Abstract
Protein mechanostability is a fundamental biological property that can only be measured by single-molecule manipulation techniques. Such studies have unveiled a variety of highly mechanostable modules (mainly of the Ig-like, β-sandwich type) in modular proteins subjected to mechanical stress from the cytoskeleton and the metazoan cell–cell interface. Their mechanostability is often attributed to a “mechanical clamp” of secondary structure (a patch of backbone hydrogen bonds) fastening their ends. Here we investigate the nanomechanics of scaffoldins, an important family of scaffolding proteins that assembles a variety of cellulases into the so-called cellulosome, a microbial extracellular nanomachine for cellulose adhesion and degradation. These proteins anchor the microbial cell to cellulose substrates, which makes their connecting region likely to be subjected to mechanical stress. By using single-molecule force spectroscopy based on atomic force microscopy, polyprotein engineering, and computer simulations, here we show that the cohesin I modules from the connecting region of cellulosome scaffoldins are the most robust mechanical proteins studied experimentally or predicted from the entire Protein Data Bank. The mechanostability of the cohesin modules studied correlates well with their mechanical kinetic stability but not with their thermal stability, and it is well predicted by computer simulations, even coarse-grained. This extraordinary mechanical stability is attributed to 2 mechanical clamps in tandem. Our findings provide the current upper limit of protein mechanostability and establish shear mechanical clamps as a general structural/functional motif widespread in proteins putatively subjected to mechanical stress. These data have important implications for the scaffoldin physiology and for protein design in biotechnology and nanotechnology.
Keywords: cellulosome, cohesin, mechanical stability, protein nanomechanics, single-molecule force spectroscopy
Mechanical forces are ubiquitous in biological systems. However, we have only recently learned how to experimentally control and measure this new biophysical parameter through single-molecule manipulation. Such studies have revealed that many proteins generate, sense, transmit, or are somehow subjected to mechanical forces during their normal functioning. A subset of these “mechanical proteins” is responsible for maintaining structural integrity in vivo and they display widely different mechanical stabilities, measured in vitro as the most probable unfolding force, Fu (after having been pulled apart, normally in the N-C direction). Such forces range from the pure entropic elasticity regime (e.g., elastomers like elastin) to a few tens of piconewtons (pN) (e.g., spectrin) and to slightly above 300 pN (titin immunoglobulin-Ig-domains) (1–5).
Mechanical proteins tend to be multimodular and the modules they are made of are often of the same type, although they commonly display distinct mechanical stabilities. These mechanical modules frequently seem to act as shock absorbers that protect critical biological interactions from mechanical stress, particularly in the metazoan cell–cell interface (3–5). Their mechanostability is often attributed to a “mechanical clamp” of secondary structure that fastens their ends and, to date, Ig modules appear to be among the most mechanically stable ones.
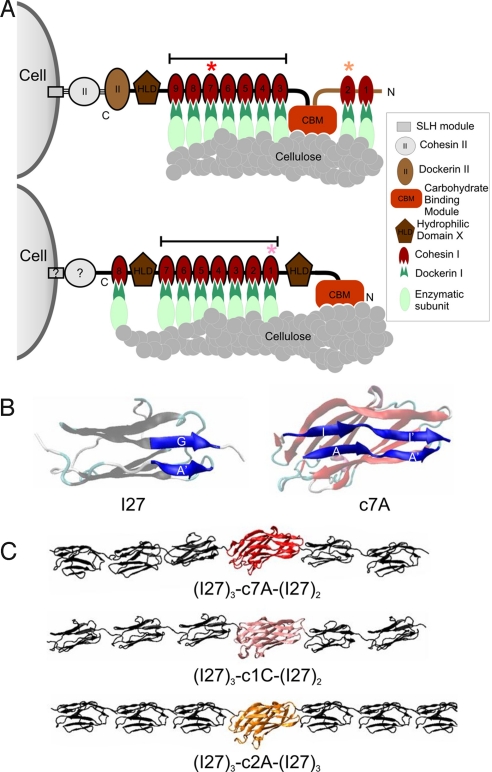
Here we have investigated the nanomechanics of scaffoldins (Fig. 1), a family of microbial modular proteins that are particularly motivating from the mechanical point of view for 3 reasons: (i) Scaffoldins are noncatalytic structural proteins of the cellulosome, a multienzyme, cell-surface complex required for adhesion and degradation of crystalline cellulose, a particularly recalcitrant substrate (Fig. 1A). These scaffolding proteins act as a molecular Lego, binding a number of cellulases through its type I cohesin (cohesin I) modules to spatiotemporally regulate the efficiency of the entire enzymatic cascade (6). Furthermore, they physically anchor the microbial cell (6, 7) to the crystalline cellulose substrate (8), although the quaternary structure of the model cellulosomes, including their linkages to the cell and substrate, is highly polymorphic and not yet well resolved (Fig. 1A; ref. 6). The bonds joining the scaffoldin system are very tenacious, which include several of the highest affinity, noncovalent bonds known in nature [interestingly, one such bond in some scaffoldins is even covalent (9)], suggesting that they may be part of a stable mechanical circuit (Fig. 1A). Like in cell adhesion proteins from metazoans (5), we hypothesize that under physiological conditions, the scaffoldin region located between the 2 key attachment points (cell and substrate) might be exposed to a greater axial mechanical stress. We call this the “connecting region,” which is a segment putatively subjected to a more intense mechanical stress than the rest of the protein, referred to as the “hanging region”. (ii) The cohesin I modules are jellyroll domains with an Ig-like, β-sandwich topology. A simple inspection of their structure reveals a mechanical topology similar to that of titin Ig domains (Fig. 1B). However, the presence of longer putative mechanical clamps of β-sheet secondary structure in cohesins suggests that they may have a higher mechanical stability. (iii) Finally, it was suggested that a linear hydrodynamic flow could mechanically disrupt the integrity of cellulosomes releasing the bound cellulases (10), thus providing indirect evidence that the mechanical integrity of this extracellular complex is important for its function.
Fig. 1.
Quaternary structure of scaffoldins and the atomic structures of the cohesin I modules studied. (A) Simplified cartoon representation of the current models for the architecture of 2 widely used model systems of closely related scaffoldins: CipA from Clostridium thermocellum (Top) and CipC from C. cellulolyticum (Bottom). Scaffoldins, the cellulase-integrating scaffolds of the cellulosome, are linked to the bacterium through anchoring proteins via their dockerin II modules, and to the substrate through a family-3, cellulose-specific, carbohydrate-binding module. The connecting region (contained in the 2 constructs analyzed in Figs. S1 and S2) is located between the 2 anchoring points and it is indicated by a black line. The 3 scaffoldin modules analyzed in this study are of the cohesion I type and they are indicated by colored asterisks: c7A, red; c2A, orange; and c1C, pink (this color code will be followed throughout this article). These modules were selected because their atomic structures were available, a prerequisite for MD studies. According to our working hypothesis, modules c7A and c1C (belonging to the connecting region) would be subjected to a greater mechanical stress than c2A (from the hanging region). (B) Mechanical topology of cohesin I modules. Atomic structures of c7A cohesin I module (Right) from C. thermocellum and, of the I27 module from human cardiac titin, a protein from the sarcomeric cytoskeleton, which is a standard in protein nanomechanics, used here for comparison (Left). The I27 module is a 7-stranded β–sandwich Ig-fold with a mechanical clamp located between the β-strands A′ and G (blue ribbons). The cohesin I module has a 9-stranded β–sandwich jellyroll fold with 2 predicted mechanical clamps located in tandem between the β–strands A-I and A′-I′ (blue ribbons). All of the atomic structures were visualized with the visual molecular dynamics (VMD) program (32). (C) The constructs used in our AFM studies. Heteromeric polyprotein fusions of the 3 cohesin I modules were synthesized with a built-in single-molecule marker [i.e., a polyprotein of the titin I27 module (2, 14, 27)]. This module was used here as both an internal control and a single-molecule marker. Constructs of the connecting region of both scaffoldins studied were also made by genetic engineering (bars in A).
Results and Discussion
We have studied 2 well-characterized model scaffoldins from motile Clostridia: CipA from Clostridium thermocellum (a thermophilic bacterium) and CipC from C. cellulolyticum (Fig. 1A) (6). According to our working hypothesis, we expect that the scaffoldin region located between the 2 anchoring points (to the bacterium and to the substrate), i.e., the connecting region, may be subjected to higher mechanical stress (Fig. 1A). Thus, we examined the 3 cohesin I modules for which an atomic structure is available: two of them from the connecting regions [module 7 from CipA (11), c7A, and the first module from CipC (12), c1C], and one from outside this region, i.e., the hanging region [module 2 from CipA (13), c2A].
Mechanical Stability of Cohesins.
Mechanical stability was measured by atomic force microscopy (AFM)-based single-molecule force spectroscopy in the length-clamp mode. For single-molecule identification we constructed fusion heteromeric polyproteins in which each cohesin I module was flanked by a marker, a polyprotein of the titin I27 module (see SI Materials and Methods; Fig. 1C; ref. 14). We found that the mechanical stability of the 2 cohesins from the connecting region (c7A: 480 ± 14pN, n = 30; c1C: 425 ± 9 pN, n = 35) was far higher than that of the cohesin from the hanging region (c2A: 214 ± 8 pN, n = 26), which is comparable to that of I27 (211 ± 3pN, n = 237), all of them probed at the same pulling speed (0.4 nm/ms; Fig. 2). In addition, sequence and mechanical analyses showed that c7A and c1C are representative of the modules present in their connecting regions (Figs. S1 and S2). Therefore, the mechanical stability (Fu) of the cohesins studied is fully consistent with their putative mechanical function in the scaffoldin physiology. Interestingly, a comparison of the modules from the connecting and hanging regions of the CipA scaffoldin shows that most of the sequence differences are concentrated in the mechanical clamps (44% identity) whereas the cellulase binding region (the dockerin I binding surface, see Fig. 1A and Fig. S3), is nearly unchanged (97% identity; Fig. S3).
Fig. 2.
Mechanical stability of cohesin I modules. AFM-based single-molecule force spectroscopy was used in the length-clamp mode (at a pulling speed of 0.4 nm/ms) to measure the mechanical stability of the representative cohesin I modules. The insert in A shows the AFM set-up configuration with a stretch of a molecule trapped. (A) Three representative force-vs.-distance traces from cohesin I modules with the unfolding force peak events from the I27 marker in black. The last force peak at the end of each curve marks the rupture of the mechanical circuit (i.e., the detachment of the molecule from either the substrate or the cantilever tip) and hence the end of the experiment. For these particular molecules, the unfolding force values were: 562 ± 5pN for c7A, 430 ± 6 pN for c1C, and 285 ± 6 pN for c2A. After adjusting to the worm-like chain model of polymer elasticity (33, 34), the calculated contour length increments (ΔLC) for the I27 were 27.3 nm in the upper trace and 28.2 nm in the other two traces. For the c7A cohesin module this value was 49.2 nm and it was 48.2 nm for the c1C and c2A modules. (B) Contour length-increment histograms (normalized). We could identify each module individually due to the different increments in contour length of the marker polyprotein. For each construct there are 2 separate populations with characteristic increments in contour length: that of the of I27 marker (27.9 ± 0.07 nm, n = 237) and that of the cohesin under study (49.3 ± 0.3 nm for c7A, n = 30; 48.5 ± 0.2 nm for c1C, n = 35; and 48.6 ± 0.2 nm for c2A, n = 26). (C) Unfolding force histograms (normalized) in which the experimental data are represented by bars. Monte Carlo (MC) simulations of the unfolding force probability are represented by lines. Solid lines represent the parameters that best fit the length-clamp experimental data (see Table 1), whereas dotted lines represent MC simulations using the potential parameters obtained from the force-ramp fittings (see Fig. 3). The dashed line in the I27 force histogram represents MC simulations by using Δx = 0.25 nm for the I27, as previously reported (35).
The results of the present study reveal that cohesins are the most mechanostable proteins reported to date, followed by the I32 module from human cardiac titin, which showed forces of 298 pN at a comparable pulling speed (in proteins, an increase in the pulling speed typically results in higher unfolding forces; ref. 15). Similar high forces have also been reported in two other systems. In the green fluorescent protein (16) the high-force peaks observed when pulling from nonterminal amino acids (548 pN at a pulling speed about one order-of-magnitude faster) can be assumed to be a pure epiphenomenon (i.e., with no physiological relevance) because this protein is not thought to be subjected to mechanical stress. In the adhesive nanofibers (likely proteinaceous) from the extracellular matrix of a fouling diatom, forces of 135–872 pN were reported (at double the pulling speed) (17). Nevertheless, in these experiments the high forces observed are interpreted as being derived from oligomers of parallel molecules in register that are pulled simultaneously. Moreover, it cannot be ruled out that these events may represent the rupture of intermolecular interactions (unbinding) rather than that of intramolecular bonds (unfolding).
Kinetic Parameters of Forced Unfolding in Cohesins.
To gain insight into the nature of the extraordinary mechanical stability of cohesin I modules, we analyzed the kinetics of the mechanical unfolding process by using the force-ramp mode of force spectroscopy and Monte Carlo simulations to fit the length-clamp unfolding force histograms (see Materials and Methods and Fig. 3). As we found no indication of intermediate states in the unfolding of cohesins, we assumed that the process follows a 2-state kinetic model. The mechanical stability of cohesin I modules correlates well with the height of the energy barrier to the process (related to the unfolding rate at zero force, α0). The potential widths obtained for cohesins are among the shortest reported to date, indicating that these structures are also particularly brittle at the experimental temperature. We have also compared mechanical stabilities with the thermal stability of the isolated modules (as determined by the melting temperature, Tm) and found no correlation. As expected, the Tm was found to be related to the optimal growth temperature of the corresponding organisms (Table 1, Fig. S4; see also SI Materials and Methods). The absence of such a correlation was also found previously for other modules (2) and is consistent with experiments showing that the thermal and mechanical unfolding pathways seem to coincide at low loading rates but not at the high loading rates used in AFM (which often drive the unfolding process very far from equilibrium) (4).
Fig. 3.
Kinetics and activation free energy diagram for the mechanical unfolding of cohesins. (A) The force-ramp mode of the AFM-based single-molecule force spectroscopy was used to directly probe the mechanical unfolding kinetics for the selected cohesins (at a pulling rate of 100 pN/s). Representative length-vs.-time traces for each construct show length steps (color coded) of 43, 45, and 42 nm for c7A, c1C, and c2A, respectively. I27 steps are ≈24 nm, in agreement with previous reports (36). There are significant differences between the lifetimes of connecting region (c7A and c1C) and hanging region (c2A) cohesins. Bottom traces show the time-course of the stretching force in which downward transients correspond to the temporary imbalance of the feedback mechanism. (B) Unfolding probability fittings (solid lines) to force-ramp experimental data (dots) for c7A, c1C, and c2A. The corresponding F1/2 values from these curves are 402 ± 4, 297 ± 4, and 134 ± 4 pN for c7A, c1C and c2A, respectively. The F1/2 value of 153 ± 4pN for I27 is in good agreement with previous reports (See Table 1 for the kinetic parameters obtained from these fittings; ref. 36). (C) Activation free-energy representation of the mechanical barriers obtained for all of the modules studied. Data points were connected by free-hand. The black line differentiates between modules with a large free-energy barrier (“moderately mechanostable”) and modules with very large free-energy barriers (“highly mechanostable”). The I27 marker lies under this line, showing the free-energy profile of this typical mechanostable protein (for details see Table 1). Solid lines represent the best Monte Carlo fittings whereas dotted lines correspond to force-ramp fittings.
Table 1.
Summary of the nanomechanical properties of the selected cohesin I modules
| Module | PDB code | Length clamp |
Monte Carlo simulations |
Force ramp |
Tm, °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FU, pN | rFU | ΔLc, nm | α0, s−1 | Δx, nm | ΔG‡, kBT | F1/2, pN | α0, s−1 | Δx, nm | ΔG‡, kBT | |||
| c7A | 1aoh | 480 ± 14 | 2.5 | 49.3 ± 0.3 | 3 × 10−4 | 0.110 | 21.9 | 300 ± 6 | (6 ± 5)10−6 | 0.131 ± 0.009 | 25.9 ± 0.8 | 84.6 ± 0.6 |
| c1C | 1g1k | 425 ± 9 | 2.1 | 48.5 ± 0.2 | 2 × 10−4 | 0.133 | 22.3 | 402 ± 6 | (1.1 ± 0.5)10−4 | 0.137 ± 0.007 | 22.9 ± 0.5 | 74.3 ± 0.2 |
| c2A | 1anu | 214 ± 8 | 1 | 48.6 ± 0.2 | 1 × 10−2 | 0.17 | 18.4 | 130 ± 6 | (2.1 ± 0.3)10−2 | 0.146 ± 0.005 | 17.7 ± 0.14 | 87.5 ± 0.6 |
| I27 | 1tit | 211 ± 3 | 1 | 27.9 ± 0.1 | 3 × 10−2 | 0.168 | 17.3 | 150 ± 6 | (5.5 ± 1.1)10−3 | 0.168 ± 0.006 | 19.0 ± 0.2 | 72.6 ± 0.1(2) |
| (4.0 ± 0.3)10−4 | 0.25 (hold) | 21.7 ± 0.08 | ||||||||||
The mechanical stability (Fu) of the cohesins studied is consistent with the proposed hypothesis of their mechanical function in the scaffoldin physiology. The values of the increase in contour length (Δ Lc) they show after mechanical unfolding indicates the presence of a single mechanical barrier fastening the ends of these modules. Mechanical stability correlates well with the spontaneous rate of mechanical unfolding (α0) and with the corresponding mechanical energy barrier of the process (ΔG‡; Figs. 2 and 3) but not with the thermal stability measured as the transition temperature, Tm in differential scanning calorimetry experiments (Fig. S4). As expected, Tm correlates with the optimal growth temperature of the corresponding bacteria: Top for C. thermocellum (c7A and c2A) is ≈60 °C and for C. cellulolyticum (c1C) is ≈37 °C (www.atcc.org). The I27 module is from human cardiac titin (Top ≈37 °C).
Steered Molecular Dynamics Simulations of Forced Unfolding of Cohesins.
Finally, we performed molecular dynamics (MD) simulations of the mechanical unfolding of cohesins to examine the atomic details of the process and pinpoint the molecular determinants of their mechanical stability (Table S1). It was previously shown that the Ig-like, β-sandwich topology to which the cohesin I modules belong is a platform that can tolerate higher axial mechanical stress than all of the others studied to date (5). The mechanical stability of modules with such topology is often determined by a patch of elements of secondary structure highly localized at the breakpoint and which represent the main mechanical barrier to stretching: the mechanical clamp. This is a secondary structural feature located near the ends of the protein and it is formed by backbone hydrogen bonds between long β-strands (and the associated packing interactions), which are shear-ruptured on pulling from the ends (5).
We used two different all-atom MD simulation methods: the “generalized Born surface area” (GBSA) (18) and the “explicit water molecules” (exW) (19). The GBSA method reproduced the hierarchies of mechanical stability in cohesins correctly, whereas the exW approximation did so more roughly (Fig. 4 and Fig. S5; Table S1). Both methods showed that rather than one mechanical clamp of backbone hydrogen bonds, as previously found in most mechanical proteins, cohesins had 2 tandemly arranged mechanical clamps with a higher number of hydrogen bonds in the modules belonging to the connecting region (A-I and A′-I′; 5 + 5 hydrogen bonds for c7A and c1C cohesins and 4 + 3 for the c2A cohesin) (Figs. S5 and S6 and Table S1). Thus, the mechanical stability of cohesins seems to be directly derived from the number of backbone hydrogen bonds present in their mechanical clamps. The presence of mechanical clamps in protein modules from bacteria suggest that this structural/functional motif is a general principle that is widespread in mechanical proteins. Nevertheless, there is more to protein mechanostability than the mechanical clamp motif. Indeed, in some protein folds the hydrophobic core also contributes to mechanical resistance [e.g., fibronectin type III modules from human tenascin (20)]. There are also exceptions to the shear mechanical clamp topology such us the green fluorescent protein (21) and ankyrin B (22). In addition to this secondary-structure-based elasticity, tertiary [e.g., the solenoid of ankyrin B (22)] and quaternary [e.g., the helical rod of Escherichia coli adhesive pili (23) and the myosin II tail (24)] structural elasticities have also been reported.
Fig. 4.
Atomic details of the process of cohesin stretching obtained through all-atom molecular dynamics simulations. Free MD trajectories and steered MD of cohesins or I27 are shown in Movie S1 and Movie S2 using both the GBSA (18, 37–40) and the exW (19) approximations. (A) Steered MD simulations of cohesin stretching by using the GBSA approximation. Different snapshots at every 200 ps were taken as the starting point of several unfolding trajectories. The averages of the force-distance traces are shown. This approximation reproduces the experimental mechanical hierarchy and the unfolding forces are in the same order of magnitude as experimental data (FI27 = 370 ± 80 pN; Fc7A = 710 ± 120 pN; Fc1C = 630 ± 70 pN; and Fc2A = 470 ± 80 pN). (B) Steered MD simulations of cohesin stretching using the exW approximation. As previously reported (19), this approximation yields unfolding forces one order-of-magnitude larger than the experimental results. Also, the relative order of the mechanical stabilities for the cohesin modules under study is less well reproduced than with the GBSA approximation at the same pulling speed (FI27 = 910 ± 50 pN; Fc7A = 1360 ± 50 pN; Fc1C = 1610 ± 30 pN; and Fc2A = 860 ± 50 pN; Table S1). In addition, 2 lower speeds were used, separated by an order of magnitude. The 3 different pulling speeds are represented from left to right (1, 0.1, and 0.01 Å/ps, respectively). (C) Histograms of the unfolding force peaks from several trajectories obtained through the GBSA approximation at 1 Å/ps are shown. (D) One trajectory obtained by the exW approximation at 0.01 Å/ps is presented.
While this study was in progress, an independent report using a theoretical model based on coarse-grained MD simulations surveyed the whole Protein Data Bank (PDB) for mechanostability (PDB; 7,510 modules, 40–150-aa long) and notably predicted the 3 cohesin I modules studied here to be among the top 30 most mechanostable proteins: c7A was 4th, c1C was 3rd, and c2A was 28th (25). However, these simulations failed to predict the correct order of the 2 modules from the connecting region (c7A and c1C). Here, we reanalyzed the details of these large-scale simulations with a refined version of the model (see SI Materials and Methods). After closer scrutiny we found that the crystal PDB file of c7A lacked several contacts for some amino acid side-chains from the mechanical clamps. By using standard fill-in software to complete the file (see SI Materials and Methods), we now conclude that the model predicted a higher mechanical stability for this cohesin than for c1C. Therefore, the reported model can indeed predict the correct order of mechanical stabilities in cohesins (the specific positions with the new model were: c7A, 3rd; c1C, 4th; and c2A, 20th; Fig. S7). This model also identified shear mechanical clamps in cohesins similar to those found in other mechanical modular proteins composed of Ig-like β-sandwiches (Fig. S7 and Table S1). Considering that the first and second positions correspond to 2 streptokinases, which are not attributed mechanical roles, the cohesins from the connecting region (ranked 3rd and 4th) are therefore predicted to be the most mechanostable modules among the mechanical proteins listed in the PDB.
Conclusions and Future Directions
Here we report the nanomechanics of the first modular adhesion protein from bacteria, findings that have key implications for several scientific fields:
In terms of protein nanomechanics, we have identified the most robust mechanical protein structures found to date: the cohesin I modules from the connecting region of scaffoldins. These modules represent the current upper limit of this important biophysical property, and its mechanical topology seems to be an extension of a common principle that involves the presence of shear mechanical clamps of secondary structure fastening the ends of mechanical proteins. It is tempting to speculate that the existence of this feature, previously described in adhesion proteins from metazoans and now reported in those from bacteria, may represent an example of convergent evolution toward a common structural solution to a similar functional problem (i.e., mechanical resistance). We suggest that this structural/functional motif may be a general principle in biology to achieve the necessary mechanostability that mechanical proteins require to maintain their structural integrity in vivo. We also present experimental demonstration of the validity of coarse-grained simulations to predict mechanical stability [this is particularly important because a massive PDB-wide survey using this method has recently been reported (25)]. However, additional experiments will be necessary to further test the robustness of these models.
In terms of cell biology, the remarkable mechanical stability of scaffoldins in the connecting region raises the possibility that this trait may have been adaptive (i.e., subjected to selection pressure), representing an adaptation to resist extremely high axial forces (which would prevent the detachment of cellulases), and it suggests that these proteins may have an additional role as mechanical scaffolds. Moreover, we propose that mechanical stability could serve as a useful parameter to test the vast number of architectural models of supramolecular (quaternary) structure proposed for the overwhelming variety of scaffoldins (i.e., to distinguish between connecting and hanging modules).
In terms of protein engineering and materials science, the extremely high mechanostability of cohesin I modules provides the basic building blocks for the introduction of this variable whenever it may be necessary in protein design for biotechnological or nanotechnological applications. Grafting of mechanical clamps has already been reported (26). In biotechnology, the control of this variable may permit more efficient cellulosomes to be designed for the industrial biodegradation of cellulose (the most abundant organic compound on Earth and hence a promising renewable source of fuel products such as bio-alcohol). Indeed, in the light of our data, it is now possible to select not only thermostable scaffoldins (to work at higher temperatures) but also highly mechanostable modules (e.g., likely resistant to strong stirring). In bio-inspired nanotechnology, the “hyperstability” of some cohesin modules (both thermal and mechanical) is an attractive property that could be used to design sturdy bio-scaffolds (6). Such properties may be exploited for the spatiotemporal coordination of specific enzymatic activities to achieve more efficient degradation of recalcitrant or complex substrates.
The results reported here raise many questions that should be addressed in future studies. For instance, it is important to know to what extent high mechanical stability is a common property of adhesion proteins from motile unicellular organisms, and whether the mechanical clamp motif is present in other bacterial proteins (i.e., both adhesion proteins and other proteins putatively subjected to mechanical stress). In addition, our mechanical hypothesis could be tested by analyzing the nanomechanics of additional cohesins from both the connecting and hanging regions of other scaffoldins, as well as the possible effect of cellulases on cohesin mechanostability. Furthermore, the putative adaptive character of this trait should be tested, measuring both the forces that are held by the connecting and hanging regions in vivo, and the mechanostability of the linkages in the scaffoldin system.
Materials and Methods
Polyprotein engineering was performed with a vector described previously (27) and the scaffoldin constructs were PCR cloned de novo from bacterial genomic DNA. Due to the high degree of conservation among its cohesin I modules (28), the C. thermocellum CipA scaffoldin construct was sequenced by using a method based on nested deletions. The AFM used in this study is a slightly modified version of a previously described home-made instrument (29) with added imaging capabilities (30). Previously established methods were followed to measure thermal stability (31) and the 3 types of MD simulations used here: coarse-grained (25) and all-atom with implicit (18) and explicit (19) solvent.
A full description methods and any associated references are available in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank J. Gómez-Herrero for help in AFM instrumentation and kindly housing our AFM, and E. Bayer, A. Oberhauser, and S. García-Manyes for critically reading the manuscript. We thank also J. Clarke (University of Cambridge, Cambridge, United Kingdom) for kindly providing the pRSETA-(I27)8 vector used for protein expression; P. Béguin (Pasteur Institute, Paris) for the pCip1 and pCip7 clones; and H.-P. Fierobe (CNRS, Marseille, France) for the pET-coh1B clone. This work was funded by from Ministerio de Ciencia e Innovación Grant BIO2007-67116, Consejería de Educación de la Comunidad de Madrid Grant S-0505/MAT/0283, and Consejo Superior de Investigaciones Científicas Grant 200620F00 (to M.C.-V.); Ministerio de Ciencia e Innovación Grant BFU2006-10288 and Consejería de Educación de la Comunidad de Madrid Grant S-BIO-0260 (to M.M.); and Ministry of Science and Higher Education Grant N N202 0852 33, and European Union within European Regional Development Fund Grant POIG.01.01.02-00-008/08 (to M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813093106/DCSupplemental.
References
- 1.Rief M, Gautel M, Oesterhelt F, Fernández JM, Gaub HE. Reversible unfolding of individual titin Ig domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 2.Carrión-Vázquez M, et al. Mechanical design of proteins studied by single-molecule force spectroscopy and protein engineering. Prog Biophys Mol Biol. 2000;74:63–91. doi: 10.1016/s0079-6107(00)00017-1. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante C, Chemla YR, Forde NR, Izhaky D. Mechanical processes in biochemistry. Annu Rev Biochem. 2004;73:705–748. doi: 10.1146/annurev.biochem.72.121801.161542. [DOI] [PubMed] [Google Scholar]
- 4.Forman JR, Clarke J. Mechanical unfolding of proteins: Insights into biology, structure and folding. Curr Opin Struct Biol. 2007;17:58–66. doi: 10.1016/j.sbi.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Oberhauser AF, Carrión-Vázquez M. Mechanical biochemistry of proteins one molecule at a time. J Biol Chem. 2008;283:6617–6621. doi: 10.1074/jbc.R700050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer EA, Belaich JP, Shoham Y, Lamed R. The cellulosomes: Multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol. 2004;58:521–554. doi: 10.1146/annurev.micro.57.030502.091022. [DOI] [PubMed] [Google Scholar]
- 7.Adams JJ, Pal G, Jia Z, Smith SP. Mechanism of bacterial cell-surface attachment revealed by the structure of cellulosomal type II cohesin-dockerin complex. Proc Natl Acad Sci USA. 2006;103:305–310. doi: 10.1073/pnas.0507109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ofir K, et al. Versatile protein microarray based on carbohydrate-binding modules. Proteomics. 2005;5:1806–1814. doi: 10.1002/pmic.200401078. [DOI] [PubMed] [Google Scholar]
- 9.Rincon MT, et al. Unconventional mode of attachment of the Ruminococcus flavefaciens cellulosome to the cell surface. J Bacteriol. 2005;187:7569–7578. doi: 10.1128/JB.187.22.7569-7578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madkour M, Mayer F. Structural organization of the intact bacterial cellulosome as revealed by electron microscopy. Cell Biol Int. 2003;27:831–836. doi: 10.1016/s1065-6995(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 11.Tavares GA, Béguin P, Alzari PM. The crystal structure of a type I cohesin domain at 1.7 Å resolution. J Mol Biol. 1997;273:701–713. doi: 10.1006/jmbi.1997.1326. [DOI] [PubMed] [Google Scholar]
- 12.Spinelli S, et al. Crystal structure of a cohesin module from Clostridium cellulolyticum: Implications for dockerin recognition. J Mol Biol. 2000;304:189–200. doi: 10.1006/jmbi.2000.4191. [DOI] [PubMed] [Google Scholar]
- 13.Shimon LJ, et al. A cohesin domain from Clostridium thermocellum: The crystal structure provides new insights into cellulosome assembly. Structure. 1997;5:381–390. doi: 10.1016/s0969-2126(97)00195-0. [DOI] [PubMed] [Google Scholar]
- 14.Li H, et al. Multiple conformations of PEVK proteins detected by single-molecule techniques. Proc Natl Acad Sci USA. 2001;98:10682–10686. doi: 10.1073/pnas.191189098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, et al. Reverse engineering of the giant muscle protein titin. Nature. 2002;418:998–1002. doi: 10.1038/nature00938. [DOI] [PubMed] [Google Scholar]
- 16.Dietz H, Berkemeier F, Bertz M, Rief M. Anisotropic deformation response of single protein molecules. Proc Natl Acad Sci USA. 2006;103:12724–12728. doi: 10.1073/pnas.0602995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dugdale TM, Dagastine R, Chiovitti A, Wetherbee R. Diatom adhesive mucilage contains distinct supramolecular assemblies of a single modular protein. Biophys J. 2006;90:2987–2993. doi: 10.1529/biophysj.105.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsui V, Case DA. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers. 2001;56:275–291. doi: 10.1002/1097-0282(2000)56:4<275::AID-BIP10024>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Isralewitz B, Krammer A, Vogel V, Schulten K. Unfolding of titin immunoglobulin domains by steered molecular dynamics simulation. Biophys J. 1998;75:662–671. doi: 10.1016/S0006-3495(98)77556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng SP, et al. Mechanical unfolding of TNfn3: The unfolding pathway of a fnIII domain probed by protein engineering, AFM and MD simulation. J Mol Biol. 2005;350:776–789. doi: 10.1016/j.jmb.2005.04.070. [DOI] [PubMed] [Google Scholar]
- 21.Dietz H, Rief M. Exploring the energy landscape of GFP by single-molecule mechanical experiments. Proc Natl Acad Sci USA. 2004;101:16192–16197. doi: 10.1073/pnas.0404549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, et al. Nanospring behaviour of ankyrin repeats. Nature. 2006;440:246–249. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- 23.Miller E, Garcia T, Hultgren S, Oberhauser AF. The mechanical properties of E. coli type 1 pili measured by atomic force microscopy techniques. Biophys J. 2006;91:3848–3856. doi: 10.1529/biophysj.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwaiger I, Sattler C, Hostetter DR, Rief M. The myosin coiled-coil is a truly elastic protein structure. Nat Mater. 2002;1:232–235. doi: 10.1038/nmat776. [DOI] [PubMed] [Google Scholar]
- 25.Sulkowska JI, Cieplak M. Mechanical stretching of proteins—A theoretical survey of the Protein Data Bank. J Phys: Condens Matter. 2007;19:283201–283261. [Google Scholar]
- 26.Borgia A, Steward A, Clarke J. An effective strategy for the design of proteins with enhanced mechanical stability. Angew Chem Int Ed Engl. 2008;47:6900–6903. doi: 10.1002/anie.200801761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steward A, Toca-Herrera JL, Clarke J. Versatile cloning system for construction of multimeric proteins for use in atomic force microscopy. Prot Sci. 2002;11:2179–2183. doi: 10.1110/ps.0212702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerngross UT, Romaniec MP, Kobayashi T, Huskisson NS, Demain AL. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of internal homology. Mol Microbiol. 1993;8:325–334. doi: 10.1111/j.1365-2958.1993.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 29.Schlierf M, Li H, Fernandez JM. The unfolding kinetics of ubiquitin captured with single-molecule force-clamp techniques. Proc Natl Acad Sci USA. 2004;101:7299–7304. doi: 10.1073/pnas.0400033101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valbuena A, et al. Quasi-simultaneous imaging/pulling analysis of single polyprotein molecules by atomic force microscopy. Rev Sci Instrum. 2007;78:113707. doi: 10.1063/1.2794732. [DOI] [PubMed] [Google Scholar]
- 31.Varea J, et al. Do sequence repeats play an equivalent role in the choline-binding module of pneumococcal LytA amidase? J Biol Chem. 2000;275:26842–26855. doi: 10.1074/jbc.M004379200. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 33.Bustamante C, Marko JF, Siggia ED, Smith S. Entropic elasticity of lambda-phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- 34.Marko JF, Siggia ED. Stretching DNA. Macromolecules. 1995;28:8759–8770. [Google Scholar]
- 35.Carrion-Vazquez M, et al. Mechanical and chemical unfolding of a single protein: A comparison. Proc Natl Acad Sci USA. 1999;96:3694–3699. doi: 10.1073/pnas.96.7.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberhauser AF, Hansma PK, Carrion-Vazquez M, Fernandez JM. Stepwise unfolding of titin under force-clamp atomic force microscopy. Proc Natl Acad Sci USA. 2001;98:468–472. doi: 10.1073/pnas.021321798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonson T. Macromolecular electrostatics: Continuum models and their growing pains. Curr Opin Struct Biol. 2001;11:243–252. doi: 10.1016/s0959-440x(00)00197-4. [DOI] [PubMed] [Google Scholar]
- 38.Bashford D, Case DA. Generalized born models of macromolecular solvation effects. Ann Rev Phys Chem. 2000;51:129–152. doi: 10.1146/annurev.physchem.51.1.129. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins GD, Cramer CJ, Truhlar DG. Pairwise solute descreening of solute charges from dielectric medium. Chem Phys Lett. 1995;246:122–129. [Google Scholar]
- 40.Hawkins GD, Cramer CJ, Truhlar DG. Parametrized models of aqueous free energies of solvation based on pairwise descreening of solute atomic charges from a dielectric medium. J Phys Chem. 1996;100:19824–19839. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.