Abstract
In this article, we provide information on patient-reported side effects from a cross-section of real-world patients. Specifically, data on side effects was tabulated for patients taking one of the following selective serotonin reuptake inhibitor antidepressants: citalopram, escitalopram, fluoxetine, paroxetine, and sertraline. Thirty-eight percent of the approximately 700 patients surveyed reported having experienced a side effect as a result of taking a selective serotonin reuptake inhibitor antidepressant; the most common side effects mentioned were sexual functioning, sleepiness, and weight gain. Only 25 percent of the side effects were considered “very bothersome” or “extremely bothersome.” Regardless of how bothersome the side effects were, however, only 40 percent of patients mentioned the side effects to their prescribing physicians.
Keywords: SSRI, antidepressant, patient-reported side effect, real-world data
Introduction
Recognizing that time for patient care by the physician is limited, it is important for practicing physicians to understand which issues to prioritize in their patient interactions. In this article, we provide information on patient-reported side effects from a cross-section of real-world patients using selective serotonin reuptake inhibitors (SSRIs).
Methods
iGuard.org, a patient drug safety monitoring service, randomly surveys enrolled patients on a continuous basis to obtain data on treatment satisfaction, efficacy, and side effects using a validated, patient-reported, outcomes instrument called the Treatment Satisfaction Questionnaire for Medications (TSQM). Data on side effects were tabulated for patients taking one of the following SSRI antidepressants: citalopram, escitalopram, fluoxetine, paroxetine, and sertraline. Please note that surveyed patients may have been taking other central nervous system (CNS) and non-CNS products concurrently with SSRI antidepressants.
Results
Thirty-eight percent of the approximately 700 patients surveyed reported experiencing one or more side effects as a result of taking an SSRI antidepressant. Figure 1 displays the side effects most commonly mentioned by patients. Of the 229 patients who listed at least one side effect, sexual functioning, sleepiness, and weight gain were the most commonly mentioned items. Only about one-quarter of these patients (26%), however, indicated that these side effects were “very bothersome” or “extremely bothersome” (Figure 2).
FIGURE 1.
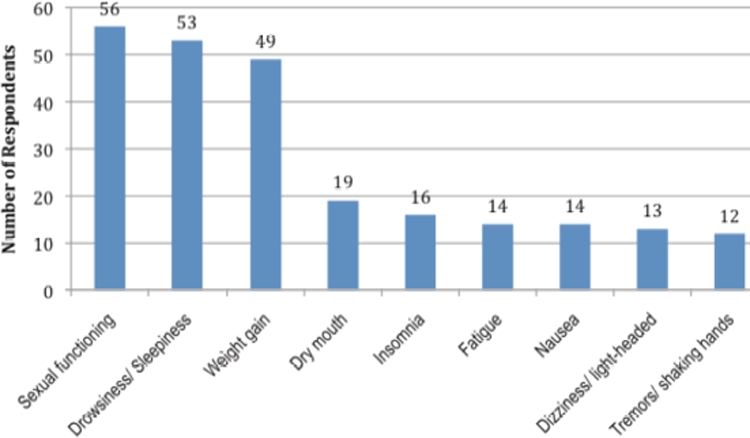
Analysis of www.iGuard.org data for citalopram, escitalopram, fluoxetine, paroxetine, and sertraline showing the most commonly patient-reported side effects
FIGURE 2.
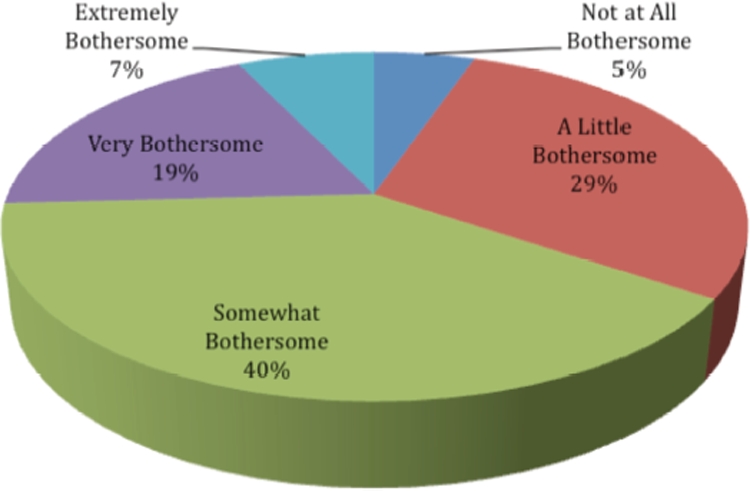
Analysis of www.iGuard.org data for citalopram, escitalopram, fluoxetine, paroxetine, and sertraline showing percentage of patients who ranked side effects as “bothersome.”
Figure 3 shows the proportion of patients who discuss side effects with their prescribing physicians. As seen in Figure 3, only 39 percent of patients reported side effects to their physicians. Interestingly, the proportion of patients reporting side effects to their physicians was the same for all patients, including those who considered the side effects “very bothersome” or “extremely bothersome” (39% vs. 37%).
FIGURE 3.
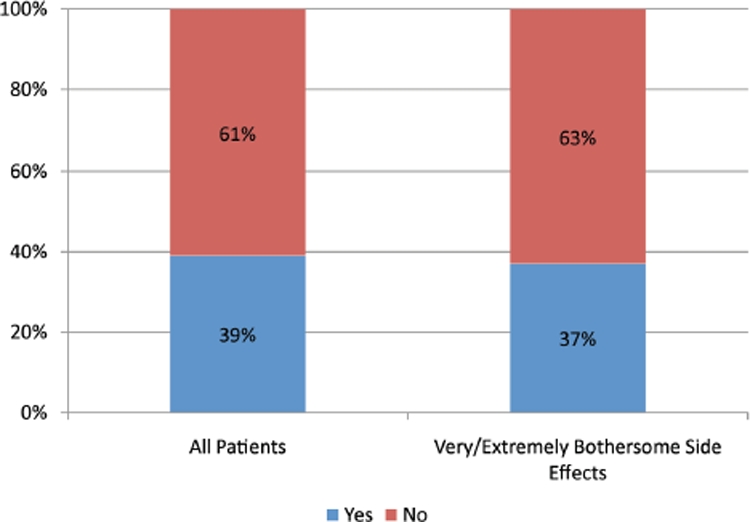
Analysis of www.iGuard.org data for citalopram, escitalopram, fluoxetine, paroxetine, and sertraline. Left bar indicates the percentage of total patients who reported side effects to their physicians; right bar indicates the percentage of the subset of patients who ranked their side effects as “very” or “extremely bothersome” and reported their symptoms to their physicians.
Expert Commentary
by Sidney H. Kennedy, MD, FRCPC
The antidepressant side-effect debate has existed for many years. It was the beneficial side-effect profile of fluoxetine that differentiated it from tricyclic antidepressants after the drug was launched in 1988. There was no doubt that the reduction in cardiac arrhythmias and other potential lethalities played a major role in opening the door to the exponential growth of antidepressant prescribing. With growing recognition of the need for long-term antidepressant use and attention to quality of life, attempts to minimize antidepressant side effects and drug discontinuation have gained a prominent focus.
The side effects reported in this article emerged from a survey of 700 enrolled participants in a drug safety monitoring service who were identified as SSRI recipients. The nine most frequently endorsed side effects were identified together with a ranking of burden. Sexual dysfunction, sleepiness, and weight gain were the most encountered side effects, and, in total, 38 percent of the patients surveyed experienced at least one side effect, while 26 percent reported a high level of burden. This likely provides a fair cross-section of side effects biased toward continuation or maintenance phases of treatment and supports other clinical trial data showing that sexual dysfunction and weight gain are the most frequent reasons cited by patients for SSRI discontinuation. The most interesting aspect of this report is the disclosure that only 39 percent of patients reported side effects to their physicians, and there was no difference in those with “very bothersome” or “extremely bothersome” side effects compared to the total sample. An analysis of data that compares various factors, such as age, gender, or drug, goes beyond the scope of this report and therefore is not included.
In general, variations in side-effect profiles are attributed to mechanistic differences within or between drug classes. Of equal importance, however, is the difference in patient profiles, ranging from genetic polymorphisms to personality dimensions. SSRIs are predominantly metabolized by cytochrome 2D6. While microarrays are commercially available to detect extensive or poor metabolizer status, this strategy has not yet become a standard component of clinical care. There is also debate in the literature about polymorphism status for the serotonin transporter influencing SSRI side-effect reporting. Neuroticism as a personality dimension has also been shown to influence side-effect reporting, even with placebo, with high levels of neuroticism increasing side effects. Other variables that contribute to drug tolerability include time of dosing, intake with or without food, and smoking status. All of these factors should be considered during antidepressant therapy to promote optimal response.
Contributor Information
Elisa Cascade, Ms. Cascade is Vice President, Quintiles Inc./iGuard, Falls Church, Virginia.
Amir H. Kalali, Dr. Kalali is Vice President, Global Therapeutic Group Leader CNS, Quintiles Inc., San Diego, California, and Professor of Psychiatry, University of California, San Diego.
Sidney H. Kennedy, Dr. Kennedy is Psychiatrist-in-Chief, University Health Network, Toronto, Ontario, Canada.


