SUMMARY
Protein Kinase D (PKD) mediates signal transduction downstream from phospholipase C and diacylglycerol (DAG). PKDs are activated by hormones and stresses in cell lines, but little is known about PKD functions, regulators and effectors in vivo. Here, we show that DKF-2, a C. elegans PKD, regulates innate immunity. Animals lacking DKF-2 are hypersensitive to killing by bacteria that are C. elegans and human pathogens. DKF-2 induces >75 mRNAs, which encode anti-microbial peptides and proteins that sustain intestinal epithelium. Induction of immune effector mRNAs by DKF-2 proceeds via PMK-1 (p38 Map-kinase)-dependent and independent pathways. TPA-1, a PKCδ homolog, regulates activation and functions of DKF-2 in vivo. DKF-2 provides a novel molecular link that couples DAG signaling to regulation of immunity. A newly-discovered intersection between DAG/TPA-1/DKF-2 and PMK-1 pathways enables integrated immune responses to multiple stimuli.
INTRODUCTION
Diacylglycerol (DAG) is generated when hormones or other stimuli activate phospholipase Cβ (PLCβ), PLCγ, or PLCε. DAG binds and promotes activation of 8 protein kinase C isoforms: PKCs α, βI, βII and γ are activated by DAG and Ca2+; PKCs δ, ε, η and θ are stimulated by DAG alone (Newton, 2003). PKCs regulate physiological processes by phosphorylating Ser or Thr hydroxyl groups in effector proteins. DAG signals are also disseminated by Ser/Thr protein kinases named protein kinase D (PKD) (Rozengurt et al., 2005; Wang, 2006). Three genes encode homologous PKD isoforms (PKDs 1–3) in mammals. Like PKCs, PKDs have a C terminal kinase domain preceded by C1 domains, which bind DAG or phorbol 12-myristate 13-acetate (PMA). Upon PLC activation, C1 domains mediate translocation of PKDs and PKCs from cytoplasm to DAG-enriched membranes. DAG-activated PKC phosphorylates the PKD activation loop (A-loop), thereby switching on D kinase catalytic activity (Rozengurt et al., 2005). Thus, PKDs are PKC effectors.
PKDs convert transient DAG signals into prolonged physiological effects because activity persists (min to h) as the kinases translocate to various intracellular locations (Rozengurt et al., 2005; Wang, 2006). Since PKDs and PKCs phosphorylate different substrates (Nishikawa et al., 1997), PKDs place novel effectors and distinct physiological processes under DAG-control.
Hormones that bind Gq coupled receptors elicit PKD activation in cell lines (Rozengurt et al., 2005), suggesting PKDs regulate many facets of mammalian physiology. In cell culture, PKDs (a) mediate pro-survival signaling (NFκB activation) induced by oxidative stress; (b) control fission of Golgi membrane vesicles; (c) modulate antigen-activated signaling in B and T cells; (d) regulate JNK/c-jun dependent proliferation; (e) govern motility and adhesion; and (f) promote nuclear export of type II histone deacetylases (HDACs) (Rozengurt et al., 2005; Vega et al., 2004; Wang, 2006). HDACs 5 and 7 are PKD substrates; their phosphorylation de-represses gene transcription.
It is not known if (a) roles assigned to PKDs are major or minor functions of D kinases in normal cells, or (b) PKDs regulate NFκB, vesicle fission etc ubiquitously or in a few tissues. For example, HDACs were not phosphorylated when PKCs were activated in PKD-deficient, avian B lymphoma cells (Liu et al., 2007). However, proliferation, oxidative stress signaling, c-jun activity and HDAC nuclear export were not affected. Thus, PKD functions remain undiscovered in a model B cell. Likewise, little is known about PKD functions and effectors in normal tissues of intact animals. Thus, elucidation of in vivo regulation, physiological roles and effectors of PKDs are major challenges in comprehending DAG-regulated signal transduction.
Problems in PKD-mediated signaling can be addressed by studying C. elegans. C. elegans employs signaling molecules, mechanisms and pathways that are conserved in mammals (Kim et al., 2002; Nicholas and Hodgkin, 2004; Schulenburg et al., 2004). Recently, we characterized a 120 kDa C. elegans PKD named DKF-2 (for D Kinase Family) (Feng et al., 2007); the corresponding gene is named dkf-2. DKF-2 (1070 amino acids) and human PKDs contain conserved regulatory (C1) and kinase domains that are 75% identical in amino acid sequence (Fig 1A). In transfected cells, DKF-2 binds DAG or PMA at plasma membrane and is activated when PKC phosphorylates two A-loop serines (Feng et al., 2007). Human and C. elegans PKDs have similar substrate specificities. Thus, DKF-2 is a prototypical PKD.
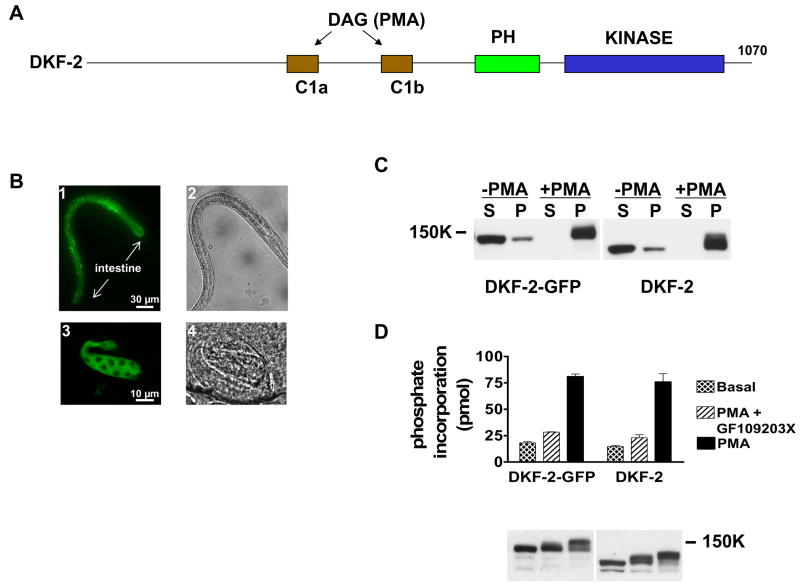
Figure 1. DKF-2-GFP Is Fully Functional and Selectively Expressed in Intestine.
A depicts locations of C1, PH and kinase domains along the DKF-2 polypeptide. B, animals expressing a dkf-2::DKF-2-GFP transgene were created. GFP-derived fluorescence was recorded using a Zeiss Axio Imager Z1 microscope and AxioVision software. Arrows mark anterior and posterior ends of the intestine. B1 (L2 larva) and B3 (late embryo) show DKF-2-GFP is dispersed in intestinal cells. B2 and B4 are Nomarski interference images of B1 and B3, respectively. C is a Western immunoblot containing cytosolic (S) and membrane (P) proteins (30 μg/lane) isolated from HEK293 cells expressing DKF-2-GFP (lanes 1–4) or DKF-2 (lanes 5–8). Cells were incubated with 0.3 μM PMA (10 min) as indicated. Cytosol and membranes were isolated as previously reported (Feng et al., 2007). The blot was probed with anti-DKF-2 IgGs and chemiluminescence signals were recorded on X-ray film. D, transfected cells expressing DKF-2-GFP or DKF-2 were treated with 0.3 μM PMA or vehicle (basal) for 10 min. Duplicate samples of cells were incubated with pan-PKC inhibitor GF109203X for 1 h prior to PMA addition. Cells were lysed, and DKF-2 proteins were immunoprecipitated and assayed for catalytic activity as described in Experimental Procedures. An immunoblot shows similar amounts of DKF-2 were used in each assay (D, lower panel). Increases in apparent Mr of DKF-2 in PMA-treated cells (C, D) are due to phosphorylation catalyzed by PKCs and other protein kinases.
C. elegans homozygous for a 1.5 kb deletion in the dkf-2 gene (dkf-2(pr3) allele) has a null phenotype: DKF-2 protein is not expressed (Feng et al., 2007). However, the animals are viable, fertile and develop normally. Consequently, null animals and null mutants re-constituted with a WT dkf-2 transgene can be exposed to stimuli to (a) discover physiological roles of DKF-2, (b) identify regulators and effectors of DKF-2 and (c) illuminate mechanisms by which DKF-2 controls biological processes.
We discovered that DKF-2 contributes to C. elegans innate immunity by inducing expression of genes that protect against pathogenic bacteria. TPA-1, a PKCδ homolog, activates DKF-2 in vivo. PMK-1, a p38 MAP-kinase homolog, is essential for anti-microbial activity of DKF-2.
RESULTS
DKF-2 Is Differentially Expressed in Intestine
A 1.4 kb segment of genomic DNA, which flanks the 5′ end of the dkf-2 structural gene, was cloned upstream from a green fluorescent protein (GFP) reporter gene in a C. elegans expression vector. The minigene is designated dkf-2::GFP (promoter/enhancer DNA is shown in lower case italics; DNA encoding protein sequence is identified by upper case lettering). Several lines of transgenic C. elegans exhibited robust dkf-2 promoter activity in 18 cells that constitute the intestine (Fig. S1). Modest GFP fluorescence was detected in 4 neurons (Fig. S1, asterisks). The dkf-2 promoter also directed synthesis of full-length DKF-2-GFP in intestinal cells of late embryos, larvae and adult C. elegans. The kinase was dispersed in cytoplasm and slightly enriched along apical surfaces of cells (Fig 1B). Weak fluorescence was detected in neurons described in Fig. S1 when exposure time was increased (not shown). DKF-2-GFP was not evident in other cells.
DKF-2-GFP was expressed in HEK293 cells. A DAG surrogate, PMA, induced transfer of DKF-2-GFP from cytoplasm to membranes and elicited a 4–5-fold increase in catalytic activity (Figs. 1C, 1D). PMA-induced kinase activation was abolished by incubating cells with GF109203X, an inhibitor of DAG-activated PKCs. Properties of DKF-2-GFP and WT DKF-2 were similar (Figs. 1C, 1D), demonstrating that the fusion protein is properly folded, correctly regulated and fully functional.
DKF-2 Regulates Responses to Ingested Bacterial Pathogens
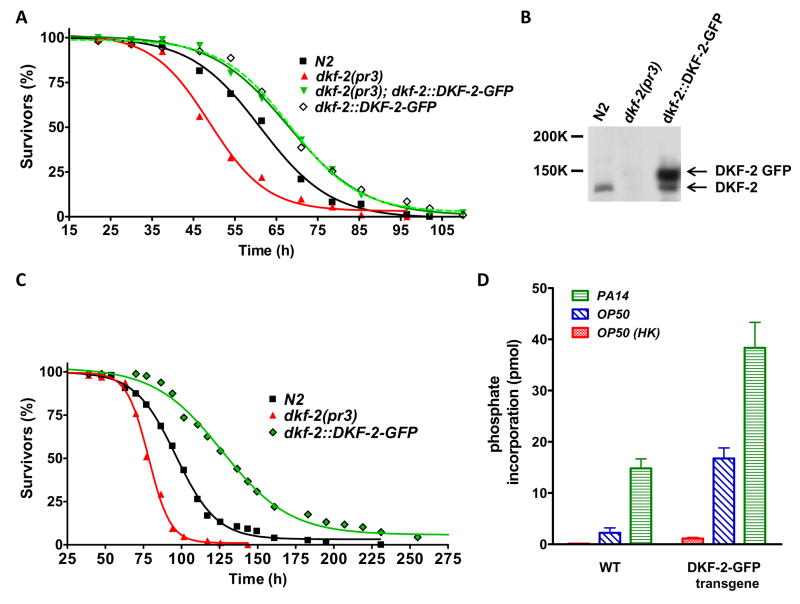
C. elegans feeds on soil microbes and uses an innate immune system to eliminate pathogenic bacteria (Nicholas and Hodgkin, 2004; Schulenburg et al., 2004). The nematode’s intestinal cells contribute many effectors of innate immunity and provide a model for studying host defense mechanisms against human pathogens. To determine if PKD affects pathogen resistance, WT, dkf-2(pr3) null animals and transgenic C. elegans expressing elevated DKF-2-GFP (WT background) were fed P. aeruginosa strain 14 (PA14). DKF-2 depleted C. elegans was hypersensitive to killing by Gram negative PA14 (Fig. 2A). Half of dkf-2 null animals died after 48 h and only 18% survived 62 h. In contrast, 79% and 50% of WT C. elegans were alive after feeding on PA14 for 48 and 62 h, respectively. An integrated transgene directed accumulation of DKF-2-GFP to a level that exceeded WT DKF-2 by ~3.6-fold (Fig. 2B). Elevated DKF-2 protected C. elegans, supporting 92% and 71% survival after animals consumed PA14 for 48 and 62 h, respectively (Fig. 2A). Expression of DKF-2-GFP in a dkf-2(pr3) null background caused a similar increase in PA14 resistance (Fig. 2A).
Figure 2. DKF-2 Deficient Animals Are Hypersensitive to Pathogenic Bacteria; Elevated DKF-2-GFP Expression Confers Pathogen Resistance.
A shows survival curves for WT (N2), dkf-2(pr3) null, transgenic (dkf-2::DKF-2-GFP and dkf-2(pr3);dkf-2::DKF-2-GFP) C. elegans fed with PA14. Assays were replicated 4 times with similar results; typical data are shown. B, proteins (30 μg/lane) from the indicated animals were separated by denaturing electrophoresis and transferred to a PVDF membrane. The blot was probed with anti-DKF-2 IgGs. C shows survival curves for animals fed with E. faecalis OG1RF. Assays were replicated 3 times with similar results. D, WT or transgenic animals were fed with OP50, OP50 HK or PA14 as indicated. Animals were disrupted in a French press, using buffer containing 1% Triton-X100. DKF-2 and DKF2-GFP were immunoprecipitated from samples containing 0.5 mg protein (from equal numbers of animals) by anti-DKF-2 IgGs. Precipitated proteins were assayed for kinase activity.
Dkf-2 null animals were hypersensitive to killing by a Gram positive pathogen, E. faecalis OG1RF. After feeding on OG1RF for 94 h, only 9% of DKF-2 depleted animals survived, whereas 57% of WT C. elegans and 93% of animals over-expressing DKF-2-GFP were alive (Fig. 2C). S50 values (hours of pathogen ingestion that yield 50% survivors) were 77, 97 and 131 h for dkf-2(pr3) null, WT and transgenic animals, respectively. Transition from DKF-2 deficiency to DKF-2 surfeit extended survival time ~70% in a toxic environment. Thus, DKF-2, a DAG-controlled PKD, upregulates innate immunity.
Pathogens Potently Activate DKF-2
WT and transgenic animals were fed PA14 (strong pathogen), E. coli OP50 (standard food, but a mild pathogen) or nonpathogenic, heat killed OP50 (OP50 HK). In both WT C. elegans and animals expressing elevated DKF-2-GFP, D kinase activity was a) extremely low when OP50 HK was consumed, b) markedly increased by a diet of living OP50 and c) induced to maximal levels by ingestion of PA14 (Fig. 2D). Signaling pathways governing DKF-2 activity were almost completely inactive when food was non-pathogenic. Weak and strong pathogens differentially induced intermediate and large increases, respectively, in catalytic activity of endogenous DKF-2. PA14 infection increased DKF-2 kinase activity 6.5-fold relative to activity in animals fed OP50 (Fig. 2D). The increase is ~100- fold relative to DKF-2 activity in animals consuming OP50 HK.
In parallel with the increase in DKF-2-GFP protein (Fig. 2B), transgenic animals fed OP50 expressed a 7-fold higher level of D kinase activity than WT, OP50-fed C. elegans (Fig. 2D). PA14 infection elicited a further 2.6-fold increase in kinase activity in animals expressing DKF-2-GFP. Maximal PA14-stimulated D kinase activity in transgenic nematodes is ~300-fold higher than DKF-2 catalytic activity in WT animals fed OP50 HK. In transgenic animals, over- expressing DKF-2-GFP, constitutive high-level expression of DKF-2 kinase activity prior to infection and further (2.6-fold) amplification of enzyme activity upon PA14 infection are linked to enhanced protection against pathogen mediated killing (Figs. 2A).
Although dkf-2(pr3) null animals are hypersensitive to PA14 mediated killing (Fig. 2A), they live 40% longer than WT C. elegans on plates containing viable OP50 or OP50 HK ((Feng et al., 2007) (Fig. S2A). In contrast, increased DKF-2 activity promoted resistance to PA14, but slightly diminished lifespan (S50 declined by 0.5–1 day) when animals were fed viable OP50 or OP50 HK (Fig. S2B). Opposing effects of diminished and increased DKF-2 activity on lifespan and pathogen resistance show that PA14 killing assays reflect the impact of DKF-2 on the defense against attacking pathogens, not DKF-2 dependent changes in aging.
DKF-2 Induces Expression of 85 Genes that Defend against Bacterial Infection
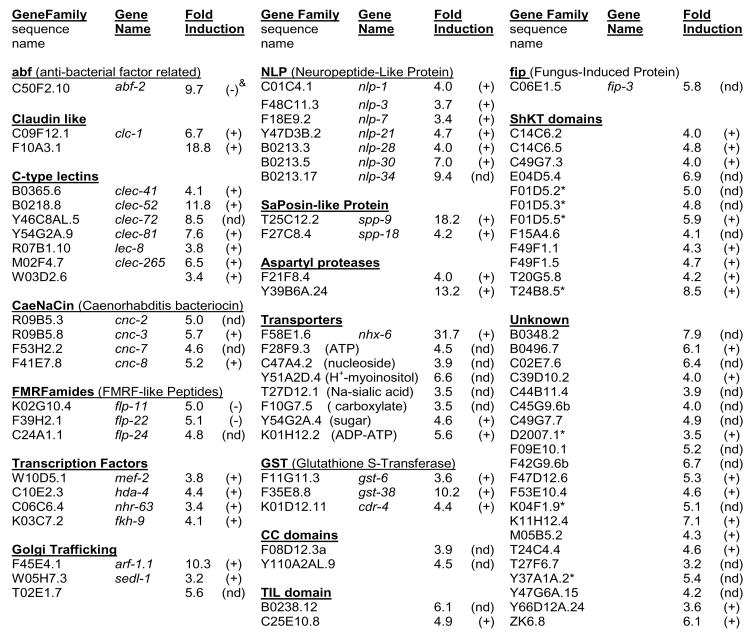
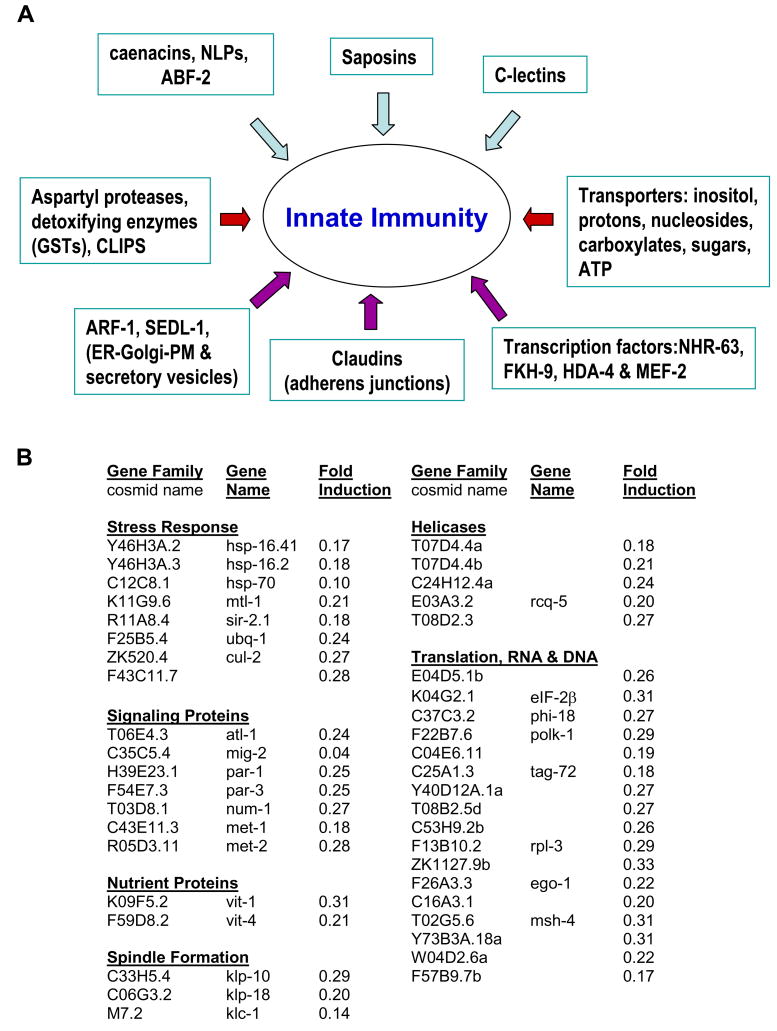
When bacterial pathogens populate the intestinal lumen, C. elegans induces gene expression that promotes survival (Nicholas and Hodgkin, 2004; Schulenburg et al., 2004; Troemel et al., 2006). A pool of activated, intestinal DKF-2-GFP enhanced pathogen resistance in transgenic animals (Figs. 1B, 1D and 2). Therefore, genome-wide microarray analysis was used to determine if DKF-2 induces mRNAs encoding immune effectors. We discovered that increased DKF-2 activity induced 3–30-fold increases in 85 mRNAs (Fig. 3). Many DKF-2 induced mRNAs encode proteins involved in innate immunity (Schulenburg et al., 2004). These proteins constitute 8 classes of effectors that apparently produce an integrated response to bacterial pathogens (Fig. 4A).
Figure 3. DKF-2 Induces 3-30-fold Increases in 85 mRNAs.
Microarray analysis measured the abundance of mRNAs in animals expressing an integrated dkf-2::DKF-2-GFP transgene (relative to mRNA levels in WT C. elegans). Animals were fed E. coli OP50. & Symbols in parenthesis indicate: (+), mRNA expressed in intestine; (nd), mRNA expression pattern not determined; (−) mRNA expressed in various cells, but not intestine. Designations are based on data provided by WormBase (wormbase.org) and the Genome Sciences Center MultiSAGE C. elegans database (elegans.bcgsc.ca).
Figure 4. DKF-2 Mobilizes an Integrated Response to Pathogens.
A, DKF-2 induced 8 categories of effectors that collectively provide an integrated immune response to bacterial infection. B, DKF-2 activation sharply diminished levels of >40 mRNAs.
DKF-2 activation diminished expression of genes controlling non-immune stress responses (Fig. 4B). mRNAs encoding heat shock proteins (HSPs 16.2, 16.41 and 70), toxic metal induced metallothionein (MTL-1), ubiquitin and ubiquitin E3 ligases (UBQ-1, CUL-2 and F43C11.7) declined 70–90%. Transcripts encoding proteins that govern DNA repair, chromatin remodeling, RNA and DNA unwinding and modification, nutrient delivery to oocytes (vitellogenins), meiotic spindle formation and protein translation were also suppressed 70–95%.
DKF-2 Induces mRNAs that Increase in PA14-infected C. elegans
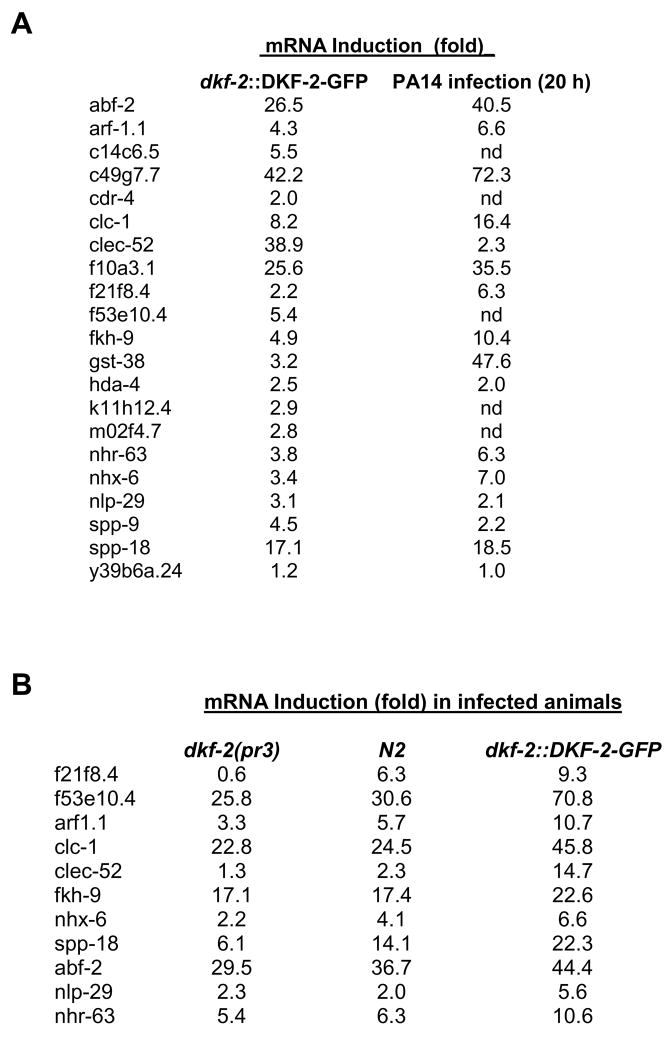
Effects of DKF-2 on gene expression were independently assessed by quantitative real-time PCR (qRT-PCR) analysis. Levels of 20 mRNAs (selected from Fig. 3) were measured in WT C. elegans and animals over-expressing DKF-2-GFP. Concentrations of selected mRNAs increased 2 to 42-fold when DKF-2 activity was elevated (Fig. 5A). Large, DKF-2 dependent increases in abundance of mRNAs encoding CLEC-52, ABF-2 and SPP-18 anti-microbial proteins were documented (Fig. 5A). Although levels vary somewhat between microarray and qRT-PCR experiments (Figs. 3, 5A), the data demonstrate that DKF-2 potently induces multiple classes of immune effector mRNAs.
Figure 5. DKF-2 Induced mRNAs Are Up-regulated by PA14 Infection; Levels of Pathogen-induced Transcripts Are Modulated by DKF-2 in Infected Animals.
A shows induction of mRNAs caused by increased DKF-2 activity or PA14 infection. qRT-PCR data are normalized to actin mRNA and expressed as fold-induction (mRNA concentration in transgenic or infected animals ÷ mRNA concentration in WT animals); nd, not determined. B shows induction of mRNAs in DKF-2 deficient, WT and transgenic (dkf-2::DKF-2-GFP) animals infected with PA14 (20 h). Normalized qRT-PCR data are expressed as fold-induction (mRNA concentration in indicated infected animals ÷ mRNA concentration in uninfected animals). Experiments in A and B were repeated twice, yielding similar results.
Are DKF-2 regulated mRNAs elevated in PA14-infected C. elegans? We measured levels of 15 candidate DKF-2/PA14-induced mRNAs by qRT-PCR. DKF-2 activation and PA14 infection increased the abundance of 12 mRNAs (Fig. 5A). Two transcripts were divergently regulated: DKF-2 elevated CLEC-52 mRNA 39-fold, whereas PA14 infection caused a 2.3-fold increase; conversely, PA14 and DKF-2 triggered 48- and 3.2-fold elevations, respectively, in GST-38 mRNA. Thus, most DKF-2 effectors contribute to host responses to PA14 infection. Some DKF-2 controlled proteins may target different pathogens or regulate distinct facets of cell physiology. A limited ability to induce GST-38 and other immune effector mRNAs (see below) suggests DKF-2 regulates expression of a subset of anti-pathogen genes.
DKF-2 regulates accumulation of PA14-induced transcripts during infection
Pathogen proliferation in intestinal lumen elicits immune responses by activating the PMK-1, DBL-1 (TGFβ) and other signaling pathways (Nicholas and Hodgkin, 2004; Schulenburg et al., 2004). Since concentrations of PA14-induced mRNAs can be controlled by two or more signaling systems, logical predictions are: (1) increased DKF-2 activity will synergize with infection to promote supra-normal accumulation of some PA14-induced mRNAs and (2) DKF-2 deficiency will reduce (but not eliminate) PA14-mediated induction of a subset of immune effector mRNAs.
Predictions were tested by quantifying levels of 11 pathogen-induced mRNAs after feeding dkf-2(pr3), WT and transgenic (dkf-2::DKF-2-GFP) C. elegans with PA14. Enhanced DKF-2 activity elicited increases in levels of all mRNAs (Fig. 5B). Elimination of DKF-2 significantly reduced the abundance of 7 PA14-induced mRNAs encoding anti-microbial proteins. Thus, DKF-2 regulates the amplitude of accumulation of immune effector mRNAs induced by bacterial pathogen.
DKF-2 Controls mRNA Levels by P38-dependent and Independent Pathways
A protein kinase cascade, composed of NSY-1, SEK-1 and PMK-1 (homologs of human ASK-1, MEK3/6 and p38 MAP-kinase, respectively), plays a critical role in innate immunity (Kim et al., 2002). Mutations in nsy-1, sek-1 or pmk-1 cause supersensitivity to killing by pathogens. Since a) DKF-2 induced several PMK-1-regulated mRNAs (Troemel et al., 2006)) (Fig. 3, asterisks), b) DKF-2-regulated mRNAs increase in PA14 infected animals (Fig. 5) and c) PMK-1 mediates immune responses to PA14, we determined if signals disseminated by DKF-2 intersect with the PMK-1 pathway.
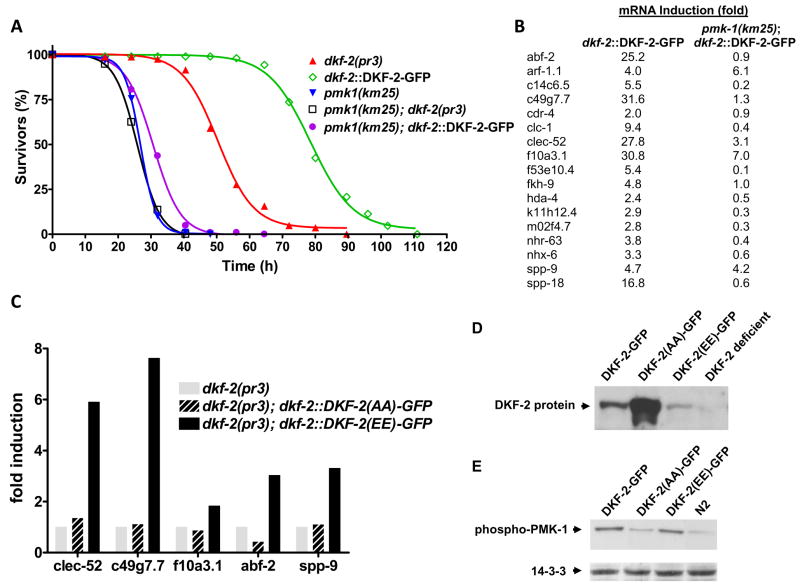
After feeding on PA14 for 72 h, 75% of animals expressing elevated DKF-2-GFP protein and activity survived, but nearly all DKF-2 depleted animals died (Fig. 6A). S50 decreased from 80 to 50 h. PA14 infection caused populations of PMK-1 deficient animals to decline more rapidly (S50 = 27h, Fig. 6A). However, dkf-2::DKF-2-GFP transgene expression only slightly altered the pmk-1(km25) null phenotype; PA14 killed all animals within 48 h (S50 = 31 h, Fig. 6A). Survival curves for pmk-1(km25) null and pmk-1(km25), dkf-2(pr3) double null mutants were similar. Thus, PMK-1 is indispensable for DKF-2 induced innate immunity. The results reveal a novel intersection between DAG-controlled and p38-mediated signal transduction pathways.
Figure 6. A-loop Phosphorylation and PMK-1 Are Essential for Induction of Immune Effector mRNAs by DKF-2.
A shows survival curves for the indicated mutant and transgenic animals upon feeding with PA14. B, amounts of 17 mRNAs were quantified (qRT-PCR) in animals expressing dkf-2::DKF-2-GFP in WT or pmk-1(km25) null backgrounds. Fold-induction is: mRNA concentration in a specified transgenic animal ÷ mRNA concentration in WT C. elegans. C shows effects of DKF-2(A925A929)-GFP and DKF-2(E925E929)-GFP expression on levels of 5 mRNAs in a dkf-2(pr3) background. qRT-PCR data are expressed as fold-induction. D, 30 μg total protein from the indicated WT, null and transgenic strains of C. elegans were assayed by Western immunoblot analysis. The blot was probed with anti-DKF-2 IgGs; DKF-2 polypeptides were detected via chemiluminescence. E, A Western blot (prepared as described in D) was probed with IgGs directed against the di-phosphorylated A-loop of PMK-1 (Promega). Phospho-PMK-1 was detected by chemiluminescence. 14-3-3 protein is a loading control. Experiments in Fig. 6 were performed 3 times, yielding similar results.
Loss of PMK-1 could ablate expression of a few critical DKF-2 effectors or suppress accumulation of many DKF-2 inducible mRNAs. To distinguish between these possibilities, we measured amounts of 17 DKF-2 regulated mRNAs in animals expressing a dkf-2::DKF-2-GFP transgene in WT or pmk-1 null backgrounds. PMK-1 deficiency eliminated or suppressed induction of 15 mRNAs (Fig. 6B). Thus, PMK-1 links DKF-2 to a majority of its downstream effectors. Knowledge of p38 signaling was advanced by adding 15 genes (Fig. 6B) to the constellation of PMK-1 effectors. DKF-2 promoted large increases in ARF-1.1, SPP-9 and F10A3.1 mRNA levels in animals lacking PMK-1. Thus, DKF-2 controls expression of immune effectors by PMK-1 dependent and independent mechanisms.
When mutations inhibit the daf-2/age-1 signaling pathway, DAF-16 (a target transcription factor) accumulates in nuclei. DAF-16 elicits gene expression that increases C. elegans lifespan and imparts resistance to pathogens (Garsin et al., 2003). DKF-2 depletion enhances accumulation of DAF-16 in gut nuclei and increases lifespan ~40% (Feng et al., 2007). If DAF-16 mediates regulation of immunity by DKF-2, then a daf-16(mgDf50) loss-of-function mutant will be hypersensitive to killing by PA14.; dkf-2(pr3) null animals should exhibit enhanced resistance to PA14. However, PA14 killed WT and daf-16(mgDf50) mutants with similar kinetics (Fig. S3). DKF-2 depleted animals are hypersensitive, not resistant, to PA14 (Fig. 2A). Survival curves for DKF-2 deficient animals and dkf-2(pr3) null, daf-16(mgDf50) double mutants were similar (Fig. S3). Moreover, elevated DKF-2 activity increased PA14 resistance in WT (Fig. 2A) and daf-16(mgDf50) mutant (not shown) backgrounds. Thus, DKF-2 induces immune effector mRNAs via PMK-1 dependent and independent pathways, but not by DAF-16 mediated transcription.
A-loop Phosphorylation Is Required for DKF-2 Mediated Induction of Anti-microbial mRNAs
Non-activated PKDs control some physiological processes by serving as scaffolds (Medeiros et al., 2005; Zhang et al., 2005). To determine if DKF-2 kinase activity is required to induce immune effectors, we characterized animals expressing dkf-2::DKF-2(A925A929)-GFP and dkf-2::DKF-2(E925E929)-GFP transgenes in a dkf-2 null background. PKC-catalyzed phosphorylation of S925 and S929 in the A-loop switches on DKF-2 catalytic activity (Feng et al., 2007). Consequently, DKF-2(A925A929))-GFP cannot be activated. DKF-2(E925E929)-GFP is constitutively active because E for S substitutions mimic phosphorylation of A-loop serines. We tested the mutant kinases’ ability to induce immune effector mRNAs. DKF-2(A925A929))-GFP was abundantly expressed (Fig. 6D), but effector mRNA levels were not altered (Fig. 6C). A low level of DKF-2(E925E929)-GFP accumulated in transgenic animals (15% relative to WT DKF-2). However, immune effector mRNA levels increased 2–7-fold in animals expressing constitutively active D kinase. Thus, A-loop phosphorylation and DKF-2 activity are required in vivo to induce expression of genes involved in combating pathogens.
DKF-2 promotes activation of PMK-1
A-loop phosphorylation at Thr191 and Tyr193 switches on PMK-1 catalytic activity. Thus, PMK-1 activity was monitored by Western immunoblot analysis, using IgGs that bind a di-phosphorylated A-loop peptide shared by human p38 MAP kinase and C. elegans PMK-1 (Fig. 6E). PMK-1 activity was low in WT C. elegans and animals over-expressing DKF-2(A925A929))-GFP (dkf-2 null background). However, elevated expression of WT DKF-2-GFP or reconstitution of a dkf-2 null mutant with DKF-2(E925E929)-GFP elicited a 3–4-fold increase in di-phosphorylated PMK-1 (Fig. 6E). Thus, a surfeit of DKF-2 activates PMK-1 and enhances resistance to PA14 (Fig. 6A).
DKF-2 depletion diminished the S50 value for PA14 mediated killing by 15 h (Fig. S4). Disruption of the pmk-1 gene reduced S50 by 30 h. The more severe phenotype evidently arises from the ability of the PMK-1 pathway to receive and integrate signals from several inputs. PMK-1 is activated by PA14 infection and (by analogy with p38 MAP kinase) stress, autocrine and paracrine signals generated as infection progresses. DKF-2 couples signals carried by DAG to up-regulation of PMK-1 activity.
TPA-1, a PKCδ homolog, regulates DKF-2 in vivo
C. elegans expresses 3 PKCs capable of regulating DKF-2. PKC-2 is activated by DAG and Ca2+; PKC-1 and TPA-1 are switched on by DAG alone (Islas-Trejo et al., 1997; Land et al., 1994; Tabuse et al., 1995). We created homozygous null animals lacking both PKC-1 and PKC-2 proteins. The double mutants develop, feed and reproduce normally. Viable, fertile C. elegans homozygous for a severe loss of function mutation in TPA-1 (tpa-1(k530) allele) was obtained from the C. elegans Genetics Center. Elimination of relevant PKCs will extinguish A-loop phosphorylation and activation of DKF-2, thereby generating a “dkf-2 null” phenotype in animals expressing normal or elevated levels of DKF-2.
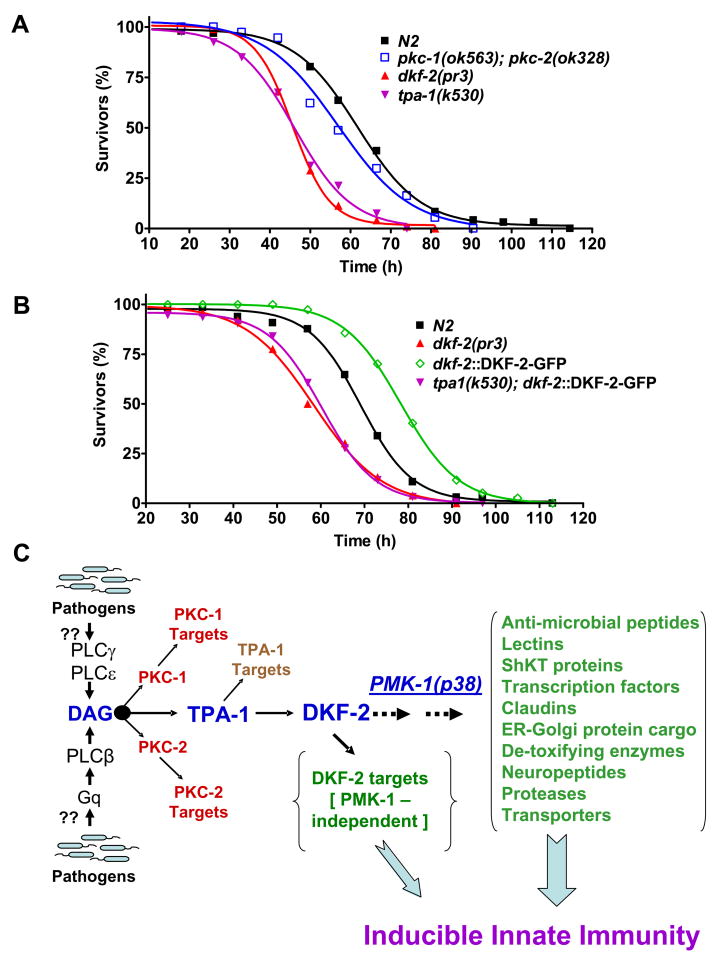
Elimination of both PKC-1 and PKC-2 did not significantly diminish survival of C. elegans fed with PA14 (Fig. 7A). Therefore, PKC-1 and PKC-2 are not critical regulators of DKF-2. Inactivation of TPA-1, a PKCδ homolog, shifted the survival curve to mimic the dkf-2(pr3) null phenotype (Fig. 7A). TPA-1 deficiency abolished enhanced innate immunity conferred by DKF-2-GFP over-expression and again recapitulated a dkf-2 null phenotype (Fig. 7B). A classical interpretation of the genetic analysis places TPA-1 downstream from DKF-2 in a common pathway. However, the epistasis data are also compatible with the idea that TPA-1 is an essential activator of endogenous WT DKF-2 and over-expressed DKF-2-GFP. Results in Fig. 7 do not reveal which kinase is the upstream regulator and which is substrate/effector. The ambiguity is resolved by extensive biochemical evidence, which demonstrates conclusively that PKCs switch on PKD (DKF-2) catalytic activity by phosphorylating the D kinase activation loop (A-loop) (Feng et al., 2007; Rozengurt et al., 2005; Wang, 2006). Thus, we conclude that TPA-1 uniquely regulates DKF-2 in intestinal cells in vivo. TPA-1 and DKF-2 are components of a signaling cascade that allows DAG to regulate host responses to pathogens (Fig. 7C).
Figure 7. TPA-1 Controls DKF-2.
A depicts survival curves for dkf-2(pr3) null, pkc-1(ok563);pkc-2(ok328) double null and tpa-1(k530) defective C. elegans mutants feeding on PA14. B shows survival curves for WT, dkf-2(pr3) and transgenic (dkf-2::DKF-2-GFP and tpa-1(k530);dkf-2::DKF-2-GFP) nematodes fed PA14. C presents a model for (partial) regulation of immunity by a DAG→TPA-1→DKF-2 signaling pathway in C. elegans. We speculate that pathogens directly/indirectly activate PLCs, thereby increasing DAG levels. DAG recruits and activates TPA-1, which phosphorylates and activates DAG-bound DKF-2. DKF-2, a PKD prototype, induces expression of immune effector mRNAs that defend intestinal cells against pathogens via PMK-1 dependent and independent mechanisms.
DISCUSSION
PKDs have been studied for >10 years, but little is known about their regulation, effectors and functions in normal cells in vivo. We discovered DKF-2 regulates inducible gene expression that defends epithelium against attack by bacterial pathogens. Thus, a PKD provides a novel link between DAG/PKC-mediated signal transduction and innate immunity.
C. elegans intestinal cells govern many facets of innate immunity. DKF-2 accumulates principally in intestine and is a critical component of a novel signaling pathway (Fig. 7C). Signals flow through the pathway when pathogens or other stimuli activate PLCs. PKC-1 (PKCε homolog), PKC-2 (PKCβ) and TPA-1 (PKCδ) are candidate DKF-2 activators in C. elegans (Feng et al., 2007). PKCs α, β, δ, ε, η and θ activate PKDs in vertebrate cell lines (Rozengurt et al., 2005; Wang, 2006), suggesting D kinases are redundantly regulated. In vivo analysis in C. elegans provided a distinct counterpoint to this concept. Gene disruption, cell-specific DKF-2 transgene expression and epistasis analysis revealed that TPA-1 exclusively regulates DKF-2 in normal intestine in vivo. Disruption of pkc-1 and pkc-2 genes did not enhance or diminish PA14 mediated killing of C. elegans. Thus, PKC-1 and PKC-2 effectors do not engage the immune system.
DKF-2 plays an essential role in innate immunity. The TPA-1/DKF-2 pathway is inactive in animals consuming non-pathogenic food. Catalytic activities of endogenous DKF-2 and recombinant DKF-2-GFP are switched on by mild pathogen (OP50) and more potently elevated by PA14 ingestion. This implies that mild and strong pathogens elicit corresponding increases in DAG concentration and TPA-1 activity. The level of PA14-induced, DKF-2 kinase activity in WT animals matches the level of D kinase activity in uninfected, transgenic animals overexpressing DKF-2-GFP (Fig. 2D). This amount of phosphotransferase activity elicits substantial inductions of immune effector mRNAs (Fig. 3). Consequently, animals expressing elevated DKF-2-GFP can accumulate a pool of anti-microbial proteins prior to infection. Kinase activity is further amplified 2.6-fold when transgenic animals are fed PA14. Pre-existing anti-microbial proteins may protect against PA14 during early phases of infection; a pathogen-induced increase in DKF-2-GFP catalytic activity could enhance nematode survival during later stages of infection. Conversely, DKF-2 deficiency causes hypersensitivity to killing by PA14. Thus, both activation and amount of DKF-2 are strongly linked to C. elegans defense against invading pathogens. We conclude that DKF-2, a PKD, is a physiologically relevant mediator of innate immunity in normal cells in vivo.
DKF-2-GFP induced 3–30-fold increases in expression of 84 anti-pathogen genes. The corresponding proteins contribute to many facets of innate immunity (Figs. 3 and 4A). Caenacins and structurally-related NLPs 28, 30 and 34 are secreted anti-microbial proteins (Pujol et al., 2008). Saposins create pores in bacterial membranes and promote catabolism of essential lipids. C-lectins bind bacterial oligosaccharides, thereby generating complexes which recruit enzymes that kill or inactivate bacteria (Nicholas and Hodgkin, 2004). Activated DKF-2 increased expression of genes involved in detoxification (glutathione-S-transferases), proteolysis and catabolite re-cycling. Aspartyl protease induction is associated with progression of necrotic death of intestinal cells in C. elegans fed with P. luminescens or E. carotovora pathogens (Wong et al., 2007). Necrosis may be part of an inducible anti-pathogen defense mechanism; however, pathogens may sometimes exploit induced aspartyl proteases to hasten the death of C. elegans. Some transporters promote ATP synthesis, whereas others translocate nucleosides, nucleotides, inositol, carboxylic acids and sugars (potentially generated by extracellular and lysosome mediated degradation of bacterial constituents) into cytoplasm where they are used for metabolism and synthesis of macromolecules. DKF-2 induced mRNAs encoding secreted proteins with trypsin inhibitor like (TIL) domains. TIL proteins may inhibit proteolytic attack on epithelium or suppress proteolytic conversion of bacterial pro-toxins to active toxins.
Activated DKF-2 induced mRNAs encoding 12 proteins that contain ShKT domains (Tudor et al., 1996). Most of these proteins have signal sequences, suggesting they are exported by exocytosis. Functions of C. elegans ShKT proteins are unknown. However, mRNAs encoding 10 ShKT proteins increased in C. elegans infected with PA14 or M. nematophilum (O’Rourke et al., 2006; Troemel et al., 2006). Five pathogen-induced ShKT transcripts (F01D5.2, F01D5.3, F01D5.5, F49F1.5 and T24B8.5) were also induced by elevated DKF-2. Large increases in mRNAs encoding 21 uncharacterized proteins were evident in transgenic animals. Predicted signal sequences and/or transmembrane domains in the “unknown” proteins support the theme that DKF-2 up-regulates secreted and integral membrane proteins.
DKF-2 activation sharply increased claudin gene expression (CLC-1 and F10A3.1, Fig. 3). Claudins, which traverse plasma membrane 4 times, stabilize cell-cell adhesion and create pores that limit the size of molecules diffusing between cells (Shin et al., 2006). Increased claudin content will inhibit penetration of intestinal epithelium by pathogens and paracellular diffusion of microbial toxins and noxious metabolites. DKF-2 elicited large increases in SEDL-1, F45E4.1 and T02E1.7 mRNAs. The encoded proteins share 50–75% amino acid sequence similarity with human TRS20, ARF-1 and SURF-4 proteins, respectively (Altan-Bonnet et al., 2004; Gecz et al., 2003; Reeves and Fried, 1995). By analogy, the C. elegans proteins may mediate transport of protein cargo from endoplasmic reticulum and Golgi membranes to plasma membrane and secretory vesicles. Since DKF-2 induces dozens of transmembrane and secreted anti-microbial proteins, augmentation of protein export machinery may be essential to mount an optimal immune response.
Elevated DKF-2 elicited increases in mRNAs encoding transcription factors. Forkhead-like FKH-9 and HNF-4 related NHR-63 may mediate DKF-2 dependent upregulation of one or more immune effector mRNAs. DKF-2 also induced MEF-2 and HDA-4, a type II HDAC. HDACs bind and inactivate MEF2 isoforms in mammalian nuclei (Vega et al., 2004). PKDs dissociate the complex by phosphorylating HDACs and de-repressed MEF2 stimulates transcription of inducible genes. HDA-4/MEF-2 complexes may dynamically regulate gene transcription in C. elegans. In one scenario, DKF-2 catalyzed HDA-4 phosphorylation (MEF-2 activation) would elicit synthesis of anti-microbial mRNAs in infected animals. Dephosphorylated HDA-4 would bind and inhibit MEF-2 after pathogen elimination.
Fifty-one genes (61%) listed in Fig. 3 are expressed in the intestine. Expression patterns of 30 genes (35%) are unknown. Three highly induced mRNAs, encoding ABF-2 and two FMRFamides (FLP-11 and FLP-22), are expressed in pharynx and neurons, respectively, but not in intestine. The data suggest DKF-2 promotes innate immunity principally through actions in intestinal cells. However, gene expression in non-intestinal cells may be stimulated by indirect mechanisms. A logical speculation is that DKF-2 activation triggers secretion of hormones from intestinal cells. Hormone binding with receptors on other cell types could elicit induction of specific mRNAs. For example, INS-7 secreted by intestinal cells controls DAF-16 mediated gene transcription in muscle and hypodermis (Murphy et al., 2007). Thus, endocrine/paracrine mechanisms might mediate induction of the anti-microbial peptide ABF-2 in pharynx. Production of pharyngeal ABF-2 would synergize with anti-pathogen defense mechanisms activated in intestine. DKF-2 mediated induction of neuropeptide-like FLPs and NLPs 1, 3, 7 and 21 (Fig. 3) could proceed in neurons via an endocrine loop. DKF-2 controlled neuronal production of NLPs and FLPs might facilitate coordination of behavioral responses to pathogens (e.g., pathogen avoidance) with production of immune effectors in gut. Levels of hypodermal NLPs 28, 30 and 34 (which are induced by certain fungal and bacterial pathogens) (Pujol et al., 2008) may also be modulated by intestinal DKF-2 via an endocrine mechanism. Whether hypodermal NLPs protect C. elegans against ingested pathogens is an open question.
Increased DKF-2 kinase activity sharply decreased levels of many mRNAs (Fig. 4B). The encoded proteins mediate non-immune stress responses, chromatin modification, DNA checkpoint regulation and repair, energy sensing, protein ubiquitinylation and oocyte differentiation. Patterns of induced and repressed gene expression suggest DKF-2 suppresses normal homeostasis while maximizing processes that favor survival during bacterial infection. Identities of diminished mRNAs reveal that enhanced pathogen resistance incurs a significant cost, increased vulnerability to environmental and biological insults.
Persistent expression of DKF-2 activity in uninfected animals could be deleterious and decrease lifespan. However, endogenous or overexpressed DKF-2 is inactive in animals fed OP50 HK. Thus, C. elegans has regulatory mechanisms that suppress detrimental DKF-2 activity. DKF-2 activity and immune effectors are rapidly and potently induced by pathogens. This ensures the short-term benefit of mobilizing anti-pathogen defenses, although animals become temporarily vulnerable to other insults.
Induction of ~75% of DKF-2 regulated mRNAs is co-dependent on PMK-1. Since epistasis experiments place DKF-2 upstream from PMK-1, several steps may intervene between TPA-1 mediated DKF-2 activation and differential mRNA accumulation. Like mammalian p38 MAP-kinase, PMK-1 probably translocates to the nucleus, where it phosphorylates and activates transcription factors (Kyriakis and Avruch, 2001). PMK-1 may also regulate proteins that control mRNA stability. Increased intestinal DKF-2 activity elicits A-loop phosphorylation/activation of PMK-1. The (previously unknown) convergence of PKD (DKF-2) signaling with the p38 MAP-kinase cascade incorporates PMK-1 into a PLC/DAG-controlled network (Fig. 7C).
The DKF-2 target in the PMK-1 pathway is unknown. In mammalian endothelial cells, PKD facilitates activation of ASK-1, an NSY-1 homolog (Zhang et al., 2005). However, neither A-loop phosphorylation nor catalytic activity of PKD was required to activate ASK-1. In contrast, DKF-2 phosphorylation/activation is essential for induction of immune effector mRNAs. DKF-2 might phosphorylate upstream pathogen recognition receptors (currently unknown in C. elegans), TIR-1, (Toll-IL-1 receptor domain protein that stimulates NSY-1) or downstream protein kinases in the PMK-1 pathway (Liberati et al., 2004). Future studies on DKF-2 induced immunity in pathogen receptor, TIR-1, NSY-1 or SEK-1 deficient animals will illuminate mechanisms that link DKF-2 to PMK-1 activation. DKF-2 might also mediate inactivation of VHP-1, a protein phosphatase that quenches PMK-1 activity (Kim et al., 2004).
DKF-2 induces several mRNAs in PMK-1 deficient C. elegans (Fig. 6B) and phosphorylates the histone deacetylase HDA-4 in vitro (unpublished results). Thus, by analogy with mammalian PKDs, the C. elegans D kinase may control expression of some genes by de-repressing MEF-2, as explained above.
Our studies indicate that DAF-16 is not a DKF-2 effector (Fig. S3). A recent report supports this conclusion (Troemel et al., 2006). Micro-array and qRT-PCR analyses revealed that PMK-1 is required for PA14-induced expression of >20 immune effector genes. DAF-16 had little impact on up-regulation of PA14/PMK-1 induced mRNAs and was not required for innate immune responses in a WT background. Increased DAF-16 activity promoted resistance to PA14 in a daf-2 mutant background. However, pathogen resistance was attributed to a general stress response (and parallel pathway) that does not affect induction of immune effector mRNAs.
Little is known about roles of PKDs in mammalian innate immunity. However, several reports support parts of the model proposed in Fig. 7C. Enteropathogenic E. coli elicits PKC activation in T84 intestinal epithelial cells (Crane and Oh, 1997). It is not known if PKDs mediate induction of immune effectors in T84 cells. After binding pathogen flagellar proteins, Toll-like receptor 5 (TLR-5) transduces signals that stimulate secretion of IL-8, an inflammatory cytokine, by epithelial cells. PKD phosphorylates (in vitro) a potential regulatory site in the TLR-5 cytoplasmic domain and a TLR-5/PKD complex was isolated from transfected HEK293 cells (Ivison et al., 2007). TLR-5 induced IL-8 secretion was dependent on PKD and p38 MAP kinase. However, PKD was not activated when TLR-5 bound flagellin. Thus, classical regulatory roles for PKCs and PKD were excluded. The possibility that PKDs mediate TLR-5 signaling in normal epithelial cells remains to be explored.
In HeLa and MDCK cells, PKDs phosphorylate and activate PI4-Kinase IIIβ (PI4KIIIβ) on Golgi membranes. This triggers fission of TGN vesicles that deliver protein cargo to plasma membrane and secretory vesicles (Hausser et al., 2005). The lipid kinase generates PI4P, which is cleaved by PLCs to yield DAG; elevated DAG elicits TGN membrane invagination and vesicle fission (Bard and Malhotra, 2006). Many DKF-2 induced mRNAs encode secreted or integral membrane proteins. DKF-2 may couple a surge in protein export to an increase in cargo transport capacity by stimulating synthesis of ARF-1, TRS20, and SURF-4. These proteins can control rates and levels of protein export from TGN. ARF-1 also governs association of PI4KIIIβ with Golgi membranes and partially activates the lipid kinase (Godi et al., 1999); subsequent phosphorylation by co-localized PKD yields maximally active PI4KIIIβ. ARF-1 activates phospholipase D, thereby generating phosphatidic acid (PA). Conversion of PA to DAG, by a lipid phosphatase, promotes further TGN invagination and PI4KIIIβ activation (Bard and Malhotra, 2006). Emerging parallels between the model in Fig. 7C and observations made using mammalian cells suggest studies on roles of PKDs in vertebrate innate immunity will be worthwhile endeavors.
EXPERIMENTAL POCEDURES
Strains
The C. elegans Genetics Center provided pmk-1(km25) null and tpa-1(k530) (severe loss of function) mutants. pkc-1(ok563), pkc-2(ok328) and dkf-2(pr3) null mutants were obtained from the C. elegans Knockout Consortium (Vancouver, BC). Animals carrying chromosomally-integrated copies dkf2::GFP, dkf2::DKF2-GFP, dkf2::DKF2(A925A929)-GFP and dkf2::DKF2(E925E929)-GFP transgenes and pkc-1(ok563);pkc-2(ok328) double null mutants were generated via standard protocols. Mutants were backcrossed (6X) into WT background.
Construction of Transgenes
A 1.4 kb segment of genomic DNA that flanks the 5′ end of the dkf-2 gene was synthesized by PCR. DNA was cloned into a C. elegans NLS-GFP reporter vector (pPD95.70) as previously described (Feng et al., 2006b) to generate dkf2::GFP. cDNA encoding DKF-2 (clone yk417d2) was obtained from the Japanese C. elegans EST project. A 3.7 kb cDNA fragment (in pBluescript), which includes a portion of exon 2 and all other dkf-2 exons, was coupled, in-frame with 1.4 kb of 5′ flanking DNA, exon I and the missing part of exon 2 via ligation at a unique XhoI site. Recombined DNA was cloned into a PstI–SmaI digested GFP reporter vector (pPD95.79), yielding dkf2::DKF-2-GFP.
Pathogen-mediated Killing
P. aeruginosa (PA14) was seeded on agar plates containing C. elegans growth medium. A group of 100 or 120 synchronized L4 animals was transferred from normal food (E. coli OP50) to PA14 pathogen plates. Plates were incubated at 25°C and the number of living worms was determined at 8 h intervals. Immobile worms unresponsive to touch were scored as dead. Statistical analyses of survival curves are presented in Table S10.
Preparation of RNA
WT, mutant and transgenic C. elegans were grown and synchronized as previously described (Land et al., 1994). Total RNA was extracted from young adult animals using TRIZOL Reagent (Invitrogen) according to the manufacturer’s protocol. RNA was purified on an RNeasy column (Qiagen).
Microarray Analysis
Probes for DNA microarray hybridization were prepared with the SuperScript Plus RT-cDNA Labeling System (Invitrogen). Total RNA (20 μg) from WT and transgenic (dkf-2::DKF-2-GFP, WT background) C. elegans were used as templates for synthesis of Cy3- and Cy5-labeled cDNAs, respectively. Microarray slides and hybridization protocols were obtained from Washington University; St. Louis, MO. cDNAs were hybridized with a slide containing 60-mer oligonucleotides representing transcripts encoded by all (~22,000) C. elegans genes. After washing, Cy5 and Cy3 fluorescence intensities were recorded with a Gene Pix 400A scanner, normalized and analyzed with Gene Pix Pro 6.0 software. High Cy5:Cy3 values identified mRNAs induced by DKF-2; low ratios revealed repressed mRNAs. Assays were replicated 3 times, using independently isolated RNA. Microarray datasets are provided in Tables S1–S7.
Quantitative Real Time PCR Analysis
RNA from various C. elegans strains was used as a template for cDNA synthesis catalyzed by SuperScript III reverse transcriptase (Invitrogen). cDNA was serially diluted and qRT-PCR was performed, using SYBR green detection in combination with the Roche LightCycler System and software. The delta Ct method was used to calculate fold-induction; the efficiency of PCR reactions was 1.89 to 1.95 (i.e., 95–98%) in all experiments. Data were normalized to the invariant level of ACT-1 mRNA. qRT-PCR primers were designed using Primer3 software and checked against C. elegans genomic DNA for specificity. Statistical data (means, SEM and p values) for qRT-PCR measurements are given in Tables S8 and S9.
Immunoprecipitation and in vitro Kinase Assays
C. elegans or transfected cells were disrupted in buffer containing 1% Triton X-100 as previously described (Feng et al., 2007). DKF-2 or DKF2-GFP was precipitated with affinity-purified antibodies and catalytic activity was quantified by measuring incorporation of 32P radioactivity from [γ-32P] ATP into Syntide-2 as previously reported (Feng et al., 2007).
Other Procedures
Descriptions of transfection experiments; characterization of gene deletions; generation and selection of transgenic animals; immunofluorescence microscopy; mutagenesis; denaturing electrophoresis; antibody production and characterization; and Western immunoblot analysis are given in previous papers (Feng et al., 2007; Feng et al., 2006a; Feng et al., 2006b).
Acknowledgments
This work was supported by NIH grant GM080615 (C. S. Rubin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J. Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol. 2004;16:364–372. doi: 10.1016/j.ceb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- Crane JK, Oh JS. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Ren M, Chen L, Rubin CS. Properties, regulation and in vivo functions of a novel protein kinase D: C. elegans DKF-2 links diacylglycerol second messenger to the regulation of stress responses and lifespan. J Biol Chem. 2007;282:31273–31288. doi: 10.1074/jbc.M701532200. [DOI] [PubMed] [Google Scholar]
- Feng H, Ren M, Rubin CS. Conserved domains subserve novel mechanisms and functions in DKF-1, a Caenorhabditis elegans protein kinase D. J Biol Chem. 2006a;281:17815–17826. doi: 10.1074/jbc.M511898200. [DOI] [PubMed] [Google Scholar]
- Feng H, Ren M, Wu SL, Hall DH, Rubin CS. Characterization of a novel protein kinase D: Caenorhabditis elegans DKF-1 is activated by translocation-phosphorylation and regulates movement and growth in vivo. J Biol Chem. 2006b;281:17801–17814. doi: 10.1074/jbc.M511899200. [DOI] [PubMed] [Google Scholar]
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- Gecz J, Shaw MA, Bellon JR, de Barros Lopes M. Human wild-type SEDL protein functionally complements yeast Trs20p but some naturally occurring SEDL mutants do not. Gene. 2003;320:137–144. doi: 10.1016/s0378-1119(03)00819-9. [DOI] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Trejo A, Land M, Tcherepanova I, Freedman JH, Rubin CS. Structure and expression of the Caenorhabditis elegans protein kinase C2 gene. Origins and regulated expression of a family of Ca2+-activated protein kinase C isoforms. J Biol Chem. 1997;272:6629–6640. doi: 10.1074/jbc.272.10.6629. [DOI] [PubMed] [Google Scholar]
- Ivison SM, Graham NR, Bernales CQ, Kifayet A, Ng N, Shobab LA, Steiner TS. Protein kinase D interaction with TLR5 is required for inflammatory signaling in response to bacterial flagellin. J Immunol. 2007;178:5735–5743. doi: 10.4049/jimmunol.178.9.5735. [DOI] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- Kim DH, Liberati NT, Mizuno T, Inoue H, Hisamoto N, Matsumoto K, Ausubel FM. Integration of Caenorhabditis elegans MAPK pathways mediating immunity and stress resistance by MEK-1 MAPK kinase and VHP-1 MAPK phosphatase. Proc Natl Acad Sci USA. 2004;101:10990–10994. doi: 10.1073/pnas.0403546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Land M, Islas-Trejo A, Freedman JH, Rubin CS. Structure and expression of a novel, neuronal protein kinase C (PKC1B) from Caenorhabditis elegans. PKC1B is expressed selectively in neurons that receive, transmit, and process environmental signals. J Biol Chem. 1994;269:9234–9244. [PubMed] [Google Scholar]
- Liberati NT, Fitzgerald KA, Kim DH, Feinbaum R, Golenbock DT, Ausubel FM. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci USA. 2004;101:6593–6598. doi: 10.1073/pnas.0308625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Scharenberg AM, Cantrell DA, Matthews SA. Protein kinase D enzymes are dispensable for proliferation, survival and antigen receptor-regulated NFkappaB activity in vertebrate B-cells. FEBS Lett. 2007;581:1377–1382. doi: 10.1016/j.febslet.2007.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, Billadeau DD, Shimizu Y. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity. 2005;23:213–226. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:19046–19050. doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas HR, Hodgkin J. Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol Immunol. 2004;41:479–493. doi: 10.1016/j.molimm.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- O’Rourke D, Baban D, Demidova M, Mott R, Hodgkin J. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 2006;16:1005–1016. doi: 10.1101/gr.50823006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, Schulenburg H, Ewbank JJ. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008;4:e1000105. doi: 10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JE, Fried M. The surf-4 gene encodes a novel 30 kDa integral membrane protein. Mol Membr Biol. 1995;12:201–208. doi: 10.3109/09687689509027508. [DOI] [PubMed] [Google Scholar]
- Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- Schulenburg H, Kurz CL, Ewbank JJ. Evolution of the innate immune system: the worm perspective. Immunol Rev. 2004;198:36–58. doi: 10.1111/j.0105-2896.2004.0125.x. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Sano T, Nishiwaki K, Miwa J. Molecular evidence for the direct involvement of a protein kinase C in developmental and behavioural susceptibility to tumour-promoting phorbol esters in Caenorhabditis elegans. Biochem J . 1995;312:69–74. doi: 10.1042/bj3120069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:1725–1739. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor JE, Pallaghy PK, Pennington MW, Norton RS. Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone. Nat Struct Biol. 1996;3:317–320. doi: 10.1038/nsb0496-317. [DOI] [PubMed] [Google Scholar]
- Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank JJ. Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 2007;8:R194. doi: 10.1186/gb-2007-8-9-r194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zheng S, Storz P, Min W. Protein kinase D specifically mediates apoptosis signal-regulating kinase 1-JNK signaling induced by H2O2 but not tumor necrosis factor. J Biol Chem. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]