Abstract
The goals of the current study were to use behavioral and pupillary measures to examine working memory on a spatial n-back task in 8-20-year-olds with youth-onset psychosis or ADHD (Combined subtype) and healthy controls to determine the contribution of different attentional factors to spatial working memory impairments, and to examine if age-related changes in performance differed across groups. Although both clinical groups had lower perceptual sensitivity on both 0- and 1-back, there was no evidence of an impairment in spatial working memory or differential order effects on the 0-back. Instead, results suggest that both clinical groups had difficulty encoding the stimuli. They also appeared to have difficulty maintaining attention and/or readiness to respond, and, to a lesser extent, recruiting resources on a trial-to-trial basis. It is likely that these attentional problems prevented the clinical groups from encoding the stimuli effectively and contributed to their general performance deficits.
Keywords: ADHD, schizophrenia, psychosis, n-back, pupillometry, encoding, variability, spatial working memory
There are intriguing similarities between schizophrenia and ADHD, including impairments in the same general cognitive domains, behavioral symptoms such as inattentiveness, and abnormalities in the same general neurotransmitter systems and brain regions (Barr, 2001; Karatekin, 2001). Yet, there have been relatively few direct comparisons between psychosis and ADHD.
Most of these comparisons have involved youth-onset schizophrenia or “multidimensionally impaired” (MDI) children with psychotic symptoms (McKenna et al., 1994). Youth-onset schizophrenia is a rare, severe and more genetically loaded form of the disorder that does not differ qualitatively from the adult-onset form on most of the dimensions examined (Asarnow et al., 2001; Asarnow et al., 2002; Bertolino et al., 1998; Frazier et al., 2007; Jacobsen & Rapoport, 1998; Nicolson et al., 2003; Rabinowitz et al., 2006; Ueland et al., 2004; Vourdas et al., 2003).
Both schizophrenia and ADHD are associated with impairments in working memory. However, tasks designated as assessing “working memory” often make demands on different aspects of attention, and it is critically important to examine the extent to which impairments on these tasks are related to working memory per se versus more general aspects of attention and how these relations may differ across disorders. The goals of the current study were to use behavioral and pupillary measures to examine working memory on a spatial n-back task in 8-20-year-olds with youth-onset psychosis or ADHD and healthy controls, to determine the contribution of attentional factors to spatial working memory impairments, and to examine if age-related changes in performance differed across groups.
N-Back
There is no established version of the n-back task, and there are also variants of continuous performance tests (CPTs) that resemble the n-back task. These tasks differ in how much demand they place on working memory, attention, and inhibition. In the current study, we were specifically interested in probing spatial working memory and the contribution of attentional deficits to working memory deficits. Therefore, we used 0- and 1-back conditions that required participants to press a button on each trial and made few demands on inhibition. On 0-back, participants were instructed to press one button if the stimulus was in a specific location on a computer screen and another one if it was anywhere else. On 1-back, they indicated whether the stimulus was in the same location as the one on the previous trial.
The 0-back condition requires participants to sustain attention to monitor a stream of stimuli; encode their location; match the current stimulus against the internal representation of the previous stimulus; and make a manual response. The 1-back condition also requires ability to maintain the stimulus location in mind via visual-spatial rehearsal, to continuously update this internal representation, and to inhibit previously relevant spatial information. The key measures on the n-back are perceptual sensitivity (e.g., d′), that is, the participant's ability to discriminate targets from non-targets, and response bias (e.g., c, β), the participant's level of willingness to respond that a target is present. Other behavioral measures include accuracy rates, RTs, and variability of RTs.
In a previous paper (Karatekin et al., 2007a), we summarized behavioral and brain imaging results of studies of 0- and 1-back conditions. Briefly, studies with adults yield mixed results on behavioral comparisons between 0- and 1-back, with most reporting a decline in performance from 0- to 1-back, and others showing no difference. Brain imaging studies comparing the two conditions have also yielded mixed results. The small sample sizes and the lack of reporting of the statistical significance of the differences between the two conditions make it difficult to reach firm conclusions about the brain bases of 1-back compared to 0-back.
Performance on the N-Back Task in Schizophrenia
Studies examining performance of individuals with schizophrenia on 0- and 1-back generally show no differences from controls in errors or perceptual sensitivity on 0-back (Abi-Dargham et al., 2002; Bertolino et al., 2003; Callicott et al., 2000, 2003; Jansma et al., 2004; Perlstein et al., 2001; Perlstein et al., 2003). Results differ across studies in whether group differences are found in response time (RT), whether any impairments are found on 1-back, and whether disproportionate impairments are found on 1-compared to 0-back. Some of the negative findings are probably due to floor effects on 0-back conditions and small sample sizes, as most of these studies have used functional brain imaging to probe the neural substrates of performance. RT variability was not measured in any of these studies.
Whether behavioral deficits or disproportionate impairments are found or not, functional brain imaging results consistently point to abnormalities in the dorsolateral prefrontal cortex (DLPFC) during task performance (e.g., Abi-Dargham et al., 2002; Bertolino et al., 2003; Callicott et al., 2000, 2003; Jansma et al., 2004; Perlstein et al., 2001, 2003). The abnormalities also include both hyper- and hypoactivation, increased D1 receptor availability, and reductions of N-acetylaspartate ratios. However, the point at which this abnormality emerges (on 0-, 1-, 2- or 3-back) differs across studies.
In a meta-analysis of these and other n-back studies involving 2- and 3-back conditions in schizophrenia, Glahn and colleagues (2005) conclude that hypofrontality in the DLPFC is a robust finding. However, they add that there is evidence for increased activation in the anterior cingulate and frontal pole as well.
Performance on the N-Back Task in ADHD
There have been relatively few studies of the n-back task in ADHD. A study of 7- to 12-year-olds found that psychostimulant-naïve children with ADHD had fewer hits than controls, but did not differ in terms of correct rejections (Shallice et al., 2002). In addition, diagnostic group did not interact with condition (0- vs. 1- vs. 2-back). Although there were group by condition interactions for RTs, the nature of these interactions was not specified. In a study by Klein and colleagues (2006), 7- to 14-year-olds with ADHD were more impaired than controls on both 0- and 1-back in terms of error rates, RTs, and variability of RTs. Diagnostic group did not interact with condition for omission or commission errors, median RTs or variability of RTs. In a functional MRI study of 0- versus 2-back in adults with ADHD and controls, Valera and colleagues (2005) found a trend toward lower perceptual sensitivity on 0-back in the ADHD group, but no group differences in response criterion or RT in either condition. However, the ADHD group showed decreased activation in the occipital, cerebellar and prefrontal regions compared to controls on the 2-back. Only the cerebellar activation remained significant when individuals with learning disabilities were excluded from the analyses.
Precursors of the Current Study
In a precursor to the current study (Karatekin et al., 2007a), we tested healthy 10-year-olds and young adults on 0- and 1-back conditions. Within each age group, a third of the participants were administered 0-back first, 1-back second, a third were administered 1-back first and 0-back second, and a third were administered two 0-back conditions only. In addition to behavioral measures, we measured pupillary dilations to the stimuli. As summarized in our previous paper, pupillary dilations constitute one of the few relatively direct measures of resource recruitment (Kahneman, 1973), and are hypothesized to reflect the momentary mental effort or recruitment of cognitive resources in accordance with task demands (reviewed in Beatty, 1982; Beatty & Lucero-Wagoner, 2000).
In both children and adults, repeated administration of 0-back led to greater RT variability, revealing a subtle order effect. Compared to 0-back, perceptual sensitivity was lower, the conservative response bias was higher, RTs were longer and more variable, and pupillary dilations to hits were larger in 1-back in both age groups. Effects of repeated administration of 0-back and differences between 0- and 1-back in perceptual sensitivity and RTs were similar between the age groups. We concluded that on this task, 10-year-olds recruited cognitive resources in a manner similar to adults. In addition, in both age groups, higher RT variability was associated with lower perceptual sensitivity in both conditions and higher RT variability in 0-back was associated with lower perceptual sensitivity in 1-back, suggesting that attentional fluctuations play a role in spatial working memory performance.
Goals of the Current Study
In the current study, the conditions were identical to the previous study. However, to investigate the role of sustained attention in spatial working memory, we administered a second 0-back condition to all participants after they completed the first two conditions in counterbalanced order. Thus, participants in each diagnostic group were administered the conditions in two orders: 1-0-0 or 0-1-0. To date, there have not been any direct comparisons of n-back performance between psychosis and ADHD. Thus, a central goal of the study was to directly compare these groups and to determine if there were group differences in age-related changes in performance. The first research question was to test if the clinical groups had impairments in spatial working memory, as reflected in a disproportionate decline in performance on 1-back compared to 0-backs.
The other questions were aimed at comparing the two clinical groups on different aspects of attention and testing if spatial working memory performance was related to attentional factors:
-
Sustained attention over relatively long periods of time:
a. Do the clinical groups show disproportionate order effects on the 0-back (i.e., a disproportionate decline in performance from the first to the second 0-back)?
b. Does decrement in performance from the first to the second 0-back predict performance on 1-back?
-
Attentional fluctuations from trial to trial/maintaining readiness to respond:
a. Do the clinical groups show greater fluctuations (RT variability) than controls?
b. Do these fluctuations predict performance on 1-back?
-
Cognitive effort/momentary recruitment of resources on each trial:
a. Do the clinical groups differ from controls in terms of resource recruitment (measured through pupillary dilations), either because they recruit too few resources or because they recruit more resources than controls?
b. Do pupillary dilations predict performance on 1-back?
Method
Participants
Table 1 lists participants' demographic and clinical characteristics, and Table 2 breaks down each sample by age. Participants with psychosis were recruited from inpatient and outpatient clinics at the University of Minnesota, mental health professionals in the community, and flyers distributed at regional mental health conferences. One participant was in day treatment, and one was in residential treatment at the time of the study. The rest were living at home. Participants in the control and ADHD groups were recruited from advertisements in the local community, and friends of other participants. ADHD participants were also recruited from support groups for ADHD.
Table 1.
Demographic and Clinical Characteristics of the Participants
| Control | Psychosis | ADHD | Diagnosis Effects | Post-Hoc Tests | |
|---|---|---|---|---|---|
| N | 56 | 29 | 32 | ||
| M:F, % | 43:57 | 52:48 | 78:22 | X22 = 10.4, p = .006 | ADHD ≠ (Psychosis, C) |
| Age in months (SD) | 152 (30) | 174 (43) | 153 (33) | F2,114 = 4.16, p = .018 | Psychosis > (C = ADHD) |
| Range | 106-228 | 101-241 | 107-225 | ||
| Socioeconomic status (Hollingshead, 1975) | 53 (9) | 41 (13) | 50 (9) | F2,108 = 11.00, p < .001 | Psychosis < (ADHD = C) |
| Estimated IQ (SD) | 114 (13) | 98 (16) | 109 (14) | F2,109 = 12.35, p < .001 | Psychosis < (ADHD = C) |
| Range | 77-144 | 74-132 | 85-141 | ||
| Handedness | 6.4 (1.7) | 5.6 (2.4) | 6.2 (1.8) | ns | |
| SANS/SAPS | |||||
| Negative symptoms | 2.7 (0.9) | ||||
| Positive symptoms | 1.9 (1.0) | ||||
| Psychotic symptoms | 2.4 (1.2) | ||||
| Medications, % | |||||
| Antipsychotic | 66 | 0 | |||
| Psychostimulant | 14 | 63 | |||
| Antidepressant | 24 | 3 | |||
| Mood stabilizer | 31 | 0 | |||
| Alpha-adrenergic | 3 | 3 | |||
| Anti-histamine | 10 | 0 | |||
| Benzodiazepine | 10 | 3 | |||
| Ethnicity, % | X82 = 19.5, p = .012 | ||||
| Caucasian | 86 | 55 | 84 | ||
| African-American | 2 | 10 | 13 | ||
| Asian | 2 | 10 | 0 | ||
| Hispanic | 3 | 0 | |||
| Mixed/Other | 11 | 21 | 3 |
Notes. C = Control. ns = not significant.
IQ was estimated from the Vocabulary and Block Design subtests from the Wechsler Intelligence Scale, 3rd ed. (WISC-III; Wechsler, 1991), the WISC-IV (Wechsler, 2003), or the Wechsler Adult Intelligence Scale, 3rd ed. (Wechsler, 1997). SANS/SAPS = Scales for the Assessment of Negative/Positive Symptoms (Andreasen, 1983, 1984). Medications taken by fewer than 5% of participants are not reported. Handedness was measured by asking children to pick up seven objects with their preferred hand; 1 point was awarded for each object picked up with the right hand.
Table 2.
Number of Participants at Each Age in Each Diagnostic Group
| Control | Psychosis | ADHD | |
|---|---|---|---|
| 8 | 1 | 2 | 0 |
| 9 | 8 | 2 | 4 |
| 10 | 7 | 3 | 6 |
| 11 | 7 | 1 | 4 |
| 12 | 5 | 1 | 4 |
| 13 | 12 | 3 | 7 |
| 14 | 6 | 2 | 2 |
| 15 | 3 | 1 | 0 |
| 16 | 2 | 7 | 1 |
| 17 | 3 | 2 | 1 |
| 18 | 1 | 3 | 3 |
| 19 | 1 | 1 | 0 |
| 20 | 0 | 1 | 0 |
Potential participants were excluded if they were not fluent in English or were color blind, if they had been premature by more than four weeks (to reduce the effects of brain damage associated with prematurity on the results), had a history of significant neurological conditions, or IQ lower than 70. Potential participants were excluded from the ADHD and control groups if they had been adopted, or had first-degree biological relatives with schizophrenia. Potential participants were excluded from the ADHD group if they were taking psychoactive medications other than psychostimulants, if their parents were not willing to discontinue psychostimulants for 24 hours prior to cognitive testing, if they had been diagnosed with or suspected of having a pervasive developmental disorder. To keep the sample relatively homogeneous, participants were also excluded from the ADHD group if they had never met criteria for the Combined subtype. However, we included two adolescents who currently met criteria for the Inattentive subtype, but who had previously met criteria for the Combined subtype and scored above 60 on the Attention Problems scale of the Achenbach Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001). Potential controls were excluded if they had ever taken psychoactive medications, been diagnosed with a major psychiatric disorder or met criteria for a current disorder, had attention problems for which they had sought help, or had first-degree biological relatives with ADHD.
Diagnoses were made using DSM-IV criteria (American Psychiatric Association, 1994), and based on semi-structured interviews (Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version, K-SADS-PL; Kaufman et al., 1996) conducted separately with participants and at least one parent/guardian.
Diagnoses are listed in Table 3. In the psychosis group, average age of onset of psychotic symptoms was 12.5 years (SD = 3.0, range = 7-17). Of the 7 participants with Psychosis Not Otherwise Specified (NOS), 6 could have been in the prodromal phase, and 4 had psychotic symptoms in the context of a complex clinical picture (these subgroups were not mutually exclusive). One participant might have met criteria for “multidimensionally impaired disorder” (McKenna et al., 1994). Most of these 7 participants had clear hallucinations, delusions, or formal thought disorder. In addition, four of these participants had first-degree biological relatives with schizophrenia, and one had a second-degree biological relative with schizophrenia and a biological sibling with psychotic symptoms.
Table 3.
Major Lifetime Diagnoses of the Participants
| Control | Psychosis | ADHD | |
|---|---|---|---|
| Schizophrenia | 52% | ||
| Schizophreniform | 7% | ||
| Schizoaffective | 17% | ||
| Psychosis Not Otherwise Specified | 24% | ||
| ADHD | |||
| Combined | 94% | ||
| Inattentive (with history of Combined) | 6% | ||
| Mood Disorders | 4% | 14% | 19% |
| Anxiety Disorders | 4% | 14% | 13% |
| Oppositional Defiant Disorder/Conduct Disorder | 0 | 21% | 34% |
| Substance Use/Abuse | 0 | 7% | 6% |
| Tic disorder | 0 | 0% | 3% |
Note. All diagnoses in the control group refer to past diagnoses.
Families were provided with monetary compensation for participation. Most families were also provided with a diagnostic report.
Further details of the diagnostic procedure can be found in Karatekin et al. (2008).
Participants were asked to refrain from taking psychostimulants for at least 24 hours prior to testing. However, on the day of testing, three participants in the ADHD and three in the psychosis group had taken psychostimulants. In the psychosis group, only one participant on antipsychotic medications was taking a typical neuroleptic (molindone). The other participants were all on atypical neuroleptics.
Two control participants, one ADHD participant, and one psychosis participant were excluded due to behavioral problems during testing. One ADHD participant was excluded due to technical problems. Two psychosis participants were excluded because they refused to be administered the task.
Procedure
The current study was part of a larger study of youth-onset psychosis and ADHD. As part of this larger study, participants were administered other tasks over two sessions, and some underwent neuroimaging (11 control and 9 ADHD participants were included in Karatekin, 2006; 10 control and 8 psychosis participants were included in White et al., 2007; 52 controls were included in Karatekin, Marcus, & White, 2007b; 34 controls, 13 ADHD and 12 psychosis participants were included in Karatekin et al., 2008; 44 controls, 23 ADHD, and 27 psychosis participants were included in Karatekin et al., in press-a; 54 controls, 31 ADHD, and 29 psychosis participants were included in Karatekin et al., in press-b; 55 controls, 32 ADHD, and 26 psychosis participants were included in Karatekin et al, in press-c). The current task was always administered first on the second day of testing.
The apparatus and the rest of the procedure were identical to those used in our prior study with healthy children and adults (Karatekin et al., 2007a), with the exception that the current study included three rather than two conditions. Participants were assigned randomly to the 0-1-0 or the 1-0-0 order of administration. The two 0-back conditions were identical.
In all conditions, participants were instructed to look at a black fixation cross (0.8° × 0.8°) at the center of the screen throughout the task, both during and between trials. To adapt the procedure to children, we included in the instructions a story about chipmunks playing “catch me if you can.” A small picture of a chipmunk (1.2° × 1.2°) appeared for 200 ms in 1 of 8 locations (4.4°, 5.8° or 7.3° from the center, distributed on an imaginary circle around the center) 300 ms after the onset of the cross. In the 0-back conditions, participants pressed the right button if the stimulus was in one of the 8 locations and the left button if it was in one of the remaining 7. The target was always in the same location, in the middle of the upper right quadrant. In 1-back, participants pressed the right button every time the stimulus appeared in the same location as in the previous trial and the left button if it did not. They were instructed to emphasize speed and accuracy equally and to press the “different” button for the first trial.
Because the target stimulus in 0-back was on the right-hand side of the monitor, we required all participants to press the right button for target-present responses to minimize the possibility of a response conflict. To keep the responses consistent across conditions, we also required all participants to press the right-hand button for target-present responses in 1-back.
There were 20 practice trials and 200 experimental trials in each condition, and targets appeared on 25% of the trials in each condition. There were 50 targets and 150 distractors in each condition. Targets and distractors were presented randomly, with the constraint that each 50-trial block in each condition contain 12 to 13 targets and 37 to 38 distractors. Stimuli were assigned randomly to the locations, subject to the constraints of the target proportions. As a result, the stimuli appeared in the same location on 25% of the trials in the 0-backs, and in the remaining 7 locations on 7 to 17% of the trials. In 1-back, the stimuli appeared at each of the 8 locations on 6 to 21% of the trials.
Inter-stimulus interval was 2 s. Each condition took approximately 8-9 minutes to administer. The instructions and practice trials were repeated if participants had difficulty following directions. Participants were provided with brief rest breaks between conditions as necessary, but we tried to avoid taking breaks longer than a few minutes' duration between conditions.
Recording of Eye Movements, Pupillary Responses, and Behavioral Data
Horizontal and vertical coordinates of the center of gaze and pupillary diameter were recorded with a video-based eye monitor (ISCAN Eye Tracking Laboratory, Model ETL-400), which has a temporal resolution of 60 Hz and a spatial resolution of 1°. The spatial resolution for measurement of pupillary diameter was 0.037 mm. Participants sat 69 cm from the monitor on which the stimuli were displayed. A camera with an attached infrared light source to illuminate the pupil were positioned in front of the screen on which the stimuli were presented, below eye level and 40 cm from the participants' eye. The camera recorded movements of the participants' left eye. Because the camera automatically compensated for small head movements, participants' heads were not restrained. However, they rested their heads against a padded head rest. The experimenter sat behind the participant, in front of the computer that controlled the video camera and collected the eye movement data.
Gaze position was calibrated for each participant at the beginning of the session by focusing the camera on his/her left eye and having him/her look at small visual stimuli in the center and four corners of the screen. These positions were recorded as the targets of gaze. Calibration was repeated between blocks of trials if necessary due to excessive head movement.
A custom-built 2-button box was used to record manual responses. The 1.5 cm2 buttons were arranged horizontally. Participants used each index finger to press the buttons. Custom software was used to present the stimuli and to record the onset and duration of the button presses.
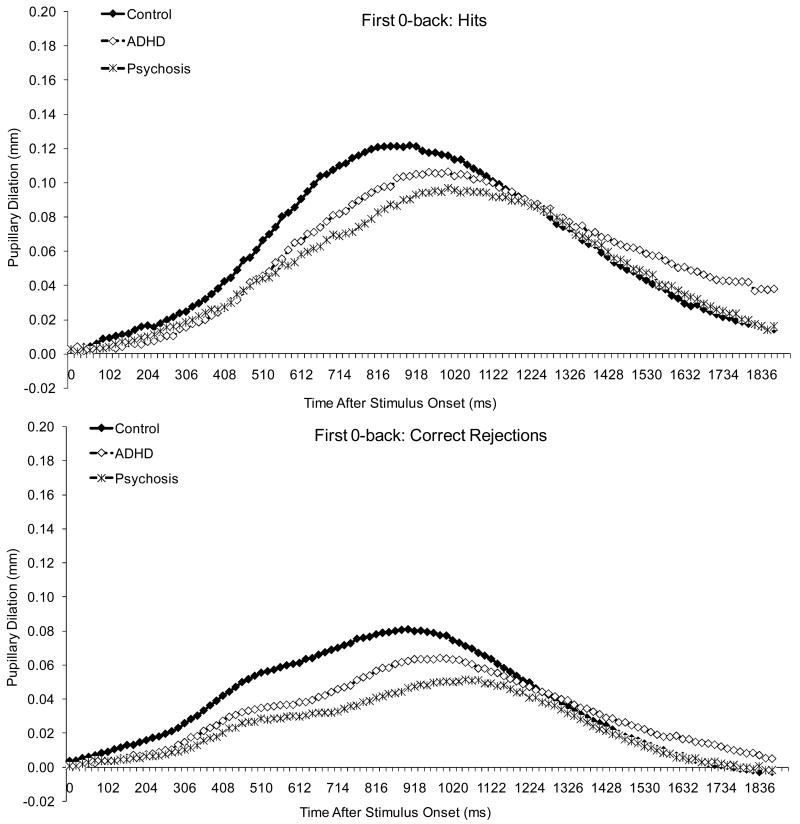
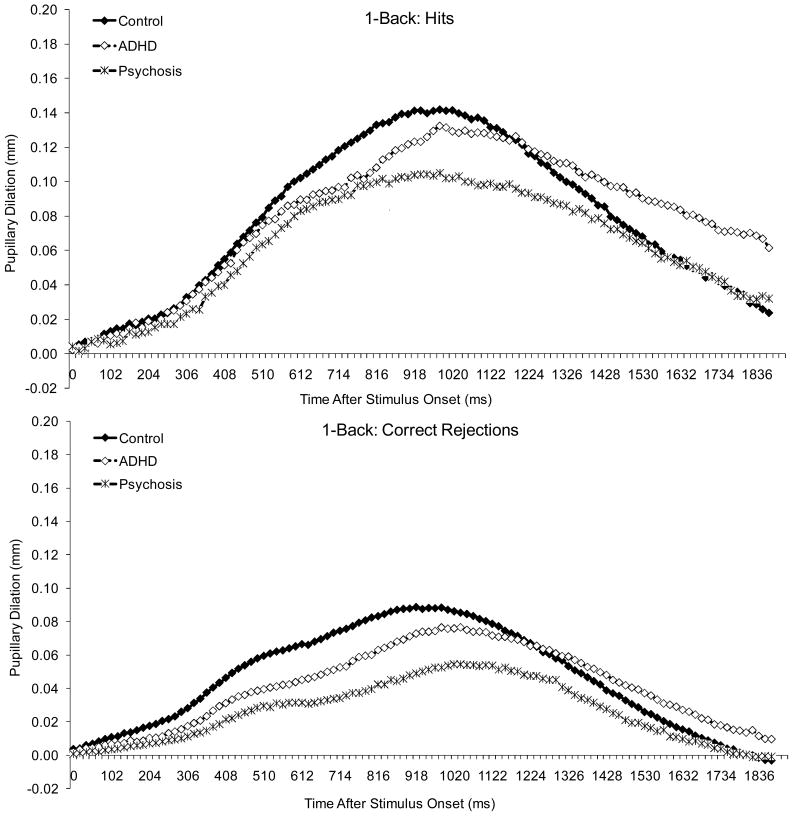
The experiment was conducted in a windowless room with fluorescent lighting. All stimuli were presented against a white background on a Dell Trinitron VGA color monitor (53 cm diagonal; resolution: 640 × 480 pixels). The luminance of the screen on which the stimuli were displayed was 140 cd/m2, and the average luminance of the white wall that was directly behind the computer was 139 cd/m2, as measured with a spot photometer. Assuming a high reflectance for the wall (0.7-0.8), we can estimate the ambient illumination in the room to be approximately 550 to 625 lux (Legge, 2007, Box 4.3). Because the target stimulus was only 1.2° × 1.2°, there was not a significant change in luminance when it was presented on the screen. As shown in Figures 1-3, there was also no pupillary constriction following the stimulus, which is consistent with the notion that there was no significant luminance change.
Figure 1.
Pupillary waveforms to hits and correct rejections in the first 0-back
Figure 3.
Pupillary waveforms to hits and correct rejections in 1-back.
Dependent Variables
Frequencies of hits (H), correct rejections, misses, and false alarms (FA)
These measures were calculated for each condition. Results are reported in Table 4 to enable comparisons to other studies, as these are the most commonly used measures on the n-back task.
Table 4.
Frequency of Hits, Correct Rejections, False Alarms and Misses in Each Condition in Each Diagnostic Group
| Control | Psychosis | ADHD | |
|---|---|---|---|
| First 0-back | |||
| Hits | 44 (4) | 38 (9) | 40 (5) |
| Correct Rejections | 142 (5) | 124 (26) | 130 (19) |
| False Alarms | 5 (4) | 13 (16) | 12 (17) |
| Misses | 5 (4) | 9 (7) | 8 (4) |
| Second 0-back | |||
| Hits | 43 (5) | 33 (10) | 37 (6) |
| Correct Rejections | 143 (4) | 122 (25) | 134 (9) |
| False Alarms | 3 (3) | 10 (16) | 5 (3) |
| Misses | 6 (5) | 12 (8) | 11 (5) |
| 1-back | |||
| Hits | 35 (10) | 24 (12) | 27 (10) |
| Correct Rejections | 142 (10) | 119 (31) | 133 (11) |
| False Alarms | 5 (10) | 16 (23) | 7 (6) |
| Misses | 15 (10) | 22 (11) | 22 (9) |
Notes. numbers in parentheses refer to standard deviations. In each condition, total possible number of hits was 50 and total possible number of correct rejections was 150.
Signal detection measures
Signal detection measures of perceptual sensitivity (d′) and response bias (c) were calculated as the key measures of accuracy in each condition (Macmillan & Creelman, 1991; Pastore et al., 2003; See et al., 1995). d′ and c were calculated according to formulae in Sorkin (1999), with adjustments made for perfect scores. Further details on the formulae can be found in Karatekin et al. (2007a).
Sensitivity is an index of the ability to discriminate between targets and non-targets, and higher values of d′ indicate better ability to discriminate between targets and distractors. When performance is error-free, d′ (after adjustment) equals 4.53. When performance is at chance level, d′ equals 0.
Response bias is an index of the participant's willingness to respond that a target is present, with lower values indicating a more “trigger-happy” attitude. We used c rather than the more commonly used β as the index of response bias because c, unlike β, is independent of d′ (Macmillan & Creelman, 1991, p. 46). Higher values indicate a more conservative response threshold (i.e., greater bias to respond that the target was absent). The range of values for c is similar to that for d′ but centered around 0 (i.e., ± 2.33).
Manual RTs
Stimulus presentation and manual RT data were merged offline with time-linked eye tracking data. An algorithm was used to calculate the speed and accuracy of manual responses relative to stimulus onset in the merged data file. Because the sampling rate of the eye monitor determined the temporal resolution of the merged data, manual RTs were accurate to 16.7 ms. RTs for hits and correct rejections occurring less than 100 ms after stimulus onset or that were longer than 3 SD from the participant's condition mean for hits or correct rejections, respectively, were excluded from the analyses. Median manual RTs for hits and correct rejections were calculated for each condition. Because there were relatively few false alarms and misses, RTs for these types of trials were not analyzed.
Coefficient of variation
Coefficient of variation of RTs (SD/mean) was used as an estimate of the variability of absolute pupillary diameters and manual RTs.
Blinks/artifacts, peak pupillary dilation, and latency to peak pupillary dilation
The procedure for identifying blinks and saccade-related artifacts in the pupillary record is described in Karatekin et al. (2007a). Pupillary dilation was defined as the magnitude of the adjusted maximum pupillary dilation during the 1500 ms after the onset of the stimulus. Pupillary dilations were analyzed in the same way as in Karatekin et al. (2007a).
To remove blink- and saccade-related artifacts from the data that may have been missed by the algorithms, we excluded from analyses all adjusted pupillary dilation values smaller than -0.11 mm or larger than 1.12 mm. These absolute threshold values for outliers were based on visual inspection of all the participants' data, without regard to condition. Average number of pupillary dilations to hits or correct rejections rejected from analyses due to outliers ranged from 1.0 to 3.2 across conditions and diagnostic groups. Adjusted pupillary dilations were calculated separately for each visual stimulus. Median pupillary dilations for hits and correct rejections for each individual's set of data were used in the analyses to minimize the effect of outliers.
Latency to peak dilation was defined as the difference in time between stimulus onset and peak pupillary dilation. Peak latencies that occurred less than 200 ms or more than 1500 ms after stimulus onset were not analyzed. Latency data, calculated separately for each visual stimulus, were averaged across hits and correct rejections in each condition.
Pupillary waveforms were created by averaging pupillary dilations for each 17-ms interval during the 2 s after stimulus onset across trials within each stimulus type within each participant, and then averaging the data across participants within each diagnostic group and condition.
Statistical Analysis
Statistical analyses were conducted with SPSS 14.0 and MacAnova 5.06 (an open-source cross-platform statistics program available for Windows, Macintosh and Linux at http://www.stat.umn.edu/macanova/).
Decisions regarding data transformations were made in the same manner as in Karatekin et al. (in press-a). Through this procedure, RTs, CVs, latencies to peak pupillary dilation, median pupillary dilations, and anticholinergic equivalents of antipsychotic medications were all transformed using logarithms, and chlorpromazine equivalents of antipsychotic medications were transformed by taking their square root.
Continuous demographic variables were analyzed with univariate ANOVAs, and significant findings were followed up with Tukey tests. Categorical demographic variables were analyzed with X2 tests. Correlations between variables within each group were calculated using Pearson correlation coefficients, controlling for age and IQ.
Repeated-measures Type III ANCOVAs, with age as the covariate, were used to examine effects of subject variables. Each ANCOVA tested linear and quadratic trends for age. IQ and SES were not used as covariates because controlling for these variables would have reduced variance due to the disorders. Huynh-Feldt-adjusted dfs were reported where applicable. For all measures, we used planned comparisons to compare the 0-backs to each other and to compare the average of the 0-backs to 1-back. Main effects or interactions were generally not followed up when there were higher-order interactions involving the same variables. Strict controls were instituted to protect against multiple testing. Please refer to Karatekin et al. (in press-a). for more detailed information on our use of ANCOVAs.
To calculate effect size, we used a measure similar to Cohen's d but that took into account the age differences among groups (this measure was devised by one of the co-authors, CB). Specifically, we divided the difference of the group means (age adjusted as appropriate) by the square root of the MSe term for the between-subjects analysis section of the ANCOVA. In cases where there was an interaction between group and age, the value reflects the size of the effect at the average age for the whole sample. When the two groups are similar in age, as in the ADHD-control comparisons, our measure of effect size and Cohen's d yield similar results.
All tests were two-tailed. Findings are reported as significant if α ≤ .05. All significant results of multi-way analyses are listed; therefore, if a main effect or interaction is not mentioned in the results, it can be inferred that it was not significant.
Results
Perceptual sensitivity (d′)
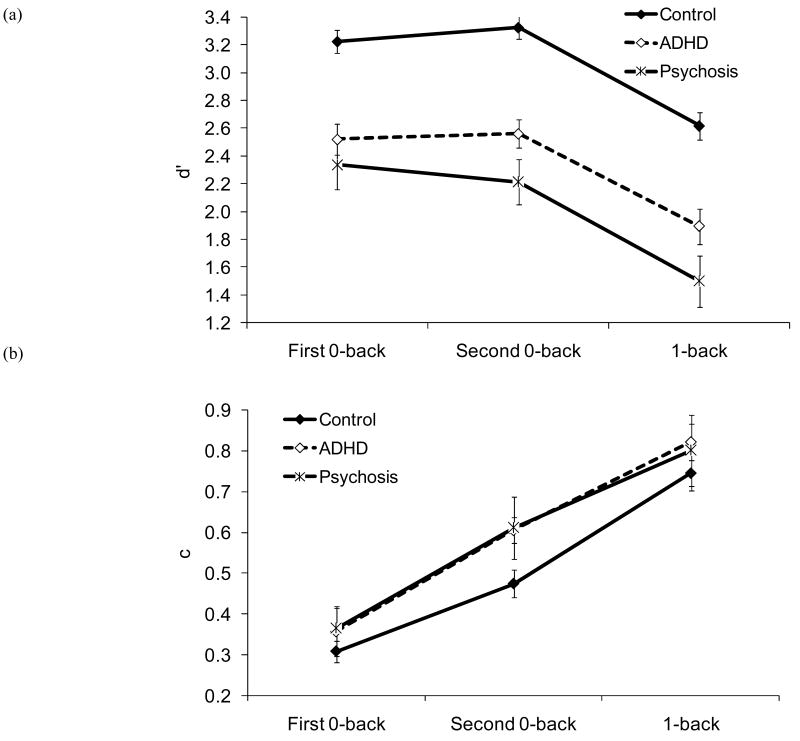
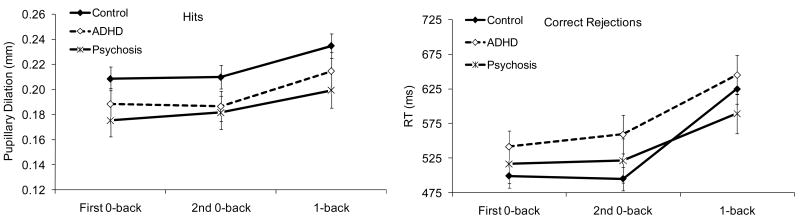
d′ increased linearly with age, F (1, 113) = 33.63, p < .001. There was an effect of condition, F (1.9, 219.7) = 114.60, p < .001: d′ did not differ between the 0-backs, but was lower in 1-back compared to 0-backs (see Figure 4-a). There was an effect of diagnosis, F (2, 113) = 41.34, p < .001; d′ was lower in the psychosis than in the ADHD group, and lower in the ADHD than in the control group. Importantly, there was no interaction between condition and diagnosis, p = .371. As can be seen in Table 5, the effect sizes of the pairwise group differences in d′, especially clinical-control differences, were quite large.
Figure 4.
(a) d′ and (b) c as a function of diagnosis and condition. Error bars refer to 95% confidence intervals in all figures.
Table 5.
Effect Sizes of Pairwise Group Differences on 0-Backs and 1-back
| Control vs. Psychosis | Control vs. ADHD | Psychosis vs. ADHD | |
|---|---|---|---|
| 0-backs | |||
| d′ | 1.96 | 1.22 | 0.76 |
| c | 0.97 | 0.57 | 0.40 |
| RT | 0.20 | 0.34 | -0.14 |
| Variability of RT | 1.47 | 1.15 | 0.33 |
| Pupillary dilation | -0.40 | -0.45 | 0.05 |
| Latency to peak dilation | 0.22 | 0.08 | 0.14 |
| Absolute pupillary diameter | 0.01 | -0.28 | 0.29 |
| 1-back | |||
| d′ | 1.79 | 0.97 | 0.82 |
| c | 0.37 | 0.22 | 0.14 |
| RT | -.30 | 0.11 | -.41 |
| Variability of RT | 1.03 | 1.01 | 0.02 |
| Pupillary dilation | -0.43 | -0.41 | -0.02 |
| Latency to peak dilation | 0.31 | 0.22 | 0.09 |
| Absolute pupillary diameter | 0.04 | -0.29 | 0.33 |
Notes. Data were averaged across 0-backs and across hits and correct rejections in all conditions. Effect size was calculated by dividing the difference between age-adjusted group means by the square root of the MSe term for the between-subjects analysis section of the ANCOVA. In cases where there was an interaction between group and age, the value reflects the size of the effect at the average age for the whole sample.
Response criterion (c)
Values of c as a function of diagnosis and condition are displayed in Figure 4 (b). One-sample t tests comparing c values to 0 indicated that, as in our previous study with healthy adults and children, there was a bias to respond conservatively in all conditions in each group (all ps ≤ .001).
The ANCOVA on c yielded a condition effect, F (1.8, 203.4) = 88.13, p < .001. As in our previous study, the conservative response bias was greater in the second than in the first administration of 0-back, and greater in 1-back than in the 0-backs (see Figure 4-b). The linear trend for age was significant, F (1, 111) = 11.61, p = .001, but there was also an interaction between condition and the linear trend for age, F (1.8, 203.4) = 3.33, p = .042. C decreased with age in the second 0-back and in 1-back but not in the first 0-back.
As shown in Figure 4-b, there was an effect of diagnosis, F (2, 111) = 5.70, p = .004: c was higher in the psychosis than in the control group. Although c was also higher in the ADHD than in the control group, the difference did not reach significance, p = .090. The diagnosis effect was qualified by an interaction between diagnosis and the linear trend for age, F (2, 111) = 5.66, p = .005. There was a linear decrease in c with age in the control and psychosis groups, but not in the ADHD group. As with d′, however, there was no indication of an interaction between condition and diagnosis, p = .606.
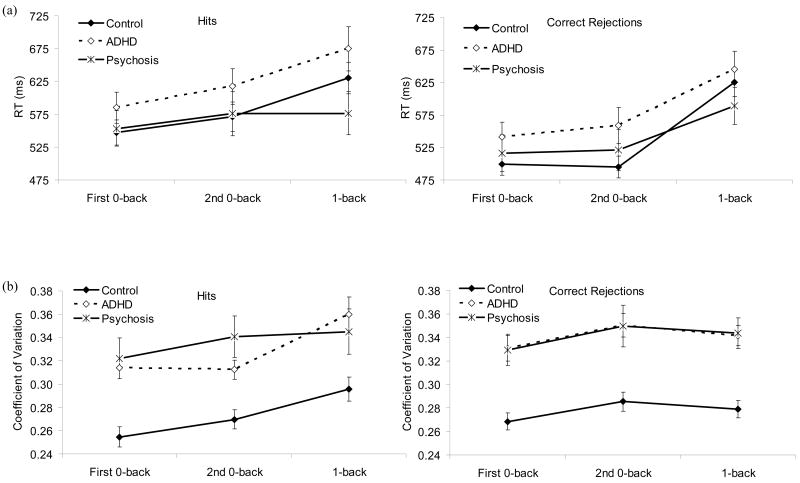
Response Times (RTs)
RTs are displayed in Figure 5 (a). RTs decreased quadratically with age, F (1, 112) = 6.28, p = .014. There was a condition effect, F (1.8, 196.8) = 21.80, p < .001: RTs did not differ between 0-backs but were longer in 1-back than in the 0-backs. There was a stimulus effect, F (1, 112) = 38.60, p < .001, an interaction between condition and stimulus, F (1.6, 175.9) = 38.09, p < .001, and a 3-way interaction among stimulus, condition, and the quadratic trend for age, F (1.6, 175.9) = 6.00, p = .006. RTs were longer to hits than to correct rejections in the 0-backs but not in 1-back. The quadratic trend for age was significant for correct rejections in the first 0-back but not for hits in this condition or for either stimulus type in the other conditions.
Figure 5.
(a) RTs (mm) and (b) coefficient of variation of RTs (SD/mean) as a function of diagnosis, condition, and stimulus type.
Condition interacted with diagnosis, F (3.5, 196.9) = 4.51, p = .003. RTs did not differ among groups in any of the conditions. As can be seen in Table 5, the effect sizes of the pairwise differences were also generally quite small. However, RTs were longer to 1-back than to the 0-backs in the control and ADHD groups, but not the psychosis group. The difference in RT between 0-backs was not significant in any diagnostic group and did not differ among the groups. Again, there was no evidence that the clinical groups were disproportionately slowed down by the demands of 1-back compared to 0-backs or on the second compared to the first administration of 0-back.
Variability of RTs
Coefficient of variation of RTs are displayed in Figure 5(b). Results showed a quadratic decrease in variability with age, F (1, 108) = 5.24, p = .024, and a condition effect, F (1.8, 196.2) = 5.94, p = .004: variability was greater in the second than in the first 0-back but did not differ between 1-back and the 0-backs. Thus, although there did not appear to be a decline in performance from the first to the second administration of 0-back in terms of d′ or RTs, a decline was apparent in terms of variability of RTs. Variability was also greater to correct rejections than to hits, F (1, 108) = 5.17, p = .025.
There was a diagnosis effect, F (2, 108) = 16.48, p < .001. As can be seen in Figure 5(b), variability was smaller in the control than in the clinical groups, who did not differ. There were also interactions between diagnosis and condition, F (3.6, 196.2) = 2.97, p = .024, condition and stimulus, F (1.8, 197.3) = 3.17, p = .049, and diagnosis, condition, and stimulus, F (3.7, 197.3) = 3.08, p = .021. In all conditions, variability of RTs to correct rejections was smaller in the control than in the clinical groups, who did not differ. In the first 0-back as well, variability to hits was smaller in the control than in the clinical groups, who did not differ. In the second 0-back, variability to hits was smaller in the control than in the psychosis group. In 1-back, variability of RTs to hits did not differ among groups.
Finally, there was a 3-way interaction among diagnosis, condition, and the quadratic trend for age, F (3.6, 196.2) = 2.65, p = .040. However, the quadratic trend was not significant in any condition in any group, and the groups did not differ in terms of the quadratic trend on any of the conditions.
Thus, these data also show no evidence of a disproportionate worsening of performance in the clinical groups on 1-back compared to 0-backs or on the second compared to the first 0-back.
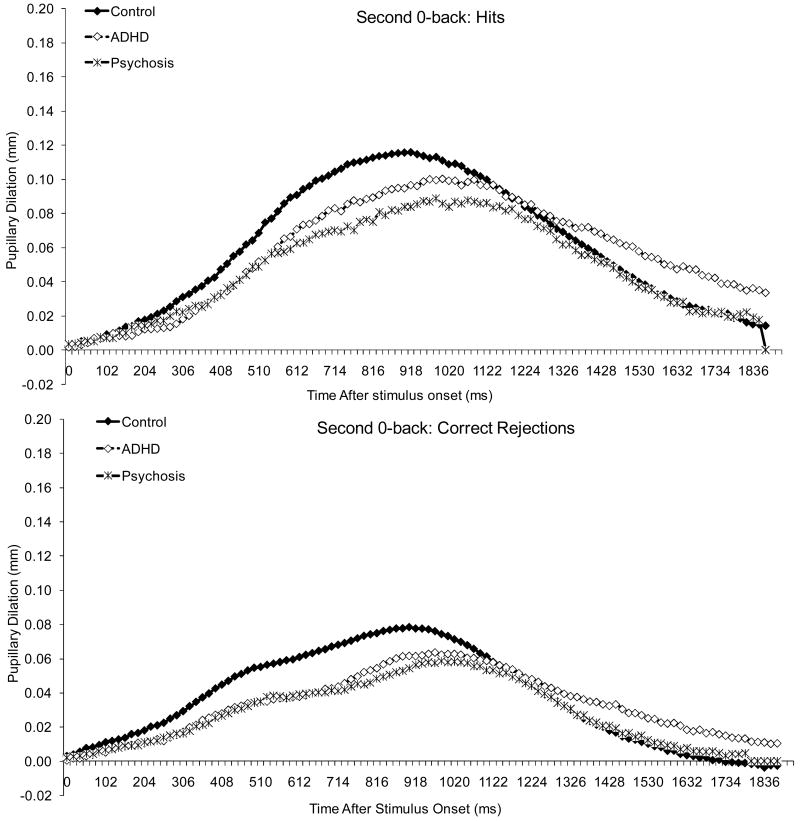
Pupillary Dilations
Figure 6 displays average pupillary dilation data, and Figures 1-3 show pupillary waveforms in the three groups as a function of condition. Pupillary dilations decreased linearly with age, F (1, 111) = 13.32, p < .001, and dilations were larger to hits than to correct rejections, F (1, 111) = 323.05, p < .001.
Figure 6.
Pupillary dilations (mm) as a function of diagnosis, condition, and stimulus type.
There was a condition effect, F (1.9, 215.8) = 18.81, p < .001, and condition by stimulus interaction, F (2.0, 221.9) = 8.89, p < .001. For both hits and correct rejections, dilations were larger in 1-back than in the 0-backs, which did not differ from each other. In addition, the difference between hits and correct rejections did not differ between 0-backs but was larger in 1-back than in the 0-backs.
Finally, there was a diagnosis effect, F (2, 111) = 3.09, p = .050, and an interaction between diagnosis, stimulus and the linear trend for age, F (2, 111) = 4.02, p = .021. Although dilations were larger in the control than in the ADHD, p = .087 and psychosis groups, p = .138, neither difference reached significance. Dilations decreased linearly with age for both hits and correct rejections in the ADHD group, but not in the other two groups. However, the function relating the linear trend for age to dilation did not differ among groups for either hits or correct rejections. As with the behavioral data, there was no evidence of a disproportionate impairment in the clinical groups on 1-back compared to 0-backs or on the second compared to the first 0-back.
Because visual inspection of the data in Figures 1-3 suggested smaller dilations in both clinical groups compared to controls, we re-analyzed the data comparing each clinical group to controls. Results showed a diagnosis effect for both the psychosis-control difference, F (1, 81) = 3.84, p = .053, and the ADHD-control difference, F (1, 85) = 4.39, p = .039. Thus, the lack of significance in the previous analysis can be attributed to the stringent corrections we had instituted for Type 1 error.
We also examined whether group differences in pupillary dilation were related to differences in latency to reach peak pupillary dilation. Latencies decreased quadratically with age, F (1, 112) = 4.66, p = .033. There was also a condition effect, F (2.0, 220.9) = 4.69, p = .010. Latencies did not differ between 0-backs, but were longer in 1-back than in 0-backs. There was an interaction between the linear trend for age and stimulus, F (1, 112) = 5.81, p = .018, and between diagnosis and stimulus, F (2, 112) = 4.19, p = .018. Latencies decreased with age for both hits and correct rejections, and the slope of the line relating age to latency was steeper for hits than for correct rejections. Latencies to hits versus correct rejections did not differ in either the control or ADHD groups, but latencies to correct rejections were longer than latencies to hits in the psychosis group. Again, there was no indication of an interaction between diagnosis and condition.
Next, we examined absolute pupillary diameters to determine if group differences in pupillary dilation were due to differences in baseline diameter. Absolute diameter decreased linearly with age, F (1, 111) = 22.98, p < .001, and there was a condition effect, F (2.0, 220.2) = 7.23, p = .001. Although there were no differences in pupillary diameter between the 1-back and 0-back conditions, diameters were smaller in the first than in the second 0-back. There was also an interaction between diagnosis and the linear trend for age, F (2, 111) = 4.69, p = .011. Diameters decreased linearly with age in the control and ADHD groups, but not in the psychosis group. The function relating age to absolute diameter did not differ between the control and ADHD groups but was shallower in the psychosis than in the control, p = .014, and ADHD groups, p = .068.
Correlations Between d′ on 1-Back and Decrement in Performance from the First to the Second 0-Back
To examine the relation between spatial working memory and ability to sustain attention over two administrations of the 0-back condition, we calculated correlations between d′ on 1-back and both the raw and proportional change in d′ and CV of RTs (averaged across hits and correct rejections) from the first to the second 0-back. None of the correlations reached significance in any group (all ps > .149).
Correlations Between RT Variability and d′
As can be seen in Table 6, greater variability of RTs was strongly correlated with lower d′ in all three conditions in the control group, as in our previous study with controls. The same pattern was observed in the ADHD and psychosis groups, although the r values were smaller in some cases.
Table 6.
Correlations Between d′ and Variability of RTs
| Control | Pychosis | ADHD | |
|---|---|---|---|
| First 0-back | -.50 | -.46 | -.50 |
| (<.001) | (.020) | (.007) | |
| Second 0-back | -.59 | -.60 | -.31 |
| (<.001) | (.001) | (.104) | |
| 1-back | -.54 | -.46 | -.31 |
| (<.001) | (.020) | (.105) |
Notes. CVs were averaged across hits and correct rejections in each condition. Numbers in parentheses refer to p levels. df = 51 for controls, 23 for psychosis, and 26 for ADHD. RT = response time (ms). CV = coefficient of variation of RTs. CR = correct rejection.
Also as in our previous study, variability of RTs in 0-backs (averaged across the two conditions) predicted d′ in 1-back in controls, r (51) = -.43, p = .001. The same association was observed in psychosis, r (23) = -.43, p = .032, but not ADHD, r (26) = -.25, p = .199. Visual inspection of the scatterplots revealed greater range restriction on both variables in ADHD compared to the other two groups. The range for d′ was 3.47 in the control group, 3.59 in the psychosis group, and 3.21 in the ADHD group. The range for log-transformed coefficients of variation in 0-backs was 0.39 in the control group 0.47 in the psychosis group, and 0.25 in the ADHD group.
Correlations Between Pupillary Dilation and Behavioral Performance
As can be seen in Table 7, there were modest but significant correlations between greater pupillary dilation and higher d′ in ADHD, but not in the other two groups.
Table 7.
Correlations Between d′ and Pupillary Dilations
| Control | Pychosis | ADHD | |
|---|---|---|---|
| First 0-back | .07 | -.01 | .41 |
| (.607) | (.950) | (.030) | |
| Second 0-back | .17 | .06 | .37 |
| (.212) | (.786) | (.050) | |
| 1-back | .113 | .18 | .37 |
| (.421) | (.401) | (.052) |
Notes. Data were averaged across hits and correct rejections in all conditions. Numbers in parentheses refer to p levels. df = 51 for controls, 23 for psychosis, and 26 for ADHD
Correlations Between Speed and Accuracy
We also tested if there was a trade-off between speed (RT) and accuracy (d′) in any condition. None of the correlations approached significance in the clinical groups. In controls, however, longer RTs were associated with higher d′ in the first 0-back, r (51) = .33, p = .017 and in 1-back, r (51) = .28, p = .045. Thus, there was no evidence for a speed-accuracy trade-off in any group.
Effects of IQ
We repeated the ANCOVAs on d′, c, RTs, variability of RTs, and pupillary measures using IQ as a covariate. For variability of RTs, only the diagnosis effect reached significance, F (2, 107) = 14.34, p < .001. For latencies, there were interactions between IQ and diagnosis, F (2, 100) = 4.73, p = .011, the quadratic trend for age and diagnosis, F (2, 100) = 3.39, p = .034, stimulus and diagnosis, F (2, 100) = 9.59, p < .001, the quadratic trend for age, diagnosis, and stimulus, F (2, 100) = 4.80, p = .010, and the linear trend for age, condition, and diagnosis, F (4.0, 199.2) = 2.43, p = .049. Higher IQ was associated with shorter latencies to reach peak dilation in the controls, but not in the clinical groups. There was a quadratic trend with age for correct rejections in the psychosis group, but not the other groups. The linear trend for age was not significant in any group for any condition. None of the other results involving main effects or interactions with diagnosis changed when IQ was covaried.
Medication Effects in the Psychosis Group
As many psychiatric medications have anticholinergic properties that can influence memory, an anticholinergic equivalent measure was calculated for each patient based on anticholinergic receptor binding affinity (Chew et al., 2006; de Leon, Canuso, White, & Simpson, 1994; Minzenberg, Poole, Benton, & Vinogradov, 2004). Correlations between anticholinergic equivalents and d′, c, RTs, variability of RTs, pupillary dilations, latency to peak dilation, and absolute diameter did not yield any significant results (to reduce the number of correlations, data were averaged across hits and correct rejections within each condition for these analyses).
In addition, each subject's current dose of antipsychotic medications was converted to a chlorpromazine equivalent, which provides an estimate of dopamine D2 receptor blocking activity (Woods, 2003). The only correlation that reached significance was positive correlation between chlorpromazine equivalent and response criterion in 0-backs, r (12) = .56, p = .039. However, given the number of correlations calculated and the lack of a correlation with c in 1-back, p = .308, it is possible that this result occurred by chance.
Discussion
Spatial Working Memory
Compared to 0-backs, the 1-back condition led to lower d′, larger pupillary dilations, and longer latency to reach peak pupillary dilation in all groups, and longer RTs in the control and ADHD groups. Results for the controls replicate those of our previous study and show that 1-back was more difficult than 0-back for all groups. As can be seen in Table 4, the clinical groups missed close to half of the targets in the 1-back condition, further suggesting that the task was difficult for them.
In all three conditions, perceptual sensitivity was lower in the psychosis than in the ADHD group and lower in the ADHD than in the control group, with large effect sizes for all pairwise differences. There was no evidence of a disproportionate decline from the 0-backs to 1-back in the clinical groups compared to the control group on any of the measures. Thus, both groups were able to cope with the additional working memory demands imposed by the 1-back condition as well as controls.
These results indicate that participants had difficulty primarily with encoding the stimuli rather than with maintaining and updating the representation of the stimuli over the 2-s inter-stimulus interval. These results also demonstrate that increasing task difficulty does not automatically result in a disproportionate decline in performance in either disorder.
Sustained Attention
Out of all the measures we used, only RT variability showed a change from the first to the second 0-back. In our previous study with healthy children and adults as well, we found an increase in RT variability from the first to the second 0-back (Karatekin et al., 2007a).
There was no evidence of a disproportionate decline from the first to the second 0-back in either clinical group. In addition, the magnitude of the decrement in performance from the first to the second 0-back did not predict performance on 1-back in any of the groups.
The lack of a disproportionate decline over time on 0–backs in either clinical group is likely to be due to the fact that the task did not tax sustained attention adequately. There were short rest breaks between conditions necessitated by the fact that we had to switch between different experimental files on the computer, and half of the participants were administered a more difficult condition between the two 0-backs. These breaks probably prevented us from detecting decrements in attention over time. In addition, the 0-back condition was not cognitively demanding (e.g., through perceptual degradation; Nuechterlein, 1983), and participants differed greatly in how much they aroused themselves (e.g., by fidgeting or talking to themselves or the experimenter). Furthermore, absolute pupillary diameters were larger in the second than in the first 0-back, which suggests that participants had become more engaged in the task instead of habituating to it.
Fluctuations in Attention/Maintaining Readiness to Respond
RT variability was greater in the clinical groups than in the control group, with no difference between the clinical groups. Furthermore, as shown in Table 5, RT variability yielded the largest or second largest effect sizes for the clinical-control differences. These results replicate those of many studies showing greater RT variability on a variety of tasks in ADHD (e.g., Castellanos et al., 2005; Klein et al., 2006; Kuntsi et al., 2001) and schizophrenia (e.g., Schwartz et al., 1989). In our previous paper, we suggested that RT variability might be related to fluctuations in attention, or, perhaps more specifically, maintaining readiness to respond during the inter-stimulus interval (e.g., Bellgrove et al., 2004; Godefroy et al., 2002; Segalowitz et al., 1997; Stern et al., 2005).
Furthermore, lower perceptual sensitivity was related to greater RT variability within all conditions in all groups, and lower perceptual sensitivity on 1-back was also related to greater RT variability on 0-backs in the psychosis and control groups (range restrictions might have prevented the same correlation from becoming significant in the ADHD group). The results, which replicate those of our previous study (Karatekin et al., 2007a), suggest that successful performance on a spatial working memory task (1-back) is related to attentional fluctuations and/or ability to maintain readiness to respond on a trial-by-trial basis on a task making no demands on working memory.
Phasic Recruitment of Cognitive Resources
As in our previous study (Karatekin et al., 2007a), pupillary dilations were larger in 1- than in 0-backs, and larger to hits than to correct rejections. Although pupillary dilations were smaller in both clinical groups than in the control group, the difference did not reach significance for either group. In addition, there were modest but significant correlations in the ADHD group, but not the other groups, between larger pupillary dilations and perceptual sensitivity on both 0- and 1-back.
Effect sizes for the control-clinical differences were in the .40s (Table 5), so our failure to find significant differences was likely due to the relatively small sample sizes and the precautions we took to avoid Type 1 errors. Nevertheless, it was clear at least that the clinical groups were not recruiting more resources or spending more effort than controls on a trial-by-trial basis to perform the task. Impairments in phasic recruitment of cognitive resources may also contribute to a difficulty in discriminating between targets and non-targets.
It should be noted that pupillary dilations increase with motor responses (e.g., Richer, Silverman, & Beatty, 1983). Therefore, pupillary dilations in the current task likely reflect purely cognitive processes related to stimulus identification and classification as well as cognitive and motor processes related to making a motor response. However, as each trial required the same motor response, differences between conditions or stimulus types cannot be attributed to purely motor factors.
Response Criterion
Previous studies on schizophrenia and ADHD show mixed results for response criterion. It is likely that response criterion depends to a greater extent than perceptual sensitivity on specific task parameters, such as the requirement to inhibit responding on non-target trials, event rate, target probability. In the current study, as in our previous study, c increased from the first to the second administration of 0-back and from 0-backs to 1-back. The increase from the first to the second 0-back likely reflects lowered expectations regarding target probability and/or fatigue, and the difference between 0-backs and 1-back is likely a function of task difficulty. Both clinical groups adopted a more conservative response threshold than the controls on all conditions, although the difference reached significance only for the psychosis group. There were no interactions between diagnosis and condition.
A reluctance to respond that the target is present can be related to fatigue, higher motivation, greater task difficulty, or lowered expectations regarding target probability. It is unlikely that the clinical groups were more motivated than the control group. Given the lack of interactions between diagnosis and condition for c or any other measure, it is also unlikely that the higher c in the clinical groups reflects fatigue or impairments in adjusting expectations in accordance with task demands. There was also no clear evidence of an interaction between diagnosis and stimulus on pupillary dilations, which might have reflected group differences in extracting target probability or stimulus relevance. Thus, we speculate that the elevated c values in the clinical groups may be related to the fact that both groups found the whole task (including 0- and 1-back conditions) more difficult than did the control group.
Age × Diagnosis Interactions
There were no age by diagnosis interactions in perceptual sensitivity, RTs, or latencies to peak pupillary dilation. However, c decreased with age in the control and psychosis groups, but not in the ADHD group. The reason for this age-related difference is not clear.
We also found that absolute pupillary diameters decreased with age in the control and ADHD groups but not in the psychosis group. Although potentially interesting (Karatekin et al., 2007a), this result awaits replication, as our results regarding age by diagnosis interactions were not consistent on two other tasks (Karatekin et al., 2008; Karatekin in press-a).
With the caveat that this was not a longitudinal study, these results suggest that most of the cognitive processes assessed by these measures mature at the same rate across the three groups.
Limitations
The temporal resolution of the eye monitor, and consequently that of individual RTs, was somewhat low (60 Hz). Therefore, some of the negative findings regarding RTs and their variability may have become significant with higher temporal resolution.
The current study was part of a larger study that included two cognitive testing sessions for all and brain imaging for some participants. This procedure likely excluded severely impaired participants and chaotic, dysfunctional or low-SES families who were unable or unwilling to invest the necessary time to participate in the study, limiting generalizability of the results. In addition, one ADHD and three psychosis participants could not be administered the whole task because of behavioral problems, and their exclusion could have further compromised the external validity of the study.
As shown in Table 1, IQs were about 0.6-1 SD above average in both the ADHD and control groups, further limiting the generalizability of the results. In the control and psychosis groups, lower IQ was associated with higher RT variability, and in the psychosis group only, lower IQ was associated with lower perceptual sensitivity. It should be noted, however, the average IQ in the psychosis group, although lower than that of the other groups, was well within the normal range. In addition, the control and ADHD groups did not differ significantly on IQ, but the performance of the ADHD group resembled that of the psychosis group on most of the analyses.
We could not assess the effects of comorbid conditions in the clinical groups adequately due to small sample sizes. Results are limited to the Combined subtype of ADHD. Additionally, we had few girls in the ADHD group, as it was difficult to find girls who met criteria for the Combined subtype.
Because there was no Bonferroni correction across terms in the ANCOVAs, some of the results, especially higher-order interactions, close to a p level of .05 may have been spurious.
Although not strictly a limitation, it should be noted that the experimenters provided structure for the participants as necessary in order for them to complete the tasks. In many cases, the experimenter sat behind the participant, but in some cases, it was essential to stand next to the participants throughout a condition to encourage them to continue. Although participants were discouraged from talking during the task, they differed in how much they spontaneously talked to the experimenter or to themselves. As noted above, these behaviors may have prevented us from assessing sustained attention adequately. There were also differences in how many reminders the experimenters gave the participants to follow the instructions. When necessary, participants received breaks between conditions, and these breaks may have differed across groups. We did not quantify differences on these variables. Had testing conditions been strictly identical across groups and had the experimenters provided less structure, we would have had more missing/invalid data but we might also have had larger group differences on the task.
Conclusions
Both clinical groups had lower perceptual sensitivity on both 0- and 1-back, with no evidence of a specific impairment in spatial working memory. We also found no evidence of differential order effects in either disorder on the 0-backs and no evidence that ability to sustain attention over two administrations of the 0-back condition contributed to 1-back performance. Instead, results suggest that both clinical groups had difficulty encoding the stimuli. They also appeared to have difficulty maintaining attention and/or readiness to respond, and, to a lesser extent, recruiting resources on a trial-to-trial basis. It is likely that these attentional problems prevented the clinical groups from encoding the stimuli effectively and contributed to their general performance deficits.
The main difference between the clinical groups was that perceptual sensitivity was lower in the psychosis than in the ADHD group, although both groups had clearly lower d′ scores than controls. Thus, the impairments we observed in this study were not specific to either disorder.
Although evidence regarding the encoding stage is scarce in ADHD, this result is highly consistent with a growing body of research in schizophrenia pointing to slower or inefficient encoding in both verbal and visual-spatial working memory. In a meta-analysis of working memory deficits in schizophrenia, Lee and Park (2005) concluded that increasing the delay period beyond 1 s did not lead to disproportionate impairments in working memory. Several studies have specifically examined the encoding stage in verbal (Cairo et al., 2006; Javitt et al., 2007; Kayser et al., 2006), visual (Fuller et al., 2005; Gold et al., 2006; Hartman et al., 2003; Hartman et al., 2002; Lencz et al., 2003; Tek et al., 2002), spatial (Badcock et al., 2008), and visual and spatial (Tek et al., 2002) working memory in schizophrenia, using behavioral, electrophysiological and neuroimaging methods. All of these studies have concluded that encoding and early maintenance processes are slower and/or less efficient in schizophrenia than in control samples, leading to deficiencies in the nature of the internal representation to be maintained during the delay period. Impairments in early stages of visual processing of simple visual stimuli, as indexed by reduced amplitudes for the P1 component of the event-related potential, have been observed in unaffected relatives of individuals with schizophrenia as well (Yeap et al., 2006).
Reduced P1 amplitude during the encoding phase of a visual delayed discrimination task has also been found in adolescents with schizophrenia. This reduction was accompanied by reduced activation of the visual cortex, measured with functional MRI, leading researchers to conclude that deficits in early visual processing contributed to the working memory deficits (Haenschel et al., 2007).
Thus, the current results highlight the importance of taking “lower-level” attentional impairments into account before interpreting impairments on “higher-level” tasks of executive functioning (cf. Marks et al., 2005; Rommelse et al., 2007; Wilcutt et al., 2005).
As for pupillary dilations, we have now analyzed data in this sample on three disparate tasks: the current n-back task, an auditory digit span task (Karatekin et al., 2008), and an antisaccade task (Karatekin et al., in press-a). On all three tasks, the pupillary dilation data suggested that participants in the clinical groups were affected by experimental manipulations in the same manner as controls. Participants with psychosis had smaller dilations than controls regardless of the task, with effect sizes in the medium range. Results for the ADHD group depended on the nature of the task: on tasks involving verbal or spatial short-term/working memory, the ADHD group had smaller dilations than the controls, with effect sizes also in the medium range. In contrast, there were no differences between the control and ADHD groups on the antisaccade task (effect size = -0.06). Furthermore, there was no evidence that latency to reach peak dilation differed between controls and the clinical groups on either the current task or the antisaccade task (latency was not measured on the digit span task), and effect sizes for differences in absolute pupillary diameter ranged from approximately 0 to small across the three tasks. Although reduced pupillary dilations are consistent with energetic models of ADHD (Sergeant, 2005), these results indicate that these models need to address the lack of specificity of energetic dysregulation to ADHD and to explain why regulation of energetic resources might be impaired on some tasks, but not others. With respect to psychosis, our findings are consistent with the hypothesis that phasic resource recruitment is impaired in youth-onset psychosis, as has been demonstrated before in adults with schizophrenia (e.g., Fish & Granholm, 2008; Steinhauer & Hakerem, 1992). However, the findings also indicate that the specificity of this impairment depends on the nature of the task.
Figure 2.
Pupillary waveforms to hits and correct rejections in the second 0-back
Acknowledgments
We thank the families and teachers for participating in the study; Afshan Anjum, Angie Guimaraes, Bonnie Houg, Cacy Miranda, Kathryn McGraw-Schuchman, and Marie Gabrielle Reed for helping with diagnostic assessments; Clay Collins, Nicholas Davenport, Anita Fuglestad, David Marcus, and Marcus Schmidt for helping with data collection and analyses; and research assistants for helping with data entry and organization. Funding was provided by NIMH (1RO3-MH063150), NIMH (K08-MH068540), National Alliance for Research on Schizophrenia and Depression (NARSAD), the Essel Foundation, and the University of Minnesota Center for Neurobehavioral Development.
Footnotes
We attempted to assess performance on a 2-back task. However, pilot testing indicated that performance was very variable in adults on this task, and debriefing the participants revealed great inter- and intra-individual variability in strategy use on this task. Thus, we omitted this condition from subsequent studies because the difference between 1- and 2-back seemed to be a qualitative, not a quantitative, one. As can be seen in Table 4, hit rates were below ceiling level in the current study in even the control group on 1-back. Klein and colleagues (2006) also report that a substantial number of the children in their ADHD group did not understand the 2-back task and that their data could not be analyzed).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Canan Karatekin, Institute of Child Development, University of Minnesota, Minneapolis, MN.
Christopher Bingham, School of Statistics, University of Minnesota, Minneapolis, MN.
Tonya White, Division of Child and Adolescent Psychiatry, University of Minnesota, Minneapolis, MN.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-Informant Assessment. ASEBA; Burlington, VT: 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Author; Washington, DC: 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Asarnow RF, Nuechterlein KH, Fogelson D, Subotnik KL, Payne DA, Russell AT, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia. Arch Gen Psychiatry. 2001;58:581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Nuechterlein KH, Subotnik KL, Fogelson DL, Torquato RD, Payne DL, et al. Neurocognitive impairments in nonpsychotic parents of children with schizophrenia and attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59:1053–1060. doi: 10.1001/archpsyc.59.11.1053. [DOI] [PubMed] [Google Scholar]
- Badcock JC, Badcock DR, Read C, Jablensky A. Examining encoding imprecision in spatial working memory in schizophrenia. Schizophr Res. 2008;100:144–152. doi: 10.1016/j.schres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Barr WB. Schizophrenia and attention deficit disorder: Two complex disorders of attention. Ann N Y Acad Sci. 2001;931:239–250. doi: 10.1111/j.1749-6632.2001.tb05782.x. [DOI] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol Bull. 1982;91:276–292. [PubMed] [Google Scholar]
- Beatty J, Lucero-Wagoner B. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd. Cambridge University Press; New York, NY: 2000. pp. 142–162. [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Kumra S, Callicott JH, Mattay VS, Lestz RM, Jacobsen L, et al. Common pattern of cortical pathology in childhood-onset and adult-onset schizophrenia as identified by proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1998;155:1376–1383. doi: 10.1176/ajp.155.10.1376. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Sciota D, Brudaglio F, Altamura M, Blasi G, Bellomo A, et al. Working memory deficits and levels of N-Acetylaspartate in patients with schizophreniform disorder. Am J Psychiatry. 2003;160:483–489. doi: 10.1176/appi.ajp.160.3.483. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J R Stat Soc [Ser B] 1964;26:211–252. [Google Scholar]
- Cairo TA, Woodward TS, Ngan ETC. Decreased encoding efficiency in schizophrenia. Biol Psychiatry. 2006;59:740–746. doi: 10.1016/j.biopsych.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinski B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of Attention-Deficit/Hyperactivity Disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew M, Mulsant B, Pollock B, Lehman M, Greenspan A, Kirshner M, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res. 2006;88:63–72. doi: 10.1016/j.schres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- De Leon J, Canuso C, White AO, Simpson GM. A pilot effort to determine benzotropine equivalents of anticholinergic medications. Hosp Community Psychiatry. 1994;45:606–607. doi: 10.1176/ps.45.6.606. [DOI] [PubMed] [Google Scholar]
- Fish SC, Granholm E. Easier tasks can have higher processing loads: Task difficulty and cognitive resource limitations in schizophrenia. J Abnorm Psychol. 2008;117:355–363. doi: 10.1037/0021-843X.117.2.355. [DOI] [PubMed] [Google Scholar]
- Frazier JA, McClellan J, Findling RL, Vitiello B, Anderson R, Zablotsky B, et al. Treatment of early-onset schizophrenia spectrum disorders (TEOSS): Demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979–988. doi: 10.1097/chi.0b013e31807083fd. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. J Abnorm Psychol. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O, Lhullier-Lamy C, Rousseaux M. SRT lengthening: Role of an alertness deficit in frontal damaged patients. Neuropsychologia. 2002;40:2234–2241. doi: 10.1016/s0028-3932(02)00109-4. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, et al. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia. Arch Gen Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Hartman M, Steketee MC, Silva S, Lanning K, Andersson C. Wisconsin Card Sorting Test performance in schizophrenia: the role of working memory. Schizophr Res. 2003;63:201–217. doi: 10.1016/s0920-9964(02)00353-5. [DOI] [PubMed] [Google Scholar]
- Hartman M, Steketee MC, Silva S, Lanning K, McCann H. Working memory and schizophrenia: Evidence for slowed encoding. Schizophr Res. 2002;59:99–113. doi: 10.1016/s0920-9964(01)00366-8. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Jacobsen LK, Rapaport JL. Childhood-onset schizophrenia: Implications of clinical and neurobiological research. J Child Psychol Psychiatry. 1998;39:101–113. [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJA, Kahn RS. Working memory capacity in schizophrenia: A parametric fMRI study. Schizophr Res. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Rabinowicz E, Silipo G, Dias EC. Encoding vs. retention: Differential effects of cue manipulation on working memory performance in schizophrenia. Schizophr Res. 2007;91:159–168. doi: 10.1016/j.schres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Prentice-Hall; Englewood Cliffs, NJ: 1973. [Google Scholar]
- Karatekin C. Improving antisaccade performance in adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD) Exp Brain Res. 2006;174:324–341. doi: 10.1007/s00221-006-0467-x. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Development of attentional allocation on the dual task paradigm. Int J Psychophysiol. 2004;52:7–22. doi: 10.1016/j.ijpsycho.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Developmental disorders of attention. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 561–576. [Google Scholar]
- Karatekin C, Bingham C, White T. Oculomotor and pupillometric indices of pro- and antisaccade performance in youth-onset psychosis and Attention-Deficit/Hyperactivity Disorder (ADHD) Schizophr Bull. doi: 10.1093/schbul/sbp035. in press-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin C, Couperus JW, Marcus DJ. Attention allocation on the dual task paradigm as measured through behavioral and psychophysiological responses. Psychophysiology. 2004;41:175–185. doi: 10.1111/j.1469-8986.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Marcus DJ, Couperus JW. Regulation of cognitive resources during sustained attention and working memory in 10-year-olds and adults. Psychophysiology. 2007a;44:128–144. doi: 10.1111/j.1469-8986.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Marcus DJ, White TJ. Oculomotor and manual indices of incidental and intentional spatial sequence learning in middle childhood and adolescence. J Exp Child Psychol. 2007b;96:107–130. doi: 10.1016/j.jecp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Karatekin C, White T, Bingham C. Divided attention in youth-onset psychosis and Attention-Deficit/Hyperactivity Disorder (ADHD) J Abnorm Psychol. 2008;117:881–895. doi: 10.1037/a0013446. [DOI] [PubMed] [Google Scholar]
- Karatekin C, White T, Bingham C. Incidental and intentional sequence learning in youth-onset psychosis and attention-deficit/hyperactivity disorder (ADHD) Neuropsychology. doi: 10.1037/a0015562. in press-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin C, White TJ, Bingham C. Shared and nonshared symptoms in youth-onset psychosis and Attention-Deficit/Hyperactivity Disorder (ADHD) J Atten Disord. doi: 10.1177/1087054709347434. in press-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime Version (K-SADS-PL) University of Pittsburgh School of Medicine; Pittsburgh, PA: 1996. [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43:237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in Attention-Deficit Hyperactivity Disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Legge GE. Psychophysics of Reading in Normal and Low Vision. Erlbaum; Mahwah, NJ: 2007. [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, et al. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User's Guide. Cambridge University Press; New York: 1991. [Google Scholar]
- Marks DJ, Berwid OG, Santra A, Kera EC, Cyrulnik SE, Halperin JM. Neuropsychological correlates of ADHD symptoms in preschoolers. Neuropsychology. 2005;19:446–455. doi: 10.1037/0894-4105.19.4.446. [DOI] [PubMed] [Google Scholar]
- McKenna K, Gordon C, Lenane M, Kaysen D, Fahey K, Rapaport J. Looking for childhood-onset schizophrenia: The first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33:636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Brookner FB, Lenane M, Gochman P, Ingraham LJ, Egan MF, et al. Parental schizophrenia spectrum disorders in childhood-onset and adult-onset schizophrenia. Am J Psychiatry. 2003;160:490–495. doi: 10.1176/appi.ajp.160.3.490. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. J Abnorm Psychol. 1983;92:4–28. doi: 10.1037//0021-843x.92.1.4. [DOI] [PubMed] [Google Scholar]
- Pastore RE, Crawley EJ, Berens MS, Skelly MA. “Nonparametric” A' and other modern misconceptions about signal detection theory. Psychon Bull Rev. 2003;10:556–569. doi: 10.3758/bf03196517. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Working memory dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Levine SZ, Häfner H. A population based elaboration of the role of age of onset on the course of schizophrenia. Schizophr Res. 2006;88:96–101. doi: 10.1016/j.schres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Richer F, Silverman C, Beatty J. Response selection and initiation in speeded reactions: A pupillometric analysis. J Exp Psychol Hum Percept Perform. 1983;9:360–370. doi: 10.1037//0096-1523.9.3.360. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, de Sonneville LMJ, Buschgens CJM, Buitelaar J, Oosterlaan J, et al. Are motor inhibition and cognitive flexibility dead ends in ADHD? J Abnorm Child Psychol. 2007;35:957–967. doi: 10.1007/s10802-007-9146-z. [DOI] [PubMed] [Google Scholar]
- Schwartz F, Carr AC, Munich RL, Glauber S, Lesser B, Murray J. Reaction time impairment in schizophrenia and affective illness: The role of attention. Biol Psychiatry. 1989;25:540–548. doi: 10.1016/0006-3223(89)90214-x. [DOI] [PubMed] [Google Scholar]
- See JE, Howe SR, Warm JS, Dember WN. Meta-analysis of the sensitivity decrement in vigilance. Psychol Bull. 1995;117:230–249. [Google Scholar]
- Segalowitz SJ, Dywan J, Unsal A. Attentional factors in response time variability after traumatic brain injury: An ERP study. J Int Neuropsychol Soc. 1997;3:95–107. [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: A critical appraisal of the cognitive-energetic model. Biol Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF. Executive function profile of children with Attention Deficit Hyperactivity Disorder. Dev Neuropsychol. 2002;21:43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Sorkin RD. Spreadsheet signal detection. Behav Res Methods Instrum Comput. 1999;31:46–54. doi: 10.3758/bf03207691. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophrenia. Ann N Y Acad Sci. 1992;658:182–204. doi: 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- Stern ER, Horvitz JC, Côté LJ, Mangels JA. Maintenance of response readiness in patients with Parkinson's Disease: Evidence from a simple reaction time task. Neuropsychology. 2005;19:54–65. doi: 10.1037/0894-4105.19.1.54. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Ueland T, Øie M, Landrø NI, Rund BR. Cognitive functioning in adolescents with schizophrenia spectrum disorders. Psychiatry Res. 2004;126:229–239. doi: 10.1016/j.psychres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Vourdas A, Pipe R, Corrigall R, Frangou S. Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophr Res. 2003;62:13–22. doi: 10.1016/s0920-9964(02)00429-2. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th. Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- White T, Kendi ATK, Lehericy S, Kendi M, Karatekin C, Guimaraes A, et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia—a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Wilcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, et al. Early visual sensory deficits as endophenotypes for schizophrenia. Arch Gen Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]