Abstract
OBJECTIVE: To systematically study the association of monoclonal gammopathy of undetermined significance (MGUS) with all diseases in a population-based cohort of 17,398 patients, all of whom were uniformly tested for the presence or absence of MGUS.
PATIENTS AND METHODS: Serum samples were obtained from 77% (21,463) of the 28,038 enumerated residents in Olmsted County, Minnesota. Informed consent was obtained from patients to study 17,398 samples. Among 17,398 samples tested, 605 cases of MGUS and 16,793 negative controls were identified. The computerized Mayo Medical Index was used to obtain information on all diagnoses entered between January 1, 1975, and May 31, 2006, for a total of 422,663 person-years of observations. To identify and confirm previously reported associations, these diagnostic codes were analyzed using stratified Poisson regression, adjusting for age, sex, and total person-years of observation.
RESULTS: We confirmed a significant association in 14 (19%) of 75 previously reported disease associations with MGUS, including vertebral and hip fractures and osteoporosis. Systematic analysis of all 16,062 diagnostic disease codes found additional previously unreported associations, including mycobacterium infection and superficial thrombophlebitis.
CONCLUSION: These results have major implications both for confirmed associations and for 61 diseases in which the association with MGUS is likely coincidental.
The authors confirmed a significant association in 14 of 75 previously reported disease associations with monoclonal gammopathy of undetermined significance, including vertebral and hip fractures and osteoporosis. Systematic analysis of all 16,062 diagnostic disease codes found additional previously unreported associations, including mycobacterium infection and superficial thrombophlebitis.
HDL = high-density lipoprotein; H-ICDA-2 = Hospital Adaptation of the International Classification of Diseases, Eighth Edition; MGUS = monoclonal gammopathy of undetermined significance
Monoclonal gammopathy of undetermined significance (MGUS) is the most common plasma cell disorder, occurring in 3% of the population older than 50 years.1 MGUS is an asymptomatic premalignant disorder, defined by a serum monoclonal immunoglobulin concentration of 3 g/dL or less and a proportion of plasma cells in the bone marrow of 10% or less in the absence of lytic bone lesions, anemia, hypercalcemia, and renal insufficiency related to the proliferation of monoclonal plasma cells.
MGUS is a known precursor of more serious diseases, such as multiple myeloma, primary amyloidosis, and WaldenstrÖm macroglobulinemia,2 but most patients with MGUS do not develop a plasma cell malignancy. However, numerous reports suggest an association of MGUS with a wide variety of other malignant and nonmalignant diseases.3-6 Some pathogenetically important associations probably exist; however, given the relatively high prevalence of MGUS in the general population (3%), most reported disease associations are likely coincidental. The prevalence of MGUS increases with age, from 1.7% in patients aged 50 to 59 years to more than 6.6% in patients aged 80 years and older.1 The screening test for MGUS, serum protein electrophoresis, is commonly performed in patients who present with a wide variety of clinical symptoms. Therefore, associations can occur coincidentally because the serum protein electrophoresis is performed more frequently in patients with certain clinical presentations (ascertainment bias).
Identification of true disease associations with MGUS is of major importance because it sheds light on the pathogenesis of both MGUS and the associated disorder. The only method to definitively address this is to screen all persons in a geographic population for the presence or absence of MGUS and then determine the diseases that are significantly associated with MGUS. To our knowledge, we report the first systematic study to determine the association of MGUS with all diseases in a large population-based cohort screened for the presence or absence of MGUS.
PATIENTS AND METHODS
Details on assembly of the study cohort and testing for MGUS have been previously reported.1 Beginning with a list of all residents of Olmsted County, Minnesota, who were aged 50 years or older as of January 1, 1995, we obtained unused serum after routine clinical tests in the Mayo Clinic Laboratory Central Processing Area, which receives all serum samples from Mayo Clinic outpatients in Rochester, MN, as well as from patients at Mayo-affiliated Saint Marys and Rochester Methodist hospitals. A letter, approved by the Mayo Clinic Institutional Review Board, was then sent to these patients asking for permission to study their serum sample. Serum samples were obtained from 77% (21,463) of the 28,038 enumerated residents in Olmsted County.1 Informed consent was obtained from patients to study 17,398 samples. The study was approved by the Mayo Clinic Institutional Review Board.
Laboratory Testing for MGUS
Electrophoresis was performed on agarose gel (Helena REP, Beaumont, TX) to test for monoclonal protein. The agarose strip was inspected by a technician and one of the authors (R.A.K.). Any serum with a discrete band or a suspect localized band was subjected to immunofixation (Sebia HYDRASYS/HYDRAGEL system, Norcross, GA).7 On the basis of serum protein electrophoresis and immunofixation, 605 MGUS cases and 16,793 negative controls were identified.
Disease Associations With MGUS and Statistical Analyses
The Mayo Clinic Medical Index was used to obtain information on all diagnoses entered between January 1, 1975, and May 31, 2006, for the identified cases and controls, for a total of 422,663 person-years of observations. Diagnoses were obtained from a Mayo extension of the Hospital Adaptation of the International Classification of Diseases, Eighth Edition (H-ICDA-2) that includes additional levels of detail in each classification rubric.8 If MGUS progressed to multiple myeloma, amyloidosis, or other plasma cell proliferative disorder, only the diagnoses up to and including the date of progression were considered.
First, we conducted a literature search and identified 75 previously reported potential disease associations.9-103 The H-ICDA-2 codes were grouped into a single diagnostic code to represent each of the previously reported associations. The patient population was stratified by age rounded to the nearest decade, sex, and MGUS diagnosis. The total occurrences of a diagnostic code and the number of person-years were determined for each stratum. Using the GLM and MASS packages provided in the base system of R,104 the number of occurrences was modeled with Poisson regression adjusting for age and sex, with the log of the total person-years as an offset. From the resulting model, incidence rates per 100,000 years were estimated assuming equal observation across all strata, regardless of the age or sex specificity of the diagnostic code. The risk ratios (MGUS cases vs controls) with 95% confidence intervals are reported.
To identify new associations previously unreported in the literature, we analyzed all H-ICDA-2 diagnostic codes at the 6th-digit level (N=16,062 codes) with the same analysis outlined previously and an additional Bonferroni correction of P values to adjust for multiple comparisons.
RESULTS
The MGUS cohort consisted of 309 men and 296 women, with a mean age at diagnosis of 70 years (range, 39-99 years). Mean follow-up (ie, the first diagnosis date to the last) was 24 years (range, 0-31 years), for a total of 14,373 person-years. The serum M component was 12% IgA, 70% IgG, 15% IgM, and 3% biclonal, and the median M protein level was 0.5 g/dL. The controls consisted of 7520 men and 9273 women, with mean follow-up of 25 years (range, 0-30 years), for a total of 408,290 person-years. Their mean age at the date of sample was 68 years (range, 52-105 years).
Previously Reported Disease Associations With MGUS
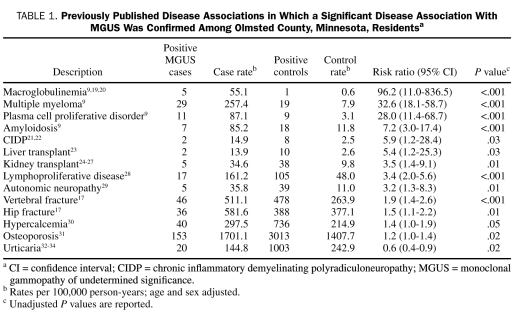
The 75 diagnoses for which a potential association with MGUS has been reported previously in the published literature are listed in Table 1 and Table 2. Of these 75 listed diagnoses, we were able to confirm a significant disease association in 14 (Table 1). As expected, 5 of the 14 disease associations were disorders known to evolve from MGUS, namely multiple myeloma, amyloidosis, lymphoproliferative disorders, macroglobulinemia, and other unclassified plasma cell proliferative disorders. More importantly, we confirmed that disorders of the bone, such as hip and vertebral fractures, osteoporosis, and hypercalcemia, are all significantly increased with MGUS, even in the absence of progression to multiple myeloma. We also confirmed known associations of MGUS with chronic inflammatory demyelinating neuropathy (relative risk, 5.9; 95% confidence interval, 1.2-28.4) and autonomic neuropathy.
TABLE 1.
Previously Published Disease Associations in Which a Significant Disease Association With MGUS Was Confirmed Among Olmsted County, Minnesota, Residentsa
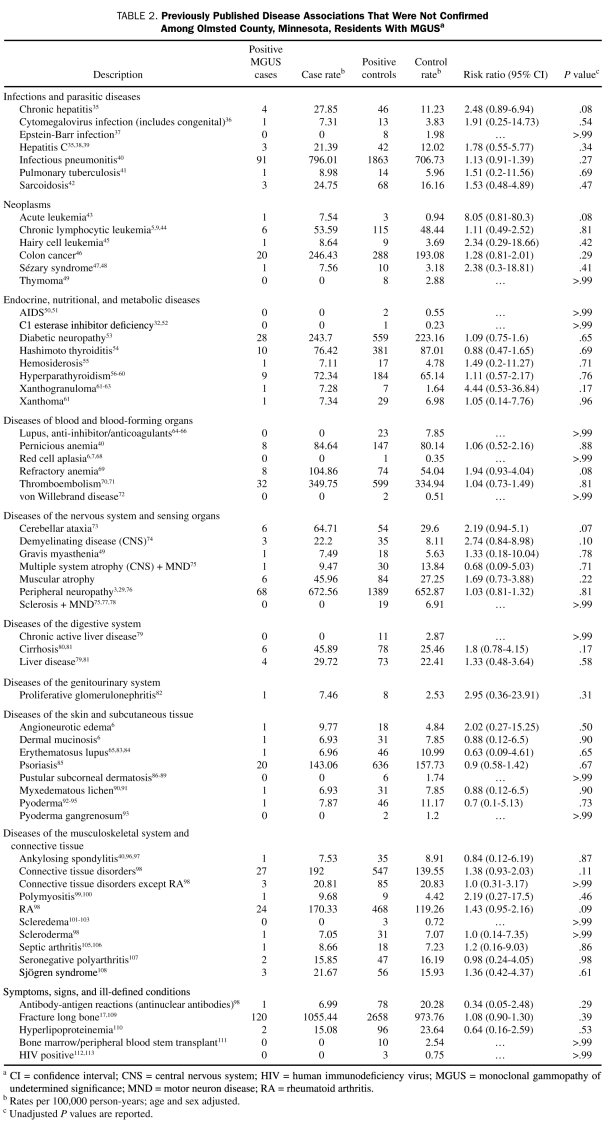
TABLE 2.
Previously Published Disease Associations That Were Not Confirmed Among Olmsted County, Minnesota, Residents With MGUSa
We found no significant association with MGUS in the 61 remaining disease diagnoses, an indication that most of these previously reported associations are either coincidental or clinically insignificant (Table 2).
New Disease Associations With MGUS
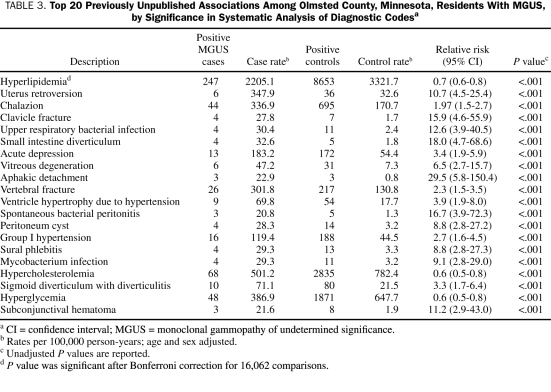
We conducted a systematic analysis of all 16,062 diagnostic codes in a screening strategy for new disease associations (see eAppendix online linked to this article). We found 5 significant associations after Bonferroni correction for 16,062 comparisons being done. Of these, 4 were for known associations that we have previously confirmed, including multiple myeloma, lymphoproliferative disease, other dysproteinemias, and plasma proliferative disorders. We also identified a new previously unreported association of MGUS with hyperlipidemia (relative risk, 0.7; 95% confidence interval, 0.6-0.8). Patients with MGUS and hyperlipidemia had a distribution of monoclonal serum proteins similar to that of the overall MGUS population, with 11% having IgA; 74%, IgG; 11%, IgM; and 4%, biclonal.
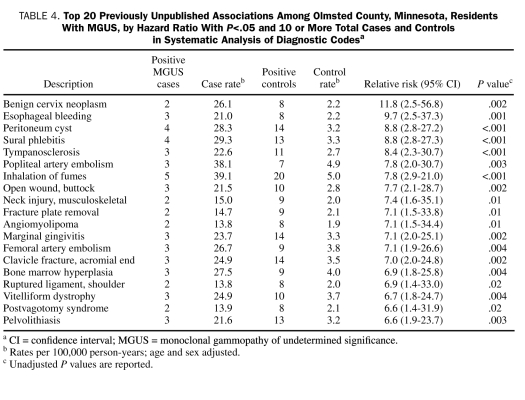
Table 3 lists 20 previously unreported associations that were significant based on the P value obtained from the systematic analysis of all diseases. Similarly, Table 4 lists 20 previously unreported associations that had the most positive associations based on the hazard ratio with a P<.05 and 10 or more total cases and controls from the systematic analysis of all diseases. Although many of the diseases listed in Table 3 and Table 4 were not statistically significant after the stringent Bonferroni correction method, they may be clinically important, previously unrecognized new associations that merit further study. Diseases such as mycobacterium infection and superficial venous thrombophlebitis are of particular interest.
TABLE 3.
Top 20 Previously Unpublished Associations Among Olmsted County, Minnesota, Residents With MGUS, by Significance in Systematic Analysis of Diagnostic Codesa
TABLE 4.
Top 20 Previously Unpublished Associations Among Olmsted County, Minnesota, Residents With MGUS, by Hazard Ratio With P<.05 and 10 or More Total Cases and Controls in Systematic Analysis of Diagnostic Codesa
DISCUSSION
Throughout the years, numerous diseases have been reported to be associated with MGUS.9 Because of the high prevalence of MGUS in the general population and the inherent bias of testing for MGUS only in patients with certain clinical symptoms, it is difficult to distinguish true pathogenetic relationships from coincidental associations. In fact, approximately 3% of patients with any given disease will be found to have MGUS based on coincidence. Therefore, the presence or absence of a true association can be determined only if the association of a disease with MGUS is significantly different from that expected in the general population. This requires screening of all persons in a geographic population for the presence or absence of MGUS and a determination of all diseases that occurred in each person over time. Moreover, because MGUS is associated with age and sex, associations need to be adjusted for these variables to eliminate bias.
The current study is based on prior screening of an entire geographically defined population for MGUS, which captured 77% of the enumerated population older than 50 years in Olmsted County.1 The study was performed with this well-defined cohort after excluding the blinded population of patients who did not provide informed consent to test passively collected serum samples. We systematically screened for association with all previously reported MGUS-disease associations in the literature, as well as all 16,062 disease diagnostic codes. Because testing for MGUS had been performed in all the study participants, ascertainment bias (which occurs in hospital- or clinical practice-based studies in which testing for presence or absence of MGUS is performed preferentially in persons with certain diseases) is not a concern.
As expected, our study shows that clonal plasma cell proliferative disorders such as myeloma, lymphoproliferative disorders, macroglobulinemia, and amyloidosis are significantly increased because they are direct progression events in patients with MGUS.9 We also found that a well-studied and suspected association between MGUS and neuropathy needs further examination. Kelly et al10 reported an MGUS prevalence of 6.7% among referred patients with idiopathic neuropathy. Many other studies have found underlying pathologic associations between neuropathies and MGUS.11-13 A direct interaction of the monoclonal protein (anti-myelin-associated glycoprotein; MAG antibody) with the peripheral nerve has been described.14 Similarly, in patients with amyloidosis, there is a direct effect of monoclonal protein-derived fibrils (amyloid fibrils) on the peripheral nerve.3 Studies have shown that treatment of the underlying plasma cell dyscrasia in patients with neuropathy results in patient improvement.15,16 Except in patients with chronic inflammatory demyelinating neuropathy and autonomic neuropathy, our results show that the vast majority of peripheral neuropathies occurred in similar frequencies in patients with and without MGUS. Because of the number of affected patients with neuropathies, this negative result is unlikely to be a result of inadequate sample size. Instead, it shows that the true proportion of cases of neuropathy that can be causally attributed to MGUS is likely to be very low.
We previously reported that the frequency of osteoporosis and bone fractures is increased in patients with MGUS, independent of progression to myeloma.17 In the current study, we confirmed that the occurrence of hip and vertebral fractures, osteoporosis, and hypercalcemia are all significantly increased with MGUS, even in the absence of progression to multiple myeloma. This has major relevance because the progression of these bony disorders may be amenable to bisphosphonates.
The reduced risk of hyperlipidemia with MGUS observed in the current study may be explained by the assays used to measure lipids. Before 1999, high-density lipoprotein (HDL) testing at Mayo Clinic was performed using a precipitation method. Apo B-containing lipoproteins were precipitated with dextran sulfate and calcium, and cholesterol concentration in the supernatant (containing only HDL lipoproteins) was measured. Since 1999, HDL cholesterol levels have been measured on the Hitachi 912 chemistry analyzer using direct HDL-cholesterol plus reagent (Roche Diagnostics, Indianapolis, IN). The reduced risk of hyperlipidemia in patients with MGUS might be explained by falsely low HDL and low-density lipoprotein concentrations reported by each assay.18
We found no significant association between MGUS and a number of other disorders that have previously been reported to be associated with MGUS (Table 2). The fact that we did not demonstrate a significant disease association with MGUS in such a large sample size is of major importance because it implies that these associations are likely not true associations, but rather coincidental ones. This has important therapeutic implications, because in some settings therapy has been administered to eradicate the monoclonal protein in the hopes that the associated disorder would be alleviated. Our study suggests that caution is needed. Some previously reported disease associations (eg, rheumatoid arthritis) that were not confirmed may still merit further study if there is continued biologic rationale for a true association to exist. Despite its large sample size, our study is limited by the fact that many diseases in which prior associations have been reported are relatively rare events, and hence it is not possible to truly exclude a statistically insignificant association with MGUS and 1 or more of the disorders listed in Table 2.
Many important new disease associations that merit further testing, such as sural thrombophlebitis and mycobacterium infection, are listed in Table 3 and Table 4. For instance, the risk of deep venous thrombosis is increased in patients with myeloma, and thrombosis is an important complication of therapy for myeloma. Studies have suggested that thrombosis is increased even in patients with MGUS.19,20 We found no association of MGUS with thromboembolism, but we did find a possible association with superficial thrombophlebitis. Similarly, there is strong rationale that chronic infection and immune stimulation may play an etiologic role in MGUS. Thus, the association of MGUS with mycobacterial infection is particularly interesting.
The current study has some specific limitations related to the use of H-ICDA-2 codes. We relied on H-ICDA-2 diagnostic codes for disease definitions, and given the sample size involved, it was not possible to verify the accuracy of the coding by manual chart review. Furthermore, there is substantial overlap in diseases classified by H-ICDA-2 codes, limiting our ability to verify or refute disease associations in many instances. A given disease may be classified by several different H-ICDA-2 codes, and the decision to merge closely related H-ICDA-2 codes is subjective. Therefore, any suspected association (or lack thereof) not discussed in this article needs further examination. This can be done by careful analysis of the eAppendix, which provides the case (MGUS) and control rate for each of the 16,062 H-ICDA-2 diagnostic codes or by performing new focused studies that involve more accurate ascertainment of disease by detailed chart review.
CONCLUSION
Our study confirms several known associations of MGUS with disorders such as vertebral and hip fractures and osteoporosis, as well as provides a list of important new associations. It refutes the reported association of MGUS with numerous other disorders as likely coincidental, a finding that may have important therapeutic implications. The positive associations will be of value in the pathogenesis of myeloma, and they provide biologic insights into mechanisms of disease. The eAppendix that has the incidence of each of the 16,062 disease codes in patients with MGUS and in controls, along with relative risks and confidence intervals, will be of immense value to investigators in various fields who study these diseases.
Supplementary Material
Footnotes
This study was supported in part by grants CA62242, CA107476, and AR30582 from the National Institutes of Health, US Public Health Service.
REFERENCES
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569 [DOI] [PubMed] [Google Scholar]
- 3.Kissel JT, Mendell JR. Neuropathies associated with monoclonal gammopathies. Neuromuscul Disord. 1996;6(1):3-18 [DOI] [PubMed] [Google Scholar]
- 4.Scutellari PN, Antinolfi G. Association between monoclonal gammopathy of undetermined significance (MGUS) and diffuse idiopathic skeletal hyperostosis (DISH) [in Italian]. Radiol Med. 2004;108(3):172-179 [PubMed] [Google Scholar]
- 5.Gregersen H, Mellemkjaer L, Salling Ibsen J, et al. Cancer risk in patients with monoclonal gammopathy of undetermined significance. Am J Hematol. 2000;63(1):1-6 [DOI] [PubMed] [Google Scholar]
- 6.Daoud MS, Lust JA, Kyle RA, Pittelkow MR. Monoclonal gammopathies and associated skin disorders. J Am Acad Dermatol. 1999;40(4):507-535 [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Katzmann JA, Lust JA, Dispenzieri A. Immunochemical characterization of immunoglobulins. In: Rose NR, Hamilton RG, Detrich B, eds. Manual of Clinical Laboratory Immunology, 6th ed.Washington, DC: ASM Press; 2002:71-91 [Google Scholar]
- 8.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54-63 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Semin Oncol. 2003;30(2):169-171 [DOI] [PubMed] [Google Scholar]
- 10.Kelly JJ, Adelman LS, Berkman E, Bhan I. Polyneuropathies associated with IgM monoclonal gammopathies. Arch Neurol. 1988;45(12):1355-1359 [DOI] [PubMed] [Google Scholar]
- 11.Chassande B, Léger JM, Younes-Chennoufi AB, et al. Peripheral neuropathy associated with IgM monoclonal gammopathy: correlations between M-protein antibody activity and clinical/electrophysiological features in 40 cases. Muscle Nerve. 1998;21(1):55-62 [DOI] [PubMed] [Google Scholar]
- 12.Quarles RH, Weiss MD. Autoantibodies associated with peripheral neuropathy. Muscle Nerve. 1999;22(7):800-822 [DOI] [PubMed] [Google Scholar]
- 13.Vrethem M, Cruz M, Wen-Xin H, Malm C, Holmgren H, Ernerudh J. Clinical, neurophysiological and immunological evidence of polyneuropathy in patients with monoclonal gammopathies. J Neurol Sci. 1993;114(2):193-199 [DOI] [PubMed] [Google Scholar]
- 14.Bollensen E, Steck AJ, Schachner M. Reactivity with the peripheral myelin glycoprotein P0 in serum from patients with monoclonal IgM gammopathy and polyneuropathy. Neurology 1988;38(8):1266-1270 [DOI] [PubMed] [Google Scholar]
- 15.Haas DC, Tatum AH. Plasmapheresis alleviates neuropathy accompanying IgM anti-myelin-associated glycoprotein paraproteinemia. Ann Neurol. 1988;23(4):394-396 [DOI] [PubMed] [Google Scholar]
- 16.Wilson HC, Lunn MP, Schey S, Hughes RA. Successful treatment of IgM paraproteinaemic neuropathy with fludarabine. J Neurol Neurosurg Psychiatry 1999;66(5):575-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melton LJ, III, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA. Fracture risk in monoclonal gammopathy of undetermined significance. J Bone Miner Res. 2004;19(1):25-30 [DOI] [PubMed] [Google Scholar]
- 18.Kadri N, Douville P, Lachance P. Monoclonal paraprotein may interfere with the Roche direct HDL-C Plus assay [letter]. Clin Chem. 2002;48(6, pt 1):964 [PubMed] [Google Scholar]
- 19.Kyle RA. Monoclonal gammopathy of undetermined significance: natural history in 241 cases. Am J Med. 1978;64(5):814-826 [DOI] [PubMed] [Google Scholar]
- 20.Heremans JF, Laurell AH, Martensson L, et al. Studies on “abnormal” serum globulins (M-components) in myeloma, macroglobulinemia and related diseases. Acta Med Scand Suppl. 1961;367:1-126 [PubMed] [Google Scholar]
- 21.Simmons Z, Albers JW, Bromberg MB, Feldman EL. Long-term follow-up of patients with chronic inflammatory demyelinating polyradiculoneuropathy, without and with monoclonal gammopathy. Brain 1995;118(pt 2):359-368 [DOI] [PubMed] [Google Scholar]
- 22.Simmons Z, Albers JW, Bromberg MB, Feldman EL. Presentation and initial clinical course in patients with chronic inflammatory demyelinating polyradiculoneuropathy: comparison of patients without and with monoclonal gammopathy. Neurology 1993;43(11):2202-2209 [DOI] [PubMed] [Google Scholar]
- 23.Pham H, Lemoine A, Salvucci M, et al. Occurrence of gammopathies and lymphoproliferative disorders in liver transplant recipients randomized to tacrolimus (FK506)- or cyclosporine-based immunosuppression. Liver Transpl Surg. 1998;4(2):146-151 [DOI] [PubMed] [Google Scholar]
- 24.Pollock CA, Mahony JF, Ibels LS, et al. Immunoglobulin abnormalities in renal transplant recipients. Transplantation 1989;47(6):952-956 [DOI] [PubMed] [Google Scholar]
- 25.Radl J, Valentijn RM, Haaijman JJ, Paul LC. Monoclonal gammapathies in patients undergoing immunosuppressive treatment after renal transplantation. Clin Immunol Immunopathol. 1985;37(1):98-102 [DOI] [PubMed] [Google Scholar]
- 26.Renoult E, Bertrand F, Kessler M. Monoclonal gammopathies in HBsAg-positive patients with renal transplants [letter]. N Engl J Med. 1988;318(18):1205 [DOI] [PubMed] [Google Scholar]
- 27.Stanko CK, Jeffery JR, Rush DN. Monoclonal and multiclonal gammopathies after renal transplantation. Transplant Proc. 1989;21(2):3330-3332 [PubMed] [Google Scholar]
- 28.Kim H, Heller P, Rappaport H. Monoclonal gammopathies associated with lymphoproliferative disorders: a morphologic study. Am J Clin Pathol. 1973;59(3):282-294 [DOI] [PubMed] [Google Scholar]
- 29.Nobile-Orazio E, Barbieri S, Baldini L, et al. Peripheral neuropathy in monoclonal gammopathy of undetermined significance: prevalence and immunopathogenetic studies. Acta Neurol Scand. 1992;85(6):383-390 [DOI] [PubMed] [Google Scholar]
- 30.John R, Oleesky D, Issa B, et al. Pseudohypercalcaemia in two patients with IgM paraproteinaemia. Ann Clin Biochem. 1997;34(pt 6):694-696 [DOI] [PubMed] [Google Scholar]
- 31.Maldonado JE, Riggs BL, Bayrd ED. Pseudomyeloma: is association of severe osteoporosis with serum monoclonal gammopathy an entity or a coincidence? Arch Intern Med. 1975;135(2):267-270 [DOI] [PubMed] [Google Scholar]
- 32.Silverman BA, Ku M, Kapur P, Schneider AT. Monoclonal gammopathy in association with allergic disorders of the skin and respiratory tract. Allergy Asthma Proc. 2006;27(2):130-139 [PubMed] [Google Scholar]
- 33.Puddu P, Cianchini G, Girardelli CR, Colonna L, Gatti S, de Pita O. Schnitzler's syndrome: report of a new case and a review of the literature. Clin Exp Rheumatol. 1997;15(1):91-95 [PubMed] [Google Scholar]
- 34.Karakelides M, Monson KL, Volcheck GW, Weiler CR. Monoclonal gammopathies and malignancies in patients with chronic urticaria. Int J Dermatol. 2006;45(9):1032-1038 [DOI] [PubMed] [Google Scholar]
- 35.Andreone P, Zignego AL, Cursaro C, et al. Prevalence of monoclonal gammopathies in patients with hepatitis C virus infection. Ann Intern Med. 1998;129(4):294-298 [DOI] [PubMed] [Google Scholar]
- 36.Ginevri F, Nocera A, Bonato L, et al. Cytomegalovirus infection is a trigger for monoclonal immunoglobulins in paediatric kidney transplant recipients. Transplant Proc. 1998;30(5):2079-2082 [DOI] [PubMed] [Google Scholar]
- 37.Badley AD, Portela DF, Patel R, et al. Development of monoclonal gammopathy precedes the development of Epstein-Barr virus-induced posttransplant lymphoproliferative disorder. Liver Transpl Surg. 1996;2(5):375-382 [DOI] [PubMed] [Google Scholar]
- 38.Mangia A, Clemente R, Musto P, et al. Hepatitis C virus infection and monoclonal gammopathies not associated with cryoglobulinemia. Leukemia 1996;10(7):1209-1213 [PubMed] [Google Scholar]
- 39.Mussini C, Ghini M, Mascia MT, et al. HCV and monoclonal gammopathies. Clin Exp Rheumatol. 1995;13(suppl 13):S45-S49 [PubMed] [Google Scholar]
- 40.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood 2008April1;111(7):3388-3394 Epub 2008 Jan 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu WC, Chen SC, Hsieh JT, Chen J, Chang HC. Penile tuberculosis associated with monoclonal gammopathy of undetermined significance. J Formos Med Assoc. 2006;105(9):753-755 [DOI] [PubMed] [Google Scholar]
- 42.Pettersson T, Koivunen E, Ilvonen M, Jouppila J, Aalto E, Wasastjerna C. Sarcoidosis and multiple myeloma: an association. Br Med J (Clin Res Ed) 1987;295(6604):958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solal-Celigny P, Desaint B, Herrera A, et al. Chronic myelomonocytic leukemia according to FAB classification: analysis of 35 cases. Blood 1984;63(3):634-638 [PubMed] [Google Scholar]
- 44.Noel P, Kyle RA. Monoclonal proteins in chronic lymphocytic leukemia. Am J Clin Pathol. 1987;87(3):385-388 [DOI] [PubMed] [Google Scholar]
- 45.Yetgin S, Olcay L, Yel L, et al. T-ALL with monoclonal gammopathy and hairy cell features. Am J Hematol. 2000;65(2):166-170 [DOI] [PubMed] [Google Scholar]
- 46.Chen HP, Carroll JA. Monoclonal gammopathy in carcinoma of the colon. Am J Clin Pathol. 1980;73(4):607-610 [DOI] [PubMed] [Google Scholar]
- 47.Kövary PM, Suter L, Macher E, et al. Monoclonal gammopathies in Sézary syndrome: a report of four new cases and a review of the literature. Cancer 1981;48(3):788-792 [DOI] [PubMed] [Google Scholar]
- 48.Venencie PY, Winkelmann RK, Puissant A, Kyle RA. Monoclonal gammopathy in Sezary syndrome: report of three cases and review of the literature. Arch Dermatol. 1984;120(5):605-608 [DOI] [PubMed] [Google Scholar]
- 49.Soppi E, Eskola J, Röyttä M, Veromaa T, Panelius M, Lehtonen A. Thymoma with immunodeficiency (Good's syndrome) associated with myasthenia gravis and benign IgG gammopathy. Arch Intern Med. 1985;145(9):1704-1707 [PubMed] [Google Scholar]
- 50.Heriot K, Hallquist AE, Tomar RH. Paraproteinemia in patients with acquired immunodeficiency syndrome (AIDS) or lymphadenopathy syndrome (LAS). Clin Chem. 1985;31(7):1224-1226 [PubMed] [Google Scholar]
- 51.Ray RA, Schotters SB. Triclonal gammopathy in a patient with AIDS [letter]. Clin Chem. 1986;32(10):2000 [PubMed] [Google Scholar]
- 52.Gelfand JA, Boss GR, Conley CL, Reinhart R, Frank MM. Acquired C1 esterase inhibitor deficiency and angioedema: a review. Medicine (Baltimore) 1979;58(4):321-328 [DOI] [PubMed] [Google Scholar]
- 53.Simmons Z, Bromberg MB, Feldman EL, Blaivas M. Polyneuropathy associated with IgA monoclonal gammopathy of undetermined significance. Muscle Nerve. 1993;16(1):77-83 [DOI] [PubMed] [Google Scholar]
- 54.Matsubayashi S, Tamai H, Nagai K, Kuma K, Nakagawa T. Monoclonal gammopathy in Hashimoto's thyroiditis and malignant lymphoma of the thyroid. J Clin Endocrinol Metab. 1986;63(5):1136-1139 [DOI] [PubMed] [Google Scholar]
- 55.Vargas V, Pedreira JD, Vilaseca J, Ramos F, Rodrigo MJ, Guardia J. Transitory IgM monoclonal gammopathy associated with brucellosis and tuberculosis (author's transl) [in Spanish]. Med Clin (Barc) 1981;77(6):247-249 [PubMed] [Google Scholar]
- 56.Arnulf B, Bengoufa D, Sarfati E, et al. Prevalence of monoclonal gammopathy in patients with primary hyperparathyroidism: a prospective study. Arch Intern Med. 2002;162(4):464-467 [DOI] [PubMed] [Google Scholar]
- 57.Dexter RN, Mullinax F, Estep HL, Williams RC., Jr Monoclonal IgG gammopathy and hyperparathyroidism. Ann Intern Med. 1972;77(5):759-764 [DOI] [PubMed] [Google Scholar]
- 58.Mundis RJ, Kyle RA. Primary hyperparathyroidism and monoclonal gammopathy of undetermined significance. Am J Clin Pathol. 1982;77(5):619-621 [DOI] [PubMed] [Google Scholar]
- 59.Rao DS, Antonelli R, Kane KR, Kuhn JE, Hetnal C. Primary hyperparathyroidism and monoclonal gammopathy. Henry Ford Hosp Med J. 1991;39(1):41-44 [PubMed] [Google Scholar]
- 60.Schnur MJ, Appel GB, Bilezikian JP. Primary hyperparathyroidism and benign monoclonal gammopathy. Arch Intern Med. 1977;137(9):1201-1203 [PubMed] [Google Scholar]
- 61.Finan MC, Winkelmann RK. Necrobiotic xanthogranuloma with paraproteinemia: a review of 22 cases. Medicine (Baltimore) 1986;65(6):376-388 [DOI] [PubMed] [Google Scholar]
- 62.Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma [published correction appears in Arch Dermatol. 1992;12(5):632] Arch Dermatol. 1992;128(1):94-100 [PubMed] [Google Scholar]
- 63.Nestle FO, Hofbauer G, Burg G. Necrobiotic xanthogranuloma with monoclonal gammopathy of the IgG lambda type. Dermatology 1999;198(4):434-435 [PubMed] [Google Scholar]
- 64.Bellotti V, Gamba G, Merlini G, et al. Study of three patients with monoclonal gammopathies and ‘lupus-like’ anticoagulants. Br J Haematol. 1989;73(2):221-227 [DOI] [PubMed] [Google Scholar]
- 65.Freestone S, Ramsay LE. Transient monoclonal gammopathy in hydralazine-induced lupus erythematosus. Br Med J (Clin Res Ed) 1982;285(6354):1536-1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams RC, Jr, Malone CC, Silvestris F, Nickerson KG. Benign monoclonal gammopathy with IgG anti-DNA, anti-Sm and anti-F(ab')2 activity. Clin Exp Rheumatol. 1997;15(1):33-38 [PubMed] [Google Scholar]
- 67.Balducci L, Hardy C, Dreiling B, Tavassoli M, Steinberg MH. Pure red blood cell aplasia associated with paraproteinemia: in vitro studies of erythropoiesis. Haematologia (Budap) 1984;17(3):353-357 [PubMed] [Google Scholar]
- 68.Resegotti L, Dolci C, Palestro G, Peschle C. Paraproteinemic variety of pure red cell aplasia: immunological studies in 1 patient. Acta Haematol. 1978;60(4):227-232 [DOI] [PubMed] [Google Scholar]
- 69.Economopoulos T, Economidou J, Giannopoulos G, et al. Immune ab normalities in myelodysplastic syndromes. J Clin Pathol. 1985;38(8):908-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer 2004;101(3):558-566 [DOI] [PubMed] [Google Scholar]
- 71.Auwerda JJ, Sonneveld P, de Maat MP, Leebeek FW. Prothrombotic coagulation abnormalities in patients with paraprotein-producing B-cell disorders. Clin Lymphoma Myeloma 2007;7(7):462-466 [DOI] [PubMed] [Google Scholar]
- 72.Lamboley V, Zabraniecki L, Sie P, Pourrat J, Fournié B. Myeloma and monoclonal gammopathy of uncertain significance associated with acquired von Willebrand's syndrome: seven new cases with a literature review. Joint Bone Spine 2002;69(1):62-67 [DOI] [PubMed] [Google Scholar]
- 73.McDonald PS, Cora-Bramble D, De Palma L. Monoclonal gammopathy of the immunoglobulin A class in a two-year-old girl with ataxia telangiectasia. Pediatr Dev Pathol. 1998;1(4):319-321 [DOI] [PubMed] [Google Scholar]
- 74.Maisonobe T, Chassande B, Verin M, Jouni M, Léger JM, Bouche P. Chronic dysimmune demyelinating polyneuropathy: a clinical and electrophysiological study of 93 patients. J Neurol Neurosurg Psychiatry 1996;61(1):36-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Younger DS, Rowland LP, Latov N, et al. Motor neuron disease and amyotrophic lateral sclerosis: relation of high CSF protein content to paraproteinemia and clinical syndromes. Neurology 1990;40(4):595-599 [DOI] [PubMed] [Google Scholar]
- 76.Baldini L, Nobile-Orazio E, Guffanti A, et al. Peripheral neuropathy in IgM monoclonal gammopathy and Waldenstrom's macroglobulinemia: a frequent complication in elderly males with low MAG-reactive serum monoclonal component. Am J Hematol. 1994;45(1):25-31 [DOI] [PubMed] [Google Scholar]
- 77.Rentzos M, Michalopoulou M, Gotosidis K, et al. Unusual association of multiple sclerosis with monoclonal gammopathy of undetermined significance (MGUS): two case reports. Funct Neurol. 2004;19(4):253-256 [PubMed] [Google Scholar]
- 78.Desai J, Swash M. IgM paraproteinemia in a patient with primary lateral sclerosis. Neuromuscul Disord. 1999;9(1):38-40 [DOI] [PubMed] [Google Scholar]
- 79.Slavin S, Zlotnick A, Levij IS, Eliakim M. Clinical implications of monoclonal gammopathy in chronic liver disease. Am J Dig Dis. 1974;19(3):223-234 [DOI] [PubMed] [Google Scholar]
- 80.Hendrick AM, Mitchison HC, Bird AG, James OF. Paraproteins in primary biliary cirrhosis. Q J Med. 1986;60(231):681-684 [PubMed] [Google Scholar]
- 81.Zawadzki Z, Edwards GA. Dysimmunoglobulinemia associated with hepatobiliary disorders. Am J Med. 1970;48(2):196-202 [DOI] [PubMed] [Google Scholar]
- 82.Meyrier A, Simon P, Mignon F, Striker L, Ramée MP. Rapidly progressive (‘crescentic’) glomerulonephritis and monoclonal gammapathies. Nephron 1984;38(3):156-162 [DOI] [PubMed] [Google Scholar]
- 83.Porcel JM, Ordi J, Tolosa C, Selva A, Castro-Salomo A, Vilardell M. Monoclonal gammopathy in systemic lupus erythematosus. Lupus 1992;1(4):263-264 [DOI] [PubMed] [Google Scholar]
- 84.Powell FC, Greipp PR, Su WP. Discoid lupus erythematosus and monoclonal gammopathy. Br J Dermatol. 1983;109(3):355-360 [DOI] [PubMed] [Google Scholar]
- 85.Humbert P, Blanc D, Laurent R, Agache P. Monoclonal IgG gammopathy in a case of pustular psoriasis: a ten year follow-up [letter]. Blut 1987;54(1):61-62 [DOI] [PubMed] [Google Scholar]
- 86.Teixeira M, Lves RA, Seloresi M. Subcorneal pustular dermatosis in association with a monoclonal IgA/k gammopathy: successful treatment with acitretin [letter]. Eur J Dermatol. 2006;16(5):588-590 [PubMed] [Google Scholar]
- 87.Kasha EE, Jr, Epinette WW. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) in association with a monoclonal IgA gammopathy: a report and review of the literature. J Am Acad Dermatol. 1988;19(5, pt 1):854-858 [DOI] [PubMed] [Google Scholar]
- 88.Lutz ME, Daoud MS, McEvoy MT, Gibson LE. Subcorneal pustular dermatosis: a clinical study of ten patients. Cutis 1998;61(4):203-208 [PubMed] [Google Scholar]
- 89.Ryatt KS, Dodman BA, Cotterill JA. Subcorneal pustular dermatosis and IgA gammopathy. Acta Derm Venereol. 1981;61(6):560-562 [PubMed] [Google Scholar]
- 90.Danby FW, Danby CW, Pruzanski W. Papular mucinosis with IgG(K) M component. Can Med Assoc J. 1976;114(10):920-922 [PMC free article] [PubMed] [Google Scholar]
- 91.James K, Fudenberg H, Epstein WL, Shuster J. Studies on a unique diagnostic serum globulin in papular mucinosis (lichen myxedematosus). Clin Exp Immunol. 1967;2(2):153-166 [PMC free article] [PubMed] [Google Scholar]
- 92.Holt PJ, Davies MG, Saunders KC, Nuki G. Pyoderma gangrenosum: clinical and laboratory findings in 15 patients with special reference to polyarthritis. Medicine (Baltimore) 1980;59(2):114-133 [PubMed] [Google Scholar]
- 93.Powell FC, Schroeter AL, Su WP, Perry HO. Pyoderma gangrenosum and monoclonal gammopathy. Arch Dermatol. 1983;119(6):468-472 [PubMed] [Google Scholar]
- 94.Setterfield JF, Shirlaw PJ, Challacombe SJ, Black MM. Pyoderma gangrenosum associated with severe oropharyngeal involvement and IgA paraproteinaemia. Br J Dermatol. 2001;144(2):393-396 [DOI] [PubMed] [Google Scholar]
- 95.van der Sluis I. Two cases of pyoderma (ecthyma) gangrenosum associated with the presence of an abnormal serum protein (beta-2-A-paraprotein). With a review of the literature. Dermatologica 1966;132(5):409-424 [PubMed] [Google Scholar]
- 96.Ghirlanda G, Perri F, Manna R, Uccioli L. Association of ankylosing spondylitis and monoclonal gammopathy: clinical case report and pathogenetic considerations. Z Rheumatol. 1984;43(1):42-45 [PubMed] [Google Scholar]
- 97.Renier G, Renier JC, Gardembas-Pain M, Chevailler A, Boasson M, Hurez D. Ankylosing spondylitis and monoclonal gammopathies. Ann Rheum Dis. 1992;51(8):951-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Broggini M, Cavallo A, Baratelli E, et al. Monoclonal gammopathy of uncertain significance in rheumatic disease [in Italian]. Recenti Prog Med. 1990;81(5):306-309 [PubMed] [Google Scholar]
- 99.Kiprov DD, Miller RG. Polymyositis associated with monoclonal gammopathy. Lancet 1984;2(8413):1183-1186 [DOI] [PubMed] [Google Scholar]
- 100.Telerman-Toppet N, Wittek M, Bacq M, Dajez P, Coërs C, Fassotte A. Benign monoclonal gammopathy and relapsing polymyositis. Muscle Nerve. 1982;5(6):490-491 [DOI] [PubMed] [Google Scholar]
- 101.McFadden N, Ree K, Soyland E, Larsen TE. Scleredema adultorum associated with a monoclonal gammopathy and generalized hyperpigmentation. Arch Dermatol. 1987;123(5):629-632 [PubMed] [Google Scholar]
- 102.Ohta A, Uitto J, Oikarinen AI, et al. Paraproteinemia in patients with scleredema: clinical findings and serum effects on skin fibroblasts in vitro. J Am Acad Dermatol. 1987;16(1, pt 1):96-107 [PubMed] [Google Scholar]
- 103.Venencie PY, Powell FC, Su WP. Scleredema and monoclonal gammopathy: report of two cases. Acta Derm Venereol. 1984;64(6):554-556 [PubMed] [Google Scholar]
- 104.R Development Core Team A Language and Environment for Statistical Computing 2.2.0 ed. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 105.Korman TM, Brodie GN, Grayson ML. Pneumococcal arthritis and monoclonal gammopathy of undetermined significance [letter]. Aust N Z J Med. 1997;27(3):342 [DOI] [PubMed] [Google Scholar]
- 106.Korman TM, Brodie GN, Grayson ML. Monoclonal gammopathy and septic arthritis [letter]. Aust N Z J Med. 1999;29(5):752 [DOI] [PubMed] [Google Scholar]
- 107.Hurst NP, Smith W, Henderson DR. IgG (kappa) paraproteinaemia and arthritis. Br J Rheumatol. 1987;26(2):142-146 [DOI] [PubMed] [Google Scholar]
- 108.Sugai S, Konda S, Shoraski Y, Murayama T, Nishikawa T. Non-IgM monoclonal gammopathy in patients with Sjogren's syndrome. Am J Med. 1980;68(6):861-866 [DOI] [PubMed] [Google Scholar]
- 109.Gregersen H, Jensen P, Gislum M, JØrgensen B, SØrensen HT, NØrgaard M. Fracture risk in patients with monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;135(1):62-67 Epub 2006 Aug 22 [DOI] [PubMed] [Google Scholar]
- 110.Spagnuolo V, Motti C, Pujia A, Gnasso A. Dyslipoproteinemia and monoclonal gammopathy: a case report [in Italian]. Ann Ital Med Int. 1996;11(3):204-207 [PubMed] [Google Scholar]
- 111.Mitus AJ, Stein R, Rappeport JM, et al. Monoclonal and oligoclonal gammopathy after bone marrow transplantation. Blood 1989;74(8):2764-2768 [PubMed] [Google Scholar]
- 112.Crapper RM, Deam DR, Mackay IR. Paraproteinemias in homosexual men with HIV infection: lack of association with abnormal clinical or immunologic findings. Am J Clin Pathol. 1987;88(3):348-351 [DOI] [PubMed] [Google Scholar]
- 113.Lefrère JJ, Debbia M, Lambin P. Prospective follow-up of monoclonal gammopathies in HIV-infected individuals. Br J Haematol. 1993;84(1):151-155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.