis, MD; Louise Thurman, MD; Richard Weisler, MD; Mary Stedman, MD; John Stoukides, MD; Leslie Taylor, MD; John Stoukides, MD; Elizabeth Reeve, MD; Mildred Farmer, MD; J. Gary Booker, MD; John S. Carman, MD; James Barbee, MD; Norman E. Rosenthal, MD; Olga Brawman-Mintzer, MD; Mark DiBuono, MD; Boadie Dunlop, MD; Kurian Abraham, MD; John E. Barkenbus, MD; Al-Li Wu Arias, MD; Naresh Emmanuel, MD; Nizar El-Khalili, MD; Anne C. Fedyszen, MD; William Fuller, MD; Bradley N. Gaynes, MD, MPH; Lawrence D. Ginsberg, MD; John K. Heussy, MD; Alexander E. Horwitz, MD; Alan M. Jonas, MD; George Joseph, MD; Vernon L. Kliewer, MD; Susan G. Kornstein, MD; David Sert Krakow, MD; Michael Lesem, MD; Michael T. Levy, MD; Craig McCarthy, MD; Matthew Menza, MD; Charles H. Merideth, MD; Irina Mezhebovsky, MD; Janice Miller, MD; Paul R. Miller, MD; Leslie Moldauer, MD; Edward R. Norris, MD; Margarita Nunez, MD; Teresa A. Pigott, MD; Alfredo N. Rivera, MD; Jennifer Rosenberg, MD; Jon Chaffee, MD; Daniel Lieberman, MD; Andrew Winokur, MD; Phebe M. Tucker, MD; and Frederick W. Reimherr, MD.
Contributor Information
Vladimir Coric, Dr. Coric is Associate Clinical Professor of Psychiatry, Yale University School of Medicine, Senior Research Scientist, Yale OCD Research Clinic, New Haven, and an employee of Bristol-Myers Squibb Company, Neuroscience Global Clinical Research, Wallingford, Connecticut.
David V. Sheehan, Dr. Sheehan is Distinguished University Health Professor, Professor of Psychiatry, Director, Depression and Anxiety Disorders Research Institute, University of South Florida College of Medicine, Tampa, Florida.
References
- 1.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [December 10, 2008]; Web-based Injury Statistics Query and Reporting System (WISQARS). www.cdc.gov/ncipc/wisqars.
- 2.American Psychiatric Association. Washington, DC: American Psychiatric Press Inc.; 2005. Practice Guideline for the Psychiatric Evaluation of Adults, Second Edition. [Google Scholar]
- 3.American Psychiatric Association. Practice Guideline for the Assessment and Treatment of Patients with Suicidal Behaviors. Washington, DC: American Psychiatric Press Inc.; 2003. [PubMed] [Google Scholar]
- 4.Posner K, Oquendo M, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;165:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammand TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):246–248. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164:1356–1363. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- 7.Bridge JA, Greenhouse JB, Weldon AH, et al. Suicide trends among youths aged 10 to 19 years in the United States, 1996–2005. JAMA. 2008;300(9):1025–1026. doi: 10.1001/jama.300.9.1025. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan DV, Lecrubier Y, Harnett-Sheehan K, et al. The MINI International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Press Inc.; 2003. [Google Scholar]
- 10.Hamilton M. The assessment of anxiety by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 11.Coric V, Stock EG, Pultz J, et al. A multicenter, randomized, double-blind, active-comparator and placebo-controlled trial of a corticotropin releasing factor receptor-1 antagonist in generalized Anxiety Disorder. 2008 doi: 10.1002/da.20695. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan ML, Asnis GM, Sanderson WC, et al. Suicide assessment: clinical interview vs. self-report. J Clin Psychol. 1994;50(2):294–298. doi: 10.1002/1097-4679(199403)50:2<294::aid-jclp2270500224>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Greist JH, Gustafson DH, Stauss FF, et al. A computer interview for suicide-risk prediction. Am J Psychiatry. 1973;130:1328–1332. doi: 10.1176/ajp.130.12.1327. [DOI] [PubMed] [Google Scholar]
- 14.Erdman HP, Greist JH, Gustafson DH, et al. Suicide risk prediction by computer interview: a prospective study. J Clin Psychiatry. 1987;48(12):464–467. [PubMed] [Google Scholar]


