Abstract
OBJECTIVE: To comprehensively evaluate clinical, economic, and patient-reported outcomes associated with various therapeutic classes of asthma controller medications.
PATIENTS AND METHODS: This observational study, which used administrative claims data from US commercial health plans, included patients with asthma aged 18 through 64 years who filled a prescription for at least 1 asthma controller medication from September 1, 2003, through August 31, 2005. Outcome metrics included the use of short-acting β-agonists (SABAs), the use of oral corticosteroids, inpatient (INP)/emergency department (ED) visits, and asthma-related health care costs. A subset of 5000 patients was randomly selected for a survey using the Mini-Asthma Quality of Life Questionnaire, the Work Productivity and Activity Impairment questionnaire, and the Asthma Therapy Assessment Questionnaire.
RESULTS: Of 56,168 eligible patients, 823 returned completed questionnaires. Compared with inhaled corticosteroids (ICSs), leukotriene modifiers (LMs) were associated with lower odds of INP/ED visits (odds ratio [OR], 0.80; P<.001), lower odds of using 6 or more SABA canisters (OR, 0.81; P<.001), and higher annual cost ($193; P<.001). In the subgroup analysis of adherent patients, LMs were associated with higher odds of INP/ED visits (OR, 1.74; P=.04), lower odds of using 6 or more SABA canisters (OR, 0.46; P<.001), and higher annual cost ($235; P<.001). Inhaled corticosteroids and LMs had a comparable impact on all patient-reported outcomes. For combination therapy, ICS plus a long-acting β-agonist consistently showed at least equivalent or better outcomes in the use of SABAs and oral corticosteroids, the risk of INP/ED visits, cost, asthma control level, quality of life, and impairment in productivity and activity.
CONCLUSION: Inhaled corticosteroids were associated with a lower risk of INP/ED visits, and a lower cost if adherence was achieved. When adherence cannot be achieved, LMs may be a reasonable alternative. Combination therapy with ICS plus a long-acting β-agonist was associated with better or equivalent clinical, economic, and patient-reported outcomes.
Inhaled corticosteroids were associated with a lower risk of inpatient/emergency department visits, and a lower cost if adherence was achieved. When adherence cannot be achieved, leukotriene modifiers may be a reasonable alternative. Combination therapy with inhaled corticosteroids plus a long-acting β-agonist was associated with better or equivalent clinical, economic, and patient-reported outcomes.
ATAQ = Asthma Therapy Assessment Questionnaire; BMI = body mass index; COPD = chronic obstructive pulmonary disease; ED = emergency department; HIPAA = Health Insurance Portability and Accountability Act; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICS = inhaled corticosteroid; INP = inpatient; LABA = long-acting β-agonist; LM = leukotriene modifier; Mini-AQLQ = Mini-Asthma Quality of Life Questionnaire; MPR = medication possession ratio; NAEPP = National Asthma Education and Prevention Program; OCS = oral corticosteroid; SABA = short-acting β-agonist; WPAI = Work Productivity and Activity Impairment
Asthma is a common and chronic inflammatory disorder of the airways that affects more than 22 million Americans.1 It is associated with considerable morbidity: in 2004 it resulted in 14.7 million outpatient visits, 1.8 million emergency department (ED) visits, and nearly 500,000 hospitalizations.1 Asthma also carries a substantial economic burden, with an estimated total cost of $18.3 billion annually, including $10.1 billion in direct costs for medications and health care services and $8.2 billion in indirect costs associated with lost productivity because of missed days of school or work.2
For editorial comment, see page 673
In addition to its clinical morbidity and economic burden, asthma is associated with adverse patient-centered outcomes, such as decreased quality of life and lost days at work or school.3,4 These outcomes are important because they reflect the patients' burden of disease rather than a simple assessment of clinical and economic perspectives. Although some clinical trials have assessed patient-centered outcomes,5-7 few such studies have been performed in real-world settings.
The current asthma management guidelines from the National Asthma Education and Prevention Program (NAEPP) Expert Panel of the National Institutes of Health (National Heart, Lung, and Blood Institute) recommend a stepwise approach to asthma management.4 However, the appropriate pharmacotherapy for asthma in real-world settings remains a subject of debate. Several studies have evaluated how effectively asthma controller medications improve clinical (eg, symptom control and exacerbation) and economic (eg, cost of care) outcomes, but the results have been inconsistent. For instance, the findings of some studies suggest that the best outcome is achieved with the use of inhaled corticosteroids (ICSs) as monotherapy,8-10 whereas other studies report discrepant findings.11,12 Several studies have found that superior outcomes are achieved by the combination of ICSs plus long-acting β-agonists (LABAs)13-15; however, others have suggested that coupling ICSs with a leukotriene modifier (LM) would be a reasonable choice.16 Few of these studies have attempted to establish the association between controller medications and patient-centered outcomes. In one study, O'Connor et al17 found that the effectiveness of fluticasone propionate/salmeterol (100/50 μg) on patient-reported outcomes was similar to that of montelukast (10 mg); however, these investigators did not study ICSs alone or other combination therapies.
The current study was designed to address the existing gap in knowledge about the effectiveness of various therapeutic classes of asthma controller medications for adult asthma patients by integrating one of the largest administrative claims databases in the United States with the results of a comprehensive survey. To our knowledge, this is the first study to comprehensively determine the association between therapeutic classes of asthma controller medications and patient-reported asthma control problems, quality of life, work productivity, and activity impairment, as well as both clinical and economic end points.
PATIENTS AND METHODS
Data Source
This observational study used administrative claims data obtained from 8 geographically dispersed US commercial health plans that represent approximately 17.5 million members. The administrative data set consisted of integrated medical claims, pharmacy claims, and eligibility files. The study database was developed in compliance with the regulations of the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Retrospective Cohort
Patient Selection. To be eligible for the study, patients had to be aged 18 through 64 years with at least 1 medical claim with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for asthma (ICD-9-CM: 493.xx) from September 1, 2002, through August 31, 2006, and at least 1 prescription claim for an asthma controller medication from September 1, 2003, through August 31, 2005 (intake period). Eligible patients had to have received either monotherapy with an ICS, a LABA, or an LM or combination therapy with an ICS and a LABA; an ICS and an LM; or an ICS, a LABA, and an LM. The class of the first controller medication for which a prescription was filled during the intake period was considered to be the index class of controller medications for each patient, and the prescription date was considered to be the index date. To account for instances of combination therapy in which prescriptions for 2 medications might be filled on different days, we used the following algorithm: the patient was considered to be taking combination therapy if a prescription for controller 2 (a different class of medication from that of the index controller) was filled and the prescription for the index controller was refilled after the fill date for controller 2 but within 90 days after the index date for controller 1.
Once an index class of controller medications and an index date had been determined, the patient remained in that index class for the rest of the study period, regardless of any switching or discontinuation of medications. Patients were required to be continuously enrolled in a health plan for at least 12 months before and after the index date. Patients were excluded from the study if they had a medical claim for chronic bronchitis or emphysema (ICD-9-CM: 491.xx-492.xx), cystic fibrosis (ICD-9-CM: 277.0x), bronchopulmonary dysplasia (ICD-9-CM: 770.7x), or other respiratory diseases (ICD-9-CM: 495.xx-519.xx) at any time during the study period.
Study Measures. Four outcomes were assessed: use of short-acting β-agonists (SABAs), use of oral corticosteroids (OCSs), inpatient (INP)/ED visits, and total cost of care attributable to asthma during the 12-month period after the index date. To account for the potential of regular prophylactic use of SABAs before exercise, we measured SABA use with a binary variable (receiving vs not receiving ≥6 canisters); this variable served as an indicator of lack of asthma control. Oral corticosteroid use was measured by the number of prescriptions filled for oral prednisone or methylprednisolone; this variable served as an indicator of asthma exacerbation. An INP/ED visit was defined as at least 1 asthma-related hospitalization or ED visit vs no such visits (binary), determined by institutional claims that contained an ICD-9-CM code for asthma. Total cost of care reflected the total allowable amount reimbursed by the health plans during the 12-month period after the index date.
Important potential confounding variables, including disease severity and adherence to controller medications, were also captured and were controlled for in analyses of the association between index controller medication and outcomes. Asthma disease severity was approximated by using 3 available claims-based severity scales, which classified patients into severity groups according to the use of asthma controllers, SABAs, and OCSs; the number of hospitalizations; and the number of ED visits during the 12-month period before the index date.18-20 The scale determined to best discriminate the groups was included in the multivariate analyses, whereas the other 2 scales were used in the sensitivity analyses to examine the consistency of the results. Adherence to controller medications was measured by using the medication possession ratio (MPR), defined as the ratio of the total-day supply of index controller medications dispensed during the period to the total number of days during the 12-month period after the index date. Patients with an MPR of 0.8 or higher were considered to be adherent to therapy.
Survey Cohort
Patient Selection. A subset of patients from the retrospective cohort was selected for the survey. Eligible patients had to be actively enrolled in a health plan on August 31, 2006 (latest data available at the time of conducting the survey), and had to have filled prescriptions for at least 2 asthma controller medications in 2006. Patients were randomly selected for the survey and were grouped according to the index controller medications determined by the retrospective cohort study. A HIPAA waiver of authorization for patient consent was obtained from a central institutional review board (Quorom Review); this waiver allowed the use of protected health information to contact patients for participation. A total of 5000 questionnaires were mailed in May and June of 2007 and were collected in compliance with HIPAA guidelines. A letter from the medical director of the regional health plan accompanied the questionnaires, and a small financial incentive was provided to encourage participation. Written consent was obtained from all respondents.
Survey Instruments. The survey was designed to collect information about age, sex, race/ethnicity, education level, income level, body mass index (BMI), smoking status, chronic obstructive pulmonary disease (COPD) status, and controller medication used at the time of survey. The validated tools included the 15-question Mini-Asthma Quality of Life Questionnaire (Mini-AQLQ), with scores ranging from 1 to 7 and higher scores indicating better quality of life21; the 4-question Asthma Therapy Assessment Questionnaire (ATAQ), with scores ranging from 0 to 4 and higher scores indicating more control problems22; and the 9-question Work Productivity and Activity Impairment (WPAI) questionnaire, with scores ranging from 0% to 100% and higher scores indicating greater impairment.23-25
Proxies of asthma disease severity scales and adherence to controller medication regimens at the time of the survey were generated by using administrative claims data from the 12 months before the survey or the end of health plan eligibility, whichever came first. As was true for analyses of data from the retrospective cohort, both measures were controlled for in the multivariate analyses so that the independent association of controller medication with patient-reported outcomes could be determined.
Statistical Analyses
All outcome measures were compared across the therapeutic classes of controller medications within the monotherapy and combination therapy cohorts. The statistical significance of differences between cohorts was assessed by using the Wilcoxon rank-sum test or the Kruskal-Wallis test for continuous variables and the Pearson χ2 test for categorical variables. In post hoc tests, Bonferroni α adjustment was used to account for multiple comparisons. An a priori 2-tailed level of significance (α value) of .05 was set for all analyses.
Multivariate analyses were performed to examine the association between index controller medication and each outcome while controlling for potential confounders. Generalized linear models were constructed with various response probability distributions and link functions, depending on the distribution of the outcomes and the goodness of fit of the models. For instance, γ distribution and log link function were used for total cost of care, whereas binomial distribution and logit link function were used for binary outcomes (eg, whether at least 6 SABA canisters were used and whether INP/ED visits occurred). Covariates were chosen a priori for all models on the basis of clinical relevance and baseline differences, which included age, sex, region, plan type (ie, health maintenance organization, preferred provider organization), season and year of index date, treatment naivety, baseline comorbid conditions (ie, allergic rhinitis, sinusitis), proxy of asthma severity, baseline medication used (ie, SABA, OCS), and other demographic information collected from the survey cohort (ie, race/ethnicity, education, income level, BMI, smoking status).
Because of a substantial difference in the level of adherence across index controller medication groups in the retrospective cohort analysis, we also conducted a subgroup analysis of data from adherent patients with an MPR of at least 0.8 during the 12-month period after the index date. All outcomes were analyzed by using multivariate analyses parallel to those used in the primary analysis above.
RESULTS
Retrospective Cohort
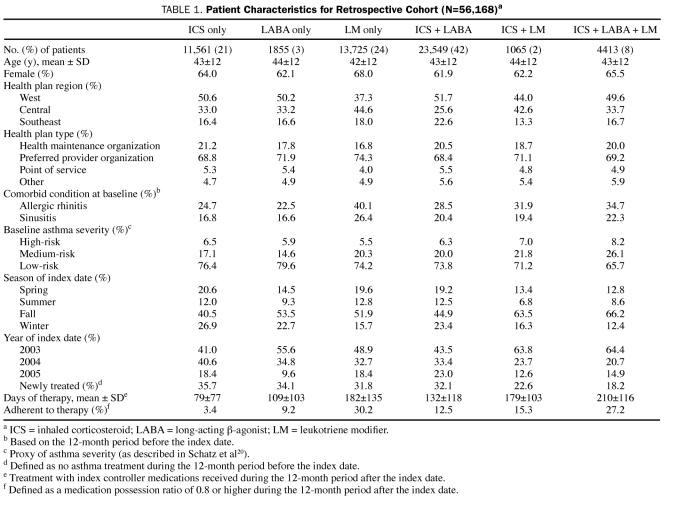
A total of 56,168 patients were eligible for the study. Of these, 11,561 (21%) used an ICS, 1855 (3%) used a LABA, and 13,725 (24%) used an LM as monotherapy, whereas 23,549 (42%) used ICS + LABA as combination therapy; 1065 (2%), ICS + LM; and 4413 (8%), ICS + LABA + LM (Table 1). Overall, the mean age of the patients was 43 years; approximately two-thirds of the cohort was female. At baseline, allergic rhinitis and sinusitis were most prevalent in the LM monotherapy group (rhinitis, 40.1%; sinusitis, 26.4%; Table 1). Most patients (65.7%-79.6%) in all controller medication groups had a low risk of asthma severity. During the 12-month period after initiation of the index controller medication, patients receiving LM monotherapy (>95% were taking montelukast) were more adherent to their index controller medication regimen than patients taking other monotherapies. On average, the LM monotherapy group received 182 days of therapy, whereas the ICS monotherapy group received 79 days of therapy. Only 9014 patients (16%) were determined to be adherent, defined as having an MPR of at least 0.8 for each controller medication used. The proportion of adherent patients was highest in the LM monotherapy group.
TABLE 1.
Patient Characteristics for Retrospective Cohort (N=56,168)a
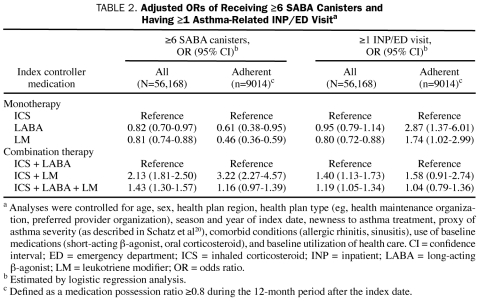
When preindex characteristics were controlled for by using a multivariable logistic regression model, the LABA and LM monotherapy groups were less likely than the ICS monotherapy group to receive at least 6 SABA canisters (LABA group: odds ratio [OR], 0.82; P=.02; LM group: OR, 0.81; P<.001) during the 12-month period after the index date (Table 2). For patients taking combination therapy, the odds of receiving at least 6 SABA canisters were 113% higher for the ICS + LM group and 43% higher for the ICS + LABA + LM than for the ICS + LABA group. The observed trend was consistent in the subanalysis of adherent patients, except that the odds of receiving at least 6 SABA canisters were similar for the ICS + LABA + LM group and the ICS + LABA group.
TABLE 2.
Adjusted ORs of Receiving ≥6 SABA Canisters and Having ≥1 Asthma-Related INP/ED Visita
In another multivariable logistic regression analysis, the odds of having at least 1 INP/ED visit during the 12-month period after the index date were 20% lower for the LM monotherapy group than for the ICS monotherapy group (Table 2). However, this lower risk did not hold true in the subanalysis of adherent patients: for these patients, the odds of such a visit were 74% higher for the LM monotherapy group than for the ICS monotherapy group. For patients receiving combination therapy, the likelihood of having at least 1 INP/ED visit was higher for the ICS + LM group (OR, 1.40; P=.002) and for the ICS + LABA + LM group (OR, 1.19, P=.005) than for the ICS + LABA group, but no significant difference was observed among adherent patients.
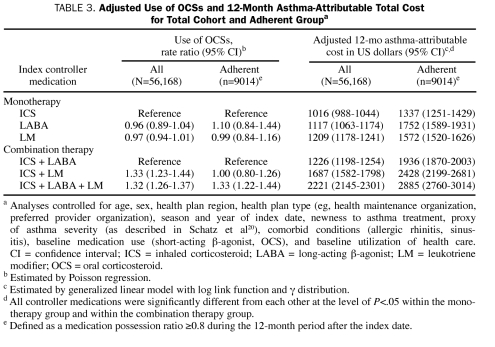
For the multivariate adjusted use of OCSs during the 12-month period after the index date, no significant difference was found among the monotherapy groups (Table 3). In contrast, differences were noted for the combination therapy groups: the use of OCSs was on average 33% higher for the ICS + LM group and 32% higher for the ICS + LABA + LM group than for the ICS + LABA group. The subanalysis of adherent patients showed a similar trend, except that no significant difference was observed between the ICS + LABA group and the ICS + LM groups.
TABLE 3.
Adjusted Use of OCSs and 12-Month Asthma-Attributable Total Cost for Total Cohort and Adherent Groupa
After the analysis was adjusted for preindex characteristics, the total asthma-attributable cost of care during the 12 months after the index period was 10% higher (P<.001) for the LABA group and 19% higher (P<.001) for the LM monotherapy group than for the ICS monotherapy group (Table 3). The adjusted mean total asthma-attributable costs per patient-year were $1016 for the ICS group, $1117 for the LABA group, and $1209 for the LM monotherapy group. For the combination therapy groups, the costs for the ICS + LM group were 38% higher (P<.001) and the costs for the ICS + LABA + LM group were 81% higher (P<.001) than those for the ICS + LABA group. The subanalysis of adherent patients showed that total asthma-attributable costs were lowest for the ICS group among the monotherapy groups and for the ICS + LABA group among the combination therapy groups.
Survey Cohort
Of the 5000 questionnaires mailed, 823 eligible responses were received (response rate, 16.5%). For each controller medication cohort, the survey patients (respondents) and the nonsurvey patients were comparable in terms of age and sex, with 2 exceptions: within the ICS + LABA combination group, survey respondents were on average 3 years older than nonsurvey patients (P<.001), and the LM monotherapy survey group included 7% more female patients than the nonsurvey group (P=.03).
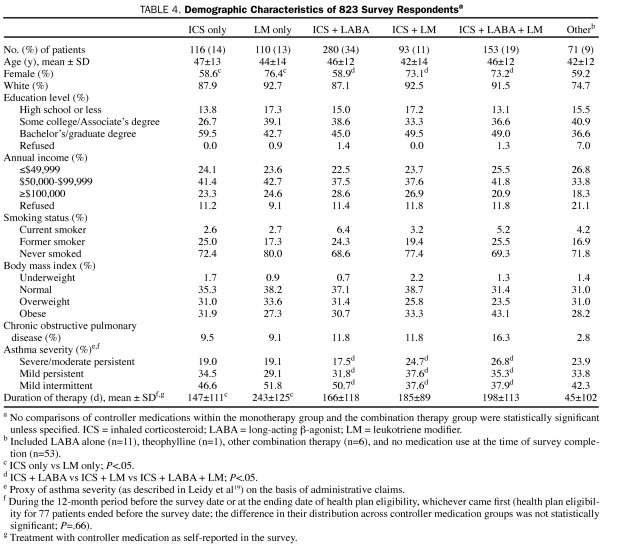
Of the 823 respondents, 116 (14%) reported using an ICS only; 110 (13%), an LM only; 280 (34%), ICS + LABA; 93 (11%), ICS + LM; 153 (19%), ICS + LABA + LM; and 71 (9%), other therapies, including a LABA (n=11), theophylline (n=1), other combination therapy (n=6), and no medication at all (n=53) at the time of survey. Most patients were female, white, educated (Bachelor's or graduate degree) nonsmokers, with a medium level of annual income ($50,000-$99,999) (Table 4). Overall, the groups using the various classes of controller medications were comparable in age, race/ethnicity, education level, income level, smoking status, BMI, and COPD status. The proportion of female patients was lower in the ICS monotherapy group (58.6%) than in the LM monotherapy group (76.4%; P=.005); among the combination therapy groups, the proportion of female patients was lowest in the ICS + LABA group (58.9%; P=.003). Most patients had “mild” asthma as determined by the Leidy severity scale.19 According to this scale, patients taking ICS + LABA + LM had more severe asthma than patients taking ICS + LABA (P=.02). During the period before the survey, patients who initiated LM monotherapy received on average 244 days of therapy with controller medications, whereas those who initiated ICS monotherapy received 147 days of therapy (P<.001). No significant difference was observed in length of therapy among the combination therapies.
TABLE 4.
Demographic Characteristics of 823 Survey Respondentsa
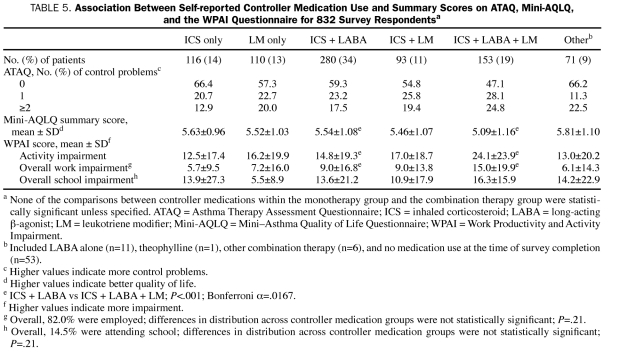
Most survey respondents reported no asthma control problem on the ATAQ (Table 5). They reported an average score of 5.48 on the Mini-AQLQ, 16.5 on WPAI-activity impairment, 9.2 on WPAI-work impairment, and 12.3 on WPAI-school impairment. No significant differences in these scores were observed across the monotherapy groups or across the combination therapy groups, except that, compared with the patients taking ICS + LABA + LM, the patients taking ICS + LABA reported higher scores on the Mini-AQLQ (5.54 vs 5.09; P<.001), lower scores on WPAI-activity impairment (14.8 vs 24.1; P<.001), and lower scores on WPAI -work impairment (9.0 vs 15.0; P<.001).
TABLE 5.
Association Between Self-reported Controller Medication Use and Summary Scores on ATAQ, Mini-AQLQ, and the WPAI Questionnaire for 832 Survey Respondentsa
When the analysis was controlled for age, sex, race/ethnicity, education level, income level, BMI, smoking status, COPD status, proxy of asthma severity (Leidy severity scale19), and adherence level, the outcomes of the ICS monotherapy group were comparable with those of the LM monotherapy group on ATAQ (13% fewer asthma control problems; P=.50), the Mini-AQLQ (0.01 better; P=.95), WPAI-activity impairment (18% less impairment; P=.27), and WPAI -work impairment (1% less impairment; P=.62). Patients receiving combination therapy with ICS + LABA reported better outcomes than those receiving ICS + LABA + LM therapy on the Mini-AQLQ (0.27 better; P=.01), WPAI-activity impairment (28% less impairment; P=.009), and WPAI -work impairment (4.6% less impairment; P=.01). Similarly, the ICS + LM group reported better outcomes than the ICS + LABA + LM group on the Mini-AQLQ (0.29 better; P=.03), WPAI-activity impairment (30% less impairment; P=.29), and WPAI -work impairment (6% less impairment; P=.01). No significant difference in outcomes was observed between the ICS + LABA group and the ICS + LM group. Multivariate adjustment was not performed for WPAI-school impairment because of the small sample size (only 14.5% of respondents were attending school).
DISCUSSION
To our knowledge, this is the first comparative effectiveness study to comprehensively evaluate clinical, economic, and patient-reported outcomes across various therapeutic classes of asthma controller medications. Among the monotherapy groups, the LM group appeared to have better clinical outcomes than the ICS group, as indicated by less SABA use and lower risk of INP/ED visits. This finding was in line with those of some previous studies12,26; however, it conflicted with the 2002 and 2007 NAEPP guidelines, which recommended ICS as the preferred monotherapy.4,27 This conflict could be due to the observation that the patients in this study were less adherent to an inhaled controller medication (ICS, LABA) regimen than to an oral controller medication (LM) regimen. This observation concurred with the findings of other studies, which indicated that adherence was poor for inhaled medications, both in general and in comparison with oral medications.28-30
As is true for other therapeutic areas (ie, hyperlipidemia, hypertension), medication adherence has been crucial to obtaining the beneficial effects of asthma treatment, and poor adherence to medication has been associated with detrimental health outcomes for patients with asthma.31,32 In this study, for patients who were adherent (MPR ≥0.8) to their controller medication regimen, the risk of INP/ED visits was lower for patients receiving ICS monotherapy than for those receiving LM. These results were consistent with the findings of clinical trials,33 in which patients were closely monitored and were more likely to adhere to medication regimens. Inhaled corticosteroid monotherapy was associated with higher use of SABAs, a marker of asthma symptom control, for both total patients and adherent patients. Although this trend concurred with the findings of some other studies,12,34 the real reasons for this trend remain unknown and require further study. The importance of this finding is reduced by the fact that this study found no differences in patient-reported measures of asthma control between the ICS monotherapy group and the LM monotherapy group.
The appropriate use of an inhaled medication remains one of the challenges in the management of asthma. Because ICS monotherapy has been proved to be efficacious in randomized clinical trials and was associated in the current study with optimal clinical (except for higher SABA use) and economic outcomes for adherent patients, care management interventions should focus on achieving better adherence to ICS regimens so that the full benefit of this controller medication can be obtained. Only 3% of patients in the ICS monotherapy group were considered adherent, a finding that underlines the urgent need for a better understanding of the barriers to patient acceptance of the most proven and effective therapy. Providing patients with better education about correct inhaler technique and reviewing correct technique at planned visits could be helpful in maximizing the benefits of inhaled medications. When ICS adherence cannot be achieved, our findings indicate that an LM may be a reasonable alternative, although at a higher cost. This suggestion is supported by the finding that the effects of ICS monotherapy and LM monotherapy on patient-reported asthma control problems, quality of life, and work productivity and activity impairment were comparable. For patients who have experienced difficulty in manipulating an inhaler, an oral tablet may be an easier option. In fact, this finding does not contradict the recommendations of the 2002 or 2007 NAEPP guidelines, which were meant to assist, not replace, the clinical decision making that is necessary for meeting individual patient needs and which consider LMs to be an alternative therapy when ICS monotherapy fails.4,27
For combination therapy, patients receiving ICS + LABA consistently showed the lowest use of SABAs, the lowest or a comparable use of OCSs, the lowest or a comparable risk of INP/ED visits, and the lowest asthma-attributable total cost of care. When ICS + LABA and ICS + LM were compared, the findings were consistent with those of other observational studies14,15 and those of a Cochrane systematic review published in 2006, which showed that adding a LABA was superior to adding an LM in preventing exacerbations that required systemic OCSs, both improving lung function and asthma symptoms and reducing the use of rescue SABAs by adults.35 In terms of patient-reported asthma control problems, quality of life, work productivity, and activity impairment, both ICS + LABA and ICS + LM were comparable in all aspects. When all clinical, economic, and patient-reported outcomes are considered, our results suggest that ICS + LABA should be the first choice when patients are switched from monotherapy to combination therapy. This finding is consistent with the recommendations of the 2002 and 2007 NAEPP guidelines, which state that ICS + LABA is the preferred combination therapy in a stepwise treatment approach.4,27
Although ICS + LABA + LM was not one of the preferred therapies listed in the 2002 and 2007 NAEPP guidelines, this combination therapy was commonly used by our study patients. As expected, the patients using this combination exhibited the highest level of disease severity, as measured by the 3 proxies of asthma severity. Combination therapy with ICS + LABA + LM was associated with inferior clinical outcomes, the highest asthma-related total cost of care, a poorer quality of life, and more severe impairment in work productivity and activity. Although we controlled the multivariate analyses for asthma severity by using proxies, residual confounding by severity may have contributed to these findings.
To assess the effect of the study methodology on the results, we performed several sensitivity analyses using different methods. These methods included analyzing the number of SABA canisters used as a continuous variable rather than as a binary variable; using a more restrictive definition of OCSs (pharmacy claims for OCS 1 day before to 3 days after an office visit that had an ICD-9-CM code for asthma) because OCSs can be used for many other conditions; using 3 different proxies of asthma severity (as described in Schatz et al,20 Leidy et al,19 and Cai et al18) in multivariate analyses; limiting the study sample to newly treated adults; analyzing ATAQ results as a continuous and as a categorical variable by using different cutoff points; and excluding patients with self-reported COPD from the analysis of the survey data. The results of all of these sensitivity analyses were consistent with the trends observed in the primary results and did not alter the overall conclusions of the study.
This study had several limitations. First, patients were not randomly assigned to treatment (controller medications) because of the nature of this observational study. Second, misclassification or measurement error could occur in administrative claims; however, it is unlikely that such errors would be systematically different across cohorts. Third, estimates of asthma severity were based on 3 claims-based proxies reported in the medical literature; these proxies may not accurately reflect disease severity as defined by the NAEPP guidelines. Fourth, although survey respondents were similar to the entire retrospective cohort in age and sex, they may not be representative of the entire retrospective cohort because of the low response rate. Although we cannot assume that our findings can be generalized to other populations, the geographical diversity and large size of the study sample increase the chances that the current results can be generalized to other insured adult populations.
CONCLUSION
To our knowledge, this study is the first to comprehensively evaluate the comparative effectiveness of various therapeutic classes of asthma controller medications on clinical, economic, and patient-reported outcomes in a real-world setting. This study factors in the perspective of the patient as well as the body of evidence of clinical and economic outcomes in its assessment of the results of asthma management. Our findings suggest that care management interventions should focus on improving adherence to ICS therapy so that optimal clinical and economic outcomes can be achieved. When adherence to ICS therapy cannot be achieved, an LM may be a reasonable choice for monotherapy. The combination therapy of ICS + LABA was associated with better or at least equivalent outcomes in all clinical, economic, and patient-reported perspectives. Therefore, ICS + LABA should be considered the first choice when patients are switched from monotherapy to combination therapy, as recommended by the 2002 and 2007 NAEPP guidelines.
Acknowledgments
The authors thank John Barron, PharmD, HealthCore, Wilmington, DE, for his review of the first draft of the manuscript, and Elizabeth Minford, HealthCore, Wilmington, DE, for her help in formatting and submission. Both are full-time employees of HealthCore and received no specific compensation for their work on this article.
Footnotes
This study was funded by WellPoint (Indianapolis, IN).
Mr Tan, Mr Sarawate, and Dr Singer are full-time employees of HealthCore, which is a subsidiary of WellPoint that provides research consultancy service to many major pharmaceutical, biotechnology, and device manufacturers. Mr Sawarate owns investment interests in Wyeth and Schering-Plough. The following authors have received honoraria for serving as a speaker: Dr Elward (Merck); Dr Smart (AstraZeneca, Pfizer, Aventis); Dr Busk (GlaxoSmithKline, AstraZeneca); and Dr O'Brien (GlaxoSmithKline). The following authors have received funding/grant support for research projects: Dr Cohen (American Lung Association-Asthma Clinical Research Center); Dr Smart (Amgen); Dr Busk (American Lung Association, Asthma Clinical Research Centers, National Institutes of Health); and Dr Schatz (GlaxoSmithKline, Merck, Genentech). Dr Schatz has served as a consultant/advisor to GlaxoSmithKline. Dr Lustig has family members employed by the National Kidney Foundation and Abbott Laboratories and is a board member of the Wisconsin Asthma Coalition. Dr Cohen is on the Airway Diseases Steering Committee of the American College of Chest Physicians.
REFERENCES
- 1.Akinbami L. Asthma prevalence, health care use and mortality: United States, 2003-05. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Web site http://www.cdc.gov/nchs/products/pubs/pubd/hestats/ashtma03-05/asthma03-05.htm 11-1-2006. Accessed April 24, 2009
- 2.Asthma and Allergy Foundation of America http://www.aafa.org/display.cfm?id=6&sub=63. Cost of asthma. 2000 Accessed August 1, 2008.
- 3.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980-1999. MMWR Surveill Summ. 2002;51(1):1-13 [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services, National Heart, Lung and Blood Institute, National Asthma Education and Prevention Program http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. 8-28-2007. Accessed April 24, 2009.
- 5.Buhl R, Kardos P, Richter K, et al. The effect of adjustable dosing with budesonide/formoterol on health-related quality of life and asthma control compared with fixed dosing. Curr Med Res Opin. 2004;20(8):1209-1220 [DOI] [PubMed] [Google Scholar]
- 6.Price DB, Williams AE, Yoxall S. Salmeterol/fluticasone stable-dose treatment compared with formoterol/budesonide adjustable maintenance dosing: impact on health-related quality of life. Respir Res. 2007;8:46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe BH, Bota GW, Fabris L, Therrien SA, Milner RA, Jacono J. Inhaled budesonide in addition to oral corticosteroids to prevent asthma relapse following discharge from the emergency department: a randomized controlled trial. JAMA 1999;281(22):2119-2126 [DOI] [PubMed] [Google Scholar]
- 8.Orsini L, Limpa-Amara S, Crown WH, Stanford RH, Kamal K. Asthma hospitalization risk and costs for patients treated with fluticasone propionate vs montelukast. Ann Allergy Asthma Immunol. 2004;92(5):523-529 [DOI] [PubMed] [Google Scholar]
- 9.Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22(2):166-174 [DOI] [PubMed] [Google Scholar]
- 10.Zeiger RS, Hay JW, Contreras R, et al. Asthma costs and utilization in a managed care organization. J Allergy Clin Immunol. 2008April12;121(4):885-892e5. Epub 2008 Mar 4 [DOI] [PubMed] [Google Scholar]
- 11.Allen-Ramey FC, Duong PT, Riedel AA, Markson LE, Weiss KB. Observational study of the effects of using montelukast vs fluticasone in patients matched at baseline. Ann Allergy Asthma Immunol. 2004;93(4):373-380 [DOI] [PubMed] [Google Scholar]
- 12.Allen-Ramey FC, Anstatt DT, Sajjan SG, Markson LE. Asthma-related health care resource use among patients starting fluticasone or montelukast therapy. Pharmacotherapy 2005;25(12):1752-1760 [DOI] [PubMed] [Google Scholar]
- 13.O'Connor RD, O'Donnell JC, Pinto LA, Wiener DJ, Legorreta AP. Two-year retrospective economic evaluation of three dual-controller therapies used in the treatment of asthma [published correction appears in Chest. 2002;122(1):387] Chest 2002;121(4):1028-1035 [DOI] [PubMed] [Google Scholar]
- 14.Delea TE, Hagiwara M, Stanford RH, Stempel DA. Effects of fluticasone propionate/salmeterol combination on asthma-related health care resource utilization and costs and adherence in children and adults with asthma. Clin Ther. 2008;30(3):560-571 [DOI] [PubMed] [Google Scholar]
- 15.Stempel DA, O'Donnell JC, Meyer JW. Inhaled corticosteroids plus salmeterol or montelukast: effects on resource utilization and costs. J Allergy Clin Immunol. 2002;109(3):433-439 [DOI] [PubMed] [Google Scholar]
- 16.Allen-Ramey FC, Bukstein D, Luskin A, Sajjan SG, Markson LE. Administrative claims analysis of asthma-related health care utilization for patients who received inhaled corticosteroids with either montelukast or salmeterol as combination therapy. J Manag Care Pharm. 2006;12(4):310-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor RD, Gilmore AS, Manjunath R, Stanford RH, Legorreta AP, Jhingran PM. Comparing outcomes in patients with persistent asthma: a registry of two therapeutic alternatives. Curr Med Res Opin. 2006;22(3):453-461 [DOI] [PubMed] [Google Scholar]
- 18.Cai B, Blais L, Suissa S, et al. Distribution of asthma severity and change in severity over time in a general population [abstract] Presented at: American Lung Association/American Thoracic Society International Conference; San Diego, CA; April23-28, 1999 [Google Scholar]
- 19.Leidy NK, Paramore LC, Watrous M, Doyle J, Zeiger RS. Development of an algorithm for estimating asthma severity from an administrative cost database [abstract PEN3]. Value Health 1999;2(5):394 [Google Scholar]
- 20.Schatz M, Nakahiro R, Jones CH, Roth RM, Joshua A, Petitti D. Asthma population management: development and validation of a practical 3-level risk stratification scheme. Am J Manag Care 2004;10(1):25-32 [PubMed] [Google Scholar]
- 21.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32-38 [DOI] [PubMed] [Google Scholar]
- 22.Vollmer WM, Markson LE, O'Connor E, et al. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160(5, pt 1):1647-1652 [DOI] [PubMed] [Google Scholar]
- 23.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4(5):353-365 [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Blanc PD, Chawla A, Hayden ML, Bleecker ER, Lee JH. Assessing productivity impairment in patients with severe or difficult-to-treat asthma: validation of the Work Productivity Activity Impairment—Asthma questionnaire [abstract 705]. J Allergy Clin Immunol. 2006;117(2)(suppl 1):S181 [Google Scholar]
- 25.Chen H, Blanc PD, Hayden M, Bleecker ER, Chawla A, Lee JH, TENOR Study Group Assessing productivity loss and activity impairment in severe or difficult-to-treat asthma. Value Health 2008;11(2):231-239 [DOI] [PubMed] [Google Scholar]
- 26.Allen-Ramey FC, Duong PT, Goodman DC, et al. Treatment effectiveness of inhaled corticosteroids and leukotriene modifiers for patients with asthma: an analysis from managed care data. Allergy Asthma Proc. 2003;24(1):43-51 [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute National Asthma Education and Prevention Program http://www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/asthmafullrpt_archive.pdf. Expert panel report: guidelines for the diagnosis and management of asthma: update on selected topics 2002. 2002 Accessed April 24, 2009. [PubMed]
- 28.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest 2000;117(2):542-550 [DOI] [PubMed] [Google Scholar]
- 29.Dorais M, Blais L, Chabot I, LeLorier J. Treatment persistence with leukotriene receptor antagonists and inhaled corticosteroids. J Asthma 2005;42(5):385-393 [DOI] [PubMed] [Google Scholar]
- 30.Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J Asthma 2003;40(1):93-101 [DOI] [PubMed] [Google Scholar]
- 31.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(3):487-497 [DOI] [PubMed] [Google Scholar]
- 32.Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288-1293 [DOI] [PubMed] [Google Scholar]
- 33.Ng D, Salvio F, Hicks G. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2004;(2):CD002314 [DOI] [PubMed] [Google Scholar]
- 34.O'Connor RD, Parasuraman B, Roberts C, Leibman C. Inhaled corticosteroids vs leukotriene receptor antagonists: health care costs across varying asthma severities. Ann Allergy Asthma Immunol. 2006;97(2):236-243 [DOI] [PubMed] [Google Scholar]
- 35.Ducharme FM, Lasserson TJ, Cates CJ. Long-acting β2-agonists versus anti-leukotrienes as add-on therapy to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2006;(4):CD003137 [DOI] [PubMed] [Google Scholar]