Abstract
The majority of retinoid (vitamin A and its metabolites) present in the body of a healthy vertebrate is contained within lipid droplets present in the cytoplasm of hepatic stellate cells (HSCs). Two types of lipid droplets have been identified through histological analysis of HSCs within the liver: smaller droplets bounded by a unit membrane and larger membrane-free droplets. Dietary retinoid intake but not triglyceride intake markedly influences the number and size of HSC lipid droplets. The lipids present in rat HSC lipid droplets include retinyl ester, triglyceride, cholesteryl ester, cholesterol, phospholipids and free fatty acids. Retinyl ester and triglyceride are present at similar concentrations, and together these two classes of lipid account for approximately three-quarters of the total lipid in HSC lipid droplets. Both adipocyte-differentiation related protein and TIP47 have been identified by immunohistochemical analysis to be present in HSC lipid droplets. Lecithin:retinol acyltransferase (LRAT), an enzyme responsible for all retinyl ester synthesis within the liver, is required for HSC lipid droplet formation, since Lrat-deficient mice completely lack HSC lipid droplets. When HSCs become activated in response to hepatic injury, the lipid droplets and their retinoid contents are rapidly lost. Although loss of HSC lipid droplets is a hallmark of developing liver disease, it is not known whether this contributes to disease development or occurs simply as a consequence of disease progression. Collectively, the available information suggests that HSC lipid droplets are specialized organelles for hepatic retinoid storage and that loss of HSC lipid droplets may contribute to the development of hepatic disease.
Keywords: Lipid Droplet, Hepatic Stellate Cell, Liver, Retinoid, Vitamin A, Triglyceride
1. Introduction
Retinoids (vitamin A and its metabolites) are required for life and act essentially in maintaining normal cell proliferation and differentiation, a healthy immune system, normal male and female reproduction, and vision [1–3]. The physiological functions of retinoids in the body, aside from in vision, are mediated by the all-trans- and 9-cis-isomers of retinoic acid, which modulate expression levels of retinoid-responsive genes [4, 5]. In excess of 500 genes are proposed to be retinoid-responsive [6]. In vision, 11-cis-retinal serves as the chromophore for the visual pigment rhodopsin [7]. The major retinoid species that are present in the body include retinyl ester, retinol, retinal and retinoic acid (see Fig. 1). Retinyl ester is considered to be an inert storage form, but it is also present in the postprandial circulation as a component of chylomicrons and their remnants [8, 9]. Retinol is transported to cells in the fasting circulation bound to its specific transport protein, retinol-binding protein (RBP) [8, 9]. The retinol transporter STRA6 is proposed to facilitate cellular uptake of retinol from the retinol-RBP complex [10–12]. Within cells, retinol is enzymatically oxidized to retinal, which in turn is oxidized to retinoic acid. Alternatively, within some cells, retinol can be enzymatically esterified to retinyl ester. Retinyl ester and retinol are normally the most abundant retinoid species within cells and tissues. Tissue concentrations of retinol and retinyl ester are 100- to 1000-fold greater than those of retinoic acid [8, 9]. All retinoid must be acquired from the diet either as preformed retinoid or from proretinoid carotenoids [8, 9]. The metabolism of retinoids is complex and involves many unique proteins and enzymes that are specific to this metabolism [13].
Fig. 1.
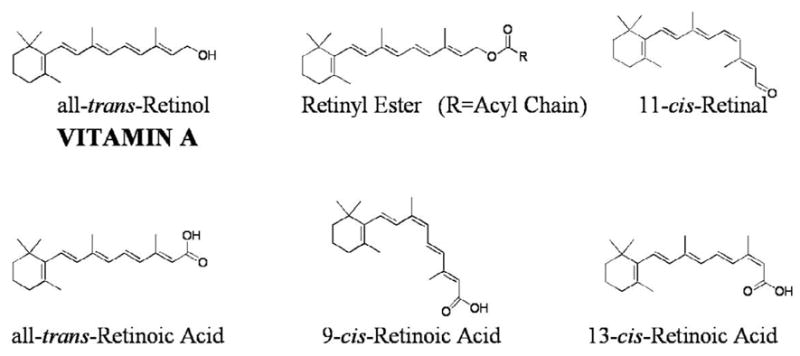
Chemical structures for the most abundant and important retinoids present in the body. All-trans-retinol, which by definition is vitamin A, is routinely referred to simply as retinol. Retinyl esters are retinoid storage forms. Outside of the eye, the preponderance of retinyl ester exists as the all-trans-isomer. It is this retinoid species that is stored in the lipid droplets of hepatic stellate cells. 11-Cis-retinal serves as the chromophore for the visual pigment rhodopsin. All-trans- and 9-cis-retinoic acids are transcriptionally active retinoids. 13-Cis-retinoic acid is a naturally occurring retinoid that lacks the potent transcriptional activity of the all-trans- and 9-cis-isomer. However, this retinoid species has proven to be clinically effective for treating skin disease (clinically it is referred to as isotretinoin or Accutane).
Hepatic stellate cells (HSCs) were first described in 1876 by Kupffer who termed the cells “Sternzellen” (as reviewed in [14]). Since Kupffer’s time, these cells have been referred to in the literature by many names, including Ito cells, lipocytes, fat-storing cells, and perisinusoidal cells. The now widely adopted and preferred nomenclature for these cells is hepatic stellate cell [15]. Like Kupffer cells and hepatic endothelial cells, HSCs are non-parenchymal cells that are located perisinusoidally in the space of Disse, in recesses between hepatocytes (parenchymal cells) [14–17]. Compared to hepatocytes, HSCs are relatively small in size and much less abundant. They comprise about 5–8% of total rat liver cells and 1% of the total liver mass [16, 17]. The most chararacteristic morphologic feature of these cells in a normal liver is the presence of multiple cytoplasmic lipid droplets. These cytoplasmic lipid droplets are readily evidenced in biopsies obtained from liver (Fig. 2).
Fig. 2.
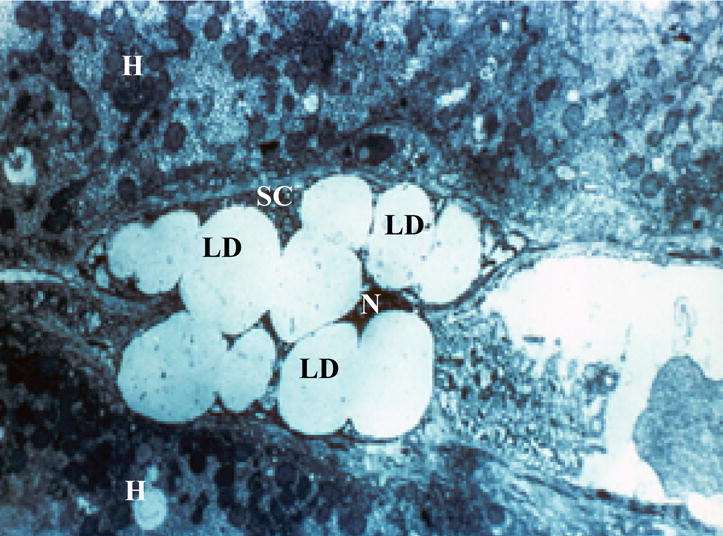
Hepatic stellate cell lipid droplets present in a biospy obtained from a human liver. The image shows the characteristic lipid droplets (LD) found in hepatic stellate cells (SC). For this electron micrograph of a human hepatic stellate cell, the nucleus (N) is compressed by the surrounding lipid droplets and very little cell cytoplasm within the hepatic stellate cell can be seen. Adjoining the hepatic stellate cell are two hepatocytes (H).
The HSCs have several important functional roles in the liver. They are the central site for retinoid storage in the liver and consequently, the body [8, 9]. In the retinoid-sufficient adult rodent, as much as 90–95% of hepatic retinoid is stored as retinyl ester in lipid droplets of HSCs [8, 9]. For a healthy adult, the liver contains approximately 70% of the total retinoid present in the body. Thus, HSC lipid droplets are the location where the majority of the body’s retinoid stores are found. HSCs are also a key cellular source of extracellular matrix synthesis (ECM) in the liver and for the synthesis of enzymes involved in ECM remodeling [15–17]. This role in ECM synthesis and remodeling renders the HSC a key cellular player in the development of hepatic fibrosis and cirrhosis. Recently published studies indicate that HSCs can act as antigen presenting cells [18, 19]. Consequently, HSCs may have an important, but previously unrecognized, role as immunoregulatory cells. The biology of the HSC has been extensively reviewed recently by Friedman [15]. Readers interested in obtaining more detailed information regarding the biology of HSCs are referred to this excellent review.
2. Structural features of hepatic stellate cell lipid droplets
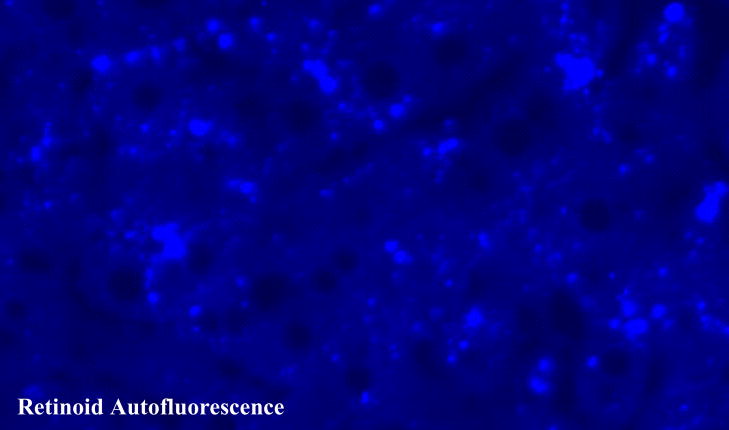
The most characteristic feature of an HSC in the intact liver is the presence of retinoid-containing lipid droplets in the cytoplasm. In unfixed tissue or cultured cells, the retinoid-containing lipid droplets exhibit a striking, rapidly fading blue-green autofluorescence when excited with light of approximately 330 nm (Fig. 3) [20, 21]. Two types of lipid droplets have been identified and described by a number of research groups [14, 22–24]. Type I lipid droplets are bound by a unit membrane and are variable in size, but usually are smaller than 2 μm in diameter. The unit membrane does not directly make contact with the lipid droplet but rather contacts a 4–8 nm thick intercalated layer that separates the unit membrane from the lipid droplet [14]. It has been proposed that type I lipid droplets are derived from “multivesicular bodies” that are suggested to be a type of lysosome [23, 24]. Type II lipid droplets are not enclosed by a membrane and are much larger than type I droplets (up to 8 μm in diameter) [14, 22–24]. The relationship between the two types of lipid droplets is not understood. Some investigators have proposed that type II lipid droplets are formed through fusion of several type I droplets [22]. However, other investigators have suggested that type II droplets are the precursors for type I droplets [23].
Fig. 3.
Fluorescence micrograph of an unfixed section showing the characteristic retinoid autofluorescence (the lightened regions) present in the lipid droplets of hepatic stellate cells of male wild type mice that had been maintained throughout life on a retinoid-containing control diet.
It is well established that the number and size of lipid droplets present in HSCs is dependent on dietary retinoid intake [14, 15]. In times of high or excessive dietary retinoid intake, the number and size of the lipid droplets increases to accommodate the retinoid. In times of insufficient dietary retinoid intake, the lipid droplet retinoid stores are mobilized to meet tissue retinoid needs and the number and size of lipid droplets correspondingly decrease. There are also marked species differences in the number of HSCs present in the liver and the number and size of lipid droplets present in HSCs [15, 25, 26]. This is most clearly seen for species, like polar bears and Arctic foxes, that live in Arctic regions and which consume diets that are very rich in retinoid [25, 26]. These Arctic species have exceedingly high capacity for retinoid storage in their relatively abundant and large HSC lipid droplets [25, 26]. Based on these observations, it has been proposed that HSC lipid droplets are specialized organelles for the storage of retinoid [14, 26]. This notion is supported strongly by biochemical data discussed below.
During liver injury, the structure of the HSCs change markedly, as these cells become “activated” [15–17]. A characteristic feature of HSC activation is the loss of the lipid droplets and the retinoid stores they contain. It is not established at the molecular level why or how the lipid droplets and their contents are lost. Importantly, it also is not known whether lipid droplet loss occurs as a result of the activation process and/or whether loss contributes causally to HSC activation and disease development [15].
3. Lipid and protein composition of hepatic stellate cell lipid droplets
The lipid composition of lipid droplets isolated from rat HSCs has been reported by a number of laboratories [27–30]. Generally, these reports are in good agreement that retinyl ester and triglyceride are present at similar concentrations in lipid droplets obtained from primary rat HSCs isolated from well nourished rats. Lipid droplets isolated from primary HSCs consist of, on a dry weight basis, 83% lipid and 17% protein [27, 30]. Collectively, retinyl ester and triglyceride account for the great majority (as much as 75%) of the lipid present in the droplets [27, 30]. Cholesterol, cholesteryl ester, phospholipids and free fatty acids are also reported to be present in rat HSC lipid droplets [27–30]. The relative abundance of retinyl ester in HSC lipid droplets is unusual. Although rat and mouse adipocytes contain retinyl ester, the concentration of retinyl ester in adipocytes isolated from healthy chow-fed rodents is less than 0.1% of the adipocyte triglyceride concentration [31, 32]. Considering the relatively low concentrations of retinyl ester elsewhere the body, it seems unlikely that lipid droplets present in cell types other than stellate cells, possibly aside from the retinal pigment epithelial (RPE) cells of the eye, will be found to have similar retinyl ester to triglyceride ratios as those found in stellate cell lipid droplets. Like HSCs, the RPE cells contain lipid droplets that are specialized for retinoid storage. These lipid droplets in the eye have been termed retinosomes and are reported to contain adipocyte differentiation-related protein (ADRP) [33, 34]. It should be noted that stellate cells are present in a number of tissues other than the liver, including pancreas and lung [14, 15, 26, 35]. These extrahepatic stellate cells possess retinoid-containing lipid droplets, however, these cells have not been as extensively studied as the HSCs.
The identification of proteins associated with HSC lipid droplets is only now starting to commence. In early 2008, Straub et al. reported immunolocalization studies showing that ADRP and TIP47 are present in HSC lipid droplets for normal mouse, bovine and human liver [36]. These investigators convincingly demonstrated that ADRP and TIP47 co-localized with the retinoid autofluorescence of HSC lipid droplets as well as with vimentin and desmin, which are established markers used to identify HSCs. Moreover, Straub et al. reported that perilipin co-localizes in HSC lipid droplets for bovine, but not mouse or human liver [36]. Thus, these members of the so-called PAT protein family, which are known to play crucial roles in the stabilization of lipid droplets in other cell types, likely act in a similar manner in HSCs. This idea is supported by earlier in vitro studies which demonstrated that adenovirus-mediated expression of ADRP in cultured HSCs resulted in an increased presence of lipid droplets in the cytoplasm of the adenovirus-treated cells [37].
In light of the relatively large retinoid concentration present in HSC lipid droplets, it would not be surprising if proteins known to be involved in retinoid metabolism or transport were also found to be associated with HSC lipid droplets. Matsuura and colleagues recently reported immunoelectron microscopic studies showing that the enzyme lecithin:retinol acyltransferase (LRAT), which is the sole enzyme in the liver responsible for retinyl ester synthesis (see below), is localized to both the rough endoplasmic reticulum and the “multivesicular bodies” present within HSCs [38]. This subcellular localization is believable and may be very important to allow for lipid droplet formation, considering that the “multivesicular bodies” have been proposed to be a precursor structure needed for the formation of type I lipid droplets [23, 24].
The triglyceride synthesizing enzyme, diacylglycerol acyltransferase 1 (DGAT1) is reported to be highly expressed in HSCs [39]. It is possible that this enzyme plays a role in synthesizing triglyceride that is needed for incorporation into HSC lipid droplets. Interestingly, in vitro studies have shown that DGAT1 can catalyze the formation of retinyl ester from retinol and fatty acyl-CoA [32, 40, 41]. However, in vivo, DGAT1 does not catalyze retinyl ester synthesis in the liver, since Lrat-deficient (Lrat−/−) mice lack significant hepatic retinyl ester [32, 42, 43]. Moreover, retinyl ester concentrations of livers from Dgat1-deficient (Dgat1−/−) mice are reported to be similar to those observed for matched wild type mice [40, 44].
4. Lipid droplet storage of retinoid
The retinyl esters that are present in HSC lipid droplets possess only long chain fatty acyl moieties [27, 28]. The most abundant retinyl ester, accounting for as much as 70–75% of the retinyl ester, is retinyl palmitate. Retinyl palmitate is followed in order of abundance by retinyl stearate, retinyl oleate and retinyl linoleate [27, 30]. Collectively, these four retinyl esters account for more than 95% of the retinyl ester present in the lipid droplets. It has long been established that the predominant retinyl esters present in the intact liver are those of the saturated fatty acids, palmitic and stearic acid [45], and the HSC lipid droplet retinyl ester distribution faithfully reflects this known hepatic composition. It is believed that this ester composition arises owing to the substrate specificity of LRAT, transferring a fatty acyl group from the A1 position of phosphatidyl choline to retinol. It is well established for the liver that saturated fatty acids represent the predominant acyl moiety present at this position [8, 45]. Very little of the free alcohol retinol is present in HSC lipid droplets (< 1% of the retinyl ester concentration). It is possible that this small amount of retinol may have been formed artifactually upon hydrolysis of retinyl ester during the processes of HSC and lipid droplet isolation.
The HSC has great capacity for the storage and metabolism of retinoid. These cells possess high levels of LRAT activity and are very enriched in cellular retinol pinding protein, type I (CRBPI) [46, 47], an intracellular protein that is needed to transport retinoid within the aqueous environment of the cytosol [48]. It has been proposed that CRBPI delivers retinol to LRAT for esterification; thus preventing acyl-CoA-dependent enzymes from catalyzing retinyl ester formation within the liver, as well as in other tissues [8, 13]. Since retinol must be mobilized from HSC lipid droplet retinyl ester stores into the blood in times of dietary retinoid-insufficiency, the lipid droplets must also possess or acquire lipases able to hydrolyze the stored retinyl ester. It is presently not clear which lipases are physiologically important for catalyzing retinyl ester hydrolysis in HSCs, but a number of lipases known to possess retinyl ester hydrolase activity in vitro, including the carboxyesterases ES-4 and ES-10, have been localized to the HSC [49, 50]. At present, it is not established how enzymes and proteins involved in retinoid metabolism interact with or contribute to the formation of lipid droplets. Yet, it is fundamentally important to understand how enzymes like LRAT and retinyl ester-hydrolyzing lipases, as well as other protein components of the retinoid-metabolizing machinery, interact with the lipid droplets and/or their precursors during lipid droplet synthesis and degradation within the HSC.
5. Effects of dietary retinoid and triglyceride on lipid droplet lipid composition
As mentioned above, the size and number of HSC lipid droplets are markedly influenced by dietary retinoid intake, and also by intraportal injection of retinol [14, 15]. These morphological observations are taken to suggest that HSC lipid droplets are specialized organelles for retinoid storage [14, 26]. Moriwaki et al. provided biochemical evidence that is consistent with this conclusion from studies of the effects of dietary retinoid and dietary triglyceride on the lipid composition of lipid droplets isolated from primary rat HSCs [30]. Groups of weanling rats were placed on one of five purified diets that differed in their contents of either retinoid (control, low or high retinol with 20.5% of calories provided by triglyceride in each diet) or triglyceride (control retinol levels in each diet with 5%, 20.5% or 45% of calories provided by triglyceride) for 8 weeks. Primary HSCs were then prepared and lipid droplets isolated from these cells. The HSC lipid droplet composition for the control group showed a mean percent lipid composition of 39.5% retinyl ester; 31.7% triglyceride; 15.4% cholesteryl ester; 4.7% cholesterol; 6.3% phosholipid; and 2.4% free fatty acids. As seen in Tables 1 and 2, both the concentration of HSC total lipid (Table 1) and lipid droplet total lipid (Table 2) were markedly altered by changes in dietary retinoid intake; whereas, the low and high triglyceride groups were similar to controls with regards to both HSC total lipid and lipid droplet total lipid concentrations. These data were taken by Moriwaki et al. to indicate that the lipid composition of HSC lipid droplets is strongly regulated by dietary retinoid status but not by dietary triglyceride intake [30].
Table 1.
Effects of dietary retinoid and triglyceride on the concentrations of retinoid and nonretinoid lipids present in freshly isolated primary rat hepatic stellate cells.a
| Lipid Mass | |||
|---|---|---|---|
| Diet Group | (n) | Retinoidb | Nonretinoidc |
| μg/106 cells | |||
| Control | 6 | 33.1 ± 6.3 | 81.0 ± 5.5 |
| Low retinold | 14 | 6.6 ± 3.7 | 76.5 ± 5.4 |
| High retinole | 6 | 358.6 ± 26.7 | 209.7 ± 37.8 |
| Low triglyceride | 6 | 31.0 ± 3.0 | 81.9 ± 5.1 |
| High triglyceride | 5 | 30.1 ± 9.0 | 82.7 ± 6.1 |
Adapted from [27].
Retinol + retinyl ester.
Triglyceride + cholesteryl ester + cholesterol + phospholipids + free fatty acids.
Providing 25% of the retinol present in the control diet.
Providing 10-times the retinol present in the control diet.
Table 2.
Effects of dietary retinoid and triglyceride on the concentrations of retinoid and nonretinoid lipids present in isolated hepatic stellate cell lipid droplets.a
| Lipid Mass | |||
|---|---|---|---|
| Diet Group | (n) | Retinoidb | Nonretinoidc |
| μg/mg dry weight | |||
| Control | 6 | 318 ± 40 | 486 ± 40 |
| Low retinold | 14 | 101 ± 35 | 679 ± 60 |
| High retinole | 6 | 527 ± 60 | 345 ± 32 |
| Low triglyceride | 6 | 284 ± 59 | 498 ± 38 |
| High triglyceride | 5 | 274 ± 50 | 515 ± 48 |
Adapted from [27].
Retinol + retinyl ester.
Triglyceride + cholesteryl ester + cholesterol + phospholipids + free fatty acids.
Providing 25% of the retinol present in the control diet.
Providing 10-times the retinol present in the control diet.
6. Role of lecithin:retinol acyltransferase (LRAT) in the formation of lipid droplets
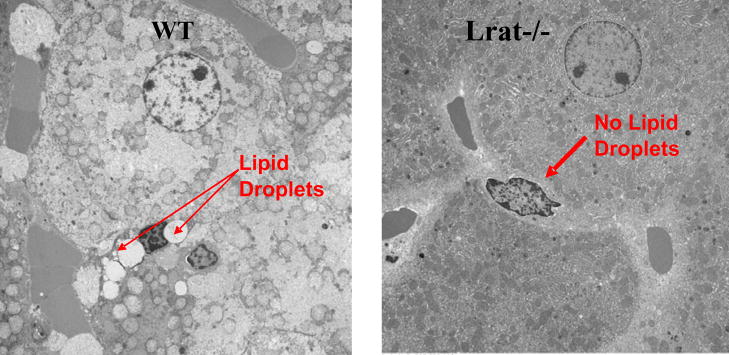
LRAT acts in an indispensable manner to allow for the formation of HSC lipid droplets. This is evidenced by Fig. 4, which shows electronmicrographs for HSCs present in situ in the livers of Lrat-deficient (Lrat−/−) and age-, gender-, diet- and genetic background-matched wild type mice. The HSCs present in the livers of Lrat−/− mice identified through staining with desmin, a commonly used marker for identifying HSCs, completely lack lipid droplets. This is accompanied by an absence of retinyl ester in livers of Lrat−/− mice (retinyl ester levels less than 0.01% of those of matched wild type mice) [32]. Prior to the generation of Lrat−/− mice, it had been proposed based on in vitro evidence that hepatic retinyl synthesis probably involved the actions of both LRAT and an unidentified acyl-CoA-dependent enzyme termed acyl-CoA:retinol acyltransferase (ARAT) [51]. These in vitro studies also provided data demonstrating that retinol bound to CRBPI served as a substrate for LRAT but not ARAT activity [52–54]. However, the published studies of Lrat−/− mice definitively establish that LRAT is the sole hepatic enzyme able to synthesize retinyl ester [32, 42, 43]. Unexpectedly, these studies also establish that either LRAT or its product retinyl ester is essentially needed for HSC lipid droplet formation. The complete absence of HSC lipid droplets in these mice raises a number of quandaries. First, why would the simple absence of a lipid component that normally may constitute only as much as 40% of the lipid present in HSC lipid droplets bring about the complete absence of the lipid droplets? It might be expected that the droplets would be smaller or fewer in number, but why would they be completely absent? Retinyl ester is an inert retinoid storage form that is not known to have any regulatory or signaling roles within the cell. Second, what role does LRAT have in the genesis of HSC lipid droplets? The literature is clear that LRAT can catalyze retinyl ester and retinyl amide formation [52–55], but there is no evidence that this enzyme is involved in the synthesis of other neutral lipids or phospholipids that may be present in HSC lipid droplets. It is possible that LRAT may have another function within the cell. There is a report in the literature that LRAT can catalyze protein fatty acylation/deacylation [56], but this finding is contradicted by another recent publication [57]. Hence, at present, it remains to be established how or in what manner LRAT and/or retinyl ester act to facilitate the formation of HSC lipid droplets.
Fig. 4.
The hepatic stellate cells of Lrat−/− mice lack lipid droplets that are a morphologic hallmark of these cells. Liver sections were prepared from 3-month-old male wild type (WT) and Lrat−/− mice. The electron micrographs show the presence of characteristic retinyl ester-containing lipid droplets in hepatic stellate cells in wild type mice (left panel) and their absence in livers from Lrat−/− mice (right panel). The arrows indicate the presence (WT) and absence (Lrat−/−) of lipid droplets in hepatic stellate cells. The large adjoining cells are hepatocytes. Taken from [32].
7. Stellate cell lipid droplets and the development of liver disease
Following liver injury, whether due to chronic alcohol consumption, viral infection, xenobiotic exposure, iron overload or another less common insult, HSCs undergo a process known as activation, which is a transition of quiescent cells into proliferative, fibrogenic and contractile myofibroblasts. This is accompanied by a series of changes in gene expression patterns that collectively give rise to responses that include cell proliferation and contractility, fibrogenesis, chemotaxis, new and altered ECM deposition, cytokine release and retinoid loss [15–17]. More detailed information, obtained from microarray studies, regarding changes in gene expression patterns in HSCs undergoing activation has been recently published [58].
Seminal work by Leo and Lieber established that the hepatic (HSC) retinoid stores of alcoholic patients are progressively lost with the development of alcoholic liver disease [59]. Hepatic retinoid levels in patients diagnosed with alcohol-induced fatty livers were approximately 20% of those control patients. Patients with alcoholic hepatitis and cirrhosis had lower levels still, approximately 10% and 5% respectively, of controls [59]. Trying to provide a basis for their observations, Leo and Lieber concluded that the reduced hepatic retinoid levels they observed in alcoholic patients were not likely due to malnutrition and suggested that they might have arisen either through enhanced degradation of the retinoid in the liver or through enhanced mobilization of the retinoid from the liver to peripheral tissues [59]. Similar observations in humans have been reported by others in the 25 years since the publication of the report by Leo and Lieber [60]. Although loss of lipid droplets and intracellular retinoid is a notable feature of HSC activation, it remains unknown whether this loss is required for HSCs to activate fully or is simply a secondary consequence of activation [15–17].
Some investigators have proposed that HSC activation results in the rapid release or burst of “toxic” transcriptionally active retinoids from the lipid droplet stores and that this contributes to the altered pattern of gene expression that is observed upon HSC activation [61–63]. This hypothesis is reasonable considering the potent transcriptional activity of retinoids [4–6]. The transcriptional regulatory actions of retinoids are mediated primarily by all-trans- and 9-cis-retinoic acid which are proposed to modulate transcription respectively through retinoic acid receptor-α, -β, or -γ (RARα, -β, and –γ) and retinoid X receptor-α, -β, and -γ (RXRα, -β, and –γ) [4, 5]. RARα, -β and -γ and RXRα, -β, and -γ are all expressed in stellate cells [64–67]. Moreover, RARγ, RXRα and RXRβ expression levels are reported to become elevated in experimentally-induced (upon CCl4 treatment) liver disease [65]. Over 500 genes are proposed to be directly responsive to retinoic acid [6], including a number of ECM genes that are expressed by HSCs (procollagens type I, III and IV, fibronectin and laminin) [65].
Still other investigators have suggested that the loss of retinoid stores in HSC lipid droplets results in a situation where these stores are no longer available to buffer against hepatic insult. Consequently, the absence of stores allows hepatic fibrosis to develop and progress [39]. Yamaguchi et al. reported that the anti-sense oligonucleotide knockdown of Dgat1 expression in rat and mouse HSCs undergoing diet-induced nonalcoholic steatohepatitis resulted in an increase in HSC Lrat expression [39]. These authors suggested that the increase in Lrat expression levels favorably altered HSC retinoid homeostasis and inhibited hepatic fibrosis. On the other hand, the absence of lipid droplets in Lrat−/− mice does not predispose 3 month-old mice to spontaneous fibrogenesis [32] suggesting that either Lrat or lipid droplets does not play a role in fibrogenesis, or that the absence of lipid droplets is not sufficient to trigger fibrosis but requires an additional “second hit”.
Although the great majority of published data suggests that the loss of HSC lipid droplet retinoid stores contributes towards the development of hepatic disease, no definitive studies are presently available that establish a causal linkage between the loss of the lipid droplets and their retinoid stores and the development of hepatic disease.
8. Summary
The lipid droplets present within HSCs are the most important site for retinoid storage within the liver and the entire body. Yet relatively little is know about the biology of these organelles. It is well established that the number and size of these lipid droplets is directly responsive to dietary retinoid intake but not to dietary triglyceride intake. Moreover, although these lipid droplets contain considerable triglyceride, total cholesterol, phospholipids, and free fatty acid, the simple absence of LRAT, an enzyme whose sole established function is the synthesis of retinyl ester, completely abolishes HSC lipid droplet formation. These observations are consistent with the idea that HSC lipid droplets are a highly specialized organelle for retinoid storage. However, the biochemical details regarding how retinoid, as retinyl ester, is incorporated into the lipid droplets are not understood. There also is little understanding of how proteins that are known or proposed to be involved in mediating retinoid metabolism and trafficking influence either HSC lipid droplet formation or degradation. Moreover, there is little understanding regarding the roles that proteins which are known to be importantly involved in lipid droplet physiology in other cell types have in HSC lipid droplets or of how these proteins may interact with retinoid-related proteins present in HSCs. These issues must be the foci for future research aimed at establishing the molecular events that are central to HSC lipid droplet formation and dissolution.
Possibly the most important information that is lacking regarding the HSC lipid droplet concerns the role that these organelles have in the development and progression of hepatic disease. It is well established that repeated hepatic insult gives rise to progressively worsening hepatic fibrosis and cirrhosis and perhaps ultimately hepatocellular carcinoma and that this is accompanied by the progressive loss of HSC lipid droplets and their retinoid stores. But it is not understood whether this loss contributes to or facilitates disease progression or whether the loss is causally unrelated to disease progression. This unresolved question must be the major focus for research aimed at understanding the physiological significance of HSC lipid droplets and the significance of their loss or absence to disease development.
Acknowledgments
The research conducted by the authors of this review has been supported by the National Institutes of Health grants R01 DK068437, R01 DK079221 and U54 CA126513.
Abbreviations
- ADRP
adipocyte-differentiation related protein
- CRBPI
cellular retinol-binding protein, type I
- DGAT1
diacylglycerol acyltransferase
- Dgat1−/−
Dgat1-deficient
- ECM
extracellular matrix
- HSC
hepatic stellate cell
- LRAT
lecithin: retinol acyltransferase
- Lrat−/−
Lrat-deficient
- RARα
retinoic acid receptor-α
- RARβ
retinoic acid receptor-β
- RARγ
retinoic acid receptor-γ
- RBP
retinol-binding protein
- RXRα
retinoid X receptor-α
- RXRβ
retinoid X receptor-β
- RXRγ
retinoid X receptor-γ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore T. Vitamin A. Elsevier Publishing Company; New York: 1957. [Google Scholar]
- 2.Goodman DS. Vitamin A and retinoids in health and disease. N Engl J Med. 1984;310:1023–1031. doi: 10.1056/NEJM198404193101605. [DOI] [PubMed] [Google Scholar]
- 3.Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids, Biology, Chemistry and Medicine. 2. Raven Press Ltd; New York: 1994. [Google Scholar]
- 4.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 5.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 6.Balmer JE, Blomhoff R. Gene expression by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Saari JC. Retinoids in mammalian vision. In: Nau H, Blaner WS, editors. The Handbook of Experimental Pharmacology. The Retinoids, Springer Verlag; Heidelberg: 1999. pp. 563–588. [Google Scholar]
- 8.Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. 2. Raven Press, Ltd; New York: 1994. pp. 229–256. [Google Scholar]
- 9.Vogel S, Gamble MV, Blaner WS. Retinoid uptake, metabolism and transport. In: Nau H, Blaner WS, editors. The Handbook of Experimental Pharmacology. The Retinoids, Springer Verlag; Heidelberg: 1999. pp. 31–96. [Google Scholar]
- 10.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wilta P, Bok D, Sun H, WS A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 11.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnbert G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinex L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary displasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Byrne SM, Blaner WS. Introduction to retinoids. In: Packer L, Obermuller-Jevic U, Kraemer K, Sies H, editors. Carotenoids and Retinoids: Molecular Aspects and Health Issues. AOCS Press; Champaign, IL: 2005. pp. 1–22. [Google Scholar]
- 14.Wake K. Perisinusoidal stellate cells (Fat-storing cells, interstitial cells, lipocytes), their related structure in and around liver sinusoids, and vitamin A storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- 15.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerts A, Bleser PD, Hautekeete ML, Niki T, Wisse E. Fat-storing (Ito) cell biology. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter D, Shafritz DA, editors. The Liver: Biology and Pathobiology. 3. Raven Press Ltd; New York: 1994. pp. 819–837. [Google Scholar]
- 17.Geerts A. Histology, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Sem Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 18.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SHE. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Winau F, Quack C, Darmoise A, Kaufmann SHE. Starring stellate cells in liver immunology. Curr Opin Immunol. 2008;20:68–74. doi: 10.1016/j.coi.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Popper H. Distribution of vitamin A in tissue as visualized by fluorescence microscopy. Physiol Rev. 1944;24:205–224. [Google Scholar]
- 21.Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissel DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wake K. Development of vitamin A-rich droplets in multivesicular bodies of rat liver stellate cells. J Cell Biol. 1974;63:683–691. doi: 10.1083/jcb.63.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto K, Ogawa K. Fine structure and cytochemistry of lysosomes in the Ito cells of the rat liver. Cell Tissue Res. 1983;223:45–57. doi: 10.1007/BF00222231. [DOI] [PubMed] [Google Scholar]
- 24.Geerts A, Bouwens L, Wisse E. Ultrastructure and function of hepatic fat-storing cells and pit cells. J Electr Micr Tech. 1990;14:247–257. doi: 10.1002/jemt.1060140306. [DOI] [PubMed] [Google Scholar]
- 25.Higashi N, Imai K, Sato M, Sato T, Kojima N, Miura M, Wold HL, Moskaug J, Berg T, Norum KR, Roos N, Wake K, Blomhoff R, Senoo H. Intralobular distribution of vitamin A-storing lipid droplets in hepatic stellate cells with special reference to polar bear and arctic fox. Comp Hepatol. 2004;3:S16. doi: 10.1186/1476-5926-2-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senoo H, Kojima N, Sato NM. Vitamin A-storing cells (stellate cells) Vitam Horm. 2007;75:131–159. doi: 10.1016/S0083-6729(06)75006-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamada M, Blaner WS, Soprano DR, Dixon JL, Kjeldbye HM, Goodman DS. Biochemical characteristics of isolated rat liver stellate cells. Hepatology. 1987;7:1224–1229. doi: 10.1002/hep.1840070609. [DOI] [PubMed] [Google Scholar]
- 28.Hendriks HFJ, Brekelmans PJAM, Buytenhek R, Brouwer A, de Leeuw AAM, Knook DL. Liver parenchymal cells differ from the fat-storing cells in their lipid composition. Lipids. 1987;22:266–273. doi: 10.1007/BF02533990. [DOI] [PubMed] [Google Scholar]
- 29.Yumoto S, Ueno K, Mori S, Takebayashi N, Handa S. Morphological and biochemical analyses, of lipid granules isolated from fat-storing cells in rat liver. Biomed Res. 1988;2:147–160. [Google Scholar]
- 30.Moriwaki H, Blaner WS, Piantedosi R, Goodman DS. Effects of dietary retinoid and triglyceride on the lipid composition of rat liver stellate cells and stellate cell lipid droplets. J Lipid Res. 1988;29:1523–1534. [PubMed] [Google Scholar]
- 31.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allen M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267:1805–1810. [PubMed] [Google Scholar]
- 32.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase. J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imanishi Y, Battan ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imanishi Y, Gerke V, Palczewski K. Retinosomes: new insights into intracellular managing of hydrophobic stustances in lipid bodies. J Cell Biol. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarroll JA, Phillips PA, Santucci N, Pirala RC, Wilson JS, Apte MV. Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89. doi: 10.1136/gut.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacker P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima M, Enjoji M, Kohjima M, Sugimoto R, Ohta S, Kotoh K, Kuniyoshi M, Kobayashi K, Imamura M, Inoguchi T, Nakamuta M, Nawata H. Adipose differentiation related protein induces lipid accumulation and lipid droplet formation in hepatic stellate cells. In vitro Cell Dev Biol Anim. 2005;41:321–324. doi: 10.1007/s11626-005-0002-6. [DOI] [PubMed] [Google Scholar]
- 38.Nagatsuma K, Hayashi Y, Hano H, Sagara H, Nurakami K, Saito M, Masaki T, Lu T, Tanaka M, Enzan H, Aizawa Y, Tajiri H, Matsuura T. Lecithin:retinol acyltransferase protein is distributed in both hepatic stellate cells and endothelial cells in normal rodent and human liver. Liver Int. 2008;9 doi: 10.1111/j.1478-3231.2008.01773.x. [epub head of print] [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Diacylglycerol acyltransferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 40.Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Orland MD, Anwar K, Cromley D, Chu CH, Chen L, Billheimer JT, Hussain MM, Cheng D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A:diacylglycerol acyltransferase 1. Biochim Biophys Acta. 2005;1737:76–82. doi: 10.1016/j.bbalip.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, van Gelder RN, Baehr W, Palczewski WK. Leicthin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 44.Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang HS, Goodman DS. Vitamin A and carotenoids. 1. Intestinal absorption and metabolism of 14C-labeled vitamin A alcohol and β-carotene in the rat. J Biol Chem. 1965;240:2839–2844. [PubMed] [Google Scholar]
- 46.Blaner WS, Hendriks HF, Brouwer A, de Leeuw AM, Knook DL, Goodman DS. Retinoids, retinoid-binding proteins, and retinyl palmitate hydrolase distributions in different types of rat liver cells. J Lipid Res. 1985;26:1241–1251. [PubMed] [Google Scholar]
- 47.Blomhoff R, Rasmussen M, Nilsson A, Norum KR, Berg T, Blaner WS, Kato M, Mertz JR, Goodman DS, Eriksson U, Peterson PA. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J Biol Chem. 1985;260:13560–13565. [PubMed] [Google Scholar]
- 48.Blaner WS, van Bennekum AM, Brouwer A, Hendriks HF. Distribution of lecithin-retinol acyltransferase activity in different types of rat liver cells and subcellular fractions. FEBS lett. 1990;274:89–92. doi: 10.1016/0014-5793(90)81336-m. [DOI] [PubMed] [Google Scholar]
- 49.Harrison EH. Lipases and carboxylesterases: Possible roles in the hepatic utilization of vitamin. A J Nutr. 2000;130:340S–344S. doi: 10.1093/jn/130.2.340S. [DOI] [PubMed] [Google Scholar]
- 50.Mello T, Nakatsuka A, Fears S, Davis W, Tsukamoto H, Bosron WF, Sanghani SP. Expression of carboxylesterase and lipase genes in rat liver cell-types. Biochem Biophys Res Commun. 2008;374:460–464. doi: 10.1016/j.bbrc.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross AC. Retinol esterfication by rat liver microsomes. Evidence for a fatty acyl coenzyme A:retinol acyltransferase. J Biol Chem. 1982;257:2453–2459. [PubMed] [Google Scholar]
- 52.MacDonald PN, Ong DE. A lecithin:retinol acyltransferase activity in human and rat liver. Biochem Biophys Res Commun. 1988;156:157–163. doi: 10.1016/s0006-291x(88)80818-0. [DOI] [PubMed] [Google Scholar]
- 53.Yost RW, Harrison EH, Ross AC. Esterification by rat liver microsomes of retinol bound to cellular retinol-binding protein. J Biol Chem. 1988;263:18693–18701. [PubMed] [Google Scholar]
- 54.Saari JD, Bredberg L. CoA- and non CoA-dependent retinol esterification in retinal pigment epithelium. J Biol Chem. 1988:8084–8090. [PubMed] [Google Scholar]
- 55.Golczak M, Imanishi Y, Kuksa V, Maeda T, Kubota R, Palczewski K. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J Biol Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 56.Xue L, Jahng WJ, Gallapalli D, Rando RR. Palmitoyl transferase activity of lecithin retinol acyl transferase. Biochemistry. 2006;45:10710–10718. doi: 10.1021/bi060897y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin M, Yuan Q, Li S, Travis GH. Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65. J Biol Chem. 2007;282:20915–20924. doi: 10.1074/jbc.M701432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 59.Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med. 1982;307:597–601. doi: 10.1056/NEJM198209023071006. [DOI] [PubMed] [Google Scholar]
- 60.Ukleja A, Scolapio JS, McConnell JP, Spivey JR, Dickson RC, Nguyen JH, O’Brein PC. Nutritional assessment of serum and hepatic vitamin A levels in patients with cirrhosis. J Parent Enter Nutr. 2002;26:184–188. doi: 10.1177/0148607102026003184. [DOI] [PubMed] [Google Scholar]
- 61.Liu C, Russell RM, Seitz HK, Wang XD. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology. 2001;120:179–189. doi: 10.1053/gast.2001.20877. [DOI] [PubMed] [Google Scholar]
- 62.Liu C, Chung J, Seitz HK, Russell RM, Wang XD. Chlormeththiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1703–1709. doi: 10.1097/01.ALC.0000037135.09289.69. [DOI] [PubMed] [Google Scholar]
- 63.Dan Z, Popov Y, Patsenker E, Preimel D, Liu C, Wang X-D, Seitz HK, Schuppan D, Stickel F. Hepatotoxicity of alcohol-induced polar retinol metabolites involves apoptosis via loss of mitochondrial membrane potential. FASEB J. 2005;19:845–847. doi: 10.1096/fj.04-2809fje. [DOI] [PubMed] [Google Scholar]
- 64.Weiner FR, Blaner WS, Czaja MJ, Shah A, Geerts A. Ito cell expression of a nuclear retinoic acid receptor. Hepatology. 1992;15:336–342. doi: 10.1002/hep.1840150226. [DOI] [PubMed] [Google Scholar]
- 65.Hellemans K, Grinko I, Rombouts K, Schuppan D, Geerts A. All-trans and 9-cis retinoic acid alter rat hepatic stellate cell phenotype differentially. Gut. 1999;45:134–142. doi: 10.1136/gut.45.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellemans K, Rombouts K, Quartier E, Dittie AS, Knorr A, Michalik L, Rogiers V, Schuit F, Wahli W, Geerts A. PPARβ regulates vitamin A metabolism-regulated gene expression in hepatic stellate cells undergoing activation. J Lipid Res. 2003;44:280–295. doi: 10.1194/jlr.M200376-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Hellemans K, Verbuyst P, Quartier E, Schuit F, Rombouts K, Chandraratna RAS, Schuppan D, Geerts DA. Differential modulation of rat hepatic stellate phenotype by natural and synthetic retinoids. Hepatology. 2004;39:97–108. doi: 10.1002/hep.20015. [DOI] [PubMed] [Google Scholar]