Abstract
Catheter ablation is a first line treatment for many cardiac arrhythmias and is generally performed under x-ray fluoroscopy guidance. However, current techniques for ablating complex arrhythmias such as atrial fibrillation and ventricular tachycardia are associated with suboptimal success rates and prolonged radiation exposure. Pre-procedure 3D CMR has improved understanding of the anatomic basis of complex arrhythmias and is being used for planning and guidance of ablation procedures. A particular strength of CMR compared to other imaging modalities is the ability to visualize ablation lesions. Post-procedure CMR is now being applied to assess ablation lesion location and permanence with the goal of indentifying factors leading to procedure success and failure. In the future, intra-procedure real-time CMR, together with the ability to image complex 3-D arrhythmogenic anatomy and target additional ablation to regions of incomplete lesion formation, may allow for more successful treatment of even complex arrhythmias without exposure to ionizing radiation. Development of clinical grade CMR compatible electrophysiology devices is required to transition intra-procedure CMR from pre-clinical studies to more routine use in patients.
Introduction
Radiofrequency (RF) catheter ablation has advanced over the last 25 years from an experimental procedure to the first line treatment for a number of cardiac arrhythmias including atrio-ventricular reentrant tachycardia, accessory pathway associated tachycardias, and typical atrial flutter [1]. These procedures are typically guided by positioning electrode catheters using x-ray fluoroscopy and using these catheters to observe the propagation of electrical activity through the heart. Successful targeting of ablation primarily to the anatomic arrhythmia substrate, as opposed to mapping and targeting ablation based on electrogram characteristics, began with recognition that common atrial flutter passes through a narrow structure known as the cavo-tricuspid isthmus [2]. By directing ablation to interrupt conduction through this region, high cure rates have been achieved with a low risk of complications [3].
The clinical indications for anatomy based catheter ablation have since expanded to more complex arrhythmias such as atrial fibrillation and scar based ventricular tachycardia [4,5]. The basis of these strategies is to target specific anatomic regions and often to create extended ablation "lines" by aligning multiple point lesions or by dragging the catheter along the endocardial surface while applying ablative energy. While the feasibility of x-ray fluoroscopy guidance has been demonstrated for these complex arrhythmias, precise targeting of ablation lesions is limited by fluoroscopy's inherently poor ability to visualize cardiovascular soft tissue anatomy. Electrospatial mapping systems, which locate the catheter tip in 3-D space relative to magnetic or electric field transmitters, were rapidly adopted to create surface maps of electrical characteristics from multiple regions of the heart and mark the location of ablation attempts so that more elaborate ablation patterns could be created (Figure 1a,b). Electrospatial mapping, however, does not provide direct visualization of the complex underlying arrhythmogenic anatomy (Figure 2a,b). The persistence of suboptimal cure rates, prolonged procedure and radiation exposure times, and the risk of serious complications have motivated new approaches to facilitate anatomy-based catheter ablation for complex arrhythmias.
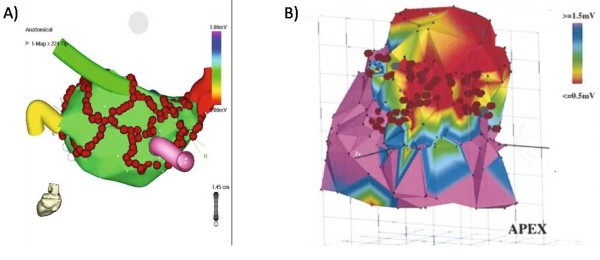
Figure 1.
Examples of electrospatial mapping guidance of complex arrhythmia ablation. A and B) Electrospatial surface maps generated by point-to-point contact mapping of the endocardial surface. The red circles are markers where ablation energy was delivered. A) Example of atrial fibrillation ablation in which ablations lesions are placed to encircle the pulmonary veins to prevent exit of arrhythmia triggering foci originating from the pulmonary veins. The pulmonary vein locations are marked by the colored "cartoon" tubes. B) Example of scar based ventricular tachycardia ablation in which linear lesions were places connecting scar (red) to normal tissue (purple) to interrupt the arrhythmia circuit. Figure 1A included with permission from The Journal of Cardiovascular Electrophysiology. (Calkins JCEP 2005; 13:53) Figure 1B included with permission from Circulation. (Marchlinski, Circ 2000; 101:1288).
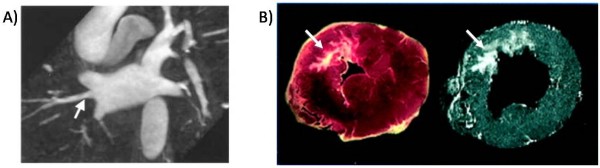
Figure 2.
Examples of arrhythmogenic anatomy depicted by CMR. A) CMR angiogram anatomy of the pulmonary veins. Note that variant pulmonary vein anatomy such as an additional right middle pulmonary vein, indicated by the white arrow, can be clearly seen by the CMR. B) The complex structure of myocardial infarction scar, indicated by white arrows, depicted by pathology on the left and delayed gadolinium enhanced CMR on the right. Figure 2A included with permission from the Journal of Cardiovascular Electrophysiology (Mansour, JCEP 2004; 15:387) Figure 2B included with permission from Circulation (Kim, Circ 1999; 100:1992).
Modern imaging techniques such as Cardiovascular Magnetic Resonance (CMR), intra-cardiac ultrasound, and x-ray computed tomography (CT) are increasingly used to approach the shortcomings of current mapping and ablation systems. CMR is a particularly flexible imaging modality that offers excellent soft tissue contrast, well characterized gadolinium enhancement techniques for myocardial scar visualization, 3-D imaging of complex cardiovascular anatomy, real-time 2-D imaging along arbitrary imaging planes, and the ability to quantify cardiac motion and blood flow. This article will review the application of CMR to current clinical procedures and ongoing advances toward full CMR guidance of electrophysiology procedures.
The present: Ablation planning and guidance using pre-procedural CMR
Atrial fibrillation
CMR has been used most extensively to assist planning and guidance of atrial fibrillation (AF) ablation procedures. AF is the most common clinically relevant arrhythmia affecting 0.4% of the general population [6]. The principle morbidities related to AF are stroke due to embolization of atrial thrombus and symptoms related to poor heart rate regulation with resting heart rates commonly over 110 beats per minute. In the early 1990's surgical modification of the atria with a series of linear incisions was found to be effective at controlling AF, but a minimally invasive catheter based procedure could not replicate these results [7,8]. It was later recognized that the triggering foci for AF frequently arise from one or more pulmonary veins (PVs) [9]. The ability to cure AF by ablating PV triggers or ablating conduction pathways exiting the PV's was promising but hampered by the risk of pulmonary vein stenosis due to injury of the vessels [8]. Electrospatial mapping technology led to the development of purely anatomic circumferential ablation strategies in which circular lesions are created further from the PV ostia to block the exit of PV triggers [4] (Figure 1a). Using this technique alone or in combination with PV isolation, a 70% to 80% success rate has been achieved [8]. However, repeat procedures are often needed to achieve this success and the success rate drops to 50% or less for the more chronic forms AF associated with ischemic, hypertensive, and valvular heart disease [8]. There also remains a 5% risk of major complications including cardiac perforation, pulmonary vein stenosis, and the rare but potentially lethal risk of atrioesophageal fistula formation [8].
In an effort to improve procedural success and reduce complications, 3-D CMR angiography (MRA) has been used to assist planning of AF ablation. Kato and colleagues used MRA to study left atrial anatomy in normal subjects and patients with paroxysmal atrial fibrillation and found that 38% of people had pulmonary vein anatomic variants [10]. Identification of these variants is important because AF triggering foci can be located within additional veins (Figure 2a). In addition, ablating near small or early branching PVs increases the risk of pulmonary vein stenosis [11]. Identification of certain anatomic variants before the procedure can also assist in catheter selection or favor using the circumferential ablation approach which is less affected by variant anatomy. 3-D imaging may also reduce the risk for complications by visualizing the relationship of the left atrium to surrounding structures including the esophagus, descending aorta, right pulmonary artery, and left circumflex coronary artery [12-15]. Knowing the location of these structures can be used to direct placement of ablation lesions to lower risk areas or guide reduction of ablation power when lesions are place close to these structures.
Scar based monomorphic ventricular tachycardia
CMR also has the potential to guide the treatment of scar based monomorphic ventricular tachycardia (MVT), a potentially lethal arrhythmia that is difficult to treat medically or with current ablation techniques. Ventricular tachycardia that results in uniform repetitive electrical activation of the heart arises from anatomically fixed arrhythmia substrate that can be targeted for ablation. Myocardial scarring due to infarction, cardiomyopathy, sarcoidosis, ARVD, or cardiac surgery is a common cause of MVT [16]. Scar related MVT typically depends on critical isthmuses of conductive tissue bounded by non-excitable scar or a valve annulus [17]. Ablating isthmus pathways can be curative but identifying the pathways using traditional mapping techniques can be difficult because these arrhythmias often lead to hemodynamic collapse. Substrate based approaches utilizing electrospatial mapping to identify reduced voltage scar border zone areas and isolated diastolic potentials within low voltage scar are now being used to identify critical portions of the arrhythmia circuit to target ablation [5,16] (Figure 1b). Still, ablation of MVT can be arduous. In addition to requiring careful point-by-point electrical mapping of the endocardium, rhythms resulting from epicardial pathways may require additional epicardial mapping and rhythms resulting from intramural pathways may be inaccessible to electrical mapping. In addition, procedures commonly last over six hours to achieve cure rates on the order of 70% even in the most experienced hands and success rates can be considerably less in lower volume centers.
The use of CMR for assisting MVT ablation is still in the investigational stages but shows promise. Late gadolinium enhancement CMR (LGE-CMR) has been used extensively to characterize regions of scar in ischemic and non-ischemic cardiomyopathy (Figure 2b). A number of clinical studies have demonstrated the association of LGE-CMR scar characteristics such as size, transmurality, and border-zone area with the risk of MVT [18-20]. Recent work suggests that high resolution LGE-CMR can be used to more directly assist in MVT ablation planning. Ashikaga and colleagues used an epicardial sock with around 300 electrodes to obtain high-resolution electrical maps during MVT in a pig infarct model [21]. These maps were registered to very-high resolution (0.39 × 0.39 × 0.39 mm) ex-vivo LGE-CMR to assess the relationship of MVT electrical propagation to scar morphology. Detailed scar imaging revealed numerous previously unseen features including 3-D tracts of viable myocardium within scar and scar within viable myocardium that visually correlated with the MVT isthmus identified by electrical activation mapping. Ciaccio et.al. complimented these findings by computationally predicting suitable locations for MVT ablation with the use of high resolution LGE-CMR scar imaging [22]. Using a model that incorporates regional scar thickness to estimate MVT excitation wavefront propagation, the MVT circuit isthmus was predicted and shown to overlap the actual isthmus, observed by electrical mapping, by around 90%. Though these experimental studies use significantly higher resolution LGE-CMR maps than the typical 1.5 × 2.5 × 8 mm pixel resolution used clinically, methods to obtain higher resolution scar images in patients are being developed and will be discussed further below [23,24]. Together with studies to identify safe MR imaging procedures for patients with implanted defibrillators [25] and clinical correlation of LGE-CMR scar morphology to successful VT ablation sites, the role of CMR for clinical VT ablation should be better defined in the near future.
The current use and limitations of pre-acquired 3D images for guiding ablation
The detailed anatomic information available from CMR is now being used as a roadmap for guiding placement of ablation lesions [26-30]. A number of techniques have been developed to register electrospatial mapping catheter coordinates to pre-acquired 3-D CMR and CT images. Dong and colleagues recently reported their technique in patients undergoing AF ablation [30]. They felt that 3-D imaging was helpful for tailoring ablations to the variant PV anatomy found in 47% of patients. They also noted that 3-D images of the atria helped to guide lesion placement in areas where stable catheter positioning was difficult, such as along the tissue ridge separating the left atrial appendage from the left pulmonary veins [11,30]. However, even when guided by 3-D image roadmaps, the study noted that circumferential ablation around the PVs prevented escape of PV triggers in only 32% of patients [30]. To completely isolate PV triggers, additional electrical mapping and ablation of specific conduction pathways was required. Others have created more extensive, riskier lesions using saline irrigated ablation catheters and still report PV isolation rates of only 50 to 60% after circumferential ablation around the PVs [31,32].
These sub-optimal results call attention to limitations of this current state-of-the-art in 3D image guided cardiac ablation. First, pre-acquired roadmap images may not correspond to the anatomy during the procedure. Changes in cardiac chamber size associated with variations in heart rate, rhythm, and volume status are not accounted for by pre-acquired imaging and could lead to catheter position registration errors [29]. Additional registration errors can result from patient motion during the exam, respiratory motion, and beat-to-beat motion of the heart, including significant motion of the PVs [33]. Second, marking attempted ablation positions and confirming reduction in the local electrogram voltage does not necessarily establish creation of a permanent ablation lesion or a continuous ablation line [30]. The electrode tissue contact area and electrode exposure to flowing blood are important factors in forming adequate ablation lesions [34] but are poorly assessed by fluoroscopy and electrospatial mapping guided procedures. Ablation lesion extent, unintentional gaps in ablation lines, and transient lesion components such as edema similarly are not well predicted by current techniques, including intra-cardiac ultrasound. These factors limit ablation accuracy and have been shown to reduce procedure efficacy [35,36].
Intracardiac echocardiography (ICE) addresses some of these shortcomings and is increasingly used in clinical practice [37]. ICE has been used to visualize electrode tissue contact, an important factor for efficient ablation lesion creation. Visualization of microbubbles at the electrode tissue contact interface during ablation has been also been used to indicate adequate electrode tissue contact while the presence of more coarse bubbles has been associated with inappropriately high tissue temperatures that could lead to tissue charring and coagulum formation. However, ICE has limitations for guiding ablation. ICE requires invasive placement and manipulation of a separate imaging catheter and physical limitations on image plane orientation and field of view limit its ability to evaluate lesion extent and characterize extended regions of ablation. Also the ability of ICE to reliably distinguish regions of ablation from surrounding viable tissue has not been established.
The future: Fully CMR guided EP ablation procedures
There are a number of reasons intra-procedure CMR is an attractive option for guiding future electrophysiology procedures. First, CMR offers a number of ablation lesion imaging techniques. In addition, the ability to obtain images in arbitrary orientations opens the potential for high quality visualization of catheters, anatomy, and electrode tissue contact. Further, the position errors introduced by registering catheter position to pre-acquired 3D images can be largely avoided because both real-time CMR images and 3D CMR images are acquired in the same coordinate system and 3-D images can be reacquired during the procedure if needed.
Over the last 15 years the basic techniques to enable fully MR guided EP procedures have been developed. Lardo and colleagues introduced the potential of CMR for guiding EP procedures in 2000 [38]. Continuous MR imaging was used to guide non-ferromagnetic EP catheter positioning from an internal jugular vein to selected locations in the right atrium and right ventricle. They also demonstrated the ability to perform and monitor ablations in the CMR scanner (Figure 3a). Delivery of RF ablation energy during imaging can cause significant image degradation, but this noise was dramatically suppressed by 10 MHz cutoff low-pass filtering of the ablation source. After ablation, imaging at the catheter location showed the lesion position and extent using both T2 weighted and gadolinium enhanced T1 weighted imaging techniques [38]. The onset of T2 changes at the ablation site was rapid enough that it could be used for lesion monitoring shortly after ablation (Figure 3b).
Figure 3.
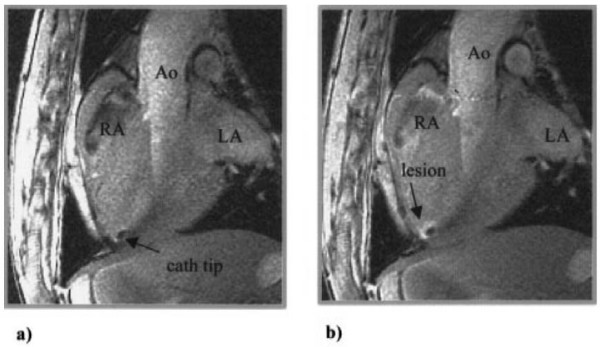
Example of CMR visualization of an ablation catheter positioned at the RV apex a) before and b) after RF ablation. Post ablation images were obtained after peripheral injection of gadolinium contrast. Figure included with permission from Circulation (Lardo Circ 2000; 102(6):698).
Subsequent work in our lab has demonstrated the ability to use real-time CMR to perform basic diagnostic EP studies [39]. Imaging was performed using an unmodified clinical scanner with interactive scan plane manipulation software to guide non-ferromagnetic catheters to standard electrogram recording sites including the high right atrium, His bundle, and right ventricular apex. Electrical interference from gradient switching was adequately suppressed by 30 Hz to 300 Hz bandpass filtering such that even the low voltage signal from the His bundle could be identified (Figure 4). Importantly, the study demonstrated that MR guided electrophysiology measurements could be performed safely in human subjects. The topic of device safety in the CMR environment is discussed further below.
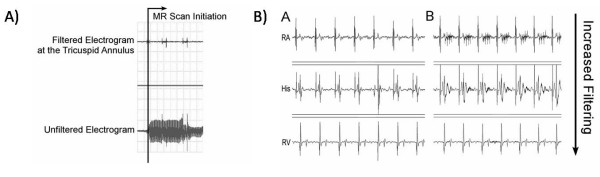
Figure 4.
A) Example of the bipolar intracardiac electrograms during scanning before filtering (bottom trace) and after filtering (top trace). B) Example of bipolar intracardiac electrograms at various locations in the heart outside the scanner (left column) and during scanning with different levels of filtering (right column). RA = Right atrium, His = His bundle, RV = right ventricular apex.
Other techniques relevant to EP procedures have also been performed using real-time CMR guidance. Trans-septal catheterization is required for left atrial catheter ablation. While generally safe, the procedure can be difficult in the setting of distorted atrial anatomy and carries the risk of serious complications such as aortic puncture. The ability of real-time CMR to guide trans-septal punctures under direct visualization of the needle, atria, fossa ovalis, and surrounding vasculature has been nicely demonstrated [40,41]. Retrograde catheterization of the left ventricle from the femoral artery is commonly required for VT ablation and has also been performed under real-time CMR guidance [42].
Recent advances promise to take MR guided EP from these initial feasibility studies to safe, efficient practice. These advances include 1) intra-procedure ablation lesion monitoring techniques, 2) faster 2-D and 3-D imaging, 3) improved device visualization, 4) intuitive 3-D anatomy and intracardiac electrogram visualization, and 5) CMR safe device construction techniques.
Intra-procedure lesion monitoring
Perhaps the most significant advantage of CMR-guided ablation therapy is the potential to visualize ablation lesions with high spatial and temporal resolution. The typical endpoint of current ablation procedures is absence of electrical conduction across the ablated region and/or an inability to re-induce the clinical arrhythmia with cardiac pacing and medications. However, propagation of electrical signals through the heart is affected by a number of factors including the tissue temperature change induced by ablation [43,44]. Some of these factors may be reversible over time leading to arrhythmia recurrence [35,36]. As described below, CMR appears capable of delineating areas permanent tissue damage caused by ablation. Using CMR lesion imaging to guide ablation could improve the procedure endpoint from assessment of potentially transient electrophysiologic changes to a more direct assessment of complete lines of permanently damaged tissue in the region of interest.
500 kHz radiofrequency (RF) current is the most commonly used ablation source used for electrophysiology procedures. Cryothermy, ultrasound, laser, and microwave ablation are also being investigated. Ablation lesions can be visualized because CMR is able to detect specific changes in proton precession and relaxation properties resulting from heating and heat induced biophysical changes in cardiac tissue including interstitial edema, hyperemia, protein conformational changes, cellular shrinkage, and tissue coagulation [38]. Acute interstitial edema is likely responsible for the hyperintense region corresponding to the area of acute RF ablation damage observed by T2-weighted FSE imaging [38,45] (Figure 5). Dickfield and colleagues found that this hyperintense region correlated well with necrotic lesion size on gross pathology and also noted that gaps between lesions on imaging corresponded with lesion gaps on pathology [46]. Lesion visualization by T2 weighted imaging has been reported as soon as 2 minutes after ablation and stable imaging characteristics have been observed from 30 minutes to 12 hours post ablation [38,46]. This could make T2 weighted CMR a tool to evaluate lesions and lesion continuity over the course of an ablation procedure.
Figure 5.
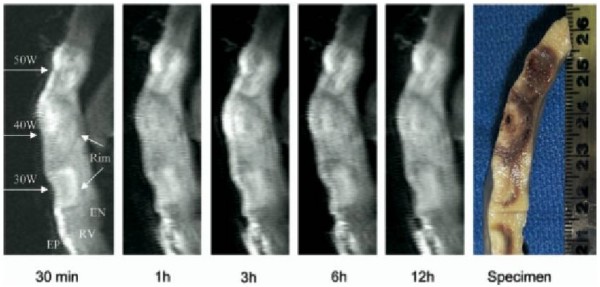
Example of non-contrast T2 weighted MR imaging of right ventricular epicardial RF ablation lesions with pathologic correlation. Stability of the imaged lesion size is demonstrated from 30 minutes to 12 hours after ablation. Figure included with permission from Heart Rhythm (Dickfeld HR 2007;4(2):215).
T1 weighted non-contrast enhanced MR imaging of RF ablation lesions has also been investigated [46]. T1 changes may be related to tissue heating, protein denaturation, and changes in intracellular and extracellular free water distribution during and following ablation [45-47]. Similar stability of imaging characteristics were reported from 30 minutes to 12 hours following ablation, though the lesion contrast by T1 weighted imaging appears to be less than for T2 weighted imaging [46].
LGE-CMR can provide better visualization of RF ablation lesions compared with non-contrast imaging techniques (Figure 6). The time to achieve full enhancement of RF ablation lesions, one to two hours, is considerably longer than for DECMR of myocardial infarct scar [48]. However, good correlation with pathologic lesion size was noted for intermediate enhancement patterns from one minute to 2 hours after contrast injection, allowing lesion extent to be assessed without waiting for full enhancement [48]. The 1 to 2 hour interval required for renal clearance between repeated dosing of gadolinium and the ceiling on total allowable gadolinium dose limits the use of this technique for serial lesion assessment during a procedure [45]. Still, gadolinium enhanced imaging may be useful for evaluating gaps in ablation lines after completion of a procedure to assess the need to place additional lesions.
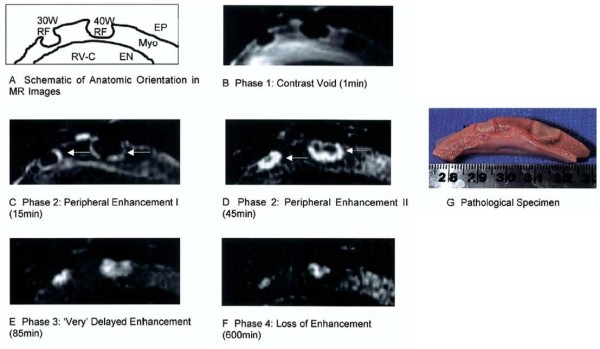
Figure 6.
Example of gadolinium enhanced T1 weighted MR imaging of right ventricular epicardial RF ablation lesions with pathologic correlation. Different lesion enhancement patterns are seen from one minute to 2 hours after contrast injection. Figure included with permission from the Journal of the American College of Cardiology (Dickfeld JACC 2006;47(2):370).
Other methods for monitoring ablation lesion formation during RF energy application are also being investigated. Proton resonance shift thermography is an CMR technique that takes advantage of the decrease in the proton resonance frequency with increasing temperature [49]. This technique has been used to follow tumor ablation in the uterus, liver, prostate, and brain using diverse energy sources including RF, high frequency ultrasound, laser, and microwave [50-55]. Its use for following RF ablation in the beating heart is being investigated. Current-vector mapping has also been described for monitoring the extent of tissue power deposition during RF ablation [56].
While most cardiac ablation lesion CMR studies have been performed in roughly 10 mm thick ventricle, imaging the less than 3 mm thick human atria is of particular clinical interest given the difficulty of achieving long term pulmonary vein isolation following atrial fibrillation ablation. Peters et.al. demonstrated 3D LGE-CMR of left atrial ablation lesions 10 to 15 minutes after contrast injection using image based respiratory gating [24]. This gating technique, also known as respiratory navigator imaging, allowed higher resolution 3D imaging to be performed without the need for prolonged breath-holding by tracking diaphragm position on fast 1-D images and collecting 3-D image data within a narrow range of diaphragm positions. Current applications have used a roughly 100 ms mid diastolic acquisition window timed to precede atrial systole to reduce atrial motion during imaging. Image resolutions of 1.25 × 1.25 × 2.5 mm (reconstructed to 0.6 × 0.6 × 1.25 mm) have been demonstrated. McGann, et.al combined this technique with 3-D visualization and quantification to assess left atrial scarring before and following atrial fibrillation ablation. They found that subjects with more than 13% left atrial delayed enhancement following ablation had a nearly 20 times higher chance of being free from atrial fibrillation than those with low amounts of post ablation delayed enhancement [57]. The technique is now being used to image immediately following the procedure to target further ablation and potentially reduce the need for repeat procedures. Interestingly, high resolution 3D LGE-CMR may also be useful for better identifying patients who will respond poorly to current ablation techniques [58]. Of note, such high-resolution imaging is not yet feasible in all patients. In the above studies, imaging took 5 to 10 minutes and 10% to 30% of patients were excluded from analysis because of poor image quality that was attributed to patient motion, significant arrhythmia, or incorrect inversion time selection. Techniques to improve the speed and reliability of high-resolution CMR require further investigation.
Faster MR imaging
While cardiac gating can be used to generate MR images with excellent spatial resolution by splitting data collection over multiple heartbeats, real-time CMR requires a more deliberate trade off between temporal and spatial resolution. To adequately visualize catheters, CMR guided EP procedures require an in-plane spatial resolution of around 2 mm2. The target temporal resolution is 7 frames per second (fps), the usual x-ray fluoroscopy frame rate for clinical EP procedures.
Since the initial 1 fps imaging used to guide the first MR guided EP procedure [38] faster, stronger gradients have increased the temporal resolution capabilities of fast gradient recalled echo sequences to the 5 fps range [59]. Improved gradient performance and B0 field homogeneity have also allowed real-time imaging to be performed with coherent steady state pulse sequences (ie. SSFP, true-FISP, or FIESTA). These sequences provide increased contrast to noise performance at a given frame rate compared with fast gradient recalled echo sequences [59]. Parallel imaging techniques can provide additional improvement in temporal resolution without sacrificing spatial resolution. These techniques accelerate imaging by covering the region of interest with multiple receive coils and using the different spatial sensitivities of these coils to correct for undersampling of image data [60]. The acceleration achieved from acquiring less data is countered by the increased processing time required for estimating coil sensitivities and for performing the parallel reconstruction [61]. Also, for a given number of receive coils, more undersampling leads to deteriorating image signal to noise or a reduced ability to suppress aliasing artifacts [62]. Balancing these issues, 15 fps true-FISP cardiac imaging with 128 phase encode lines can be performed using an 8 channel receive coil array and optimized reconstruction hardware [63]. Commercial CMR systems now commonly have multi-channel receivers and parallel imaging options. The performance of these systems is currently in the range of what is needed to perform CMR guided EP procedures at 5 fps with acceptable image quality [61].
While the current imaging rates are adequate for a single 2-D image plane, ideal visualization of the device, target anatomy, and surrounding reference anatomy may require multiple 2-D image planes or even 3-D imaging. Other techniques that can improve imaging speed while balancing imaging quality include non-Cartesian k-space sampling, temporal data sharing between images, and adjusting the tradeoff between temporal and spatial resolution [59]. These techniques may be particularly useful to accelerate imaging of reference anatomy views that are not depended on for device tracking. Use of 32 channel receive arrays to perform more rapid 3-D cardiac imaging and parallel transmission techniques to permit more efficient parallel data collection are also under active investigation [63-65].
Device Visualization and Navigation
While fluoroscopy provides projection images where the entire catheter body and tip are easily visualized, 2-D MR images typically depict a slice through the body that is around 5 to 10 mm thick. Curved devices such as catheters may pass in and out of the MR imaging plane leading to misinterpretation of the device tip position. We have noted in preclinical studies that poor delineation of the tip position can result in tissue contact trauma, such as local hemorrhage. In addition, for electrophysiology ablation procedures the device tip contains the energy source. Misestimating the tip/tissue contact region can lead to inaccurate placement of ablation lesions.
During our feasibility studies, tip location has mostly been performed using interactive real-time sequences with a user interface that permits adjustment of the scan plan during image acquisition. Part of the catheter is first identified on some imaging plane and the plane is manually adjusted until the tip is located. For vascular procedures where the device is constrained to a co-planar segment of blood vessel, manual plane manipulation is acceptable since only minor image plane translations are needed to visualize the device tip and relevant anatomy. For navigation in cardiac chambers where the device tip location is less constrained, the frequent need for manual plane manipulation necessitates a skilled operator for image plane manipulation and can distract from efficient procedure workflow.
One approach to this problem is to automatically direct imaging to the device location using position sensors located in the catheter. 15 years ago Dumolin et.al. described using 1-D projection MR imaging along the x, y, and z directions to identify the 3-D position of small receiver coils located in a catheter tip [66]. This position tracking technique has since been interleaved within real-time imaging sequences to automatically move the image plane position to the catheter tip location during device manipulation [61,67]. While a single tracking coil is sufficient to simply shift the image position, multiple tracking points are needed to maximize visualization of the device body or to orient imaging relative to the catheter tip direction. Multi-coil designs and tracking algorithms have been developed to reduce the need for separate matching circuits in space constrained catheter lumens [68,69]. Other magnetic field and electric field based position finding techniques have been developed for medical device tracking that could also be used for catheter tracking in the CMR scanner [70-72]. Some of these systems generate both position and orientation information for each sensor assembly [72,73]. These systems may provide more accurate and catheter space efficient options for device tracking. They can also reduce the performance penalty and avoid the scanner specific complexities associated with interleaving tracking and real-time imaging sequences.
An alternative technique for device visualization uses non-slice selective imaging to produce an effect similar to projection x-ray fluoroscopy [74,75]. The non-slice selective catheter imaging plane can be intersected with slice selective images containing the target anatomy to assist guidance of the catheter tip. This technique may be used to provide a "fluoroscopy" view of devices using a number of catheter antenna designs [69,76-78] (Figure 3a).
Another factor that affects device navigation in the CMR environment is the physical constraint of performing procedures near and within the narrow CMR bore. The availability of shorter, wider bore, high-field CMR scanners are making this less of an issue. Remote catheter steering is also gaining interest in the EP field to facilitate point-by-point electrical mapping of the cardiac chambers and assist stable device placement during ablation [79,80]. Some of these techniques may be amenable to use in the CMR environment. A robotic catheter manipulation system that uses steerable sheaths with multiple pull wires was recently used to perform atrial fibrillation ablation in patients [81]. A magnetic remote steering technique has also been described that utilizes the torque generated by current carrying coils in the static CMR magnetic field to deflect a catheter tip [82,83].
3-D Anatomy and IEGM visualization
The ability to generate real-time images with arbitrary orientations in addition to anatomically detailed 3-D images with flexible tissue contrast makes CMR well suited for navigating complex arrhythmia anatomy and delineating complex ablation patterns. This flexibility also introduces the potential for disorientation and information overload. Appropriate displays and user-interfaces tailored to the workflow of an EP procedure are needed to manage this flexibility. Because thin-slice real-time imaging can intersect anatomy in unfamiliar ways, 3-D visualizations that plot real-time images oriented relative to reference images can be helpful (Figure 7). A basic imaging interface for MR-guided EP procedures would provide a convenient way to "bookmark" and access reference cardiac views, switch between real-time and lesion visualization sequences during the procedure, appropriately present lesion images for ablation line continuity assessment, and display the relationship of stored images, catheter position, and intracardiac electrograms characteristics in 3-D.
Figure 7.
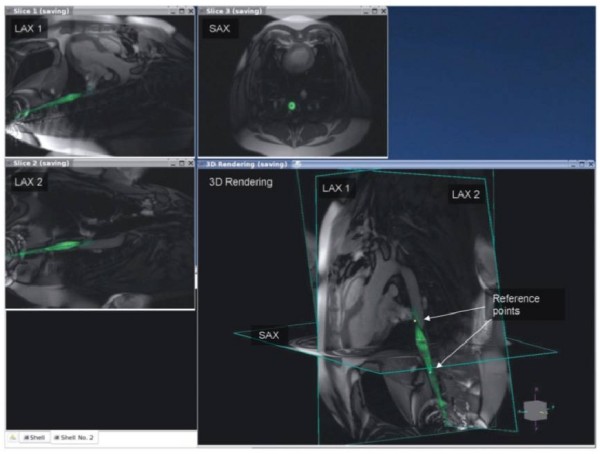
Example of using automatic catheter highlighting and reference image planes to navigate complex 3-D anatomy using real-time CMR. The anatomic location of the catheter position on the image labeled LAX2 is better appreciated when overlaid with long and short axis images of the heart. Figure included with permission from The Journal of Magnetic Resonance Imaging (Guttman JMRI 2007; 26:1429).
Interventional CMR device safety
The most important consideration for any new diagnostic or therapeutic approach is safety. While a number of studies have been performed to determine the safety of conventional CMR with regard to electromagnetic energy exposure and tissue heating [84,85], interventional procedures add additional considerations and raise new safety concerns [86-89]. The most straightforward aspect to CMR device safety is the avoidance of ferromagnetic materials that could experience significant forces when brought close to the scanner. Though CMR unsafe objects, such as ferromagnetic scissors and needle drivers, may be needed during the preparatory phase of a procedure, until CMR compatible alternatives are available a system must be in place to methodically track and remove such objects before approaching the scanner. The electric current associated with defibrillation can also lead to strong displacement forces in high magnetic fields and should be performed with the defibrillator pads maintained a safe distance from the scanner bore [90]. Similar attention is needed to address the ferromagnetic properties and CMR electromagnetic interference compatibility of other equipment associated with electrophysiology procedures including physiology monitoring equipment, ablation and pacing sources, and anesthesia apparatus. Clear marking of high field areas and secure placement of objects that may experience magnetic forces is mandatory so that appropriate pieces of equipment are kept at a safe distance [91].
An additional safety concern particular to CMR guided cardiovascular procedures is the significant heating that can result from RF transmission induced current in extended metallic objects such as guidewires, wired electrodes, and metal braided catheters [87,88]. This induction is more pronounced when portions of the device are located close to the RF transmit body coil housed within the edge of the scanner bore. Device length is an important factor in efficient coupling and heating; however, many other parameters can influence the sudden onset of significant heating in the setting of an interventional procedure [88,92]. The simplest way to avoid this problem is to construct devices from non-metallic components when possible. Polymer materials for catheter braiding, such as Dacron and Kevlar, and composite materials for guidewires, such as glass fiber reinforced plastics, can be used to achieve device functional characteristics such as torqueability, stiffness, and kink resistance [38,93]. Several approaches have also been developed to avoid significant induction heating in structures that require conductivity. Wires made from high resistance alloys and gold sputtered thread have been used to obtain intracardiac electrograms during imaging with significant reduction of electrode heating [94]. For structures where efficient power transfer is required, such as pacing or ablation electrodes, high frequency RF chokes can allow passage of signal lower than a few MHz while blocking unwanted MR transmit frequency currents [95,96] A promising heating suppression technique for position tracking and intravascular imaging coils that need to pass differential mode signals at the same frequencies as unwanted common mode induced currents is to place thin transmission line transformers in the signal carrying cables [77,97,98]. Other strategies such as detuning and decoupling of circuits prone to heating [78,99], fiberoptic transmission of signals [100], and use of inductively coupled resonators for wireless device tracking [101] are also considerations for new device design [25,90]. Using surface coils instead of the higher power body coil for RF transmission has also been proposed as a way to reduce RF current induction in devices [102]. Now that academic sites and imaging companies are focusing on heating safe device development, more rapid progress in this area is expected.
Transitioning proof of concept studies to clinical electrophysiology procedures requires collaboration between academic centers, imaging and device companies, and regulatory agencies. The first CMR guided electrophysiology procedure in a patient was performed using custom catheters made to clinical specifications by a clinical catheter manufacturer [39]. Prior to use in a human an investigational device exemption was obtained from the FDA. As part of this device exemption, catheter heating and reduction of heating using RF filtering was demonstrated in a series of device positions and orientations within the scanner using high SAR imaging protocols [39]. In addition, safe use of the catheter in animals was documented prior to human studies. Though catheters with embedded imaging coils provided improved device visualization in the animal studies, these devices were not approved for or used in patients. Before generally applying CMR guidance to interventional electrophysiology, more standardized CMR compatible catheter safety guidelines and device testing protocols need to be developed.
The compatibility of CMR with implanted devices such as pacemakers and implantable cardiac defibrillators (ICDs) is also an important consideration in performing interventional CMR studies in the electrophysiology patient population. Particular concerns include static magnetic field induced movement of the device and scanning induced programming changes, device inhibition, activation of tachyarrhythmia therapies, and lead currents leading to heating or cardiac stimulation [103-106]. Modern devices address some of these concerns with the use of less ferromagnetic material and improved resistance to electromagnetic interference [107]. A number of devices have been carefully studied during in-vitro and animal MR imaging and experience is growing for safe CMR in patients with selected devices under controlled scanning conditions [25,107,108]. This experience includes use of sequences relevant to modern CMR with SAR characteristics similar to those used for real-time CMR [25]. Still, the number of patients and devices studied thus far is limited and further work is needed to develop manufacturer protocols for establishing conditional CMR safety of pacemakers and ICDs. Carefully designed protocols for patient selection, monitoring, and scanning also need to be developed before imaging of patients with devices can be more routinely performed. The impact of device related artifacts on cardiac image interpretation also needs to be more carefully studied.
Conclusion
Increasing knowledge of the anatomic basis for cardiac arrhythmias has extended the role of catheter ablation to curing even complex rhythms such as atrial fibrillation and scar based ventricular tachycardia. CMR has demonstrated a number of uses for procedural planning, particularly for treatment of atrial fibrillation. The use of LGE-CMR for planning VT ablation procedures also shows promise. Real-time CMR combined with intra-procedural lesion imaging could allow physicians to accurate guide devices and establish completeness of ablation lines without concern for radiation exposure. This could significantly improve the way current ablation procedures are performed and open the way to ablative cure of arrhythmias such as permanent atrial fibrillation that currently respond poorly to minimally invasive approaches [7].
Competing interests
Kolandaivelu:
Imricor: Options
Lardo:
Zoll Medical: Consultant
Imricor: Equity
Surgivision: Equity
Halperin:
Zoll Medical: Consultant, Grant
Imricor: Royalties
Surgivision: Equity
Authors' contributions
AK was responsible for manuscript integration and co-author of all sections. ACL was responsible for lesion visualization and fast imaging. HRH was responsible for real time guidance and lesion visualization.
Contributor Information
Aravindan Kolandaivelu, Email: akoland@jhmi.edu.
Albert C Lardo, Email: alardo1@jhmi.edu.
Henry R Halperin, Email: hhalper@jhmi.edu.
References
- Calkins H, Yong P, Miller JM, Olshansky B, Carlson M, Saul JP, Huang SK, Liem LB, Klein LS, Moser SA, Bloch DA, Gillette P, Prystowsky E. Catheter ablation of accesso ry pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation. 1999;99(2):262–270. doi: 10.1161/01.cir.99.2.262. [DOI] [PubMed] [Google Scholar]
- Shah DC, Jais P, Haissaguerre M, Chouairi S, Takahashi A, Hocini M, Garrigue S, Clementy J. Three-dimensional mapping of the common atrial flutter circuit in the right atrium. Circulation. 1997;96(11):3904–3912. doi: 10.1161/01.cir.96.11.3904. [DOI] [PubMed] [Google Scholar]
- Natale A, Newby KH, Pisano E, Leonelli F, Fanelli R, Potenza D, Beheiry S, Tomassoni G. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol. 2000;35(7):1898–1904. doi: 10.1016/S0735-1097(00)00635-5. [DOI] [PubMed] [Google Scholar]
- Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102(21):2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101(11):1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- Wazni OM, Tsao HM, Chen SA, Chuang HH, Saliba W, Natale A, Klein AL. Cardiovascular imaging in the management of atrial fibrillation. J Am Coll Cardiol. 2006;48(10):2077–2084. doi: 10.1016/j.jacc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Cox JL, Boineau JP, Schuessler RB, Ferguson TB, Jr, Cain ME, Lindsay BD, Corr PB, Kater KM, Lappas DG. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991;266(14):1976–1980. doi: 10.1001/jama.266.14.1976. [DOI] [PubMed] [Google Scholar]
- Dong J, Calkins H. Technology insight: catheter ablation of the pulmonary veins in the treatment of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2005;2(3):159–166. doi: 10.1038/ncpcardio0137. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Kato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, Angkeow P, LaCorte J, Bluemke D, Berger R, Halperin HR, Calkins H. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003;107(15):2004–2010. doi: 10.1161/01.CIR.0000061951.81767.4E. [DOI] [PubMed] [Google Scholar]
- Mansour M, Refaat M, Heist EK, Mela T, Cury R, Holmvang G, Ruskin JN. Three-dimensional anatomy of the left atrium by magnetic resonance angiography: implications for catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(7):719–723. doi: 10.1111/j.1540-8167.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- Tsao HM, Wu MH, Higa S, Lee KT, Tai CT, Hsu NW, Chang CY, Chen SA. Anatomic relationship of the esophagus and left atrium: implication for catheter ablation of atrial fibrillation. Chest. 2005;128(4):2581–2587. doi: 10.1378/chest.128.4.2581. [DOI] [PubMed] [Google Scholar]
- Cury RC, Abbara S, Schmidt S, Malchano ZJ, Neuzil P, Weichet J, Ferencik M, Hoffmann U, Ruskin JN, Brady TJ, Reddy VY. Relationship of the esophagus and aorta to the left atrium and pulmonary veins: implications for catheter ablation of atrial fibrillation. Heart Rhythm. 2005;2(12):1317–1323. doi: 10.1016/j.hrthm.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Kenigsberg DN, Lee BP, Grizzard JD, Ellenbogen KA, Wood MA. Accuracy of intracardiac echocardiography for assessing the esophageal course along the posterior left atrium: a comparison to magnetic resonance imaging. J Cardiovasc Electrophysiol. 2007;18(2):169–173. doi: 10.1111/j.1540-8167.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Jais P, Hocini M, Sanders P, Rotter M, Rostock T, Sacher F, Jais C, Clementy J, Haissaguerre M. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol. 2005;16(10):1104–1107. doi: 10.1111/j.1540-8167.2005.50124.x. [DOI] [PubMed] [Google Scholar]
- Stevenson WG. Catheter ablation of monomorphic ventricular tachycardia. Curr Opin Cardiol. 2005;20(1):42–47. [PubMed] [Google Scholar]
- de Chillou C, Lacroix D, Klug D, Magnin-Poull I, Marquie C, Messier M, Andronache M, Kouakam C, Sadoul N, Chen J, Aliot E, Kacet S. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002;105(6):726–731. doi: 10.1161/hc0602.103675. [DOI] [PubMed] [Google Scholar]
- Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45(7):1104–1108. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115(15):2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112(18):2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikaga H, Sasano T, Dong J, Zviman MM, Evers R, Hopenfeld B, Castro V, Helm RH, Dickfeld T, Nazarian S, Donahue JK, Berger RD, Calkins H, Abraham MR, Marban E, Lardo AC, McVeigh ER, Halperin HR. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res. 2007;101(9):939–947. doi: 10.1161/CIRCRESAHA.107.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio EJ, Ashikaga H, Kaba RA, Cervantes D, Hopenfeld B, Wit AL, Peters NS, McVeigh ER, Garan H, Coromilas J. Model of reentrant ventricular tachycardia based on infarct border zone geometry predicts reentrant circuit features as determined by activation mapping. Heart Rhythm. 2007;4(8):1034–1045. doi: 10.1016/j.hrthm.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma-Carbayo MJ, Kellman P, Arai AE, McVeigh ER. Motion corrected free-breathing delayed-enhancement imaging of myocardial infarction using nonrigid registration. J Magn Reson Imaging. 2007;26(1):184–190. doi: 10.1002/jmri.20957. [DOI] [PubMed] [Google Scholar]
- Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243(3):690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, Weiss RG, Berger RD, Bluemke DA, Halperin HR. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114(12):1277–1284. doi: 10.1161/CIRCULATIONAHA.105.607655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickfeld T, Calkins H, Zviman M, Kato R, Meininger G, Lickfett L, Berger R, Halperin H, Solomon SB. Anatomic stereotactic catheter ablation on three-dimensional magnetic resonance images in real time. Circulation. 2003;108(19):2407–2413. doi: 10.1161/01.CIR.0000093191.05433.B0. [DOI] [PubMed] [Google Scholar]
- Dickfeld T, Calkins H, Zviman M, Meininger G, Lickfett L, Roguin A, Lardo AC, Berger R, Halperin H, Solomon SB. Stereotactic magnetic resonance guidance for anatomically targeted ablations of the fossa ovalis and the left atrium. J Interv Card Electrophysiol. 2004;11(2):105–115. doi: 10.1023/B:JICE.0000042348.13084.04. [DOI] [PubMed] [Google Scholar]
- Malchano ZJ, Neuzil P, Cury RC, Holmvang G, Weichet J, Schmidt EJ, Ruskin JN, Reddy VY. Integration of cardiac CT/MR imaging with three-dimensional electroanatomical mapping to guide catheter manipulation in the left atrium: implications for catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(11):1221–1229. doi: 10.1111/j.1540-8167.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Malchano ZJ, Holmvang G, Schmidt EJ, d'Avila A, Houghtaling C, Chan RC, Ruskin JN. Integration of cardiac magnetic resonance imaging with three-dimensional electroanatomic mapping to guide left ventricular catheter manipulation: feasibility in a porcine model of healed myocardial infarction. J Am Coll Cardiol. 2004;44(11):2202–2213. doi: 10.1016/j.jacc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- Dong J, Dickfeld T, Dalal D, Cheema A, Vasamreddy CR, Henrikson CA, Marine JE, Halperin HR, Berger RD, Lima JA, Bluemke DA, Calkins H. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(5):459–466. doi: 10.1111/j.1540-8167.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- Hocini M, Sanders P, Jais P, Hsu LF, Weerasoriya R, Scavee C, Takahashi Y, Rotter M, Raybaud F, Macle L, Clementy J, Haissaguerre M. Prevalence of pulmonary vein disconnection after anatomical ablation for atrial fibrillation: consequences of wide atrial encircling of the pulmonary veins. Eur Heart J. 2005;26(7):696–704. doi: 10.1093/eurheartj/ehi096. [DOI] [PubMed] [Google Scholar]
- Mantovan R, Verlato R, Calzolari V, Baccillieri S, De Leo A, Turrini P, Pastore G, Crosato M, Ramondo A, Stritoni P. Comparison between anatomical and integrated approaches to atrial fibrillation ablation: adjunctive role of electrical pulmonary vein disconnection. J Cardiovasc Electrophysiol. 2005;16(12):1293–1297. doi: 10.1111/j.1540-8167.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- Lickfett L, Dickfeld T, Kato R, Tandri H, Vasamreddy CR, Berger R, Bluemke D, Luderitz B, Halperin H, Calkins H. Changes of pulmonary vein orifice size and location throughout the cardiac cycle: dynamic analysis using magnetic resonance cine imaging. J Cardiovasc Electrophysiol. 2005;16(6):582–588. doi: 10.1046/j.1540-8167.2005.40724.x. [DOI] [PubMed] [Google Scholar]
- Wittkampf FH, Nakagawa H. RF catheter ablation: Lessons on lesions. Pacing Clin Electrophysiol. 2006;29(11):1285–1297. doi: 10.1111/j.1540-8159.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111(2):127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, Geunther J, Potenza D, Martin DO, Cummings J, Burkhardt JD, Saliba W, Schweikert RA, Natale A. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112(5):627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- Asirvatham SJ, Bruce CJ, Friedman PA. Advances in imaging for cardiac electrophysiology. Coron Artery Dis. 2003;14(1):3–13. doi: 10.1097/00019501-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Lardo AC, McVeigh ER, Jumrussirikul P, Berger RD, Calkins H, Lima J, Halperin HR. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation. 2000;102(6):698–705. doi: 10.1161/01.cir.102.6.698. [DOI] [PubMed] [Google Scholar]
- Nazarian S, Kolandaivelu A, Zviman MM, Meininger GR, Kato R, Susil RC, Roguin A, Dickfeld TL, Ashikaga H, Calkins H, Berger RD, Bluemke DA, Lardo AC, Halperin HR. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation. 2008;118(3):223–229. doi: 10.1161/CIRCULATIONAHA.107.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arepally A, Karmarkar PV, Weiss C, Rodriguez ER, Lederman RJ, Atalar E. Magnetic resonance image-guided trans-septal puncture in a swine heart. J Magn Reson Imaging. 2005;21(4):463–467. doi: 10.1002/jmri.20262. [DOI] [PubMed] [Google Scholar]
- Raval AN, Karmarkar PV, Guttman MA, Ozturk C, Desilva R, Aviles RJ, Wright VJ, Schenke WH, Atalar E, McVeigh ER, Lederman RJ. Real-time MRI guided atrial septal puncture and balloon septostomy in swine. Catheter Cardiovasc Interv. 2006;67(4):637–643. doi: 10.1002/ccd.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman RJ, Guttman MA, Peters DC, Thompson RB, Sorger JM, Dick AJ, Raman VK, McVeigh ER. Catheter-based endomyocardial injection with real-time magnetic resonance imaging. Circulation. 2002;105(11):1282–1284. doi: 10.1161/01.CIR.0000012425.71261.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S, Lynch C, 3rd, Whayne JG, Haines DE. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implications for catheter ablation. Circulation. 1993;88(4 Pt 1):1826–1831. doi: 10.1161/01.cir.88.4.1826. [DOI] [PubMed] [Google Scholar]
- Nath S, Redick JA, Whayne JG, Haines DE. Ultrastructural observations in the myocardium beyond the region of acute coagulation necrosis following radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1994;5(10):838–845. doi: 10.1111/j.1540-8167.1994.tb01122.x. [DOI] [PubMed] [Google Scholar]
- Schmidt EJ, Reddy VK, Ruskin JN. Nonenhanced magnetic resonance imaging for characterization of acute and subacute radiofrequency ablation lesions. Heart Rhythm. 2007;4(2):215–217. doi: 10.1016/j.hrthm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Dickfeld T, Kato R, Zviman M, Nazarian S, Dong J, Ashikaga H, Lardo AC, Berger RD, Calkins H, Halperin H. Characterization of acute and subacute radiofrequency ablation lesions with nonenhanced magnetic resonance imaging. Heart Rhythm. 2007;4(2):208–214. doi: 10.1016/j.hrthm.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso M, Yui Y, Kakishita M. Effects of thermal denaturation on the longitudinal relaxation time (T1) of water protons in protein solutions: study of the factors determining the T1 of water protons. Magn Reson Imaging. 1988;6(1):17–25. doi: 10.1016/0730-725X(88)90519-X. [DOI] [PubMed] [Google Scholar]
- Dickfeld T, Kato R, Zviman M, Lai S, Meininger G, Lardo AC, Roguin A, Blumke D, Berger R, Calkins H, Halperin H. Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2006;47(2):370–378. doi: 10.1016/j.jacc.2005.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AH, Hynynen K, Colucci V, Oshio K, Cline HE, Jolesz FA. Optimization of spoiled gradient-echo phase imaging for in vivo localization of a focused ultrasound beam. Magn Reson Med. 1996;36(5):745–752. doi: 10.1002/mrm.1910360513. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- McDannold N, Tempany CM, Fennessy FM, So MJ, Rybicki FJ, Stewart EA, Jolesz FA, Hynynen K. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240(1):263–272. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen KK, Jarrard J, Rieke V, Frisoli J, Daniel BL, Butts Pauly K. In vivo porcine liver radiofrequency ablation with simultaneous MR temperature imaging. J Magn Reson Imaging. 2006;23(4):578–584. doi: 10.1002/jmri.20528. [DOI] [PubMed] [Google Scholar]
- Rieke V, Kinsey AM, Ross AB, Nau WH, Diederich CJ, Sommer G, Pauly KB. Referenceless MR thermometry for monitoring thermal ablation in the prostate. IEEE Trans Med Imaging. 2007;26(6):813–821. doi: 10.1109/TMI.2007.892647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura K, Morikawa S, Murakami K, Sato K, Shiomi H, Naka S, Kurumi Y, Inubushi T, Tani T. An easy-to-use microwave hyperthermia system combined with spatially resolved MR temperature maps: phantom and animal studies. J Surg Res. 2006;135(1):179–186. doi: 10.1016/j.jss.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kahn T, Harth T, Kiwit JC, Schwarzmaier HJ, Wald C, Modder U. In vivo MRI thermometry using a phase-sensitive sequence: preliminary experience during MRI-guided laser-induced interstitial thermotherapy of brain tumors. J Magn Reson Imaging. 1998;8(1):160–164. doi: 10.1002/jmri.1880080128. [DOI] [PubMed] [Google Scholar]
- Shultz K, Pauly J, Scott G. Feasibility of Full RF Current-Vector Mapping for MR Guided RF Ablations. Paper presented at: Proceeding of the Joint Annual Meeting ISMRM-ESMRMB, Berlin, 2007. 2007. Berlin.
- McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52(15):1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgort DR, Duerk JL. A review of technical advances in interventional magnetic resonance imaging. Acad Radiol. 2005;12(9):1089–1099. doi: 10.1016/j.acra.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Niendorf T, Sodickson DK. Parallel imaging in cardiovascular MRI: methods and applications. NMR Biomed. 2006;19(3):325–341. doi: 10.1002/nbm.1051. [DOI] [PubMed] [Google Scholar]
- Bock M, Muller S, Zuehlsdorff S, Speier P, Fink C, Hallscheidt P, Umathum R, Semmler W. Active catheter tracking using parallel MRI and real-time image reconstruction. Magn Reson Med. 2006;55(6):1454–1459. doi: 10.1002/mrm.20902. [DOI] [PubMed] [Google Scholar]
- Ohliger MA, Grant AK, Sodickson DK. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn Reson Med. 2003;50(5):1018–1030. doi: 10.1002/mrm.10597. [DOI] [PubMed] [Google Scholar]
- McVeigh ER, Guttman MA, Kellman P, Raval AN, Lederman RJ. Real-time, Interactive MRI for cardiovascular interventions. Acad Radiol. 2005;12(9):1121–1127. doi: 10.1016/j.acra.2005.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakos WE, Hoge WS, Mitsouras D. Generalized encoding through the use of selective excitation in accelerated parallel MRI. NMR Biomed. 2006;19(3):379–392. doi: 10.1002/nbm.1047. [DOI] [PubMed] [Google Scholar]
- Niendorf T, Hardy CJ, Giaquinto RO, Gross P, Cline HE, Zhu Y, Kenwood G, Cohen S, Grant AK, Joshi S, Rofsky NM, Sodickson DK. Toward single breath-hold whole-heart coverage coronary MRA using highly accelerated parallel imaging with a 32-channel MR system. Magn Reson Med. 2006;56(1):167–176. doi: 10.1002/mrm.20923. [DOI] [PubMed] [Google Scholar]
- Dumoulin CL, Souza SP, Darrow RD. Real-time position monitoring of invasive devices using magnetic resonance. Magn Reson Med. 1993;29(3):411–415. doi: 10.1002/mrm.1910290322. [DOI] [PubMed] [Google Scholar]
- Elgort DR, Wong EY, Hillenbrand CM, Wacker FK, Lewin JS, Duerk JL. Real-time catheter tracking and adaptive imaging. J Magn Reson Imaging. 2003;18(5):621–626. doi: 10.1002/jmri.10402. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wendt M, Aschoff AJ, Lewin JS, Duerk JL. A multielement RF coil for MRI guidance of interventional devices. J Magn Reson Imaging. 2001;14(1):56–62. doi: 10.1002/jmri.1151. [DOI] [PubMed] [Google Scholar]
- Zuehlsdorff S, Umathum R, Volz S, Hallscheidt P, Fink C, Semmler W, Bock M. MR coil design for simultaneous tip tracking and curvature delineation of a catheter. Magn Reson Med. 2004;52(1):214–218. doi: 10.1002/mrm.20108. [DOI] [PubMed] [Google Scholar]
- Gepstein L, Hayam G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation. 1997;95(6):1611–1622. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- Wittkampf FH, Wever EF, Derksen R, Wilde AA, Ramanna H, Hauer RN, Robles de Medina EO. LocaLisa: new technique for real-time 3-dimensional localization of regular intracardiac electrodes. Circulation. 1999;99(10):1312–1317. doi: 10.1161/01.cir.99.10.1312. [DOI] [PubMed] [Google Scholar]
- Nevo E. Method and apparaus to estimate location and orientation of objects during magnetic resonance imaging. 2003. (WO/2000/013586)
- Ben-Haim SA. System for determining the location and orientation of objects during magnetic resonance imaging
- Peters DC, Lederman RJ, Dick AJ, Raman VK, Guttman MA, Derbyshire JA, McVeigh ER. Undersampled projection reconstruction for active catheter imaging with adaptable temporal resolution and catheter-only views. Magn Reson Med. 2003;49(2):216–222. doi: 10.1002/mrm.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman MA, Ozturk C, Raval AN, Raman VK, Dick AJ, Desilva R, Karmarkar P, Lederman RJ, McVeigh ER. Interventional cardiovascular procedures guided by real-time MR imaging: An interactive interface using multiple slices, adaptive projection modes and live 3D renderings. J Magn Reson Imaging. 2007;26(6):1429–1435. doi: 10.1002/jmri.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burl M, Coutts GA, Herlihy DJ, Hill-Cottingham R, Eastham JF, Hajnal JV, Young IR. Twisted-pair RF coil suitable for locating the track of a catheter. Magn Reson Med. 1999;41(3):636–638. doi: 10.1002/(SICI)1522-2594(199903)41:3<636::AID-MRM30>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kreuger S, Lips O, Ruhl KM, Schmitz S, Weiss S, Wirtz D, Beucker A. RF-safe intravascular imaging using self-visualizing transformer line. Paper presented at: Joint Annual Meeting ISMRM-ESMRMB, Berlin. 2007.
- Ocali O, Atalar E. Intravascular magnetic resonance imaging using a loopless catheter antenna. Magn Reson Med. 1997;37(1):112–118. doi: 10.1002/mrm.1910370116. [DOI] [PubMed] [Google Scholar]
- Pappone C, Vicedomini G, Manguso F, Gugliotta F, Mazzone P, Gulletta S, Sora N, Sala S, Marzi A, Augello G, Livolsi L, Santagostino A, Santinelli V. Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol. 2006;47(7):1390–1400. doi: 10.1016/j.jacc.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Aryana A, d'Avila A, Heist EK, Mela T, Singh JP, Ruskin JN, Reddy VY. Remote magnetic navigation to guide endocardial and epicardial catheter mapping of scar-related ventricular tachycardia. Circulation. 2007;115(10):1191–1200. doi: 10.1161/CIRCULATIONAHA.106.672162. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Neuzil P, Malchano ZJ, Vijaykumar R, Cury R, Abbara S, Weichet J, McPherson CD, Ruskin JN. View-synchronized robotic image-guided therapy for atrial fibrillation ablation: experimental validation and clinical feasibility. Circulation. 2007;115(21):2705–2714. doi: 10.1161/CIRCULATIONAHA.106.677369. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Hassenzahl WV, Hetts SW, Arenson RL. Remote control of catheter tip deflection: an opportunity for interventional MRI. Magn Reson Med. 2002;48(6):1091–1095. doi: 10.1002/mrm.10325. [DOI] [PubMed] [Google Scholar]
- Settecase F, Sussman MS, Wilson MW, Hetts S, Arenson RL, Malba V, Bernhardt AF, Kucharczyk W, Roberts TP. Magnetically-assisted remote control (MARC) steering of endovascular catheters for interventional MRI: a model for deflection and design implications. Med Phys. 2007;34(8):3135–3142. doi: 10.1118/1.2750963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys. 1998;74(4):494–522. [PubMed] [Google Scholar]
- Shellock FG. Radiofrequency energy-induced heating during MR procedures: a review. J Magn Reson Imaging. 2000;12(1):30–36. doi: 10.1002/1522-2586(200007)12:1<30::AID-JMRI4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Knopp MV, Essig M, Debus J, Zabel HJ, van Kaick G. Unusual burns of the lower extremities caused by a closed conducting loop in a patient at MR imaging. Radiology. 1996;200(2):572–575. doi: 10.1148/radiology.200.2.8685360. [DOI] [PubMed] [Google Scholar]
- Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13(1):105–114. doi: 10.1002/1522-2586(200101)13:1<105::AID-JMRI1016>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Konings MK, Bartels LW, Smits HF, Bakker CJ. Heating around intravascular guidewires by resonating RF waves. J Magn Reson Imaging. 2000;12(1):79–85. doi: 10.1002/1522-2586(200007)12:1<79::AID-JMRI9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Atalar E. Radiofrequency safety for interventional MRI procedures. Acad Radiol. 2005;12(9):1149–1157. doi: 10.1016/j.acra.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Raman VK, Lederman RJ. Interventional cardiovascular magnetic resonance imaging. Trends Cardiovasc Med. 2007;17(6):196–202. doi: 10.1016/j.tcm.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi R, Hill DL, Keevil SF, Miquel ME, Muthurangu V, Hegde S, Rhode K, Barnett M, van Vaals J, Hawkes DJ, Baker E. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003;362(9399):1877–1882. doi: 10.1016/S0140-6736(03)14956-2. [DOI] [PubMed] [Google Scholar]
- Armenean C, Perrin E, Armenean M, Beuf O, Pilleul F, Saint-Jalmes H. RF-induced temperature elevation along metallic wires in clinical magnetic resonance imaging: influence of diameter and length. Magn Reson Med. 2004;52(5):1200–1206. doi: 10.1002/mrm.20246. [DOI] [PubMed] [Google Scholar]
- Kreuger S, Schmitz S, Ruhl KM, Speuntrup E, Katoh M, Linssen M, Schade H, Weiss S, Beucker A. Evaluation of an MR-compatible guidewire made in a novel micro-pultrusion process. Paper presented at: Proceeding of the Joint Annual Meeting ISMRM-ESMRMB, Berlin. 2007.
- Wirtz D, Lips O, David B, Krueger S, Wiess S. Diagnostic MR-electrophysiology catheter with highly reisistive wires for reduction of RF-heating. Paper presented at: Joint Annual Meeting ISMRM-ESMRMB, Berlin. 2007.
- Ladd ME, Quick HH. Reduction of resonant RF heating in intravascular catheters using coaxial chokes. Magn Reson Med. 2000;43(4):615–619. doi: 10.1002/(SICI)1522-2594(200004)43:4<615::AID-MRM19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Atalar E. Safe coaxial cables. Paper presented at: 7th Annual Meeting ISMRM, Philadelphia, PA. 1999.
- Vernickel P, Schulz V, Weiss S, Gleich B. A safe transmission line for MRI. IEEE Trans Biomed Eng. 2005;52(6):1094–1102. doi: 10.1109/TBME.2005.846713. [DOI] [PubMed] [Google Scholar]
- Weiss S, Vernickel P, Schaeffter T, Schulz V, Gleich B. Transmission line for improved RF safety of interventional devices. Magn Reson Med. 2005;54(1):182–189. doi: 10.1002/mrm.20543. [DOI] [PubMed] [Google Scholar]
- Wong EY, Zhang Q, Duerk JL, Lewin JS, Wendt M. An optical system for wireless detuning of parallel resonant circuits. J Magn Reson Imaging. 2000;12(4):632–638. doi: 10.1002/1522-2586(200010)12:4<632::AID-JMRI17>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Fandrey S, Weiss S, Muller J. Miniature Optical Signal Transmission System for an Active Intravascular Device. Paper presented at: Joint Annual Meeting ISMRM-ESMRMB, Berlin. 2007.
- Quick HH, Kuehl H, Kaiser G, Bosk S, Debatin JF, Ladd ME. Inductively coupled stent antennas in MRI. Magn Reson Med. 2002;48(5):781–790. doi: 10.1002/mrm.10269. [DOI] [PubMed] [Google Scholar]
- Scott G. A Vector Modulation Transmitt Array System. Paper presented at: 14th Annual Meeting ISMRM, Seattle, WA. 2006.
- Hayes DL, Holmes DR, Jr, Gray JE. Effect of 1.5 tesla nuclear magnetic resonance imaging scanner on implanted permanent pacemakers. J Am Coll Cardiol. 1987;10(4):782–786. doi: 10.1016/s0735-1097(87)80270-x. [DOI] [PubMed] [Google Scholar]
- Loewy J, Loewy A, Kendall EJ. Reconsideration of pacemakers and MR imaging. Radiographics. 2004;24(5):1257–1267. doi: 10.1148/rg.245045014. discussion 1267–1258. [DOI] [PubMed] [Google Scholar]
- Shellock FG, Crues JV. MR procedures: biologic effects, safety, and patient care. Radiology. 2004;232(3):635–652. doi: 10.1148/radiol.2323030830. [DOI] [PubMed] [Google Scholar]
- Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28(4):326–328. doi: 10.1111/j.1540-8159.2005.50024.x. [DOI] [PubMed] [Google Scholar]
- Shellock FG, Fischer L, Fieno DS. Cardiac pacemakers and implantable cardioverter defibrillators: in vitro magnetic resonance imaging evaluation at 1.5-tesla. J Cardiovasc Magn Reson. 2007;9(1):21–31. doi: 10.1080/10976640600897237. [DOI] [PubMed] [Google Scholar]
- Roguin A, Zviman MM, Meininger GR, Rodrigues ER, Dickfeld TM, Bluemke DA, Lardo A, Berger RD, Calkins H, Halperin HR. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110(5):475–482. doi: 10.1161/01.CIR.0000137121.28722.33. [DOI] [PMC free article] [PubMed] [Google Scholar]