Abstract
Here we describe modifications that allow the bone diagnostic instrument (BDI) [P. Hansma et al., Rev. Sci. Instrum. 79, 064303 (2008); Rev. Sci. Instrum. 77, 075105 (2006)], developed to test human bone, to test the femora of mice. These modifications include reducing the effective weight of the instrument on the bone, designing and fabricating new probe assemblies to minimize damage to the small bone, developing new testing protocols that involve smaller testing forces, and fabricating a jig for securing the smaller bones for testing. With these modifications, the BDI was used to test the hypothesis that short-term running has greater benefit on the mechanical properties of the femur for young growing mice compared to older, skeletally mature mice. We measured elastic modulus, hardness, and indentation distance increase (IDI), which had previously been shown to be the best discriminators in model systems known to exhibit differences in mechanical properties at the whole bone level. In the young exercised murine femora, the IDI was significantly lower than in young control femora. Since IDI has a relation to postyield properties, these results suggest that exercise during bone development increases post yield mechanical competence. We were also able to measure effects of aging on bone properties with the BDI. There was a significant increase in the IDI, and a significant decrease in the elastic modulus and hardness between the young and old groups. Thus, with the modifications described here, the BDI can take measurements on mouse bones and obtain statistically significant results.
INTRODUCTION
The bone diagnostic instrument (BDI)1, 2 is a relatively new instrument developed to determine the mechanical properties of bone and possibly serve as a predictor of fracture risk. The BDI uses a force generator to move a test probe relative to a reference probe (Fig. 1). A computer is used to run a custom LABVIEW program, called OSTEOPROBE II™, to cycle the current to the force generator and thus move the test probe relative to the reference probe into the sample. The force and distance are monitored by transducers, which are then analyzed through OSTEOPROBE II™ software. Each cycle the test probe indents into the sample with a force set by the user, holds briefly at maximum force, and then retracts to its initial position. The purpose of the hold at maximum force is to monitor creep effects and to minimize the effect of the remaining creep during the linear decrease in load. This type of hold at the maximum load is used in instrumented indentation analysis, as discussed by Oliver and Pharr,3 for obtaining valid retraction slopes for elastic modulus calculation.
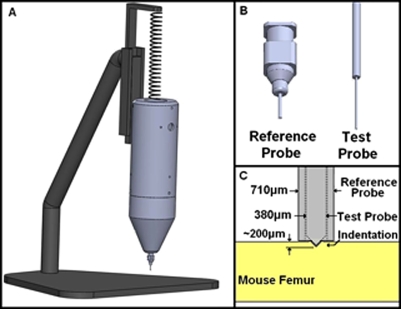
Figure 1.
(A) The BDI is supported by a spring to reduce the weight from 1.58 to 0.39 kg and held in place by a track, allowing only vertical movement. (B) The reference probe can be seen with a blunt end (left), and the test probe (right) has a 25 μm tip radius with a 90° conical end. (C) The blunt reference probe rests on the femur surface while the test probe indents into the bone to a total distance of approximately 200 μm.
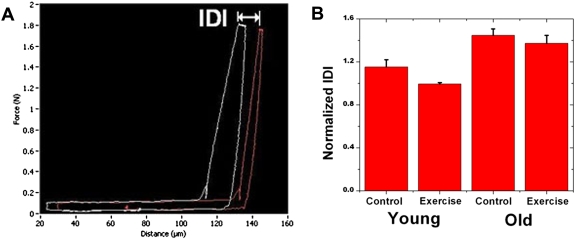
The indentation distance increase2 (IDI) is a valuable and unique parameter that the BDI can determine. The IDI is defined as the increase in the indentation distance in the last cycle relative to the indentation distance in the first cycle [Fig. 2a]. IDI is a measure of the bone’s ability to resist additional deformation with repetitive loading (i.e., a local post yield measure). The IDI is larger for bone in which the depth of the indentation at maximum load continues to increase with successive indentation cycles. The IDI discriminates between bones in model systems that exhibit differences in mechanical properties at the whole bone level.1, 2
Figure 2.
(A) The force vs distance curves show the IDI, which is the indentation from the first loading cycle to the last loading cycle. The maximum force reached is 1.8 N. (B) The normalized IDI is shown to the right. It is normalized with respect to IDI values for standard PMMA samples. The normalized IDI is significantly smaller (p<.05) for exercise compared to control groups for the young mice, but not the old. Smaller IDI has been shown to be a characteristic of more fracture resistant bone in previous studies. Thus the decreases in normalized IDI with exercise in young mice may indicate that exercise increases fracture resistance. Normalized IDI is also significantly smaller (p<.05) for the young compared to old groups, indicating fracture resistance is greater for the young group.
With modifications made to the BDI, we were able to study the effects of exercise and aging on murine femora. There are many animal models that exist to study the effects of exercise on bone structure and bone properties. Multiple exercise-based rodent models have shown a link between the loading of bone and changes in the bone mechanical properties4, 5, 6, 7 but none have investigated the effects of exercise and aging on the local postyield measure of IDI.
EXPERIMENTAL METHODS
Animals and treatment
The mice were the same sex and strain (male C57Bl∕6). There were a total of 20 femora tested, divided evenly into two age groups: old and young. The young group consisted of mice that were sacrificed at the age of 19 weeks, while the mice in the old group were sacrificed at the age of 19 months. Each age group consisted of bones from five exercise and five control mice, a subset of bones from a larger exercise study.8 Exercise mice ran on a treadmill for 30 min∕day, 7 days∕week for the last 21 days of the study. The animals were sacrificed the day after the end of exercise and the left femora were harvested. The average length of the femora was 12.1±0.8 mm with an average diameter of 1.8±0.1 mm in the medial-lateral direction at the mid-diaphysis. After removal of the soft tissue, femora were wrapped in gauze soaked in a Ca2+-buffered saline solution and stored at −80 °C. Before mechanical testing with the BDI, the bones were soaked in Hank’s solution (pH=7.4) and brought to room temperature.
Mechanical testing
To test the femora, the BDI1, 2 required several modifications. Due to the small size of the femur, the BDI had to be held by a spring supporting some of the weight of the instrument. The spring pulled on top of the instrument to reduce the effective weight from 15.5 N to 3.8 N (from 1.58 to 0.39 kg mass) [Fig. 1a]. The test sample was always tested at the same height on the stand so the stretch length of the spring and thus the effective weight of the instrument would remain constant from sample to sample. The reference probe was not a standard sharp hypodermic needle but rather a blunted hypodermic needle (22 G), cutoff square and lightly sanded to better distribute the (reduced) weight of the BDI on the small mouse femora. To prevent extensive damage to the femora, the test probe was blunted to a 25 μm radius, rather than the usual radius of less than 5 μm [Figs. 1b, 1c]. The femur was then mounted and clamped to a block and submerged in Hank’s solution (Fig. 3). The femur was oriented so that the tests would be run on the flexor surface.
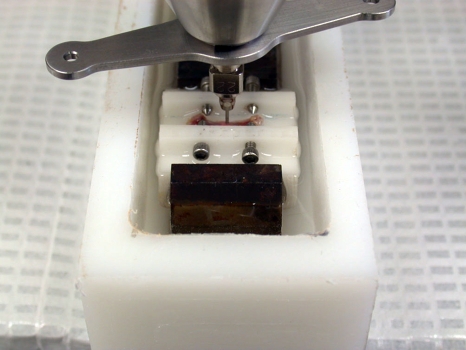
Figure 3.
The femur is clamped to a block to provide stability for testing and the probe assembly is lowered to rest on the femur which is submerged in Hank's solution.
A novel protocol was developed for testing the samples. The maximum applied loading force was reduced from the standard 12 to 1.85 N. In each test there were a total of ten indentation cycles at a frequency of 2 Hz. The total distance the test probe moves into the bone is approximately 200 μm depending on the bone properties [Fig. 1c]. For each femur tested the BDI ran ten tests along the femoral diaphysis with a spacing of approximately 1.3 mm between indents. The direction of the tests would run from the greater trochantor toward the femoral condyle. There were three tests before and after each mouse sample on polymethylmethacrylate (PMMA) for normalization. The data acquired from the BDI was analyzed through OSTEOPROBE II™ software and then normalized with respect to the PMMA values for each sample. The normalization process takes the average IDI of the sample divided by the average value of the PMMA.
EXPERIMENTAL RESULTS
A relatively brief 21-day period of running induced significant changes in the IDI in femora from young mice but not old mice. The IDI of the young control group is significantly higher (p<.0004) than the young exercise group [Fig. 2b]. The normalized IDI values are shown in Table 1. High IDI has been shown to follow the same trends as other mechanical properties that are hypothesized to be predictors of fracture risk.1, 2 This shows that short term exercise in young developing bone may reduce the risk of fracture.
Table 1.
Experimental values for normalized IDI.
| Experiment ID | Normalized IDI | Standard deviation |
|---|---|---|
| Young-control | 1.15 | 0.29 |
| Young-exercise | 0.99 | 0.12 |
| Old-control | 1.45 | 0.06 |
| Old-exercise | 1.37 | 0.07 |
The IDI was also significantly higher (p<0.0003) for the old groups compared to the young groups (see Table 1 for values). This is consistent with previous research showing significant deterioration in fracture toughness with age.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The difference in IDI values were noticeable but not significantly different for the old control bones compared to the old exercise bones, which shows that older skeletally mature mice are not as responsive to short term exercise. Ultimately the IDI proved to be the most discriminating parameter in this experiment between exercise and control femora from young mice.
The elastic modulus and hardness show no significant differences between the exercise and control for both age groups. A previous study agreed with our results that there was no significant difference in tibial elastic modulus between control and exercised mice.20, 21 There was a significant decrease between the young and old control femora for the hardness and elastic modulus. Although the effects are not statistically significant, there is a similar trend between the exercise young and old groups; the old groups display properties of slightly weaker bones. This difference, although small, follows intuition and the trend established in the control groups.
CONCLUSION
With the modifications described in this report, the BDI1, 2 is capable of measuring mechanical properties of murine femora, proving that the device can be used to test small bones. This allows for the device to be used in laboratory experiments on bone health in small animals. IDI, which has previously been shown to be smaller for less easily fractured bone, showed significant decreases with short term exercise in young mice indicating that exercise in developing bone may lead to fracture resistance. IDI was also significantly greater for old compared to young bones, indicating that the bone became mechanically compromised with age. Neither the elastic modulus nor the hardness showed significant changes with exercise in either age group, although there were significant changes due to aging. Ultimately, with the modifications made to the BDI, we determined skeletally developed femora are not as responsive to exercise as younger developing femora and the mechanical properties studied become compromised with age.
ACKNOWLEDGMENTS
We thank the NIH for funding this research under Grant No. RO1 GM 065354 and Robert Recker, Tamara Alliston, Deepak Vashishth and Adolfo Diez Perez for stimulating discussions.
References
- Hansma P., Turner P., Drake B., Yurtsev E., Proctor A., Mathews P., Lelujian J., Randall C., Adams J., Jungmann R., Garza-de-Leon F., Fantner G., Mkrtchyan H., Pontin M., Weaver A., Brown M. B., Sahar N., Rossello R., and Kohn D., Rev. Sci. Instrum. 79, 064303 (2008). 10.1063/1.2937199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma P., Turner P. J., and Fantner G. E., Rev. Sci. Instrum. 77, 075105 (2006). 10.1063/1.2221506 [DOI] [Google Scholar]
- Oliver W. C. and Pharr G. M., J. Mater. Res. 19, 3 (2004). 10.1557/jmr.2004.19.1.3 [DOI] [Google Scholar]
- Umemura Y., Ishiko T., Yamauchi T., Kurono M., and Mashiko S., J. Bone Miner. Res. 12, 1480 (1997). 10.1359/jbmr.1997.12.9.1480 [DOI] [PubMed] [Google Scholar]
- Iwamoto J., Yeh J. K., and Aloia J. F., J. Bone Miner. Res. 15, 1842 (2000). 10.1359/jbmr.2000.15.9.1842 [DOI] [PubMed] [Google Scholar]
- Notomi T., Okimoto N., Okazaki Y., Tanaka Y., Nakamura T., and Suzuki M., J. Bone Miner. Res. 16, 166 (2001). 10.1359/jbmr.2001.16.1.166 [DOI] [PubMed] [Google Scholar]
- Huang T., Lin S. C., Chang F. L., Hsieh S. S., Liu S. H., and Yang R. S., J. Appl. Physiol. 95, 300 (2003). [DOI] [PubMed] [Google Scholar]
- Kohn D., Sahar N. D., Wallace J. M., Golcuk K., and Morris M. D., Cells Tissues Organs 189, 33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. U., Yeni Y. N., and Norman T. L., J. Biomed. Mater. Res. 49, 380 (2000). [DOI] [PubMed] [Google Scholar]
- Currey J. D., Brear K., and Zioupos P., J. Biomech. 30, 1001 (1997). 10.1016/S0021-9290(97)82890-4 [DOI] [Google Scholar]
- Rho J. -Y., Kuhn-Spearing L., and Zioupos P., Med. Eng. Phys. 20, 92 (1998). 10.1016/S1350-4533(98)00007-1 [DOI] [PubMed] [Google Scholar]
- Zioupos P., Currey J. D., and Hamer A. J., J. Biomed. Mater. Res. 45, 108 (1999). [DOI] [PubMed] [Google Scholar]
- Phelps J. B., Hubbard G. B., Wang X., and Agrawal C. M., J. Biomed. Mater. Res. 51, 735 (2000). [DOI] [PubMed] [Google Scholar]
- Wang X., Shen X., Li X., and Agrawal C. M., Bone (N.Y.) 31, 1 (2002). 10.1016/S8756-3282(01)00697-4 [DOI] [PubMed] [Google Scholar]
- Wang X., Li X., Shen X., and Agrawal C. M., Ann. Biomed. Eng. 31, 1365 (2003). 10.1114/1.1623488 [DOI] [PubMed] [Google Scholar]
- Yeni Y. N. and Norman T. L., Bone (N.Y.) 27, 327 (2000). 10.1016/S8756-3282(00)00322-7 [DOI] [Google Scholar]
- Currey J. D., Brear K., and Zioupos P., J. Biomech. 29, 257 (1996). 10.1016/0021-9290(95)00048-8 [DOI] [PubMed] [Google Scholar]
- Diab T., Condon K. W., Burr D. B., and Vashishth D., Bone (N.Y.) 38, 427 (2006). 10.1016/j.bone.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Diab T. and Vashishth D., Bone (N.Y.) 37, 96 (2005). 10.1016/j.bone.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Li K. C., Zernicke R. F., Barnard R. J., and Li A. F., J. Appl. Physiol. 70, 554 (1991). 10.1063/1.349655 [DOI] [PubMed] [Google Scholar]
- Wallace J. M., Rajachar R. M., Allen M. R., Bloomfield S. A., Robey P. G., Young M. F., and Kohn D. H., Bone (N.Y.) 40, 1120 (2007). 10.1016/j.bone.2006.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]