Abstract
Adaptations in one sex may impair fitness in the opposite sex. Experiments with Drosophila melanogaster have shown that seminal fluid from the male accessory gland triggers a series of postmating responses in the female, including increased egg laying rate and lower remating propensity, but that accessory gland proteins also increase female death rate. Here, we tested the relationships among the longevity of females mated to males from 51 chromosome-extracted D. melanogaster lines, male-mating ability, and sperm-competitive ability. We found significant differences in longevity of females mated to males of different genotypes, and all mated females showed a higher death rate than control virgin females shortly after mating. Both the age-independent mortality parameter (the intercept of the female's survival function) and the slope of the mortality rate curve were significantly correlated with the proportion of progeny sired by the first male to mate relative to tester males (sperm-defense ability, P1). No significant correlation was found between the proportion of progeny sired by the second-mating male relative to tester males (sperm-offense ability, P2) and any mortality parameter. Our results support the hypothesis of a tradeoff between defensive sperm-competitive ability of males and life-history parameters of mated females.
Previous studies have shown that, for Drosophila, mating can be hazardous to females. The cost of mating goes beyond a possible increased exposure to predators during mate search or physical damage because of interactions with conspecific males. When exposure to males, egg production, and egg hatchability are held constant, high mating frequencies decrease female lifespan, suggesting that mating itself could be detrimental to female survival (1). Studies have shown that seminal fluid proteins rather than sperm are responsible for the increased mortality of mated females (2, 3). Only males transferring accessory gland secretions showed the ability to reduce female lifespan when compared with males lacking accessory gland secretions and to control nonmated females (3).
These observations seem surprising because males causing a detrimental effect on females would be expected to have a lower reproductive fitness. However, when Drosophila melanogaster females were prevented from coevolving with males, males responded by increasing their ability to mate with females previously mated to control males (not exposed to females), to reduce female's willingness to remate, and to resist sperm displacement (defense) from a second male (4, 5). When the female genome was not allowed to coevolve with the male, mating with such experimental males resulted in a lower survival of their mates. Rice (5) suggested that an increase in the toxicity of the experimental males' seminal fluids might be a pleiotropic effect resulting from their increased mating success. The deleterious effects of mating on females was found to be lower under conditions that forced monogamous matings compared with polygamous matings (6), again suggesting a genetic correlation between male-competitive success and deleterious consequences to females.
The use of transgenic flies producing no accessory gland products has demonstrated the necessity of these secretions for sperm storage in mated females (7). Acp36DE null mutant males produce and transfer normal levels of sperm but are less successful than wild-type males in inducing retention of their sperm by mated females (8). The observation that singly mated females produce more progeny than females mated to a second male that transfers only accessory gland secretion led Harshman and Prout (9) to suggest a mechanism of sperm competition by which substances in the second male ejaculate incapacitate sperm from the first mate. However, in a survey of sperm-competitive ability where males from 152 different chromosome-extracted lines of D. melanogaster were tested against control males, none of the seven accessory gland genes surveyed showed a significant association with the male's ability to outcompete resident sperm (offense ability). Only molecular variation in Acp26A, Acp29B, Acp36DE, and Acp53E were significantly associated with the ability of sperm to resist being displaced by the ejaculate from a second male (defensive ability) (10).
Despite the growing evidence that accessory gland proteins play a role in sperm-competitive ability and that they are at least partly responsible for increased female mortality (2, 3, 9), the identification of which accessory gland protein(s) are responsible for the lower lifespan of mated females has yet to be established. Only a small number of accessory gland proteins have been characterized, but a possible candidate for the toxicity effect is Acp62F. The protein product of Acp62F enters the hemolymph of females after mating (11) and exhibits significant sequence similarity to a spider neurotoxin that is lethal when injected into house flies (12–15).
In this study, we show that there is significant variation in female longevity when females from an isogenic line are mated to males from different chromosome-extracted lines. Females mated to males from any of 51 different chromosome-extracted lines showed a higher mortality rate shortly after mating than control virgin females. Only the sperm-defensive ability of males from these lines, estimated as the proportion of progeny (P1) they fathered when they are first to mate with a female who subsequently remates, significantly correlated with the parameters of the female mortality functions. Our results lend support to previous findings of significant genetic variation in sperm-defense ability and suggest that the connection between the cost of mating and male sperm-competitive ability is not simply mediated by a general effect of seminal fluid toxicity.
Materials and Methods
Drosophila Stocks and Mating Protocol.
Stocks used in this study included 51 different homozygous chromosome-extracted lines from Beltsville, MD (provided by Brian Charlesworth), Winters, CA (provided by David Begun), and Raleigh, NC (provided by Trudy Mackay) and a cn bw (bearing the recessive alleles cinnabar and brown) laboratory stock.
For each stock, half-pint bottles containing cornmeal medium were started with 30 flies of each sex. Parents were allowed to lay eggs for 5 days and were then discarded. Virgin cn bw females and virgin males from the different chromosome-extracted lines were collected and kept in vials in groups of 20 per vial. Matings between males from each of the 51 lines and cn bw females were done en masse by shaking together groups of 20 of each sex. Matings were started in the morning and allowed to proceed for 6 h, at which point flies were lightly anesthetized with CO2 and females were placed in vials containing fresh medium. Vials were checked for dead females every 24 h. Surviving females were transferred twice a week to vials containing fresh medium. Survival was also tested for a control group of unmated cn bw females.
Estimation of Mortality and Sperm-Competitive Ability.
For each group of females, lifespan was recorded as the number of days from mating to death. An average lifespan or longevity was then calculated for control virgin females and those mated to males from the different lines. Alternatively, the mortality rate (MR) of each group of females was calculated as −ln[Nt+1/Nt] (16), where Nt+1 is the number of females alive at day t + 1, and Nt is the number of females alive at day t. The logarithm of the mortality rate was plotted against the squared age at death so that a linear function could be fitted to the data. Estimates of the intercept and slope were obtained by regression from each group of females.
Data on P1 and P2, the proportions of progeny sired by males when first or second to mate, from each of the 51 chromosome-extracted lines in double-mating experiments, as well as estimates of female's fecundity and their remating and refractoriness to remate, were obtained from Clark et al. (10).
Statistical Analysis.
Heterogeneity in average longevity among groups of cn bw females mated to males from different lines was tested by analysis of variance using the SAS statistical package. The day of death was squared to obtain adequate fit to normal distribution. Pearson product–moment correlation between different parameters of the male-mating and sperm-competitive ability and the female average longevity and parameters of the mortality curve were obtained by using SYSTAT. Sequential Bonferroni-adjusted probability of each correlation coefficient was used to test its significance.
Results
Differences in Longevity Among Females.
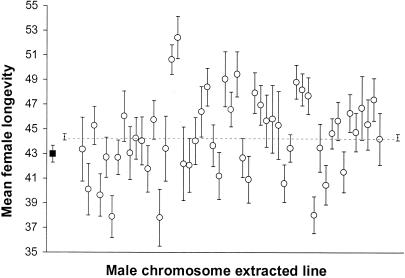
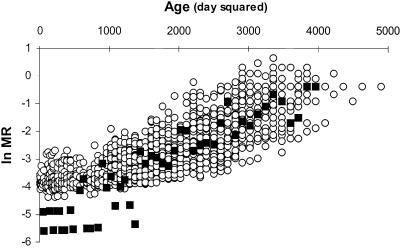
Females throughout this experiment were from the same stock, yet their mortality rate was significantly affected by a mere 6-h exposure to males, and this effect varied widely depending on the male's genotype. Analysis of variance revealed highly significant variation among the 51 lines of males in their effects on postmating female lifespan (F50,2237 = 3.94; P < 0.001). A comparison between mated and control virgin females showed no significant differences in average survival between the two groups (t(350; 0.05) = 1.88; P > 0.05) (Fig. 1), in part because of the great heterogeneity among mated females, especially late in life. Although control and mated females showed no significant differences in average survival, an age-specific difference between control and mated females is evident when a linear function is fitted to the mortality rate of flies as a function of age. Control females have a lower age-independent mortality parameter (the intercept of the female survival function) and a higher slope. This implies that mated females die at a faster rate at younger ages than do unmated females (see Materials and Methods), but the rates become indistinguishable when flies become older (Fig. 2).
Figure 1.
Mean longevity and standard errors of virgin females (■) and females mated to males from 51 different chromosome-extracted lines (○). The dashed line is the average longevity of all mated females (plotted at each end with associated standard error).
Figure 2.
Mortality of control virgin females (■) and females mated to males from different chromosome-extracted lines (○) along days. Age is plotted as the square of day at death. The MR is plotted as the logarithm of the function MR defined in Materials and Methods. Mortality rate increases as values in the y axis become less negative.
Female Mortality Rate and Male-Mating and Sperm-Competitive Ability.
Males from the lines used in this study were previously tested for their mating and sperm-competitive ability (10). We tested correlations among average female longevity, age-independent mortality, and the slope of the mortality rate vs. the male-mating and sperm-competitive parameters. None of the male parameters correlated with the average longevity of the females, but they were correlated with parameters of the female mortality rate function (Table 1).
Table 1.
Correlation between female mortality and male-mating and sperm-competitive ability parameters
| P1 | P2 | Fecundity-1 | Fecundity-2 | Remating ability | Refractoriness | |
|---|---|---|---|---|---|---|
| Average longevity | 0.117 (0.446) | 0.054 (0.720) | 0.099 (0.510) | 0.023 (0.881) | 0.216 (0.150) | −0.209 (0.159) |
| Intercept mortality rate function | 0.315* (0.035) | 0.116 (0.441) | 0.068 (0.652) | 0.199 (0.180) | −0.136 (0.368) | 0.290* (0.048) |
| Slope mortality rate function | −0.400** (0.006) | −0.161 (0.284) | −0.214 (0.149) | −0.243 (0.100) | 0.042 (0.781) | −0.226 (0.127) |
P1 and P2 are proportions of progeny sired by tested males when they are first or second to mate in a design where they are competed against cn bw males. Fecundity-1 is the fecundity of doubly mated females when the tested male is first to mate. Fecundity-2 is the fecundity of doubly mated females when the tested male is second to mate. Remating ability is the fraction of males that successfully mated with females previously mated to cn bw males. Refractoriness is the fraction of females mated to tester males that did not remate with cn bw males. *, P < 0.05; **, P < 0.01. Sequential Bonferroni-adjusted probabilities are given in parentheses.
A significant and positive correlation was found between the intercept of the mortality rate curve and the ability of male sperm to resist being outcompeted by a subsequent male (P1). The fact that the slope of the mortality rate shows a negative and significant correlation with P1 implies that strong sperm defenders cause higher mortality in females. This result is robust to several transformations and to application of quadratic regression of ln(MR) on age (2). Interestingly, the male's ability to outcompete resident sperm (P2) does not seem to have an association with any aspect of female survival (Table 1). The significant positive correlation found with the females' refractoriness to remating could relate to the fact that refractoriness and P1 have been found previously to be significantly correlated with each other (10). Both correlations suggest that a male that is better at defending his sperm by inducing females to produce more of his offspring or by making her more reluctant to mate with other males also impairs her survival.
Discussion
The nature of male × female-mating interactions has been considered in terms of conflict between the sexes, where components of seminal fluid that confer mating advantages to males become toxic and physiologically costly to the females (3–5). Natural selection acting on such sexually antagonistic attributes could explain the rapid divergence of reproductive traits. At the molecular level, high rates of interspecific differentiation have been detected for proteins expressed both in the female and male gonads and for genes of sex-related function (17, 18). Two accessory gland protein genes, Acp26A and Acp29B, that have shown significant associations between molecular variation and sperm-competitive ability (10) have also shown an excess of amino acid replacement, suggesting that directional selection has shaped their evolution (19–22).
D. melanogaster females are capable of mating and storing sperm from different males during their lifespan. Multiple mating with overlapping sperm storage provides the opportunity for complex interactions between the sexes that might determine paternity success. We know that the number of offspring sired by a male depends on both the genotype of his mate and on the genotypes of the other males competing for the same female (23, 24). Such interactions could become balanced within species and be responsible for the observed levels of polymorphism in sperm-competitive ability and sperm discrimination (10, 25). But does this elaborate male–female interaction affecting sperm use also have an effect on the flies' survival?
We have found that females mated to males from different lines showed significant variation in their average longevity, but they did not differ in mean longevity from control virgin females. However, examination of age-specific mortality rates shows that mated females die faster than control virgin females at younger ages, and the rate becomes indistinguishable later in life. This result is in agreement with previous studies showing that the detrimental effect of mating in both females and males is short lasting (26, 27). However, in our case the detrimental effect of mating is seen even after only 6 h of females' exposure to males. It has been previously shown (2, 3) that accessory gland secretions transferred during copulation lower the survival rate of mated females. The shorter lifespan we detected in mated females could be a consequence of an acceleration in the rate of egg production impairing survival or a direct toxic effect of the male ejaculate. The evolutionary dynamics of this system depend on the existence of pleiotropic effects, and deleterious effects on female survival might be retained if these effects are correlated with advantages to the male in mating and sperm-competitive abilities.
Rice (4, 5) reported that, in artificial selection experiments, males with high remating success and ability to induce reluctance by females to remate also significantly reduced the female's survival time, and this was concluded to be a consequence of a general toxicity effect of the male's ejaculate. Although we did not find significant associations between these two male characters and the female's average longevity, our results are not inconsistent with Rice's observations, as he only measured female mortality soon after females have mated. The detrimental effect of mating on female survival is only detectable shortly after mating. The lack of a significant correlation between overall female fecundity and any of the mortality rate parameters seems to indicate that the elevated female mortality is not a consequence of an increase in egg production. However, we detected an effect on female lifespan only shortly after mating. Then, it is possible that the significant correlation found between components of the mortality rate function and the male sperm-defensive ability (P1) might result from males fathering more progeny by fertilizing more eggs shortly after mating, which could be in turn detrimental to female survival. Interestingly, the only clear molecular functional evidence links accessory gland gene products with effects on sperm-storage capability (7, 8) and a short-term increased egg-laying rate (28, 29).
Our results suggest that antagonistic pleiotropic effects of male mating and sperm-competitive ability on female survival are strong but only temporary and depend on the male's ability to defend his sperm by inducing females to sire more of his progeny and/or possibly by making them more reluctant to remate.
Acknowledgments
We thank Paula Brooke, Brent Simmons, and Nicole Pacifico for scoring flies, and Brian Lazzaro, Federica Verra, Kristi Montooth, and Manolis Dermitzakis for their comments on the manuscript. This work was supported by National Science Foundation Grant DEB-9527592.
Abbreviation
- MR
mortality rate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 12953.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230305397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230305397
References
- 1.Fowler K, Partridge L. Nature (London) 1989;338:760–761. [Google Scholar]
- 2.Chapman T, Hutchings J, Partridge L. Proc R Soc London Ser B. 1993;253:211–217. doi: 10.1098/rspb.1993.0105. [DOI] [PubMed] [Google Scholar]
- 3.Chapman T, Liddle L F, Kalb J M, Wolfner M F, Partridge L. Nature (London) 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 4.Rice W R. Nature (London) 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 5.Rice W R. Proc Natl Acad Sci USA. 1998;95:6217–6221. doi: 10.1073/pnas.95.11.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland B, Rice W R. Proc Natl Acad Sci USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tram U, Wolfner M F. Genetics. 1999;153:837–844. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neubaum D M, Wolfner M F. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harshman L G, Prout T. Evolution. 1994;48:758–766. doi: 10.1111/j.1558-5646.1994.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 10.Clark A G, Aguadé M, Prout T, Harshman L G, Langley C H. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lung O, Wolfner M F. Insect Biochem Mol Biol. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 12.Rezende L J, Cordeiro M N, Oliveira E B, Diniz C R. Toxicon. 1991;29:1225–1233. doi: 10.1016/0041-0101(91)90195-w. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro M N, Richardson M, Gilroy J, Gomes de Figuereido S, Beirao P S L, Diniz C R. J Toxicol Toxin Rev. 1995;14:309–326. [Google Scholar]
- 14.Wolfner M F. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 15.Wolfner M F, Harada H A, Bertram M J, Stelick T J, Kraus K W, Kalb J M, Lung Y O, Neubaum D M, Park M, Tram U. Insect Biochem Mol Biol. 1997;27:825–834. doi: 10.1016/s0965-1748(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 16.Mueller L D, Nusbaum T J, Rose M R. Exp Gerontol. 1995;30:553–569. doi: 10.1016/0531-5565(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 17.Civetta A, Singh R S. J Mol Evol. 1995;41:1085–1095. doi: 10.1007/BF00173190. [DOI] [PubMed] [Google Scholar]
- 18.Civetta A, Singh R S. Mol Biol Evol. 1998;15:901–909. doi: 10.1093/oxfordjournals.molbev.a025994. [DOI] [PubMed] [Google Scholar]
- 19.Aguadé M. Mol Biol Evol. 1997;14:544–549. doi: 10.1093/oxfordjournals.molbev.a025791. [DOI] [PubMed] [Google Scholar]
- 20.Aguadé M. Genetics. 1998;150:1079–1089. doi: 10.1093/genetics/150.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsaur S C, Wu C-I. Mol Biol Evol. 1998;15:1040–1046. doi: 10.1093/oxfordjournals.molbev.a026002. [DOI] [PubMed] [Google Scholar]
- 22.Aguadé M. Genetics. 1999;152:543–551. doi: 10.1093/genetics/152.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark A G, Begun D J, Prout T. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- 24.Clark A G, Dermitzakis E T, Civetta A. Evolution. 2000;54:1030–1035. doi: 10.1111/j.0014-3820.2000.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 25.Clark A G, Begun D J. Genetics. 1998;149:1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partridge L, Andrews R. J Insect Physiol. 1985;31:393–395. [Google Scholar]
- 27.Partridge L, Fowler K, Trevitt S, Sharp W. J Insect Physiol. 1986;32:925–929. [Google Scholar]
- 28.Chen P S, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- 29.Herndon L A, Wolfner M F. Proc Natl Acad Sci USA. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]