Abstract
Bacterial tracheitis is due to a secondary bacterial infection of the trachea, resulting in the formation of mucopurulent exudates that may acutely obstruct the upper airway, resulting in a life-threatening condition. Bacterial tracheitis should be considered in the differential diagnosis of any child with acute upper airway obstruction. This diagnosis should also be considered in any child with viral croup that is nonresponsive to conventional therapy. The only definitive way to diagnose bacterial tracheitis is by direct visualization of the trachea via bronchoscopy; however, this may not be required in all cases. Management includes close observation and monitoring, early initiation of broad spectrum antibiotics, pain management and aggressive airway clearance techniques. The decision to intubate should be individualized based on the severity of symptoms, age of child and accessibility of personnel skilled at emergency intubation techniques. If diagnosed and treated early, complete recovery is expected.
Keywords: Bacterial tracheitis, Management, Upper airway obstruction, Stridor
Abstract
La trachéite bactérienne est causée par une infection bactérienne secondaire de la trachée qui entraîne la formation d’exsudats mucopurulents susceptibles de causer une obstruction aiguë des voies respiratoires supérieures mettant en jeu le pronostic vital. La trachéite bactérienne devrait être envisagée dans le diagnostic différentiel d’un enfant présentant une obstruction aiguë des voies respiratoires supérieures. Elle devrait également l’être chez un enfant atteint d’un croup viral qui ne réagit pas au traitement classique. Le seul moyen de diagnostiquer la trachéite bactérienne de manière définitive consiste à obtenir une visualisation directe de la trachée par bronchoscopie. Cependant, cette intervention n’est pas nécessaire dans tous les cas. La prise en charge inclut une observation et une surveillance étroites, l’administration précoce d’antibiotiques à large spectre, la prise en charge de la douleur et des techniques énergiques de clairance des voies respiratoires. La décision d’intuber doit être personnalisée selon la gravité des symptômes, l’âge de l’enfant et l’accessibilité de personnel expérimenté dans les techniques d’intubation d’urgence. Si cette maladie est diagnostiquée et traitée rapidement, une guérison complète est prévue.
Bacterial tracheitis is an uncommon cause of upper airway obstruction that can potentially result in a life threatening situation. Bacterial tracheitis was initially described in the medical literature in the late 1920s, when it was generally referred to as acute laryngotracheobronchitis (1). Although investigators described this as a bacterial illness in the early studies (2–4), the name bacterial tracheitis was not coined until much later when Jones et al (5) reported a case series of infants with the disorder. Membranous croup, pseudomembranous croup, bacterial croup, purulent tracheobronchitis and membranous laryngotracheobronchitis are other medical terms applied to this disease complex in the literature (6). Bacterial tracheitis most frequently affects preschool and early school-aged children, however, it has also been reported in infants as well as adults (5,7). Bacterial invasion of the tracheal mucosa results in the formation of mucopurulent exudates which can cause acute upper airway obstruction. The clinical features of bacterial tracheitis are similar to those of both common viral croup and epiglottitis. In this review, the important distinguishing features and the pathophysiology will be presented. The differential diagnosis and approach to treatment of the child with bacterial tracheitis will be discussed.
PATHOPHYSIOLOGY
In bacterial tracheitis, pathogenic bacteria invade the trachea and stimulate both local and systemic inflammatory responses. Locally, this results in production of thick, mucopurulent exudates, ulceration and sloughing of the tracheal mucosa (5,8). This can result in a variable degree of upper airway obstruction. The severity of the upper airway obstruction depends on the location and extent of the damage to the tracheal mucosa, the age and underlying medical condition of the patient and the size of the airway.
Bacterial tracheitis often presents as a secondary illness following an acute respiratory viral infection (3,5,9). The viral illness is believed to cause transient local or systemic alterations in the immune response that predispose the host to bacterial infection (3,8,10,11). This theory is strongly supported by the observation that bacterial tracheitis cases occur most often during upsurges of winter viral epidemics in the community, including that of viral croup (9,12,13). The isolation of both viral and bacterial pathogens in the tracheal secretions of children with bacterial tracheitis has been reported by a number of investigators. Edwards et al (14) reported on the coexistence of Staphylococcus aureus, Group A Streptococcus and Influenza B/Hong Kong virus in one child with bacterial tracheitis and S aureus with parainfluenza type 2 in another. A separate and larger case series published around the same time described the coexistence of parainfluenza type 1 with several pathogenic bacterial species including S aureus, α-Hemolytic Streptococcus species, and Haemophilus influenzae type b (9). More recently, Bernstein et al (12) isolated influenza A virus in tracheal secretions in 72% of children with bacterial tracheitis.
Microscopic evaluation of the tracheal secretions usually reveals neutrophilia and a positive bacterial identification. In the preantibiotic era, Group A Streptococcus was the most common bacterial pathogen identified. Following the development of effective antimicrobial agents, S aureus became the most common causative organism for many years (5,8,15). More recently, Moraxella catarrhalis (M catarrhalis was previously named Branhamalla catarrhalis; for purpose of clarification, we will refer to this organism as M catarrhalis throughout the article), has been identified with increased frequency as a cause of bacterial tracheitis (8,15). In the most recently published case series of children with bacterial tracheitis, M catarrhalis was identified in tracheal secretions more often than S aureus, and was associated with more severe disease (12).
Other common causative bacterial agents include Streptococcus pneumoniae and H influenzae. Although previously reported as a cause of bacterial tracheitis in children, the rate of infection with H influenzae type b has been significantly reduced since the introduction of routine vaccination against this organism. The role of anaerobic bacteria in the pathophysiology of bacterial tracheitis is unclear. In one retrospective case review, anaerobic bacteria were identified in 57% of all isolates and were the only identified pathogen in 21% of these cases (16). Clinically, on presentation there were no significant differences between patients with anaerobic infection and those with only aerobic pathogens. The author suggested that tracheal aspirates obtained in patients with suspected bacterial tracheitis should be analyzed for both aerobic and anaerobic bacteria (16). As previously discussed, viral co-infection is common, particularly with parainfluenza type 1 (the most frequent cause of viral croup) and influenza virus (9,12,14).
EPIDEMIOLOGY
As expected, given the relationship with viral epidemics, bacterial tracheitis is more common in the winter months. Although it may affect adults, it is primarily a disease of children between the ages of six months and 14 years, with a peak incidence around three to eight years of age (5,8,9,12,17–20). The reported male to female ratio is quite variable, between 1:1 and 5:1. Most published case series, however, clearly show males are more commonly affected than females, with only a single large series showing no sex differences (5,8,9,12,17–19). There are no specific risk groups identified to date. Bacterial tracheitis is an uncommon cause of hospital admissions, with an overall reported incidence of 0.4 per 1000 paediatric admissions (18). The incidence rate at the authors’ institution over the last 10 years has ranged from one to two cases per 1000 paediatric admissions. These data are based on discharge diagnoses entered into a global computerized database and do not explicitly include bronchoscopy-proven cases. This may account for the discrepancy with published data. Alternatively, it is also possible that the illness is now more frequently recognized by physicians dealing with paediatric patients. It is our impression that the disease incidence fluctuates with time, level of clinical suspicion and diagnostic thresholds.
CLINICAL FEATURES
Children with bacterial tracheitis usually present with a history of a viral respiratory illness, such as croup. Rhinorrhea, cough, fever and sore throat are frequently reported symptoms that may be present for up to one week before presentation. Presentation for medical attention is often precipitated by an acute deterioration characterized by severe upper airway obstruction, high fever and toxicity. The physical examination at this time may reveal nonspecific findings such as a high fever and toxic appearance. More specific findings include stridor, hoarseness, cough and tachypnea (3,12,18). Clinicians familiar with this disorder describe marked tracheal tenderness and reluctance to cough due to associated pain as common findings which have not been described in the literature to date. In one large case series, cough was noted in 40 of 46 children (86%) with bacterial tracheitis, and stridor was noted in 25 (54%) (12). This is consistent with the description of cough (50% to 100%) and stridor (65% to 95%) as the most common presenting clinical features of bacterial tracheitis in other case series (5,9,18).
An important clinical distinction between bacterial tracheitis and the more common clinical entity, viral croup, is the poor response to conventional medical therapy of croup, including administration of racemic epinephrine and systemic corticosteroids. Children with bacterial tracheitis also generally appear more toxic and have higher body temperatures (5,8,9,17,18). Unlike the classic description of children with epiglottitis, those with bacterial tracheitis are usually able to swallow their oral secretions and therefore do not present with drooling. Assumption of the ‘sniffing position’ to maximize airway patency is also uncommon. A summary of important clinical distinguishing features related to these diagnoses is presented in Table 1. Other clinical conditions that may present with upper airway obstruction are presented in Table 2.
TABLE 1.
Typical features of croup, epiglottitis and bacterial tracheitis
| Croup | Epiglottis laryngotracheobronchitis | Bacterial tracheitis | |
|---|---|---|---|
| Incidence | Common | Rare | Less common |
| Age | 6 months – 3 years | 2 – 7 years | 6 months – 14 years |
| Etiology | Parainfluenza type 1 | Haemophilus influenza type b | Staphylococcus aureus, Moraxella catarrhalis, Streptococcus pneumoniae |
| Onset of stridor | Insidious, common | Very rapid, less common | Rapid, common |
| Fever | Low grade | High | High |
| Cough | Common | Less common | Common |
| Voice | Hoarse | Muffled | Very hoarse |
| Drooling | Absent | Present | Absent |
| Preferred position | Supine | Tripod | Supine |
| Respiratory distress | Common | Common | Common |
| CXR | Normal | Normal or abnormal | Abnormal in >50% |
| Neck x-ray (AP) | Steeple sign† | Normal | Steeple sign |
| *Neck x-ray (Lateral) | Normal | Thumb print sign‡ | Haziness in subglottic area and soft tissue |
| Endoscopic findings | Normal supraglottic structures, edema of airways | Erythema and edema supraglottis (includes the epiglottis) | Normal supraglottis, mucopurulent secretions, erythema of subglottis |
| Response to racemic epinephrine | Very good | No response | No/partial response |
Radiographs are not indicated in the initial management of a child with suspected epiglottitis;
Narrowing of subglottic area;
Thickening and rounding of epiglottis. AP Anteroposterior; CXR Chest x-ray
TABLE 2.
Clinical features of other causes of acute upper airway obstruction
| Fever | Cough | Voice | Stridor | Specific features | |
|---|---|---|---|---|---|
| Peritonsillar abscess | Present | Absent | Muffled | +/− |
|
| Retropharyngeal abscess | Present | Absent | +/− muffled | +/− |
|
| Angioedema | Absent | Absent | Normal | +/− |
|
| Laryngeal foreign body | Absent | Croupy | Dysphonia | Present |
|
| Laryngeal diphtheria | Low grade | +/− | Hoarse +/− progression to loss of voice | Present |
|
+/− May or may not be present
INVESTIGATIONS
The diagnosis of bacterial tracheitis is most often made on the basis of the history and physical examination, combined with findings from laboratory and bronchoscopic examination. Some authors have used diagnostic criteria that included clinical signs of upper airway obstruction and at least two of the following: radiographic evidence of intra-tracheal membranes; laryngotracheal inflammation and mucopurulent secretions noted by direct visualization during bronchoscopy; or a tracheal aspirate positive for leukocytes on gram staining and a positive bacterial culture (12,18).
Results of laboratory investigations including the complete blood count with differential and blood cultures are generally nonspecific. Leukocytosis may or may not be present; however, most case series do report an increase in immature cells on the differential. Blood cultures infrequently yield positive identification of the responsible bacterium (5,9,12,17).
RADIOGRAPHIC FINDINGS
The anteroposterior radiograph of the cervical airway in children with bacterial tracheitis frequently shows narrowing of the subglottic area (5,13,17,20). Less frequently, diffuse haziness and irregularity of the anterior wall of the trachea may be seen on the lateral neck radiograph (candle-dripping sign). This finding, although less common, is more specific for bacterial tracheitis (5,13,17,20). Radiographs are contraindicated in any child suspected of having epiglottitis until the diagnosis has been confirmed and the airway stabilized.
Chest radiograph abnormalities are common in children with bacterial tracheitis. In most case series, at least 50% had radiological findings suggestive of pneumonia (13). Concurrent pneumonia is often associated with increased disease severity; the largest case series reported that only those patients with pulmonary infiltrates on chest radiograph required endotracheal intubation (12,16,21). Lobar atelectasis due to mucous plugging with compensatory hyperinflation is another, less frequent, radiographic finding (22).
LARYNGOBRONCHOSCOPY
Direct visualization of the airway is the most definitive way to diagnose bacterial tracheitis. Bronchoscopy is also helpful for the exclusion of other diagnoses such as epiglottitis. The typical bronchoscopic findings of bacterial tracheitis include subglottic narrowing, diffuse erythema and mucopurulent exudates that may partially occlude the airway. Exudates may also be seen extending into the right and/or left main bronchi. Importantly, the epiglottis usually appears normal or only slightly inflamed.
Pulmonary toilet can be effectively performed via the bronchoscope, and secretions can be easily obtained and sent for culture and sensitivity to allow for targeted antibiotic therapy. Identification of the pathogen is usual (62% to 87%) presuming no prior treatment with antibiotics (12).
The decision to perform bronchoscopy should be individualized. Factors to consider include the severity of upper airway obstruction, the level of clinical suspicion for the presence of epiglottitis, accessibility of skilled broncho-scopists and the age of the child.
TREATMENT
Broad spectrum intravenous antibiotics should be initiated as soon as the clinical diagnosis of bacterial tracheitis is made. Empirical antimicrobial agents should be targeted to the most frequently isolated organisms including S aureus, M Catarrhalis, S pneumoniae and H influenzae. A third generation cephalosporin agent combined with a beta-lacta-mase resistant penicillin (eg, cloxacillin) is appropriate for first line therapy. If methicillin-resistant S aureus is a concern at the admitting institution, coverage should be extended to cover this organsim (eg, vancomycin). If the anaerobic culture is positive, additional or alternative therapy such as clindamycin or metronidazole may be considered, particularly in a child who is not responding to first line therapy. However, contamination of tracheal aspirate secretions with oral anaerobes is not uncommon when the specimens are obtained via bronchoscopy. Antibiotic coverage can then be modified according to culture and sensitivity results. A total 10 to 14 day course of antibiotics (intravenous plus oral) is recommended based on previous case series; however, there have been no formal studies to establish the optimum duration of antimicrobial therapy for bacterial tracheitis. Intravenous therapy is recommended until the child is afebrile for at least 48 h, the tracheal tenderness has resolved and the voice has recovered normal quality.
Endotracheal intubation is frequently required for stabilization of the airway, management of acute respiratory failure, or pulmonary toilet. The decision to intubate should be individualized and will depend on the severity of the upper airway obstruction as well as the likelihood of further deterioration while awaiting a clinical response to antimicrobial therapy. Clearance of airway secretions is more effective with an endotracheal tube in situ because suctioning can be done frequently and aggressively, preventing occlusion of the airway by thick exudates. Younger children are more likely to require intubation due to the small size of their airway (20). The level of comfort of the attending physician and availability of ready access to a skilled bronchoscopist also play major roles in the decision regarding intubation.
Overall rates of intubation for airway management of bacterial tracheitis reported in published case series are variable and range from 38% to 100%, depending on the centre (5,8,9,17–20). When intubation is indicated, the endotracheal tube size needs to be modified to a smaller size than that normally required for the age to account for the airway inflammation present. In Bernstein et al’s case series (12), 26 of 46 children (54%) were managed with intubation using endotracheal tubes that were smaller than that normally required for the age (mean of −1.7 mm) (12). In this particular series, the mean duration of intubation was 3.2 days. However, there was a high yield of positive viral cultures in this patient population, raising the possibility that some subjects may not have had true bacterial tracheitis. Indeed, the diagnostic criteria used for this case series did not necessarily include positive bronchoscopic findings, and although all their patients underwent bronchoscopy, these findings were not discussed in the paper. Other case series report a mean duration of intubation of between 1.5 to 7.6 days (8,12,18,19).
Indications for extubation include indicators of general clinical improvement such as reduced fever, decreased tracheal secretions, resolution of toxic appearance and the development of a leak around the endotracheal tube. Typically, once the airway inflammation begins to subside, a leak around the endotracheal tube will be apparent, however, this is not always clinically evident. Tracheotomy was commonly performed in the past for the management of children with bacterial tracheitis but is now rarely required (12).
Children managed without endotracheal intubation need close observation in a centre with expertise in paediatric airway management. This most often requires transfer of the child to a tertiary care center and frequent communication with the accepting specialist. Adequate pain management is particularly important to avoid cough suppression with resultant failure to clear secretions, increasing the risk of sudden airway obstruction. Physiotherapy to encourage coughing and secretion clearance is also a valuable part of treatment. Although this has not been recommended in the medical literature to date, it is our experience that this strategy is particularly helpful for older children who may be suppressing cough due to discomfort.
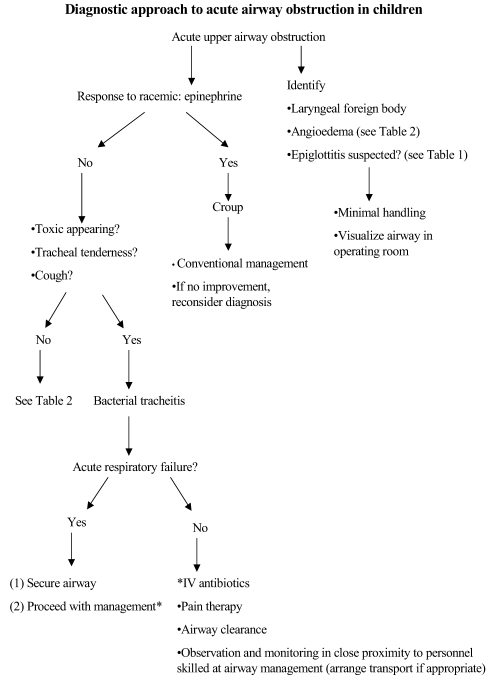
Unlike viral croup, there is currently no data to suggest that inhaled or systemic steroids provide any clinical benefit in the treatment of bacterial tracheitis. Concern regarding adverse effects in the absence of documented clinical efficacy prohibits the recommendation that steroids be used for routine management. Racemic epinephrine, used commonly to treat symptoms of upper airway obstruction in children with viral croup, is not very effective in relieving airway obstruction related to bacterial tracheitis (9,12,17–19). An algorithm outlining the approach to a child with acute upper airway obstruction is presented in Figure 1.
Figure 1).
Diagnostic approach to acute airway obstruction in children. IV Intravenous
COMPLICATIONS AND PROGNOSIS
Full recovery with no long-term morbidity is expected in the vast majority of children with bacterial tracheitis, especially if there are no complications during the acute illness. The mean length of stay in hospital varies with reports between three and 12 days (5,8,12,18,19). One small case series of children with bacterial tracheitis reported a mean duration of hospitalization of 20.8 days (17). The authors speculated that this was due to complications associated with a protracted recovery in several patients (17).
The most frequent complication associated with the acute phase of illness is pneumonia. Other less common complications include acute respiratory distress syndrome, septic shock, toxic shock syndrome, pulmonary edema, pneumothorax, and rarely, cardiorespiratory arrest (21). The mortality rate due to bacterial tracheitis has decreased dramatically with time. In the early twentieth century, the mortality rate was 10% to 40% (11). This has declined slowly with time, and the most recent and largest case series reported no mortality associated with the illness (9,12,18,19). The improvement in mortality rate is likely due to the early recognition and improved treatment of the disorder with aggressive airway clearance techniques and early initiation of broad spectrum antibiotics.
Long-term morbidity associated with bacterial tracheitis is minimal. As treatment in the acute phase of the illness frequently requires insertion of an endotracheal tube into an inflamed airway, the potential for the subsequent development of subglottic stenosis is recognized. This complication, indeed, has been reported in two papers. Gallagher (18) reported that three of 18 patients had subglottic stenosis, and Kasian (19) reported one case in 14 patients.
CONCLUSION
Bacterial tracheitis is a potentially life-threatening condition, however, when diagnosed and treated early, complete recovery is usual. Primary care physicians need to be familiar with this condition and how the clinical presentation of bacterial tracheitis differs from croup.
Acknowledgments
The authors wish to thank Dr Ruth Connors and Dr Abdulla Al-Shamrani for their thoughtful review of the manuscript and helpful editorial comments.
REFERENCES
- 1.Baum HL. Acute laryngotracheobronchitis. JAMA. 1928;91:1097–102. [Google Scholar]
- 2.Orton HB, Smith EL, Bell HO. Acute laryngotracheobronchitis. Arch Otolaryngol. 1941;33:926–60. [Google Scholar]
- 3.Nelson W. Bacterial croup: A historical perspective. J Pediatr. 1984;105:52–5. doi: 10.1016/s0022-3476(84)80356-x. [DOI] [PubMed] [Google Scholar]
- 4.Neffson AH. Acute laryngotracheobronchitis: A 25 year review. Am J Med Sci. 1944;208:524–47. [Google Scholar]
- 5.Jones R, Santos J, Overall J., Jr Bacterial tracheitis. JAMA. 1979;242:721–6. [PubMed] [Google Scholar]
- 6.Stern RC. Acute laryngotracheobronchitis. 3rd ed. Philadelphia: WB Saunders; 1983. [Google Scholar]
- 7.Johnson J, Liston S. Bacterial tracheitis in adults. Arch Otolaryngol Head Neck Surg. 1987;113:204–5. doi: 10.1001/archotol.1987.01860020096021. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly B, McMillan J, Weiner L. Bacterial tracheitis: Report of eight new cases and review. Rev Infect Dis. 1990;12:729–35. doi: 10.1093/clinids/164.5.729. [DOI] [PubMed] [Google Scholar]
- 9.Liston SL, Gehrz RC, Siegel LG, Tilelli J. Bacterial tracheitis. Am J Dis Child. 1983;137:764–7. doi: 10.1001/archpedi.1983.02140340044012. [DOI] [PubMed] [Google Scholar]
- 10.Davidson S, Barzilay Z, Yahav J, Rubinstein E. Bacterial tracheitis –a true entity? J Laryngol Otol. 1982;96:173–5. doi: 10.1017/s0022215100092380. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson J, Maltby C. Bacterial tracheitis in children. J Otolaryngol. 1989;18:101–4. [PubMed] [Google Scholar]
- 12.Bernstein T, Brilli R, Jacobs B. Is bacterial tracheitis changing? A 14-month experience in a pediatric intensive care unit. Clin Infect Dis. 1998;27:458–62. doi: 10.1086/514681. [DOI] [PubMed] [Google Scholar]
- 13.Han BK, D J, Striker TW. Membranous laryngotracheobronchitis (membranous croup) AJR Am J Roentgenol. 1979;133:53–8. doi: 10.2214/ajr.133.1.53. [DOI] [PubMed] [Google Scholar]
- 14.Edwards K, Dundon M, Altemeier W. Bacterial tracheitis as a complication of viral croup. Pediatr Infect Dis. 1983;2:390–1. doi: 10.1097/00006454-198309000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Wong V, Mason W. Branhamella catarrhalis as a cause of bacterial tracheitis. Pediatr Infect Dis J. 1987;6:945–6. doi: 10.1097/00006454-198710000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Brook I. Aerobic and anaerobic microbiology of bacterial tracheitis in children. Pediatr Emerg Care. 1997;13:16–8. doi: 10.1097/00006565-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Sofer S, Duncan P, Chernick V. Bacterial tracheitis – An old disease rediscovered. Clin Pediatr. 1983;22:407–11. doi: 10.1177/000992288302200602. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher P. An approach to the diagnosis and treatment of membranous laryngotracheobronchitis in infants and children. Pediatr Emerg Care. 1991;7:337–42. doi: 10.1097/00006565-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kasian G, Bingham W, Steingberg J, et al. Bacterial tracheitis in children. CMAJ. 1989;140:46–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman E, Jorgensen K, Healy G, McGill T. Bacterial tracheitis –Two-year experience. Laryngoscope. 1985;95:9–11. doi: 10.1288/00005537-198501000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Britto J, Habibi P, Walters S, Levin M, Nadel S. Systemic complications associated with bacterial tracheitis. Arch Dis Child. 1996;74:249–50. doi: 10.1136/adc.74.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seigler RS. Bacterial tracheitis. An unusual radiographic presentation. Clin Pediatr. 1994;33:374–7. doi: 10.1177/000992289403300612. [DOI] [PubMed] [Google Scholar]