Abstract
Babies experience hypoxia (H) and ischemia (I) from stroke. The only approved treatment for stroke is fibrinolytic therapy with tissue-type plasminogen activator (tPA). However, tPA potentiates H/I-induced impairment of responses to cerebrovasodilators such as hypercapnia and hypotension, and blockade of tPA-mediated vasoactivity prevents this deleterious effect. Coupling tPA to RBCs reduces its CNS toxicity through spatially confining the drug to the vasculature. Mitogen activated protein kinase (MAPK), a family of at least 3 kinases, is upregulated after H/I. In this study we determined if RBC-tPA given before or after cerebral H/I would preserve responses to cerebrovasodilators and prevent neuronal injury mediated through the ERK MAPK pathway. Animals given RBC-tPA maintained responses to cerebrovasodilators at levels equivalent to pre-H/I values. CSF and brain parenchymal ERK MAPK was elevated by H/I and this upregulation was potentiated by tPA, but blunted by RBC-tPA. U 0126, an ERK MAPK antagonist, also maintained cerebrovasodilation post H/I. Neuronal degeneration in CA1 hippocampus and parietal cortex after H/I was exacerbated by tPA, but ameliorated by RBC-tPA and U 0126. These data suggest that coupling tPA to RBCs may offer a novel approach towards increasing the benefit/risk ratio of thrombolytic therapy for CNS disorders associated with H/I.
Keywords: autoregulatory vasodilation, autoregulation, cerebral arteries, dilation, cerebral arteries, cerebral ischemia and/or reperfusion, ischemia, cerebral vascular response to carbon dioxide, cerebral vascular biology, cerebrovascular reactivity, cerebral vasculature, pig, experimental system, neonates, experimental system, hypercapnia, reperfusion injury, injury, ERK (extracellular signalregulated kinase), kinases, MAPK (mitogen-activated protein kinase), microvasculature, pial artery, pial vessels, global cerebral ischemia, ischemia
Introduction
Ischemic stroke is an important contributor to morbidity and mortality. Pediatric stroke may occur in as many as 1 in 4000 births (Nelson and Lynch, 2004) and complications due to hypoxia/ischemia are common (Ferriero 2004). Maternal and perinatal coagulopathy predispose to pediatric stroke (Gunther et al 2000; Kraus and Acheen 1999) with 30% of such events being due to thrombosis (DeVeber and Andrew 2001). The thrombolytic agent tissue-type plasminogen activator (tPA) remains the only approved treatment for acute stroke (Kim et al 1999), but its use in children has been limited and its benefit remains unclear (Benedict et al 2007; Janjua et al 2007). Indeed, the brief therapeutic window of tPA and the high incidence of post-treatment complications, including intracranial hemorrhage (ICH), have constrained the actual clinical use of tPA to approximately 3–8% of all patients eligible for such therapy (Lapchak and Araujo 2001; Lapchak 2002). Patients often remain hospitalized after an initial ischemic event because of the risk of recurrent cerebrovascular thrombosis, indicating a clear unmet need for safer and more effective treatment and thromboprophylaxis.
Balancing the risks and benefits of tPA is one of the central issues in managing patients with stroke and other cerebrovascular disorders accompanied by thrombosis and ischemia. Although the beneficial effects of tPA reducing the clot burden are apparent, the drug may also increase the volume of injured tissue after stroke, as exemplified in tPA null mice, provoke ICH and exacerbate excitotoxic neuronal death by enhancing signaling through the N-methyl-D-Aspartate glutamate receptor (Wang et al 1998; Nicole et al 2001).
Recent studies of brain ischemia implicate signaling through the LRP-receptor in the pathological effects of plasminogen activators on the cerebrovasculature (Armstead et al 2008). Our group has shown that a plasminogen activator inhibitor (PAI)-1 derived peptide (EEIIMD) inhibits tPA- and low density lipoprotein (LRP)-mediated signal transduction without compromising catalytic activity (Armstead et al 2005a; Akkawi et al 2006; Nassar et al 2004). Specifically, EEIIMD blocks the deleterious effects of exogenous tPA on stroke volume, ICH and edema in models of rat middle cerebral artery occlusion and embolic stroke models and markedly prevents neuronal cell loss after traumatic brain injury (TBI) in piglets without inhibiting tPA’s fibrinolytic acitivity (Armstead et al 2006). These data indicate that it may be possible to improve clinical outcome of stroke and TBI by separating the favorable and harmful activities of tPA.
In animal models of stroke, tPA contributes to adverse outcome through mechanism(s) involving the activation of matrix metalloproteinases (MMPs) (Tsui et al 2005). MMPs are upregulated after brain injury (Wang et al 2002; Laher and Zhang 2001), in part, by activating mitogen activated protein kinase (MAPK), a family of at least 3 kinases (extracellular signal-related kinase - ERK -, p38, and c-Jun-N-terminal kinase – JNK). Our recent studies show that urokinase plasminogen activator (uPA) contributes to impaired stimulus-induced cerebrovascular dilation following cerebral hypoxia/ischemia (H/I) in the newborn pig through LRP and upregulation of ERK MAPK (Armstead et al 2008).
Contemporaneous studies from our group demonstrate that anchoring tPA on red blood cells (RBC) endows the resultant complex, RBC-tPA, with dramatically prolonged circulation time (many hours vs minutes for tPA), while spatially constraining it to the intravascular space (Murciano et al 2003; Ganguly et al 2005; Zaitsev et al 2006). In rodent models of cerebrovascular thrombosis and traumatic brain injury, treatment with this RBC-tPA complex provided effective thromboprophylaxis, rapid reperfusion, neuroprotection, and reduction in mortality all without causing ICH (Danielyan et al 2008; Stein et al, in press). These studies suggest that RBC carriage may offer a unique opportunity to increase the benefit risk ratio of tPA within the CNS.
The present study was designed to determine if RBC-tPA given before or after cerebral hypoxia/ischemia would help preserve stimulus-induced cerebrovasodilation and prevent neuronal injury mediated through the ERK MAPK pathway.
Methods
Closed cranial window technique and cerebral hypoxia/ischemia
Newborn pigs (1–5 days, 1.2–1.8 Kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were sedated with isoflurane (1–2 MAC). Anesthesia was maintained with a-chloralose (30–50 mg/kg. supplemented with 5 mg/kg/h i.v.). A catheter was inserted into a femoral artery to monitor blood pressure and to sample for blood gas tensions and pH. Drugs to maintain anesthesia were administered through a second catheter placed in a femoral vein. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37° – 39° C, monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37° C and had the following chemistry: pH 7.33, pCO2 46 mm Hg, and pO2 43 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measurped with a microscope, a camera, a video output screen and a video microscaler.
Total cerebral ischemia was accomplished by infusing artificial CSF into a hollow bolt in the cranium to maintain an intracranial pressure 15 mm Hg greater than the numerical mean of systolic and diastolic arterial blood pressure. Intracranial pressure was monitored via a sidearm of the cranial window. To prevent the arterial pressure from rising inordinately (Cushing response), venous blood was withdrawn as necessary to maintain mean arterial blood pressure no greater than 100 mm Hg. As the cerebral ischemic response subsided, the shed blood was returned to the animal. Cerebral ischemia was maintained for 20 min. Hypoxia (PO2 of approximately 35 mm Hg) was produced for 10 min before ischemia by decreasing the inspired O2 via inhalation of N2, which was followed immediately by the total cerebral ischemia. Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 ml blood/Kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25 and 45%, respectively). Such drops in blood pressure were maintained constant for 10 min by titration of additional blood withdrawal or blood reinfusion. Two levels of hypercapnia (low and high) were induced via inhalation of graded levels of a 10% CO2-21% O2-balance N2 gas mixture for 10 min to produce levels of pCO2 of 50–60 mm Hg for the low exposure and 70–80 mm Hg for the high exposure.
Protocol
Two types of pial vessels, small arteries (resting diameter, 120–160 μm) and arterioles (resting diameter, 50–70 μm) were examined to determine whether segmental differences in the effects of hypoxia/ischemia could be identified. Typically, 2–3 ml of artificial CSF were flushed through the window over a 30s period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μl of the total cranial window volume of 500 μl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
Thirteen experimental groups were studied (all n=6): (1) sham control, vehicle treated, (2) hypoxia/ischemia, vehicle pre-treated, (3) hypoxia/ischemia pre-treated with tPA (2mg/kg iv), (4) hypoxia/ischemia pre-treated with tPA (0.1 mg/kg iv), (5) hypoxia/ischemia pre-treated with RBC-tPA (0.1 mg/kg iv), (6) hypoxia/ischemia pre-treated with the RBC-vehicle, (7) hypoxia/ischemia pre-treated with U 0126 (1mg/kg iv), (8) hypoxia/ischemia, vehicle post-treated, (9) hypoxia/ischemia post-treated with tPA (2mg/kg iv), (10) hypoxia/ischemia post-treated with tPA (0.1 mg/kg iv), (11) hypoxia/ischemia post-treated with RBC-tPA (0.1 mg/kg iv), (12) hypoxia/ischemia post-treated with the RBC-vehicle, and (13) hypoxia/ischemia post-treated with U 0126 (1mg/kg iv). Pre-treatment time was 30min prior to insult, while post-treatment time was 2h post insult. The vehicle for all agents was 0.9% saline, except for the MAPK inhibitor, which used dimethyl sulfoxide (100 μl) diluted with 9.9 ml 0.9% saline. In sham control animals, responses to hypercapnia, hypotension, and isoproterenol (10−8, 10−6 M) were obtained initially and then again 1 and 4h later in the presence of the agent vehicle. In hypoxia/ischemia animals, responses to vasoactive stimuli were obtained initially and then again 1 and 4h post insult in the presence of the agent vehicle. In drug treated hypoxia/ischemia animals, drugs were administered either 30 min before or 2h after hypoxia/ischemia and the insult protocol followed as described above.
Preparation of RBC-tPA
RBCs were isolated by centrifugation from fresh anti-coagulated (heparin, 1000U/Kg) animal blood. Biotinylated tPA was coupled to biotinylated RBC via streptavidin, producing RBC-tPA complexes possessing 5×104 tPA molecules per RBC, as described previously (Murciano et al 2003; Ganguly et al 2005).
ELISA
Commercially available ELISA Kits were used to quantity CSF ERK MAPK (Assay Designs) concentration. Phosphorylated ERK MAPK enzyme values were normalized to total form and then expressed as percent of the control condition.
Immunohistochemistry
Four hours after cerebral hypoxia/ischemia, the animal was sacrificed and 2 thin slices of parietal cortex were cut parallel to the brain surface. These slices were placed in 4% paraformaldehyde for 24h at 4° C and then subjected to Paraffin sectioning. Paraffin sections of the parietal cortex from piglet brains after hypoxia/ischemia and from uninjured sham control animals were unwaxed, were incubated in 10mM sodium citrate buffer pH 6.0 inside a food steamer (sub boiling temperature) for 10 minutes to unmask the antigen, endogenous peroxidase was blocked with 0.3 % H2O2, and stained with anti-Phospho-p44/42 MAPK rabbit monoclonal antibody which recognize the phosphorylated forms of both p42 and p44 kinases (ERK1 and ERK2) (1 μg/ml, Cell Signaling #4376), or with mouse IgG1 as a negative control, secondary biotinylated anti-mouse IgG (1:200), followed by incubation with HRP-conjugated streptavidin. Peroxidase was detected using the avidin-biotin complex ABC Kit (Vector Lab) counterstained with hematoxylin. Positive staining is visualized by the brown colored [3,3-diaminobenzidine (DAB) reaction product.
Histologic preparation
Brains were perfused with heparinized saline, followed by 4% paraformaldehyde and then phosphate buffered saline. Coronal sections were prepared at 0.5 cm intervals for gross examination and photography. For histopathology, staining was done on both frozen and paraffin embedded slides, blocks from some animals were cryoprotected in sucrose and serial sections were cut at 40 μm intervals from the front face of each frozen block and mounted on microscope slides. Sections (10 μm) were stained with hematoxylin and eosin (H + E). Six animals from each of the groups were studied.
Histologic analysis of degeneration of neurons
All procedures were performed by investigators blinded to treatment. Preliminary examinations of injured brains revealed that the parietal lobe and the hippocampus suffered the most extensive pathologic changes. As such, more detailed histopathologic analyses were performed in these regions. The specific anatomic location used to select sections was based on the Stereotaxic Atlas of the Pig Brain (Felix et al 1999). To determine the extent of neuron degeneration in the cortex, 6 HE-stained sections from both hemispheres from each animal were examined and the number of dying neurons was counted manually. Fifteen frames from each slide (1× 1.2mm/per frame) encompassing a total area of 18 mm2 were chosen to estimate the number of degenerating neurons in the parietal cortex using light microscopy at 100X magnification. To determine the extent of neuron degeneration in the hippocampus, 6 HE-stained sections from both hemispheres from each animal were examined and the number of dying neurons in the CA1 subfield were counted manually. This subfield has been found previously to display the most extensive neuronal degeneration within the hippocampus in this model. Three frames (1× 1.2mm/per frame) encompassing a total area of 3.6 mm2 in each slide from each hippocampal CA1 region were analyzed at 100X magnification. The mean number of degenerating neurons in the cortical and hippocampal CA1 regions from both hemispheres in each group of animals was determined.
Statistical analysis
ERK MAPK staining was semi quantitatively determined through the use of a five point scale (0, no stain; 5, maximal stain) by an investigator blinded to treatment. Pial artery diameter, CSF ERK MAPK, and degenerating neuron values were analyzed using ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An α level of p<0.05 was considered significant in all statistical tests. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value.
Results
High dose tPA and RBC-tPA cause comparable pial artery vasodilation after cerebral H/I
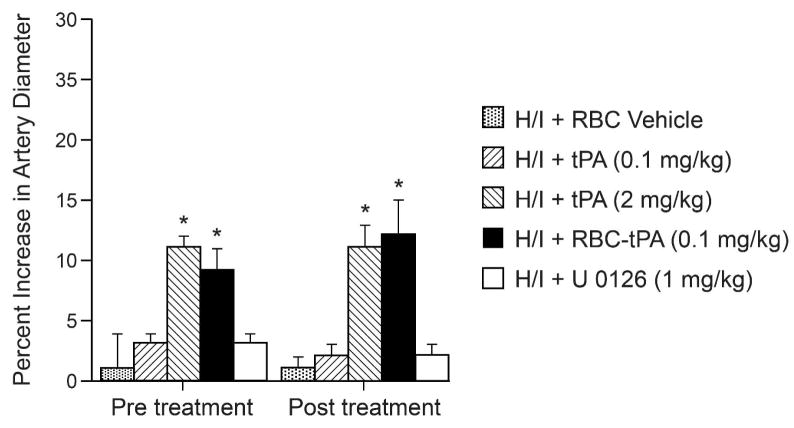
H/I produces pial artery vasoconstriction (Armstead et al 2008) and reduction in cerebral blood flow is thought to contribute to neuronal cell injury post insult. Animals were treated 30 min prior to H/I (pre-treatment) or 2h post insult (post-treatment) and the effect on pial artery diameter induced by H/I was measured. Neither pre-insult nor post-insult treatment with either tPA (0.1 mg/kg iv) or with the MAPK inhibitor U0126 (1 mg/kg iv) altered the effect of H/I on pial artery diameter (Fig 1). In contrast, RBC-tPA (0.1 mg/kg iv) or tPA (2 mg/kg iv) given pre-insult or post-insult caused a comparable increase in pial artery diameter in the setting of H/I (Fig 1). RBC carriage enhanced the potency of tPA induced vasodilation approximately 10- fold based on the dose administered.
Figure 1.
Influence of RBC vehicle, tPA (0.1 mg/kg iv), tPA (2 mg/kg iv), RBC-tPA (0.1mg/kg iv) and U 0126 (1 mg/kg iv) on pial artery diameter after H/I, n=4–6. *p<0.05 compared to H/I + RBC vehicle.
RBC-tPA prevents, whereas tPA aggravates, H/I induced impairment of hypercapnic and hypotensive pial artery dilation
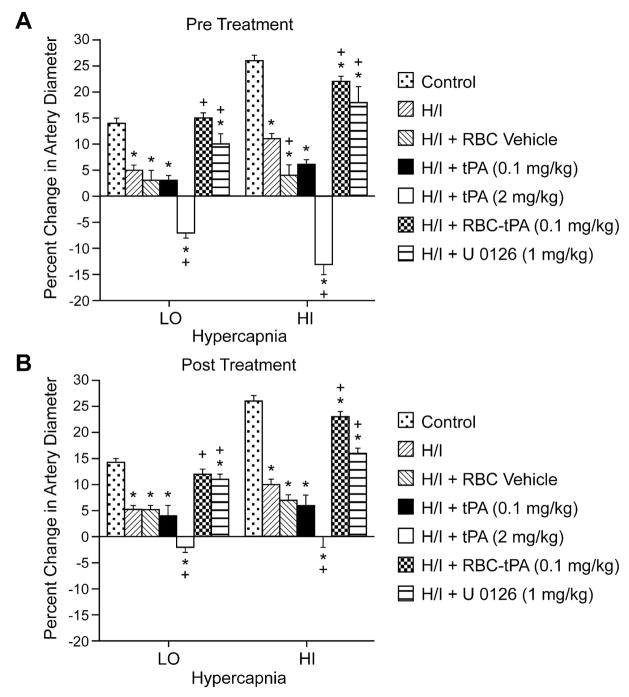
Hypercapnia, hypotension, and isoproterenol elicited reproducible dilation of pial small arteries and arterioles (data not shown). Dilation of small pial arteries in response to hypercapnia was blunted after H/I, but reversed to vasoconstriction in pigs treated before (30 min) or after (2h) insult with tPA (2 mg/kg iv) (Fig. 2). Pre- or post-treatment with either tPA (0.1 mg/kg iv) or RBC vehicle did not significantly affect the impaired responses to hypercapnia seen after H/I (Fig. 2). In contrast, dilation was maintained at levels nearly equivalent to pre-H/I values in animals given RBC-tPA (0.1 mg/kg iv) pre- or post-insult. Similarly, the ERK MAPK inhibitor U 0126 (1 mg/kg iv) preserved vasodilation in the setting of hypercapnia when given post insult (Fig. 2).
Figure 2.
Soluble tPA administration aggravates H/I induced impairment of hypercapnic pial artery dilation, whereas RBC-tPA prevents hypercapnic dilator impairment. Influence of hypercapnia (lo, hi; pCO2 of 50–55 and 70–75 mm Hg) on pial artery diameter before (control), 2.5h after hypoxia/ischemia (H/I), after H/I treated with RBC vehicle, after H/I treated with tPA (0.1 mg/kg iv), after H/I treated with tPA (2 mg/kg iv), after H/I treated with RBC-tPA (0.1mg/kg iv), and after H/I treated with U 0126 (1 mg/kg iv), n=4–6. A: pretreatment 30min before H/I B: post treatment 2h after H/I *P<0.05 versus corresponding control value +P<0.05 versus corresponding non treated H/I value
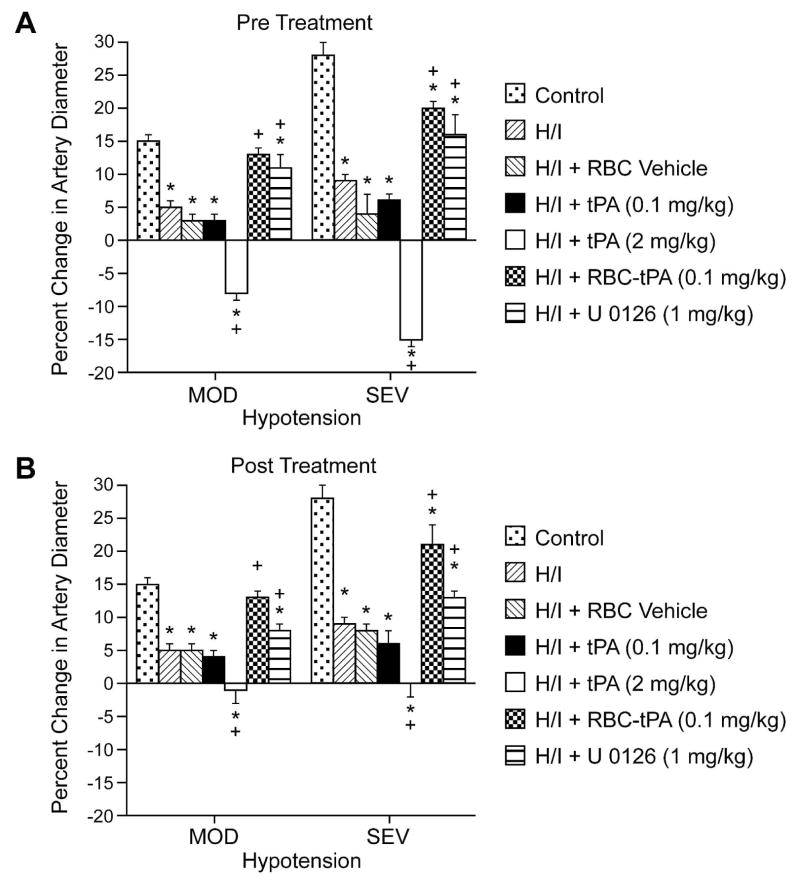
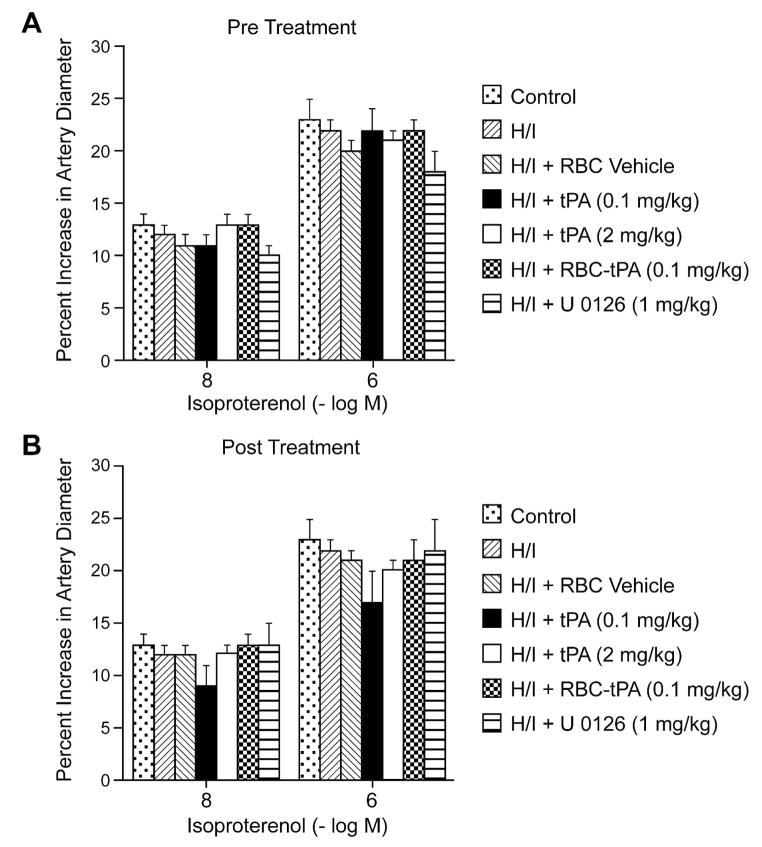
Exogenous tPA (2 mg/kg iv) further impaired dilation of small pial arteries in response to hypotension caused by H/I, whereas RBC-tPA (0.1 mg/kg iv), but not the RBC vehicle used to deliver tPA (0.1 mg/kg iv) and the ERK MAPK inhibitor both prevented (pre-insult) or restored (post-insult) vasodilation (Fig. 3). Thus, RBC-tPA and tPA caused opposite effects on pial artery vasoreactivity impairment of hypercapnia and hypotension. It is of interest that pretreatment with tPA (2 mg/kg iv) impaired responses to these two stimuli to a greater extent than what was seen when tPA was given after the H/I insult (Fig. 2 and 3). These data indicate that prophylactic administration of tPA may produce a greater impairment of cerebral hemodynamics. Vasodilation of small pial arteries in response to isoproterenol was unaffected by H/I, tPA, RBC-tPA and U 0126 (Fig. 4). Similar observations were made in pial arterioles (data not shown).
Figure 3.
Soluble tPA administration aggravates H/I induced impairment of hypotensive pial artery dilation, whereas RBC-tPA prevents hypotensive dilator impairment. Influence of hypotension (mod,sev; 25 and 45% reductions in mean arterial blood pressure for 10 min) on pial artery diameter before (control), 2.5h after hypoxia/ischemia (H/I), after H/I treated with RBC vehicle, after H/I treated with tPA (0.1 mg/kg iv), after H/I treated with tPA (2 mg/kg iv), after H/I treated with RBC-tPA (0.1mg/kg iv), and after H/I treated with U 0126 (1 mg/kg iv), n=4–6. A: pretreatment 30 before H/I B: post treatment 2h after H/I *P<0.05 versus corresponding control value +P<0.05 versus corresponding non treated H/I value.
Figure 4.
Soluble tPA and RBC-tPA has no effect on isoproterenol induced pial artery dilation. Influence of isoproterenol (10−8, 10−6M ) on pial artery diameter before (control), 2.5h after hypoxia/ischemia (H/I), after H/I treated with RBC vehicle, after H/I treated with tPA (0.1 mg/kg iv), after H/I treated with tPA (2 mg/kg iv), after H/I treated with RBC-tPA (0.1mg/kg iv), and after H/I treated with U 0126 (1 mg/kg iv), n=4–6. A: pretreatment 30 before H/I B: post treatment 2h after H/I.
RBC-tPA blunts, whereas tPA augments, H/I induced phosphorylation of ERK MAPK
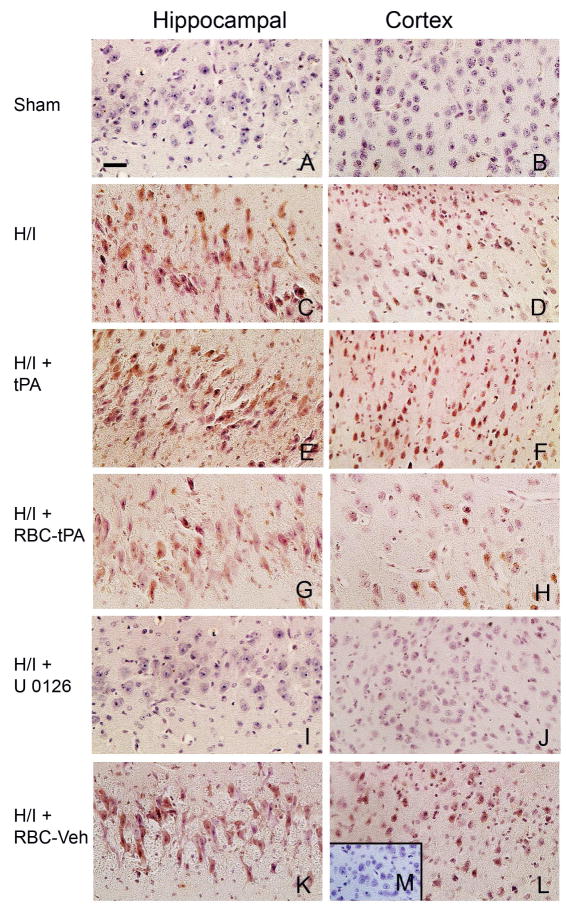
We next investigated the mechanism by which RBC-tPA preserved vasodilation beginning with its effect on phosphorylation of ERK MAPK after ischemia (Laher and Zhang 2001). Cerebral hypoxia/ischemia was induced in piglets. Two hours later the animals were given tPA (2 mg/kg iv), RBC-tPA (0.1 mg/kg iv), RBC-vehicle, or U 0126 (1 mg/kg iv). Two hours post-treatment (4h post cerebral H/I), phospho (activated) ERK MAPK was analyzed using an immunohistochemical approach (Fig. 5). Abundant phospho ERK MAPK (4–5 on a 5 point scale) was observed in the hippocampus and parietal cortex in animals given tPA after cerebral H/I (Fig. 5, panels E,F). tPA increased the amount of phospho ERK MAPK compared with animals exposed to H/I that remained untreated (Fig 5, panels C,D) (Armstead et al 2008). The comparable section from a sham control animal was unremarkable (Fig. 5, panels A,B) (Armstead et al 2008). In contrast, less phospho ERK MAPK was detected in the parietal cortex and hippocampus of animals treated with RBC-tPA (Fig. 5, panels G,H) compared to animals exposed to H/I that were treated with tPA (Fig. 5, panels E,F). Detection of phospho ERK MAPK in animals treated with the RBC-vehicle (lacking coupled tPA) (Fig. 5, panels K,L) was comparable to untreated animals post H/I (Fig 5, panels C,D) (Armstead et al 2008). U 0126 blocked the upregulation of phospho ERK MAPK after H/I (Fig. 5, panels I, J). In contrast, no staining was seen with non-immune IgG in the hypoxic/ischemic animal (Fig. 5, panel M insert).
Figure 5.
tPA aggravates H/I induced ERK MAPK phosphorylation, while RBC-tPA blunts insult associated increases in ERK MAPK phosphorylation. Immunohistochemical data for phospho (activated) ERK MAPK obtained from piglets 4h after sham control (Panels A,B) or cerebral H/I and post-treatment 2 h after injury with either tPA (2 mg/kg), RBC-tPA (0.1 mg/kg), RBC-vehicle, or U 0126 (1 mg/kg). Abundant phospho ERK MAPK (4–5 on a 5 point scale) was observed in the hippocampus and parietal cortex with tPA administration following cerebral H/I (Panels E,F). Comparison with post insult non treated with tPA (Panels C,D) evidences a greater upregulation with tPA post-treatment. However, RBC-tPA post treatment shows much less upregulation of phospho ERK MAPK in the parietal cortex and hippocampus (Panels G,H). In contrast, upregulation of phospho ERK MAPK in animals treated with the RBC-vehicle (lacking coupled tPA) (Panels K,L) was no different than that observed with cerebral H/I alone (Panels C,D). U 0126 blunted phospho ERK MAPK upregulation (Panels I,J), supportive of it being an efficacious ERK MAPK antagonist. The insert of panel M serves as a negative control in that there was minimal IgG staining. These data indicate that post-treatment with tPA and RBC-tPA modulates activated ERK MAPK upregulation in both the parietal cortex and hippocampus after H/I.
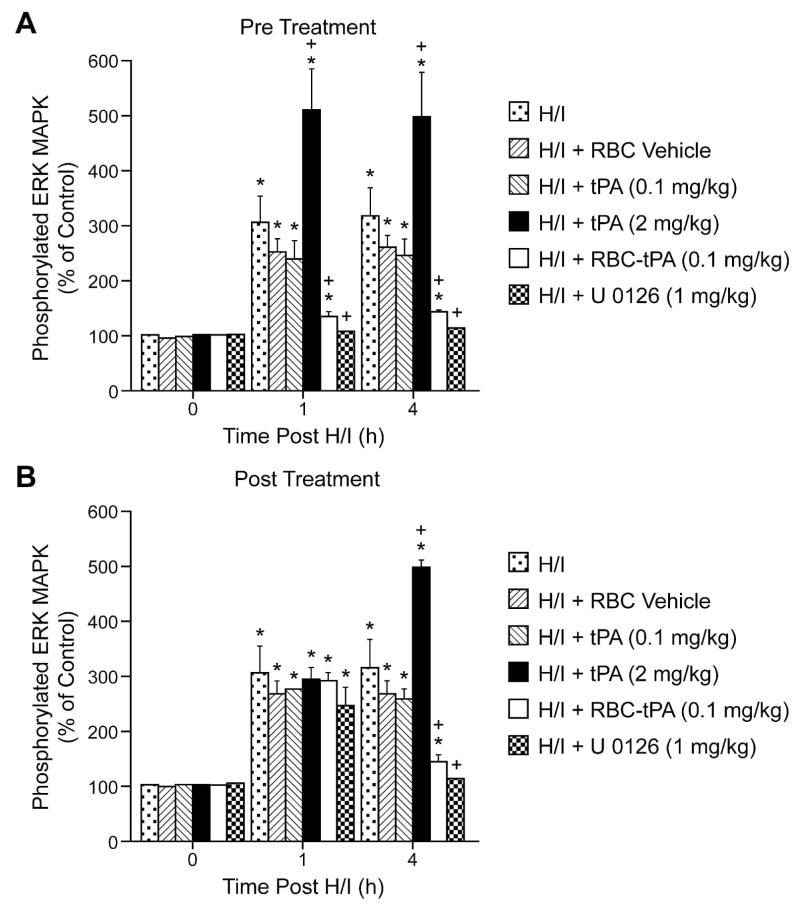
To estimate ERK MAPK in brain tissue, total and phosphorylated ERK MAPK in cortical periarachnoid CSF was measured by ELISA at baseline and after H/I. The activation (phosphorylation) state of the ERK MAPK isoform was determined by expressing the data as a percent of control (total). H/I induced a marked phosphorylation (activation) of ERK MAPK within 1h post injury (Fig. 6). Exogenous tPA (2mg/kg iv) administered 30 min prior to, or 2h after, H/I potentiated the phosphorylation of ERK MAPK (Fig. 6). In contrast, administration of RBC-tPA (0.1 mg/kg iv) pre- or post-injury blunted insult-induced phosphorylation of CSF ERK MAPK. Of note, RBC-tPA not only blocked the potentiation of CSF ERK MAPK release observed with tPA, but almost completely restored the values to those measured under sham control conditions (Fig. 6). Administration of RBC vehicle or 0.1 mg/kg iv tPA pre or post insult failed to modify ERK MAPK phosphorylation (Fig. 6). U 0126 (1mg/kg iv), a purported ERK MAPK antagonist, blocked ERK MAPK phosophorylation, as expected (Fig. 6).
Figure 6.
Phosphorylation of ERK MAPK in cortical periarachnoid CSF prior to H/I (0 min) and as a function of time (hour) after H/I in RBC vehicle, H/I and tPA (0.1 mkg/kg iv), in H/I + tPA (2 mg/Kg iv), H/I + RBC coupled to tPA (0.1 mg/Kg iv), and H/I + U 0126 (1 mg/kg iv) animals, n=3–6. Data expressed as percent of control by ELISA determination of phospho MAPK and total MAPK isoforms and subsequent normalization to total form. A: pretreatment 30 min prior to H/I B: post treatment 2h after H/I. *P<0.05 compared with corresponding 0 time value +P<0.05 compared with corresponding H/I non treated value.
Post-injury treatment with RBC-tPA and U 0126 attenuates histopathologic changes in the parietal cortex and hippocampus associated with H/I
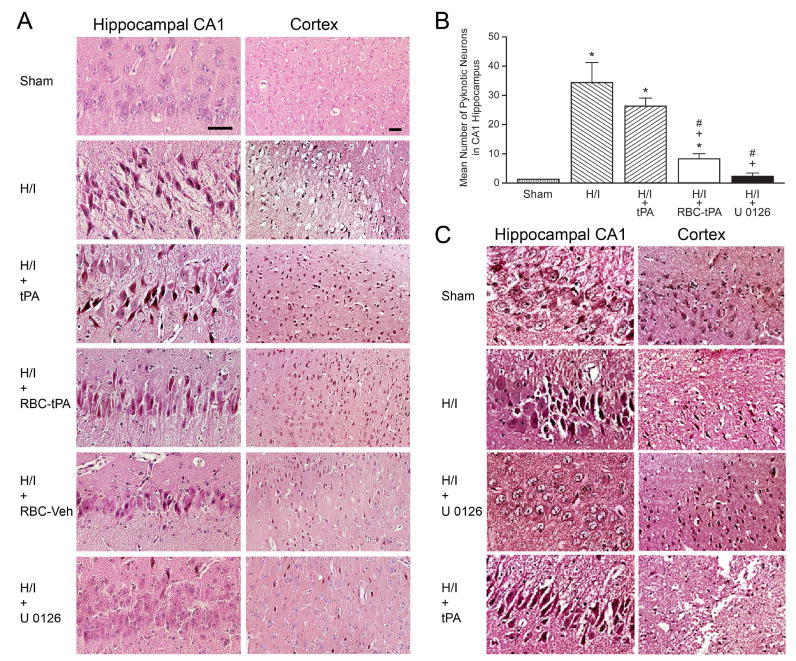
Figure 7A shows H + E staining for the hippocampal CA1 region and parietal cortex under sham, H/I, H/I + tPA, H/I + RBC-tPA, H/I + RBC vehicle, and H/I + U0126 post-treated conditions 4 after cerebral H/I injury. Numerous necrotic neurons in the cortex and CA1 regions were seen in animals given tPA (2 mg/kg iv) after cerebral H/I (Fig. 7A). In contrast, far fewer histopathological abnormalities were seen in animals treated post H/I with RBC-tPA or U 0126 (Fig. 7A). Animals given the RBC-vehicle evidenced large numbers of degenerating neurons, similar to that observed with H/I alone. The increase in the number of degenerating (pyknotic) neurons in the CA 1 region after H/I was statistically significant (Fig. 7B). tPA given after H/I caused greater neuronal necrosis than was seen in sham control animals (Fig. 7B), but this value was not statistically greater than in untreated animals post H/I. Importantly, RBC-tPA and U 0126 administration post insult caused a significant reduction in the number of degenerating neurons compared to animals treated with tPA (Fig. 7B). Administration of tPA 30 min prior to injury increased the severity of histologic damage compared to untreated H/I animals (Fig. 7C). In contrast, administration of U 0126 30 min prior to injury ameliorated H/I- induced histologic damage (Fig 7C).
Figure 7.
A: Post-treatment with RBC-tPA and U 0126 blunt parietal cortex and hippocampal histopathologic changes associated with H/I. H + E stained brain sections from the CA 1 hippocampus and parietal cortex of sham, hypoxia/ischemia (H/I), H/I + tPA (2 mg/kg iv), H/I + RBC-tPA (0.1 mg/kg iv), H/I + RBC-vehicle, and H/I + U 0126 (1mg/kg iv) post-treatment animals (agents delivered 2h post injury), n=3. B: Quantitative determination that post-treatment with RBC-tPA and U 0126 blunt hippocampal histopathologic changes associated with H/I. Mean ± SEM of pyknotic neurons in the hippocampal CA1 region in sham, H/I, H/I + tPA (2 mg/kg iv), H/I + RBC-tPA (0.1 mg/kg iv), and H/I + U 0126 (1 mg/kg iv) post –treated (2h after H/I) animals, n = 3–6. * p<0.05 compared with sham +p<0.05 compared with H/I alone #p<0.05 compared with H/I + tPA. C: Pre-treatment with tPA aggravates parietal cortex and hippocampal changes associated with H/I. H + E stained brain sections from the CA1 hippocampus and parietal cortex of sham, H/I, and H/I + tPA (2 mg/kg iv) pre-treatment animals (tPA administered 30 min pre injury), n=3.
Blood Chemistry
Blood chemistry values were collected before and after all experiments. There were no statistically significant differences between sham control, H/I, and H/I and drug treated animals. Hypoxia decreased pO2 to 35 ± 5 mm Hg. Low levels of hypercapnia raised pCO2 to 58 ± 8 and high levels of hypercapnia raised pCO2 to 78 ± 9 mm Hg. Carbon dioxide levels were kept constant during periods of hypoxia and oxygen levels were kept constant during periods of hypercapnia.
Discussion
The data in this paper show that coupling of tPA to carrier RBCs protects against cerebral injury caused when tPA is given in the setting of cerebral hypoxia/ischemia as occurs in stroke. Specifically, we found that impairment of cerebrovascular responses to hypercapnia and hypotension associated with hypoxia/ischemia was largely prevented by RBC-tPA. In prior studies, we observed that pial artery dilation in response to these two stimuli was blunted post insult, reversed to pial artery vasoconstriction with administration of tPA prior to the injury, and re-reversed to vasodilation with the PAI-1 inhibitor, EEIIMD (Armstead et al 2005b). Since impaired responsiveness to cerebovasodilator stimuli is thought to contribute to adverse outcome, RBC-tPA may offer a novel approach towards treatment of CNS injury associated with hypoxia/ischemia.
These findings have been extended in the present study by the observation that tPA and RBC-tPA have diametrically opposite effects on the responsiveness of pial arteries to vasodilator stimuli post insult. Administration of tPA (2 mg/kg iv) two hours post injury exacerbates impairment of cerebrovascular responses to hypercapnia and hypotension. In contrast, administration of RBC-tPA (0.1 mg/kg iv) given at the same time in the same setting prevents impairment of dilation to hypercapnia and hypotension post insult. These data indicate that coupling tPA to RBC enhances its pro-vasodilation effect in the piglet CNS exposed to hypoxia/ischemia, likely due to prolongation of the half-life in the circulation and retention in the intravascular compartment. Since tPA and RBC-tPA elicited comparable pial artery vasodilation, RBC carriage enhanced the potency of tPA induced vasodilation approximately 10- fold based on the dose administered. The protective effect on stimulus-induced vasodilation provided by RBC-tPA appears selective for several reasons. First, coupling of the vehicle did not cause vasodilation nor did it prevent impairment in dilator responses post insult. Second, cerebral hypoxia/ischemia does not exert a non-specific global impairment in the vasculature as responses to isoproterenol were unchanged post insult, as reported previously (Armstead et al 2005b; 2008). Third, RBC-tPA did not alter the dilation in animals given isoproterenol, excluding a non-specific enhancement of cerebrovasodilator responsiveness. The observation that RBC-tPA preserves cerebrovasodilation whether given pre- or post-hypoxia/ischemia suggests its utility both as a treatment for cerebral ischemia and a means to prevent delayed injury in settings where the risk of recurrent ischemic occlusion is high.
Three additional conclusions can be drawn from our findings. First, the clinical relevance of preserving cerebral vasodilation is supported by the finding that administration of RBC-tPA at a ten fold lower dose pre or post H/I reduced neuronal degeneration in the parietal cortex and CA1 hippocampus in contrast to the deleterious effects of tPA, although our studies do not formally prove that preservation of vasodilation promotes blood flow and thereby prevents neuronal injury. Second, the neuronal cell loss between animals treated with tPA, RBC-tPA or not treated at all, indicates that spatial containment of tPA within the vasculature limits extravasation into brain parenchyma to promote neuronal cell injury. Third, the data also help affirm our previous findings that tPA regulates cerebral vascular tone by acting within the vasculature (Armstead et al 2005b; 2008).
Blockade of ERK MAPK upregulation preserved vasodilation in animals given RBC-tPA. This extends previous findings which indicate that upregulation of ERK MAPK impairs responses to stimulus-induced cerebrovasodilation (Armstead et al 2008). Plasminogen activator induced vascular activity and signal transduction is thought to be mediated at least in part through LRP (Armstead et al 2008; Bu et al 1992). Hypoxia/ischemia associated upregulation of ERK MAPK is potentiated by exogenous urokinase plasminogen activator and inhibited by the LRP antagonist RAP, an anti-LRP-1 antibody, and by U 0126, a purported ERK MAPK selective antagonist (Armstead et al 2008). U 0126 functions as an ERK MAPK antagonist in this experimental model based on Western analysis and ELISA (Armstead et al 2008). The immunohistochemical and quantitative ELISA data in the present study confirm and extend prior observations by showing that the injury caused by administering tPA before or after cerebral hypoxia/ischemia is associated with upregulation of ERK MAPK in the cerebral cortex and hippocampus. In contrast, RBC-tPA decreased upregulation of ERK MAPK and attenuated injury. The specificity of this relationship is supported by the findings that U 0126 blocked the increased expression of ERK MAPK post hypoxia/ischemia, while the vehicle for the RBC conjugate had no effect. An unexpected finding was that the RBC-tPA actually reduced the upregulation of ERK MAPK to the level seen under sham control conditions. By inference, spatial constraint of tPA to the vasculature might limit the ability of tPA to interact with LRP and/or cells in the brain parenchyma where ERK MAPK is upregulated. Alternatively, the lower dose of tPA used when coupled to RBC may also have contributed to the result observed. Whether the MAPK isoform expression profile is also altered is under investigation. We hypothesize that the capacity of RBC-linkage to attenuate upregulation of ERK MAPK expression by tPA provides a mechanistic link between cerebral hemodynamic responsiveness and neuronal cell integrity in the setting of cerebral hypoxia/ischemia. However, additional study will be needed to establish a cause-effect relationship.
The dose of free tPA was selected based on clinical usage, 2 mg/kg. For comparison and to justify the dose of RBC-tPA chosen, our previous studies in rats showed thrombolytic efficacy of 0.05 mg/kg in the setting of traumatic brain injury (Sherman et al, in press), fibrinolysis of clots formed ex vivo (Murciano et al 2003), and safety of this dose in an ICH model (Danielyan et al, 2008). In mice, RBC-tPA (0.2 mg/kg) showed a thrombolytic effect in a stroke model (Danielyan et al, 2008) and 0.5 mg/kg showed fibrinolysis in pulmonary embolism and carotid occlusion models. Further, RBC-tPA at doses of 0.4 and 0.08 mg/kg also caused thrombolysis in a mouse pulmonary embolism model (Ganguly et al 2005). We therefore judged that 0.1 mg/kg RBC-tPA was likely an effective dose based on studies of thrombosis in rats and mice. Since free tPA and RBC-tPA are in essence different drugs, we compared their effective doses. Finally, we experimentally determined fibrinolysis for the newborn pig in vitro and observed that indeed the relative order of fibryinolysis is human>pig>mouse>rat (unpublished data).
Many studies of cerebral ischemia have been performed in rodent models. Piglets offer a unique advantage in elucidating pathways involved in CNS ischemic injury by virtue of having a gyrencepahalic brain that contains substantial white matter similar to humans, which is more sensitive to ischemic damage than grey matter (Shaver et al 1996). On the basis of interspecies extrapolation of brain growth curves (Dobbing 1974), the age of the newborn pig used in these studies roughly approximated the newborn-infant time period in the human. Although the incidence of cerebral ischemic events in the pediatric population is relatively low compared to the adult (Janjua et al 2007), the magnitude of the condition is amplified by the potential long-term loss of quality of life years for children with CNS ischemic disorders.
Indeed, the 2001 workshop report of the National Institute of Neurological Disorders and Stroke noted a deficiency in research in pediatric stroke related to the paucity of animal models and basic research investigation into ischemic disorders of the CNS in the pediatric population (Lynch et al 2002). The use of a piglet model importantly allows for study of physiologic variables and is more clinically relevant than rodent models. The identification of a molecular target (ERK MAPK) for modifying neuropathologic injury is in itself an important observation and potentially clinically relevant as there are, to date, no clinically proven neuroprotective interventions other than hypothermia for neonatal hypoxic/ischemic brain injury. The findings in the present study regarding tPA are equally important as more attention is focused on treating stroke in infants and children. The important finding is that tPA alone may exacerbate neuronal injury in the setting of hypoxia/ischemia. This observation was made in adult models of focal arterial-occlusive stroke years ago, but its significance was diminished, indeed outweighed, by the overwhelming clinical trial evidence of benefit due to fibrnolysis. Yet, the use of tPA for adult acute stroke is highly constrained so as to minimize the risk of hemorrhage. To date there have been no safety or efficacy trials for tPA in the treatment of infants and children with acute stroke. Many individual children however are receiving tPA based on the assumption that studies in adults are generalizable to children. The data from this study provide additional evidence that both safety and efficacy of tPA – whether it be traditional IV or intrarterial tPA, or RBC-tPA – must be evaluated systematically in children before being widely adopted in clinical care.
Since neonates are at risk for intraventricular hemorrhage, it could be seen as counterintuitive to administer a plasminogen activator. However, the fact that endogenous plasminogen activator release occurs after cerebral hypoxia/ischemia (Armstead et al 2008) makes our non thrombotic model relevant to investigation of regulation of cerebrovasodilator mechanisms and histopathology.
Using a model dominated by thrombosis could well be criticized as likely to yield ambiguous results, as the salutary effects of fibrinolysis will be variably offset by the deleterious effects of tPA on vascular tone depending on the specific experimental setting. We seek to delineate the mechanism by which fibrinolytic therapy causes CNS damage and to help re-engineer these molecules through use of novel delivery systems or use selective inhibitors of the signal transduction pathway(s) they initiate to increase their benefit/risk ratio. The non-thrombotic model of cerebral hypoxia/ischemia is well suited for this purpose. Nonetheless, clinically, cerebral hypoxia/ischemia is often associated with neonatal stroke (Ferriero 2004). Therefore, investigation of the potential for tPA to have deleterious effects in the setting of cerebral hypoxia/ischemia, and their amelioration with RBC-tPA, in a piglet model might have considerable translational relevance. Although the present studies might have implications for the pediatric population, our results may also have wider applicability given the observed beneficial action of RBC-tPA in adult animal models of stroke and traumatic brain injury (Danielyan et al 2008; Stein et al in press).
In summary, our results indicate a protective effect of coupling tPA to RBC in the setting of cerebral ischemia. The observation that RBC-tPA preserves cerebrovasodilation whether given pre- or post-H/I suggests its utility both as a treatment for cerebral ischemia and a means to prevent further injury in settings where the risk of recurrent ischemic occlusion is high. Our studies suggest that RBC-tPA may increase the benefit ratio of thrombolytic interventions and extend the benefits to additional patients in the pediatric age group with stroke and ischemic disorders involving the CNS.
Acknowledgments
Sources of Financial Support: This research was supported by grants from the National Institutes of Health, NS53410 and HD57355 (WMA), HL76406, CA83121, HL76206, HL07971, and HL81864 (DBC), HL77760 and HL82545 (AARH), HL66442 and HL090697 (VRM), the University of Pennsylvania Research Foundation (WMA and VRM), the University of Pennsylvania Institute for Translational Medicine and Therapeutics (DBC), and the Israeli Science Foundation (AARH).
Footnotes
Disclosure/Conflict of Interest The authors have nothing to declare.
References
- 1.Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AAR. LRP and avB3 mediate tPA- activation of smooth muscle cells. AJP. 2006;291:H1351–H1359. doi: 10.1152/ajpheart.01042.2005. [DOI] [PubMed] [Google Scholar]
- 2.Armstead WM, Cines DB, Bdeir K, Kulikovskaya I, Stein SC, Higazi AAR. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res. 2008;1231:121–131. doi: 10.1016/j.brainres.2008.06.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Dev Brain Res. 2005a;156:139–146. doi: 10.1016/j.devbrainres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005b;36:2265–2269. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- 5.Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi AAR. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nature Neuroscience. 2006;9:1150–1157. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- 6.Benedict SL, Ni OK, Schloesser P, White KS, Bale JF. Intra-arterial thrombolysis in a 2-year-old with cardioembolic stroke. J Child Neurol. 2007;22:225–227. doi: 10.1177/0883073807300296. [DOI] [PubMed] [Google Scholar]
- 7.Bu G, Williams S, Strickland DR, Schwartz AL. Low density lipoprotein receptor- related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Nat Acad Sci. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielyan K, Ganguly K, Ding B, Atochin D, Zaitsev S, Murciano JC, Huang PL, Kasner SE, Cines DB, Muzykantov VR. Cerebrovascular thromboprophylaxis by erythrocyte coupled tPA. Circulation. 2008;118:1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVeber G, Andrew M. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417– 423. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 10.Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6. [PubMed] [Google Scholar]
- 11.Felix B, Leger ME, Albe-Fessard DA. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 12.Ferriero DM. Neonatal brain injury. New Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 13.Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines D, Muzykantov V, Murciano JC. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharm Exp Ther. 2005;312:1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 14.Gunther G, Junker R, Strater R, Schobess R, Kurnik K, Kosch A, Nowak-Gottl U. Symptomatic ischemic stroke in full-term neonates. Stroke. 2000;31:2437–2441. doi: 10.1161/01.str.31.10.2437. [DOI] [PubMed] [Google Scholar]
- 15.Janjua N, Nasar A, Lynch JK, Qureshi AI. Thrombolysis for ischemic stroke in children. Data from the nationwide inpatient sample. Stroke. 2007;38:1850–1854. doi: 10.1161/STROKEAHA.106.473983. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Park JH, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science. 1999;284:647–50. doi: 10.1126/science.284.5414.647. [DOI] [PubMed] [Google Scholar]
- 17.Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies, and cerebral palsy. Hum Pathol. 1999;30:759–769. doi: 10.1016/s0046-8177(99)90136-3. [DOI] [PubMed] [Google Scholar]
- 18.Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. JCBFM. 2000;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:1–6. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- 20.Lapchak PA, Araujo DM. Reducing bleeding complications after thrombolytic therapy for stroke: clinical potential of metalloproteinase inhibitors and spin trap agents. CNS Drugs. 2001;15:819–829. doi: 10.2165/00023210-200115110-00001. [DOI] [PubMed] [Google Scholar]
- 21.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 22.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Pro- phylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21:891–896. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 23.Nassar T, Akkawi S, Shin A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman SN, Higazi AA. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897– 202. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 24.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- 25.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nature Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 26.Shaver EG, Duhaime AC, Curtis M, Gennarelli LM, Barrett R. Experimental acute subdural hematoma in infant piglets. Pediatric Neurosurgery. 1996;125:123–129. doi: 10.1159/000121109. [DOI] [PubMed] [Google Scholar]
- 27.Stein SC, Ganguly K, Belfeld CM, Xu X, Swanson EW, Chen XH, Browne KD, Johnson VE, Smith DH, LeBold DG, Cines DB, Muzykantov VR. Erthrocyte bound tissue plasminogen activator (tPA) is neuroprotective in experimental traumatic brain injury. J Neurotrauma. doi: 10.1089/neu.2008.0720. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Mori T, Jung JC, Fini ME, Lo EH. Secretion of matrix metalloproteinase-2 -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma. 2002;19:615–625. doi: 10.1089/089771502753754082. [DOI] [PubMed] [Google Scholar]
- 30.Wang YF, Tsirka SE, Strickland S, Stiege PE, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 31.Zaitsev S, Danielyan K, Murciano JC, Krasik T, Taylor RP, Pincus S, Jones SM, Cines DB, Muzykantov VR. CR-1-directed targeting of tPA to circulating erythrocytes for pro- phylactic fibrinolysis. Blood. 2006;108:1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]