Abstract
Optical-resolution photoacoustic microscopy (OR-PAM) has been validated as a valuable tool for label-free volumetric microvascular imaging. More importantly, the advantages of noninvasiveness and measurement consistency suggest the use of OR-PAM for chronic imaging of intact microcirculation. Here, such chronic imaging is demonstrated for the first time by monitoring the healing process of laser-induced microvascular lesions in a small animal model in vivo. The central part of a 1 mm by 1 mm region in a nude mouse ear was treated under a continuous-wave laser to create a microvascular lesion for chronic study. The region of interest was imaged before the laser treatment, immediately after the treatment, and throughout the healing process using both the authors’ OR-PAM system and a commercial transmission-mode optical microscope. Three-dimensional microvascular morphology and blood oxygenation information were imaged simultaneously at capillary-level resolution. Transmission-mode optical microscopic images were acquired for comparison. OR-PAM has potential important applications in microcirculatory physiology or pathophysiology, tumor angiogenesis, laser microsurgery, and neuroscience.
Keywords: optical-resolution photoacoustic microscopy, chronic imaging, label-free, noninvasive, wound healing, hemoglobin oxygen saturation
INTRODUCTION
Chronic imaging of microcirculation in vivo permits direct visualization of long-term microhemodynamics, which is closely associated with disease progression,1, 2 neural dynamics,3 and functional recovery from pathological states.4 However, existing high-resolution microvascular imaging techniques suffer in different regards when employed for chronic studies. For example, invasive procedures5 and fluorescence labeling6, 7 generally disturb the normal physiology of the microcirculation, discourage longitudinal studies, and impede clinical translations.
To overcome these difficulties, we have developed a bright-field photoacoustic microscopy with optically diffraction-limited lateral resolution, called OR-PAM, for in vivo microvascular imaging down to the capillary level.8 OR-PAM is highly sensitive to the physiologically specific optical absorption of hemoglobin, thereby bypassing the limitations of invasiveness and phototoxicity. By keeping the microcirculation intact, OR-PAM is ideal for chronic studies. In this Letter, we report on the first demonstration of using OR-PAM for functional chronic imaging. The healing process of a laser-induced microvascular lesion was monitored over a period of 12 days. Chronic OR-PAM imaging permits direct visualization of the morphological and functional recovery of microcirculation after laser destruction.
METHODS
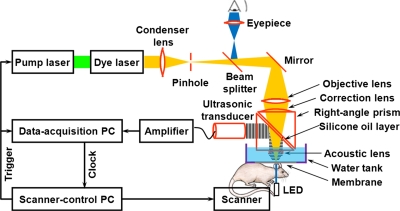
In our OR-PAM system (Fig. 1), the animal tissue is irradiated with a short-pulsed laser beam generated by a wavelength-tunable laser set, consisting of an Nd:YLF pump laser (INNOSLAB, Edgewave, Wuerselen, Germany) and a dye laser (CBR-D, Sirah, Kaarst, Germany). Wideband ultrasonic waves—referred to as photoacoustic waves—are induced as a result of transient thermoelastic expansion due to the laser excitation, collected via an acoustic lens, and then detected by a high-frequency ultrasonic transducer [V2022 (BC), Olympus NDT, Kennewick, WA]. To maximize the sensitivity for in vivo capillary imaging, the optical illumination and the ultrasonic detection in our system are configured confocally by an acoustic-optical beam splitter. In this simple design, two right-angle prisms form a cube with a thin silicone oil layer in between. The glass and the silicone oil have similar optical refractive indices (1.1 in ratio) but very dissimilar acoustic impedances (12.7 in ratio), which makes the layer optically transmissive but acoustically reflective. The lateral resolution of this acoustic-optical confocal configuration is determined by the product of the two point-spread functions of the optical illumination and the acoustic detection. Since acoustic resolution is difficult to achieve down to the capillary level due to the frequency-dependent ultrasonic absorption, a microscopic objective lens (RMS4X, Thorlabs, Newton, NJ) is employed to achieve diffraction-limited optical resolution. For chronic study, a transmission-mode optical microscope is integrated into our system by adding a light-emitting diode beneath the animal holder. Utilizing the reverse optical path of the OR-PAM illumination, the imaging region can be viewed under an eyepiece. This addition helps us quickly target the same region of interest (ROI) during multiday monitoring. The detailed system description and performance can be found in our previously published paper.8
Figure 1.
Schematic of the OR-PAM system.
The advantages of OR-PAM include the following: (i) Endogenous optical absorption contrast enables direct imaging of microvessels down to capillaries, with a high signal-to-noise ratio (SNR);8 (ii) multiwavelength measurement permits vessel-by-vessel mapping of blood oxygenation,9, 10, 11 holding the potential for functional studies such as brain function mapping;12 (iii) time-resolved ultrasonic detection provides depth information without depth scanning; (iv) working in reflection mode noninvasively makes the technique applicable to more anatomical sites in vivo, without disturbing the microcirculatory function.
RESULTS
The above advantages, along with measurement consistency, permit OR-PAM to monitor long-term microhemodynamics. One promising application lies in the realm of laser microsurgery, where OR-PAM can provide fruitful structural and functional information about the targeted microvascular lesions, such as their morphology, precise location, and blood oxygenation, which will facilitate accurate diagnosis and proper treatment. More importantly, OR-PAM enables noninvasive monitoring of the healing process of the microvascular lesions after laser microsurgery, which has been desired for a long time.13
A mouse ear model was chosen to demonstrate this ability in vivo because it is among the few anatomical sites that are readily imaged by transmission-mode optical microscopy, which can be used to partially validate OR-PAM. All experimental animal procedures were carried out in conformance with the laboratory animal protocol approved by the School of Medicine Animal Studies Committee of Washington University in St. Louis.
One day before the beginning of the chronic study, the hair on the left ear of a nude mouse (Hsd:Athymic Nude-Foxn 1NU, Harlan Co., Indianapolis, IN ∼25 g) was gently removed with human hair-removing lotion (Surgi cream, Ardell International, Los Angeles, CA). For daily monitoring, a dose of 87 mg∕kg ketamine and 13 mg∕kg xylazine was administered intraperitoneally to anesthetize the animal right before transferring it to a stereotaxic imaging stage. During the experiment, a dual-wavelength (570 and 578 nm) OR-PAM measurement was utilized to extract the total hemoglobin concentration (HbT) and the hemoglobin oxygen saturation (sO2). 570 nm is an isosbestic point, and the photoacoustic signal acquired at this wavelength reflects HbT. 578 nm is a local absorption peak of oxyhemoglobin, which helps differentiate it from deoxyhemoglobin. A detailed description of the method used for computing sO2 can be found in a previously published paper.9 Throughout each experiment, anesthesia was maintained using vaporized isoflurane (1.0%–1.5% isoflurane with an airflow rate of 1 l∕min), and the body temperature of the animal was maintained at 37 °C with a temperature controlled heating pad. At the end of the chronic study, the animal was euthanized by an intraperitoneal administration of pentobarbital at a dosage of 100 mg∕kg.
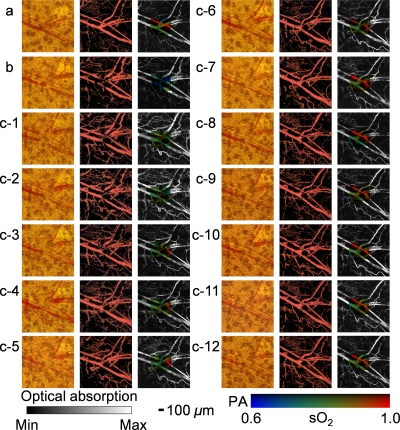
At the beginning of the chronic imaging, we selected and photographed a 1 mm by 1 mm region in the mouse ear under a commercial transmission-mode optical microscope. Then we imaged the ROI using OR-PAM [Fig. 2a], after which we switched the excitation source to a continuous-wave (cw) laser (output power: 150 mW; wavelength: 532 nm) and removed the pinhole to enable the laser to create a microvascular lesion for study. The central part of the ROI (0.25 mm by 0.25 mm) was then scanned with the focused cw laser beam (diameter: ∼30 μm) for ∼10 min. The ROI was imaged immediately after the cw laser treatment [Fig. 2b] and in the subsequent 12 days [Fig. 2c, (c-1)–(c-12)] using both our OR-PAM system and the commercial transmission-mode optical microscope. Our results clearly show a four-step wound healing process:14 (i) Vessel regression and hemostasis occurred right after the laser destruction [Fig. 2b]; (ii) inflammation, the second phase of wound healing, was exhibited in the form of vasodilation 24 h after the injury and lasted for about 5 days [Fig. 2c, (c-1)–(c-5)]. Hypoxia facilitated the synthesis of the vascular endothelial growth factor to trigger angiogenesis [Figs. 2b, 2c, (c-1)–(c-5)]; (iii) about 3 days after the wound occurred, the ingrowth of new capillaries started to restore the microcirculation [Fig. 2c, (c-3)], which was previously supplied by the damaged arteriole; (iv) after 12 days, the damaged arteriole-venule pair was almost completely recovered to normal status [Fig. 2c, (c-12)]. The microvascular morphology imaged by OR-PAM is partially validated by the commercial transmission-mode optical microscope; however, capillary networks are only visible to OR-PAM. The consistency in microvascular morphology during the multiday monitoring implies the robustness of our technique.
Figure 2.
OR-PAM monitoring of the healing process of a laser-induced microvascular lesion. (a) Before laser destruction. (b) Immediately after laser destruction. (c) On each of the subsequent 12 days. The left image in each part [(a)–(c-12)] is a photograph taken by a commercial transmission-mode optical microscope; the middle one is the front view of the 3D microvascular morphology acquired by OR-PAM at 570 nm; the right one is the maximum-amplitude projection (MAP) image overlaid by the sO2 mapping of the laser-damaged region.
CONCLUSION AND DISCUSSION
In this work, we demonstrated the capability of OR-PAM for functional chronic imaging of microhemodynamics in vivo noninvasively. However, this technique has potentially broader applications. In cancer research, OR-PAM can monitor tumor angiogenesis and evaluate tumor therapy.1 In drug development for microvascular dysfunctions, such as stroke, OR-PAM can trace drug functioning and evaluate drug efficacy. In the physiological study of angiogenesis, OR-PAM can help understand the signal transduction pathway by perturbing it and detecting the difference before and after perturbation. In laser microsurgery, surgical lasers can be readily integrated into our OR-PAM system to perform on-site high-precision microsurgery with presurgery diagnosis and postsurgery evaluation. In neuroscience, the noninvasive feature and fine imaging resolution make OR-PAM ideal for chronic studies of cortical plasticity15 as well as neurovascular coupling16 at the capillary level.
ACKNOWLEDGMENTS
The authors appreciate Professor James Ballard’s close reading of the manuscript. This work was sponsored by National Institutes of Health Grant Nos. R01 EB000712, R01 NS46214 (Bioengineering Research Partnerships), R01 EB008085, and U54 CA136398 (Network for Translational Research). L.V.W. has a financial interest in Endra, Inc., which, however, did not support this work.
References
- Jain R. K., Munn L. L., and Fukumura D., “Dissecting tumour pathophysiology using intravital microscopy,” Nat. Rev. Cancer 2, 266–276 (2002). 10.1038/nrc778 [DOI] [PubMed] [Google Scholar]
- Fukumura D. and Jain R. K., “Imaging angiogenesis and the microenvironment,” APMIS 116, 695–715 (2008). 10.1111/j.1600-0463.2008.01148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K., Feng G., Majewska A. K., Miesenbock G., Ting A., and Schnitzer M. J., “Next-generation optical technologies for illuminating genetically targeted brain circuits,” J. Neurosci. 26, 10380–10386 (2006). 10.1523/JNEUROSCI.3863-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda A., Arias C., and Sengpiel F., “Optical imaging of intrinsic signals: Recent developments in the methodology and its applications,” J. Neurosci. Methods 136, 1–21 (2004). 10.1016/j.jneumeth.2004.02.025 [DOI] [PubMed] [Google Scholar]
- Iga A. M., Sarkar S., Sales K. M., Winslet M. C., and Seifalian A. M., “Quantitating therapeutic disruption of tumor blood flow with intravital video microscopy,” Cancer Res. 66, 11517–11519 (2006). 10.1158/0008-5472.CAN-06-1743 [DOI] [PubMed] [Google Scholar]
- Laemmel E., Genet M., Le Goualher G., Perchant A., Le Gargasson J. F., and Vicaut E., “Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy,” J. Vasc. Res. 41, 400–411 (2004). 10.1159/000081209 [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Mitra P. P., Helmchen F., and Denk W., “Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex,” Proc. Natl. Acad. Sci. U.S.A. 95, 15741–15746 (1998). 10.1073/pnas.95.26.15741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov K., Zhang H. F., Hu S., and Wang L. V., “Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries,” Opt. Lett. 33, 929–931 (2008). 10.1364/OL.33.000929 [DOI] [PubMed] [Google Scholar]
- Zhang H. F., Maslov K., Sivaramakrishnan M., Stoica G., and Wang L. V., “Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy,” Appl. Phys. Lett. 90, 053901 (2007). 10.1063/1.2435697 [DOI] [Google Scholar]
- Zhang H. F., Maslov K., Stoica G., and Wang L. V., “Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging,” Nat. Biotechnol. 24, 848–851 (2006). 10.1038/nbt1220 [DOI] [PubMed] [Google Scholar]
- Zhang H. F., Maslov K., and Wang L. V., “In vivo imaging of subcutaneous structures using functional photoacoustic microscopy,” Nature Protocols 2, 797–804 (2007). 10.1038/nprot.2007.108 [DOI] [PubMed] [Google Scholar]
- Malonek D. and Grinvald A., “Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: Implications for functional brain mapping,” Science 272, 551–554 (1996). 10.1126/science.272.5261.551 [DOI] [PubMed] [Google Scholar]
- Anderson R. R. and Parrish J. A., “Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation,” Science 220, 524–527 (1983). 10.1126/science.6836297 [DOI] [PubMed] [Google Scholar]
- Chin G. A., Diegelmann R. F., and Schultz G. S., in Wound Healing, edited by Falabella A. F. and Kirsner R. S. (Talyor & Francis, Boca Raton, 2005), pp. 17–37. [Google Scholar]
- Masino S. A. and Frostig R. D., “Quantitative long-term imaging of the functional representation of a whisker in rat barrel cortex,” Proc. Natl. Acad. Sci. U.S.A. 93, 4942–4947 (1996). 10.1073/pnas.93.10.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pang Y., Ku G., Xie X., Stoica G., and Wang L. V., “Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain,” Nat. Biotechnol. 21, 803–806 (2003). 10.1038/nbt839 [DOI] [PubMed] [Google Scholar]