Abstract
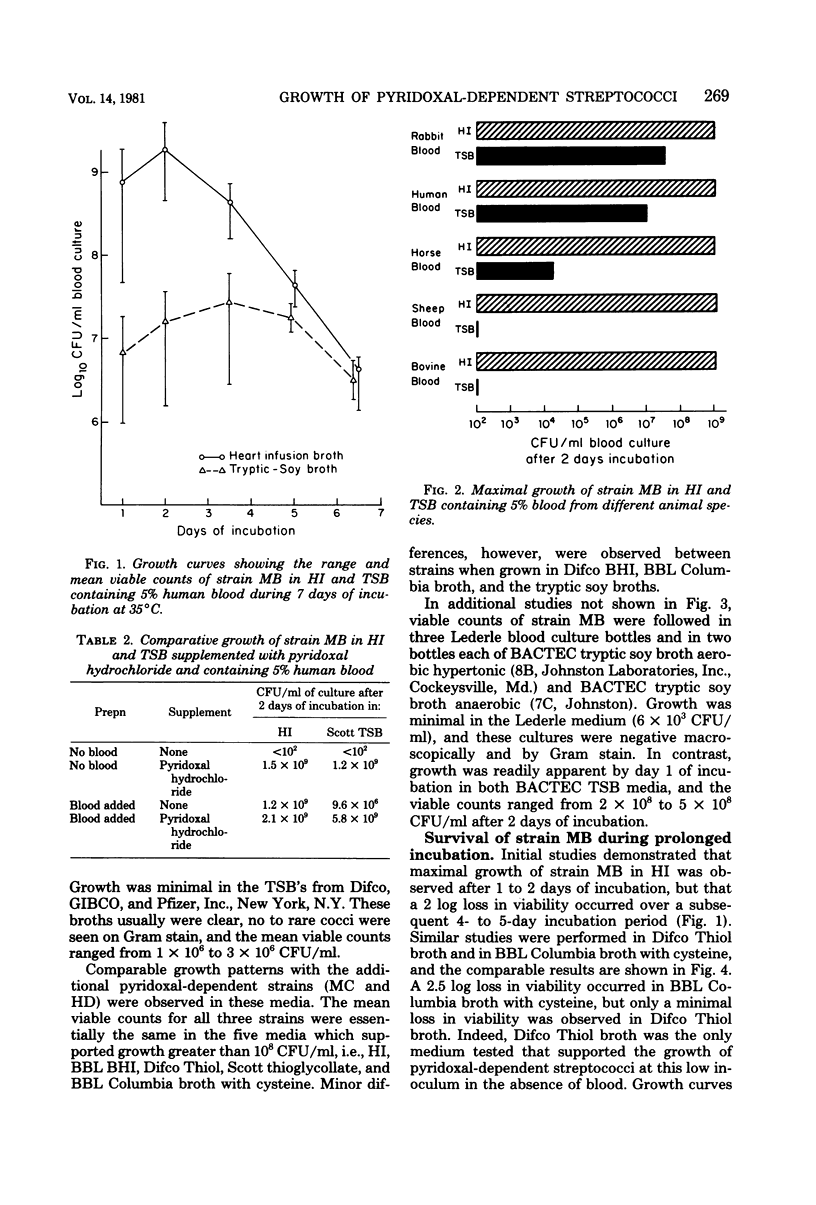
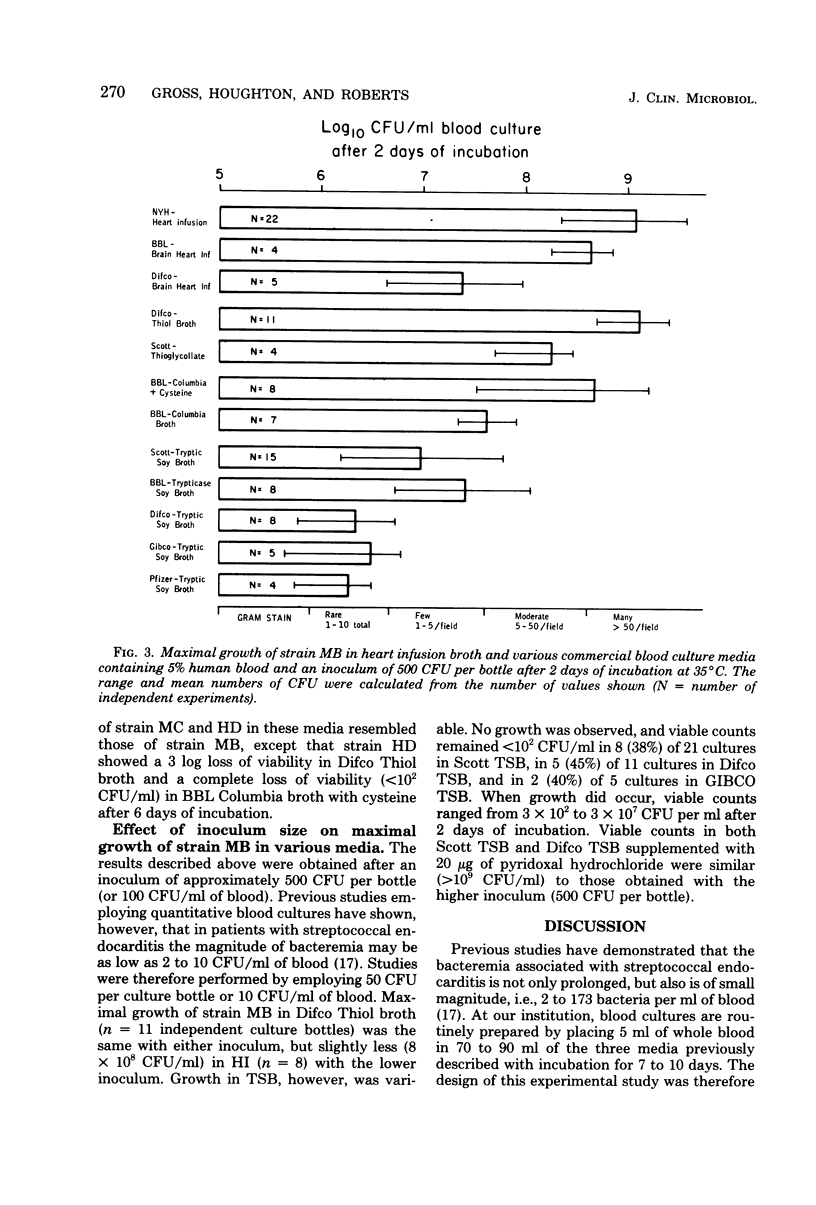
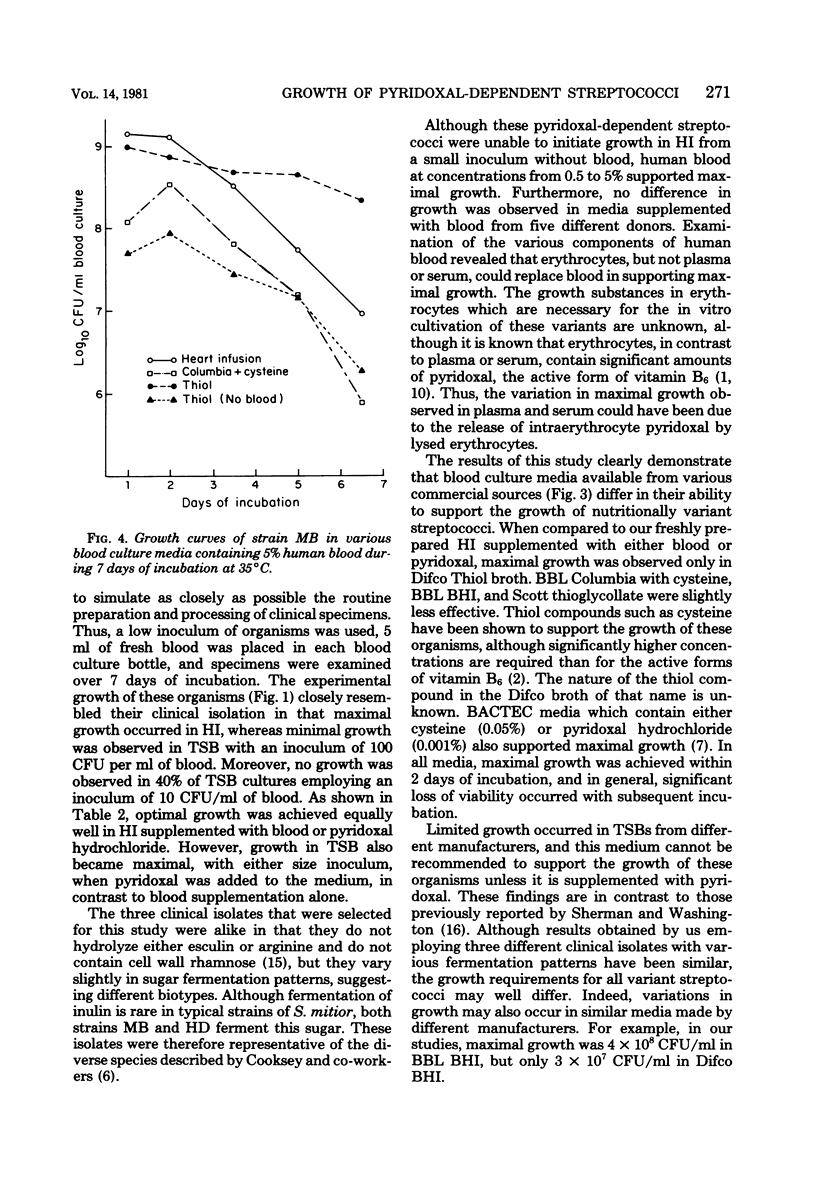
Nutritional variant streptococci identified as pyridoxal-dependent Streptococcus mitior (mitis) account for 5 to 6% of streptococcal endocarditis and may be a cause of "culture-negative" endocarditis. Hence, growth of three variant strains in 11 commercial blood culture broths was compared to that in fresh heart infusion broth. For simulation of clinical specimens, culture bottles were injected with 5 ml of human blood, inoculated with approximately 500 colony-forming units (CFU) per bottle, and monitored for 7 days with Gram stains and viable counts. Only Thiol broth (Difco Laboratories, Detroit, Mich.) supported growth without blood at this low inoculum. In media containing blood, maximal growth of 10(9) CFU/ml was reached within 2 days of incubation, and heavy turbidity was consistently observed in only heart infusion broth, Thiol broth, and media supplemented with pyridoxal hydrochloride. Columbia broth (BBL Microbiology Systems, Cockeysville, Md.) with increased cysteine, thioglycollate broth, and one brain heart infusion broth produced moderate growth (1 x 10(8) to 5 x 10(8) CFU/ml), whereas Columbia broth, another brain heart infusion broth, and two brands of tryptic soy broth showed fair growth (1 x 10(7) to 4 x 10(7) CFU/ml). The poor growth (1 x 10(6) to 3 x 10(6) CFU/ml) observed in three other brands of tryptic soy broth was often not apparent macroscopically or by Gram stain. Furthermore, on growth occurred in 40% of tryptic soy broth cultures inoculated with 50 CFU. Therefore, to ensure isolation of these variant streptococci from clinical blood cultures, a medium containing thiol compounds or supplemented with pyridoxal should be used. Subcultures should be made within 2 days of incubation to blood agar enriched with pyridoxal or containing a Staphylococcus sp. streak for satellitism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. B., Fulford-Jones C. E., Child J. A., Beard M. E., Bateman C. J. Conversion of vitamin B 6 compounds to active forms in the red blood cell. J Clin Invest. 1971 Sep;50(9):1901–1909. doi: 10.1172/JCI106682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. B., Brause B. D., Roberts R. B. Antimicrobial therapy of vitamin B6-dependent streptococcal endocarditis. Ann Intern Med. 1977 Aug;87(2):150–154. doi: 10.7326/0003-4819-87-2-150. [DOI] [PubMed] [Google Scholar]
- Carey R. B., Gross K. C., Roberts R. B. Vitamin B6-dependent Streptococcus mitior (mitis) isolated from patients with systemic infections. J Infect Dis. 1975 Jun;131(6):722–726. doi: 10.1093/infdis/131.6.722. [DOI] [PubMed] [Google Scholar]
- Cayeux P., Acar J. F., Chabbert Y. A. Bacterial persistence in streptococcal endocarditis due to thiol-requiring mutants. J Infect Dis. 1971 Sep;124(3):247–254. doi: 10.1093/infdis/124.3.247. [DOI] [PubMed] [Google Scholar]
- Cooksey R. C., Thompson F. S., Facklam R. R. Physiological characterization of nutritionally variant streptococci. J Clin Microbiol. 1979 Sep;10(3):326–330. doi: 10.1128/jcm.10.3.326-330.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENKEL A., HIRSCH W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature. 1961 Aug 12;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- Feder H. M., Jr, Olsen N., McLaughlin J. C., Bartlett R. C., Chameides L. Bacterial endocarditis caused by vitamin B6-dependent viridans group Streptococcus. Pediatrics. 1980 Aug;66(2):309–312. [PubMed] [Google Scholar]
- George R. H. The isolation of symbiotic streptococci. J Med Microbiol. 1974 Feb;7(1):77–83. doi: 10.1099/00222615-7-1-77. [DOI] [PubMed] [Google Scholar]
- Hines J. D., Love D. S. Determination of serum and blood pyridoxal phosphate concentrations with purified rabbit skeletal muscle apophosphorylase b. J Lab Clin Med. 1969 Feb;73(2):343–349. [PubMed] [Google Scholar]
- McCarthy L. R., Bottone E. J. Bacteremia and endocarditis caused by satelliting streptococci. Am J Clin Pathol. 1974 May;61(5):585–591. doi: 10.1093/ajcp/61.5.585. [DOI] [PubMed] [Google Scholar]
- Narasimhan S. L., Weinstein A. J. Infective endocarditis due to a nutritionally deficient streptococcus. J Pediatr. 1980 Jan;96(1):61–62. doi: 10.1016/s0022-3476(80)80327-1. [DOI] [PubMed] [Google Scholar]
- Roberts K. B., Sidlak M. J. Satellite streptococci. A major cause of "negative" blood cultures in bacterial endocarditis? JAMA. 1979 May 25;241(21):2293–2294. doi: 10.1001/jama.241.21.2293. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Gross K. C. The species of viridans streptococci associated with microbial endocarditis: incidence and antimicrobial susceptibility. Trans Am Clin Climatol Assoc. 1978;89:36–48. [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Sherman S. P., Washington J. A., 2nd Pyridoxine inhibition of a symbiotic streptococcus. Am J Clin Pathol. 1978 Oct;70(4):689–690. doi: 10.1093/ajcp/70.4.689. [DOI] [PubMed] [Google Scholar]
- Werner A. S., Cobbs C. G., Kaye D., Hook E. W. Studies on the bacteremia of bacterial endocarditis. JAMA. 1967 Oct 16;202(3):199–203. [PubMed] [Google Scholar]


