Abstract
We completed a phase 1/2 trial to evaluate the safety and potential efficacy of direct intravesical instillation of a botulinum type A toxin/dimethyl sulfoxide (DMSO) solution for treatment of idiopathic detrusor overactivity in women. Twenty-five women with medication-resistant, urodynamic-confirmed idiopathic detrusor overactivity were enrolled. A total of 9 patients were treated in phase 1 of the study. Three patients were given a 66% dosing of solution; 22 patients received the full 300 units of botulinum toxin and 50 mL of DMSO (50% concentration). Adverse effects, 24-hour pad weights, episodes of incontinence, postvoid residuals, and scores on the Blaivas-Groutz anti-incontinence scale, Indevus Urgency Severity Scale, Incontinence Impact Questionnaire-short form, and Urogenital Distress Inventory (6 items) questionnaire were recorded at baseline, 1 month, and 3 months after instillation. No serious adverse effects or clinically important increases in postvoid residual occurred. Among the 21 women who completed phase 2 of the study, the median number of incontinent episodes decreased from 4 at baseline to 2 at 1 month (P=.004) and increased to 4 at 3 months (P=.81). Median scores improved from baseline to 1 month on the Incontinence Impact Questionnaire (from 13 to 7; P=.007) and Urogenital Distress Inventory (from 10 to 5; P=.003). Although 11 women (52%) reported severe urgency based on the Indevus Urgency Severity Scale at baseline, only 1 (5%; P<.001) and 3 (14%; P=.004) women reported severe scores at 1 and 3 months, respectively. Direct instillation of botulinum toxin/DMSO solution is safe. Its promising clinical effect warrants further evaluation in a randomized, placebo-controlled phase 3 setting.
AE = adverse effect; DMSO = dimethyl sulfoxide; IDO = idiopathic detrusor overactivity; IUSS = Indevus Urgency Severity Scale; PVR = postvoid residual; UDI = Urogenital Distress Inventory
Establishment of effective methods for treating overactive bladder symptoms resistant to conventional therapy (eg, anticholinergic medications and neuromodulation) has proven challenging. Encouraging results of intravesical injections of botulinum toxin type A (BOTOX, Allergan, Irving, CA) for patients with neurogenic detrusor overactivity has logically led to interest in botulinum toxin for idiopathic detrusor overactivity (IDO). As a result, multiple investigators have demonstrated the safety and efficacy of intravesical injections of botulinum toxin for IDO since 2003.1,2 Main drawbacks to intravesical botulinum toxin A are the requirement of cystoscopic injection with individualized anesthetic administration and cost of the agent. We present results of a phase 1/2 prospective study that demonstrated safety and early evidence of clinical efficacy of an intravesical instillation of a botulinum toxin/dimethyl sulfoxide (DMSO) solution to encourage resource allocation for future phase 3 studies.
PATIENTS AND METHODS
Solution Instillation
All patients suitable for enrollment underwent intravesical instillation of the botulinum toxin solution by one of the authors (S.P.P.). The patients were placed in a dorsal lithotomy position, and the urethra was sterilized in the standard fashion. A 14-F red rubber catheter was placed in the bladder, and the bladder was drained. Then, 50 mL of the solution (300 units of botulinum toxin and 50 mL of 50% DMSO in aqueous solution: each milliliter of solution contained 0.54 g of DMSO) was placed into the bladder via the red rubber catheter. The catheter was slowly removed from the urethra in an antegrade manner. The assisting nurse compressed the urethra with her index finger for 30 minutes and then allowed the patient to void. Vital signs were checked at baseline, 15 minutes, and 30 minutes after instillation. The patients were reevaluated at 45 minutes and 2 hours after instillation before being discharged home.
Study Design
After Mayo Clinic Institutional Review Board approval, we completed a prospective phase 1/2 pilot study from November 2006 to March 2009 to investigate the safety and efficacy of a botulinum toxin/DMSO intravesical solution for treatment of medication-refractory IDO. Candidates for the study were women older than 18 years with overactive bladder syndrome, as defined by the International Continence Society. Women were eligibile for the study if at least 2 anticholinergic medications had failed to resolve symptoms of overactive bladder. The patients were instructed to discontinue use of anticholinergic medications before undergoing instillation.
Inclusion criteria were urodynamic-demonstrated IDO and the absence of stress incontinence on urodynamics and physical examination. Patients were excluded from the study if they had a neurologic diagnosis consistent with neurogenic bladder dysfunction, a history of pelvic radiation, interstitial cystitis, a serious comorbid illness, a bladder tumor, or chronic pelvic pain. Pregnant or breastfeeding women were ineligible.
Phase 1 of the study involved sequential recruitment of 3 sets of 3 patients. Each set of patients was evaluated for severe adverse effects (AEs) for 1 month before the next set of 3 patients was treated. The first 3 patients underwent instillation of the solution with a reduced dose, 66% of the target botulinum toxin dose (200 units). Patients in phase 1 were assessed for AEs immediately, 4 hours, 24 hours, and 7 days after instillation in addition to the standard study assessments at 2 hours, 1 month, and 3 months. After all 9 phase 1 patients had been followed up for 3 months without experiencing any severe AEs, the remaining 16 patients were recruited. The final 22 patients received the full dose of botulinum toxin/DMSO.
Safety and Efficacy
Safety was evaluated by recording AEs within 2 hours, 4 hours, 24 hours, and 7 days after instillation (phase 1), as well as at the 1-month and 3-month follow-up visits. Catheterized postvoid residuals (PVRs), urine cultures, and urinalysis were performed at baseline, 1 month, and 3 months.
To evaluate the clinical effect of our full botulinum toxin dose on IDO symptoms in our study patients, we collected measures of urinary incontinence, urinary urgency, and quality of life at baseline and at the 1-month and 3-month follow-up visits. As an objective evaluation of urinary incontinence, we measured pad weights and the number of incontinent episodes for the 24-hour period before the baseline, 1-month, and 3-month visits. In addition, we used responses on the Blaivas-Groutz anti-incontinence scale3 as a measure of urinary incontinence. The Blaivas-Groutz anti-incontinence score combines information on the number of incontinent episodes in a 24-hour period, 24-hour pad weights, and a qualitative rating by the patient into a single score ranging from 0 to 6. This score is then used to categorize incontinence as none (0), mild (1-2), moderate (3-4) or severe (5-6). To assess urinary urgency, we used responses on the Indevus Urgency Severity Scale (IUSS)4 at our 3 study points. Similar to the Blaivas-Groutz anti-incontinence score, responses categorize patient's urgency as none, mild, moderate, or severe. Finally, to assess any changes in urinary-associated quality of life, we collected information using the Incontinence Impact Questionnaire-short form5 and the 6-item Urogenital Distress Inventory (UDI-6).5 The items on both these forms were scored from 0 (not at all) to 3 (greatly), with total scores ranging from 0 to 21 and 0 to 18, respectively, on the 2 instruments. Along with the UDI-6 instrument was the question: “How badly does loss of urinary control bother you?” with response on a 0 to 10 scale, and patients were asked to estimate the average number of pads used per day.
Statistical Analyses
Continuous data are summarized as median and interquartile range; categorical data, as pro portions and percentages. To evaluate changes in study measures from baseline to 1 month and baseline to 3 months, we used Wilcoxon signed rank tests and sign tests, when appropriate. Given the exploratory nature of this study, we made no adjustments for multiple testing in these analyses, although resulting P values were interpreted cautiously in light of the number of tests performed. All statistical tests were performed using S-PLUS software (version 8.0 for Unix; Insightful Corporation; Seattle, WA).
RESULTS
Twenty-five women met inclusion criteria and were treated. The median age for all the women who completed the study was 74 years (minimum, 37 years; maximum, 86 years), and the median body mass index (calculated as weight in kilograms divided by height in meters squared) was 26.6 kg/m2 (minimum, 18.4 kg/m2; maximum, 40.0 kg/m2). One patient in the phase 2 portion of the study withdrew because of unrelated health reasons after treatment and is not included in the efficacy analysis.
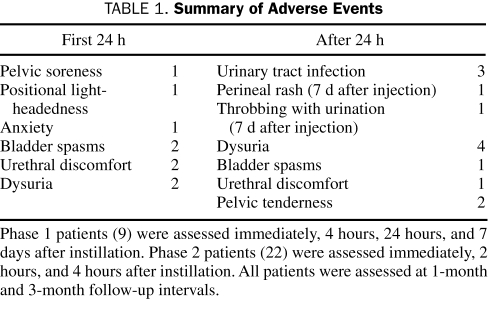
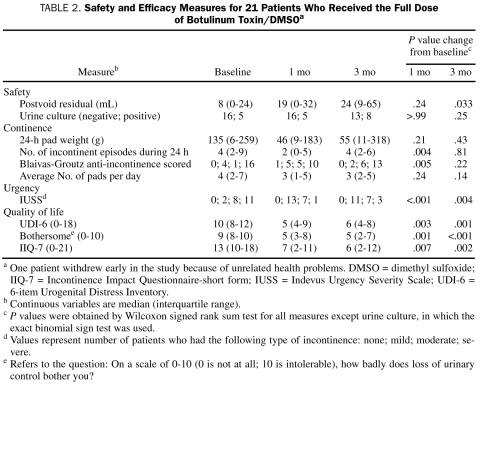
No systemic AEs were noted with the 200-unit or 300-unit dose (Table 1). No patient reported generalized muscle weakness. Of the 25 patients, 18 (72%) reported 1 or more AE during follow-up. Nine patients (36%) reported an AE within the first 24 hours after injection. The median PVR increased from baseline (8 mL) to 1 month (19 mL) and 3 months (24 mL) (Table 2), although not to a degree that was clinically important. No patient required catheterization after instillation. Results of urine culture after treatment were similar to baseline results (Table 2).
TABLE 1.
Summary of Adverse Events
TABLE 2.
Safety and Efficacy Measures for 21 Patients Who Received the Full Dose of Botulinum Toxin/DMSOa
Efficacy measures for the 21 women who completed the full dose portion of our investigation are shown in Table 2. We noted a reduction in median 24-hour pad weight from baseline (135 g) to 1 month (46 g) and 3 months (55 g); however, neither of these reductions were statistically significant. The median number of incontinent episodes reported for the previous 24-hour period decreased from 4 at baseline to 2 at 1 month; however, it increased to 4 at 3 months. On the basis of the scores obtained from responses on the Blaivas-Groutz anti-incontinence scale, 16 (76%) of the 21 women reported severe incontinence at baseline; this number decreased to 10 women (48%) at 1 month and increased to 13 (62%) at 3 months. With regard to our measures of urinary urgency obtained via responses on the IUSS questionnaire, we noted significant improvements in scores at both 1 month and 3 months. Indeed, 11 (52%) of the 21 women reported severe IUSS scores at baseline compared with only 1 (5%) and 3 (14%) at 1- and 3-month follow-up, respectively.
The 3 quality-of-life measures at baseline, 1 month, and 3 months are summarized in Table 2. For each measure, we noted significant improvements from baseline to 1 month and from baseline to 3 months. Indeed, median scores improved from baseline to 1 month on the Incontinence Impact Questionnaire-short form (from 13 to 7) and UDI-6 (from 10 to 6). Moreover, these improvements in self-reported urinary quality-of-life measures remained apparent at 3 months for each of the measures.
DISCUSSION
Interest in intravesical injections of botulinum toxin for IDO has been increasing because of the demonstrated improvement in incontinent episodes and quality of life and the decrease in urinary urgency in patients in whom medical therapy has failed.6-8 Investigators have reported a mean duration of treatment effect after intravesical injection that ranges from 5.3 months to 10.5 months.1 It appears that repeated cystoscopic intravesical injections are required to maintain effect.1,9 Given this requirement, a catheter-based delivery system has inherent appeal.
Botulinum toxin has been administered intravesically in spinal cord-injured rat bladders pretreated with intravesical administration of protamine sulfate (an agent that enhances absorption of botulinum toxin).10 In a study of humans, researchers have safely instilled botulinum toxin into the bladder using a normal saline solution but without improved clinical effect.9 Because of the failure of the human study group to achieve clinical response with the botulinum toxin/normal saline solution and the noted response in the rat model using protamine, we examined use of DMSO as a delivery agent. In the urologic literature, DMSO is well known as a nonspecific anti-inflammatory agent that can penetrate and/or permeate the glycosaminoglycan layer of the bladder with direct effect on the detrusor muscle. In addition, DMSO may contain some inherent anticholinesterase and neuromuscular blocking properties.11 After topical application, DMSO is absorbed and generally distributed into the tissues and body fluids. Because of the mechanism of action of DMSO, its current use in urology is approved by the Food and Drug Administration for bladder inflammatory disorders such as interstitial cystitis.12 Investigators using an animal model reported that DMSO as a transfer agent could effectively alter the permeability and secondary delivery of intravesical agents.11 As an example, in the animal model, by using intravesical instillation of cisplatin, DMSO was able to safely increase intramuscular concentration of cisplatin 3-fold.13 In the bladder muscle, DMSO causes a concentration-dependent absorption, with a peak effect occurring at more than 90% DMSO concentration.14 For the current study, we used a 50% concentration because it results in complete desquamation of the entire epithelial and basal layer but does not alter the lamina propria.14 The intact lamina propria helps to markedly diminish the potential for extravasation outside of the bladder (into the bladder serosa).
Preliminary analysis by Schurch et al15 in 2000 suggested that botulinum A toxin detrusor injection can suppress bladder overactivity and increase bladder filling; the most effective dose without AEs was determined to be between 200 and 300 units. For that reason, we chose the dose of 300 units when developing the solution.
Meta-analysis of intravesical injections of botulinum toxin for IDO has shown low morbidity with systemic AEs being almost nonexistent.1,2,9 The most common local AEs after injection are urinary tract infection (approximately 5% of cases) and hematuria (1.6% of cases).1 We reported a low number of AEs, and those we observed were consistent with AEs reported by previous authors of trials of injectable botulinum toxin.1 No systemic AEs or reports of generalized muscle weakness occurred with the 200-unit or 300-unit doses. The most commonly reported AE after instillation was urethral discomfort and dysuria (9/25 women; 36%). Overall rates of urinary retention or necessity of intermittent catheterization after intravesical injection of botulinum toxin for all indications are relatively poorly reported and may depend on the dose injected and the location of the injection.16 Urinary retention after intravesical injections of botulinum toxin for IDO has been reported in approximately 5% of cases.1 Although we noted a statistically significant increase in PVRs from baseline to the 3-month follow-up period, it was not clinically important. No patient had urinary retention or required catheterization during the 3-month follow-up period.
Continence is regained in 33% to 91% of patients after intravesical injections of botulinum toxin for IDO and may have a duration of effect of 5 to 9 months.1 In the current trial, 24-hour pad weights and average number of pads per day decreased from baseline at 1-month and 3-month follow-up, but the decreases were not statistically significant. In addition, the number of incontinent episodes per 24-hour period decreased at 1-month follow-up but returned to near baseline at 3 months. Although results of the current phase 1/2 study cannot be compared with results of published studies of botulinum toxin injections, the number of long-term incontinent episodes after instillation should be examined in future studies.
The objective of this phase 1/2 study was to evaluate the safety profile and clinical effect of catheter-based instillation of botulinum toxin/DMSO. The patient-generated clinical response data are promising and provide a clear rationale to further examine this novel treatment method. Improvement in urinary quality of life was noted. Although the quality of life of these women is of primary concern, the more subjective nature of these measures means they are particularly susceptible to placebo effects. As such, a randomized, placebo-controlled trial will better determine the magnitude of the placebo effect. The decision regarding whether it is appropriate to pursue this treatment in further studies needs to take into account the whole profile of outcome measures considered. We think that these results have shown sufficient evidence of efficacy to warrant future study of this novel mode of treatment.
CONCLUSION
Direct instillation of a botulinum toxin/DMSO solution is safe. Given the promising results of this study in treating women with IDO, further evaluation of this novel technique in a randomized, placebo-controlled phase 3 setting is justified.
REFERENCES
- 1.Dmochowski R, Sand PK. Botulinum toxin A in the overactive bladder: current status and future directions. BJU Int. 2007;99(2):247-262 [DOI] [PubMed] [Google Scholar]
- 2.Kim DK, Thomas CA, Smith C, Chancellor MB. The case for bladder botulinum toxin application. Urol Clin North Am. 2006;33(4):503-510 [DOI] [PubMed] [Google Scholar]
- 3.Groutz A, Blaivas JG, Rosenthal JE. A simplified urinary incontinence score for the evaluation of treatment outcomes. Neurourol Urodyn. 2000;19(3):127-135 [DOI] [PubMed] [Google Scholar]
- 4.Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174(2):604-607 [DOI] [PubMed] [Google Scholar]
- 5.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA, Continence Program for Women Research Group Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995;14(2):131-139 [DOI] [PubMed] [Google Scholar]
- 6.Rapp DE, Lucioni A, Katz EE, O'Connor RC, Gerber GS, Bales GT. Use of botulinum-A toxin for the treatment of refactory overactive bladder symptoms: an initial experience. Urology 2004;63(6):1071-1075 [DOI] [PubMed] [Google Scholar]
- 7.Werner M, Schmid DM, Schüssler B. Efficacy of botulinum-A toxin in the treatment of detrusor overactivity incontinence: a prospective nonrandomized study. Am J Obstet Gynecol. 2005;192(5):1735-1740 [DOI] [PubMed] [Google Scholar]
- 8.Schmid DM, Sauermann P, Werner M, et al. Experience with 100 cases treated with botulinum-A toxin injections in the detrusor muscle for idiopathic overactive bladder syndrome refractory to anticholenergics. J Urol. 2006;176(1):177-185 [DOI] [PubMed] [Google Scholar]
- 9.Rackley R, Abdelmalak J. Urologic applications of botulinum toxin therapy for voiding dysfunction. Curr Urol Rep. 2004;5(5):381-388 [DOI] [PubMed] [Google Scholar]
- 10.Vemulakonda VM, Somogyi GT, Kiss S, Salas NA, Boone TB, Smith CP. Inhibitory effect of intravesically applied botulinum toxin A in chronic bladder inflammation. J Urol. 2005;173(2):621-624 [DOI] [PubMed] [Google Scholar]
- 11.Swanson BN. Medical use of dimethyl sulfoxide (DMSO). Rev Clin Basic Pharm. 1985;5(1-2):1-33 [PubMed] [Google Scholar]
- 12.Shirley SW, Stewart BH, Mirelman S. Dimethyl sulfoxide in treatment of inflammatory genitourinary disorders. Urology 1978;11(3):215-220 [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld RH, Belville WD, Jacob WH, Buck AS, Dresner ML, Insalaco SJ, Ward GS. The effect of Dimethyl Sulfoxide on the uptake of cisplatin from the urinary bladder of the dog: a pilot study. J Am Osteopath Assoc. 1983;82(8):570-573 [PubMed] [Google Scholar]
- 14.Borzelleca JF, Harris TM, Bernstein S. The effect of dimethylsulfoxide on the permeability of the urinary bladder. Invest Urol. 1968;6(1):43-52 [PubMed] [Google Scholar]
- 15.Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164(3, pt 1):692-697 [DOI] [PubMed] [Google Scholar]
- 16.Shaban AM, Drake MJ. Botulinum toxin treatment for overactive bladder: risk of urinary retention. Curr Urol Rep. 2008;9(6):445-451 [DOI] [PubMed] [Google Scholar]