Abstract
Loss of bladder control (urge incontinence) is common in elderly; the cause is usually unknown. Functional imaging has revealed the brain network controlling responses to bladder filling. Age-related changes in this network might predispose to urge incontinence. We sought such changes in 10 continent, healthy women aged 30 – 79 years who underwent fMRI while fluid (20 ml) was repeatedly infused into and withdrawn from the bladder. Data were collected in 4 measurement blocks with progressively increasing bladder volumes and were analyzed by SPM2, using the contrast infuse-withdraw to quantify response to bladder infusion. Effective connectivity was examined by physiophysiological interaction (PhPI; see interpretation in Supplementary Material), with right insula (RI) and dorsal anterior cingulate cortex (dACC) as seed regions. Dependence on age and bladder volume (=block number) was assessed. Bladder infusion evoked expected activations. Activation decreased with age in bilateral insula and dACC. PhPI revealed connectivity with RI and dACC in regions that included bilateral putamen and R pontine micturition center. Interaction (connectivity) tended to increase with age in regions including L insula, L paracentral lobule and PAG. Consistent with a special role in maintaining continence, medial prefrontal cortex (mPFC) showed a trend to deactivation on bladder infusion that became more prominent in old age, and a trend to negative interaction (connectivity) that weakened significantly with age. Thus, with increasing age, weaker signals in the bladder control network as a whole and/or changes in mPFC function or connecting pathways may be responsible for the development of urge incontinence.
Keywords: bladder, fMRI, effective connectivity, physiophysiological interaction, medial prefrontal cortex, aging
Introduction
Voluntary control of the urinary bladder and its sphincter is learned as a child but is frequently lost in old age, resulting in urge incontinence – urine leakage associated with a sudden compelling desire to pass urine (Abrams et al., 2002). This problem is conventionally ascribed to involuntary bladder contractions (detrusor overactivity (Abrams et al., 2002)) or abnormal bladder afferents (Fowler et al., 2008) but unless there is overt neurological disease the underlying cause is usually unknown and treatment, whether by pharmaceuticals or behavioral measures, remains unsatisfactory. Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have revealed the network of brain regions involved in normal bladder control (Fowler et al., 2008). Among the most firmly established of these regions are: the periaqueductal gray (PAG), believed to receive bladder afferents from the spinal cord; the insula, especially on the right (RI), activation of which is linked to subjective visceral sensation (‘feeling states’); and the dorsal anterior cingulate cortex (dACC), responsible for emotional response, motivational behaviors (Mayer et al., 2006) and concurrent efferent autonomic control (Critchley et al., 2003). In these regions, and in the insula in particular, response to bladder filling during urine storage becomes stronger with increasing bladder volume and sensation (Griffiths et al., 2007). This network is similar to that involved in the brain-gut axis (Mayer et al., 2006). In addition, the prefrontal cortex (PFC) is involved in decisions about voiding; the medial part in particular is believed to be responsible for decisions such as whether to void or not, based on the social situation (Adolphs, 1999). Finally, if voluntary voiding occurs, there is activation of the genual ACC (Blok et al., 1997), and activation of the pontine micturition center (PMC) that results in coordinated urethral sphincter relaxation and detrusor contraction, and thus urination (Blok et al., 1998).
fMRI measurements have shown that the responses of this control network to bladder filling are abnormal in subjects with urge incontinence and suggest that the connectivity of the network is abnormal also (Tadic et al., 2008). It is thus possible that cerebral changes may cause or contribute to incontinence. In the elderly, changes in cortical pathways (white matter hyperintensities on MRI) are particularly common (Wu et al., 2006). Moreover, clinical studies after brain injury and stroke suggest that damage to white matter in frontal cortical regions or to gray matter in medial prefrontal regions is likely to impair bladder control (Andrew et al., 1964)(Griffiths et al., 2008a). Furthermore, SPECT brain studies of the elderly suggest that urge incontinence is associated with reduced perfusion of frontal cortex (Griffiths et al., 1994). Therefore it is plausible that age-associated changes in connecting pathways may predispose to loss of bladder control in older people. The aim of this study was thus to examine, in normal subjects, age-associated changes in the control network, including changes in its connectivity, and whether they might predispose to urge incontinence. Particular attention was paid to the physiological interpretation of the chosen method of determining effective connectivity (physiophysiological interaction, PhPI, see Supplementary Material). We chose to examine female subjects because incontinence is more common in women and is not confounded by the effects of possible prostatic obstruction.
Materials and methods
Subjects
This was a secondary analysis of fMRI data from 10 continent female volunteers aged 30 to 79 years who had taken part in a previous study (Tadic et al., 2008). They had no history of urge incontinence, no symptoms suggestive of lower urinary tract dysfunction, and no detrusor overactivity on urodynamic study prior to scanning. All signed written informed consent. The University of Pittsburgh Institutional Review Board approved all research procedures.
Imaging methods and analysis
As described previously (Griffiths et al., 2005; Griffiths et al., 2007; Tadic et al., 2008), two 8 F urethral catheters were introduced into the bladder, for filling/emptying and for measurement of bladder pressure to rule out detrusor overactivity. After recording a structural MRI, 50-100 ml of saline solution were introduced into the empty bladder, without scanning. Small amounts of saline solution were then repeatedly infused into and withdrawn from the bladder while the fMRI BOLD (blood oxygen level dependent) signal, representing neuronal activity, was recorded. Data were acquired from a GE Signa scanner with 3 T magnet, using transverse relaxation time (T2*)-weighted single-shot spiral scans, with a 20 cm field of view, 3.1 mm resolution, echo time (TE) = 26 ms, and flip angle (FA) = 60°, allowing for one whole brain scan per 1.5s (TR). Each infusion/withdrawal cycle comprised the following sequence: pause (10.5 s); infusion (22 ml in 10.5 s); pause (10.5 s); withdrawal (15 ml in 10.5 s). Four cycles followed by a 10.5 s run-out period formed one measurement block. After performing 2 such measurement blocks, the bladder was filled further, without scanning, until the subject signaled strong bladder sensation with a pushbutton. If she agreed, further measurement blocks were then performed, with scanning, until sensation became uncomfortable. In most subjects this resulted in a further 2 to 4 measurement blocks (i.e. 4 to 6 blocks in total). Thus bladder volume increased in successive measurement blocks, in part because more was infused than withdrawn, in part because of urine production, and because of deliberate bladder filling between blocks 2 and 3.
Image preprocessing and further analyses were performed using Statistical Parametric Mapping (SPM2) (Wellcome Department of Imaging Neuroscience, 2003) and an alignment toolbox (INRIAlign, 2000). For main effects, regional brain responses to bladder filling were determined by comparing the BOLD signal (deconvolved with the SPM hemodynamic response function) during fluid infusion into the bladder with the signal attained during fluid withdrawal (contrast = infusion minus withdrawal) (Griffiths et al., 2005; Griffiths et al., 2007; Tadic et al., 2008).
Main-effects analyses were performed in each subject for each of 4 measurement blocks (the first, second, third and last blocks), which corresponded to increasing bladder volume and increasing sensation of desire to void. In subsequent analyses, volume was treated as a categorical variable with integer values 1 to 4 (the block numbers). We performed a standard random-effects analysis in the whole group (Holmes et al., 1998), see Figure 1. We examined the effects of varying bladder volume and age separately, using simple correlations based on all 4 measurement blocks. We also performed multiple regression on age, volume (block number) and their interaction to rule out significant interaction in the regions where the correlation with age was significant.
Figure 1.
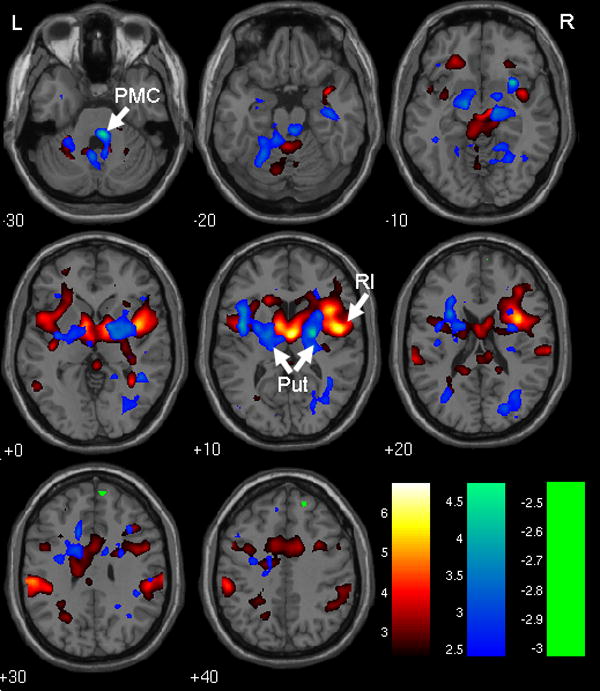
Main effect of bladder filling and pattern of effective connectivity with RI and dACC. Red/yellow: regions responding to bladder filling with activation (see Table 1). Green: region responding to bladder filling with deactivation (see Table 1). Blue: regions where PhPI shows positive connectivity with RI and dACC (see Table 3). Color bars show how t-values are color coded. Note that blue obscures red/yellow where they overlap. RI = right insula, dACC = dorsal anterior cingulate cortex, Put = putamen, PMC = pontine micturition center. R = right, L = left, numbers show z-coordinate in mm.
We then used physiophysiological interaction (PhPI) as implemented in SPM2 to analyze effective connectivity in the bladder control network. PhPI identifies brain regions that are effectively connected to two seed regions chosen a priori (see Supplementary Material, Figure S1): we selected the right insula (RI) and the dorsal part of the anterior cingulate cortex (dACC), because they showed consistent activation in response to bladder filling in nearly every subject and are known to play an important role in regulation of bladder (Griffiths et al., 2007) and gut (Mayer et al., 2006). We used for RI a spherical region of interest with radius 12 mm, centered at Montreal Neurological Institute (MNI) coordinates (38, 16, -6); for dACC the region of interest was centered at (2, 12, 42) with radius 18 mm.
For group connectivity results we performed a second-level t-test of the first-level PhPI results, using all blocks (each calculated separately) and without distinguishing between subjects. To determine dependence of connectivity on bladder volume (block number) and age we performed simple correlations on these variables, with multiple regression to test for significant interaction effects.
Locations are specified by MNI coordinates. Data are reported for clusters of 16 or more contiguous voxels (exceeding threshold significance of P<0.01), which were significant at cluster level (P<0.05) after correction for multiple comparisons. Significance at set level (P<0.05) is reported if present. Clusters attaining a less stringent threshold (P<0.01 uncorrected) are reported as showing a trend to significance. In one case, identified in the text and tables, the threshold was relaxed to P < 0.05 (uncorrected). For display purposes, threshold significance was set at P < 0.01 (uncorrected).
Results
Five of the 10 subjects were 30 – 46 y old and five were 67 – 90 y old, reflecting a special effort to include both young and old.
Bladder filling (based on results in all 4 blocks combined) led to activation in well-known regions (Griffiths et al., 2007), including PAG and inferior parietal lobule, as well as the right insula and dorsal ACC that are the seed regions of the PhPI analysis (see Figure 1 and Table 1). Negative responses (“deactivation”, see discussion in Supplementary Material) were rare, but parts of the medial prefrontal cortex and a posterior region showed a trend to deactivation (Figure 1). Detrusor overactivity was never observed during scanning.
Table 1.
Principal regions that responded with activation or deactivation to bladder filling, with special attention to medial prefrontal/genual ACC area. Bold = in significant cluster; italics = trend only. & = in same cluster as previous row
| Region | Coordinates | Z-value | Cluster size | |
|---|---|---|---|---|
| Activation | thalamus | 4, -6, 8 | 5.47 | 16855 |
| R insula (RI) | 34, 8, 16 | 5.26 | & | |
| L insula | -46, 0, 4 | 3.90 | & | |
| L inferior parietal lobule | -60, -26, 28 | 4.51 | 1734 | |
| R inferior parietal lobule | 62, -22, 26 | 4.19 | 1238 | |
| dACC | 0, 6, 30 | 3.54 | 16855 | |
| Deactivation | medial prefrontal | 6, 62, 24 | 2.85 | 445 |
| genual ACC | 4, 38, -2 | 2.10 | 34 |
As expected, there was a trend to positive association with bladder volume in R insula (MNI coordinates x = 44 mm, y = 8 mm, z = 8mm; Z-value = 4.16), and there were similar but weaker trends elsewhere, including medial frontal cortex (-8, 52, 6; Z = 3.21) and PAG (10, -10, -4; Z = 3.69). The whole set of clusters was significant at set level. No region showed significant negative association between response and bladder volume. Interaction between age and volume showed no significant clusters but a trend to significant negative interaction in sublobar gray matter at -6, 8, -8 (Z = 5.36), not important for the results shown in Table 2.
Table 2.
Principal regions where response was negatively associated with age, with special attention to medial prefrontal/genual ACC area. Bold = in significant cluster.
| Region | Coordinates | Z-value | Cluster size |
|---|---|---|---|
| R insula (RI) | 38, 8, 10 | 4.45 | 5542 |
| L insula | -42, 4, 8 | 3.77 | 1586 |
| dACC | 0, 6, 34 | 3.74 | 5542 |
| medial prefrontal | 18, 50, 8 | 3.24 | 628 |
Response to bladder filling was negatively associated with age in R and L insula and dACC (Figure 2 and Table 2) and also in medial prefrontal cortex. There was no clear evidence of age-associated decrease of response in PAG. Response was not positively associated with age in any region.
Figure 2.
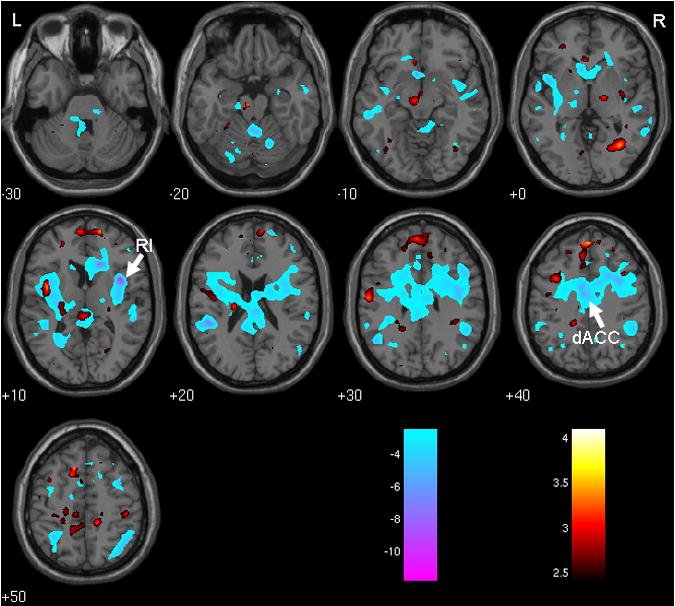
Effects of age. Blue: regions where response to bladder infusion diminished with age (see Table 2). Red: regions where connectivity coefficient increased with age (see Table 4). Color bars show how t-values are color coded. RI = right insula, dACC = dorsal anterior cingulate cortex. R = right, L = left, numbers show z-coordinate in mm.
There was extensive positive connectivity (i.e. positive correlation with RI×dACC), notably in the putamen bilaterally and the pontine micturition center (PMC), and also in L insula, R cuneus and R parahippocampal gyrus (see Figure 1 and Table 3). Negative connectivity was rare, but there was a negative trend in the superior parietal lobule. In the medial prefrontal and genual cingulate cortices the connectivity coefficient was not significant but relaxation of the threshold for significance suggested it was unlikely to be positive (Table 3). There was a trend to negative association between connectivity and bladder volume, with no significant clusters, in precuneus at (-2, -46, 54) (Z = 3.53) and in medial prefrontal cortex at (4, 50, 12) (Z = 3.13). Interaction between age and volume showed no significant clusters and a trend only toward positive interaction in the L caudate body at (20, 22, 10) (Z = 3.74), a region not important for the results shown in Table 4. In contrast, there were widespread age-associated differences in connectivity which reached significance at set level (Figure 2 and Table 4). In particular there was significant positive association between the connectivity coefficient and age in medial frontal cortex, L PAG, and L insula. There was no evidence of significant decrease of the connectivity coefficient with age.
Table 3.
Principal regions with effective connectivity with RI and dACC, with special attention to medial prefrontal/genual ACC area. Bold = in significant cluster; italics = trend only. & = in same cluster as previous row.
| Region | Coordinates | Z-value | Cluster size | |
|---|---|---|---|---|
| Positive connectivity coefficient | R cuneus | 28, -68, 16 | 3.45 | 1546 |
| R putamen | 22, -6, 10 | 3.82 | 2280 | |
| L putamen | -28, -4, 8 | 3.28 | 3129 | |
| L insula | -44, 0, 10 | 3.61 | & | |
| R parahippocampal gyrus | 18, -46, -6 | 3.19 | 1546 | |
| pontine micturition center | 4, -34, -32 | 3.99 | 478 | |
| Negative connectivity coefficient | superior parietal lobule | 30, -52, 52 | 2.93 | 120 |
| genual ACC¶ | 2, 40, 0 | 2.29 | 16¶ | |
| medial prefrontal¶ | 6, 56, -10 | 2.07 | 16¶ |
threshold relaxed to P < 0.05 uncorrected
Table 4.
Principal regions where connectivity coefficient increased with age. The ensemble of clusters was significant at set level; bold = in significant cluster; italics = trend only.
| Region | Coordinates | Z-value | Cluster size |
|---|---|---|---|
| superior frontal cortex | -12, 48, 36 | 3.32 | 1204 |
| medial prefrontal cortex | -6, 60, 10 | 2.91 | 1204 |
| L thalamus | -22, -20, 16 | 3.71 | 175 |
| L insula | -42, 0, 12 | 3.35 | 442 |
| PAG | -6, -18, -18 | 3.33 | 134 |
| R lingual gyrus | 32, -62, -4 | 3.44 | 354 |
| L paracentral lobule | -16, -38, 44 | 3.10 | 599 |
Since the medial prefrontal cortex (mPFC) was expected to be particularly important for continence, relevant findings in that region were summarized in Figure 3 and contrasted with findings in non-frontal regions (see Table 5).
Figure 3.
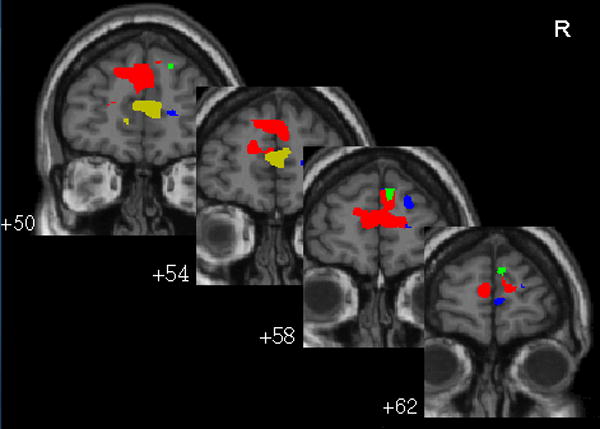
Regions of medial prefrontal cortex that show the following. Blue: negative response to bladder filling (deactivation); green: response negatively correlated with age. Red: connectivity coefficient positively correlated with age; yellow: connectivity coefficient negatively correlated with bladder volume. Negative connectivity coefficient is significant only at P < 0.05 uncorrected (Table 3) and therefore not seen in this display. Numbers show y-coordinate in mm.
Table 5.
Overview of sign of responses (activation or deactivation), sign of connectivity coefficient, and sign of correlation with age or volume, in seed regions RI and dACC and in non-frontal and medial frontal cortical regions. Based on Tables 1-4.
| RI / dACC | Other non-frontal regions | Medial frontal regions | |
|---|---|---|---|
| Response to infusion | ++ / + | ++ | − |
| Response vs volume | + | + | + |
| Response vs age | − − | − | − − |
| Connectivity coefficient | ++ | −¶ | |
| Connectivity vs volume | − | − | |
| Connectivity vs age | + | ++ |
++, − − significant at cluster level, P < 0.05 corrected
+, − trend to significance, P<0.01 uncorrected
trend to significance only at P<0.05 uncorrected
Discussion
Age has a significant influence on the working of the brain-bladder control network, even in women with normal bladder function. As previously reported (Griffiths et al., 2007), most non-frontal parts of the brain respond to bladder infusion with activation (Table 5), and there is extensive age-associated decrease in this activation, in particular in the right and left insula, which appears to be the neural correlate of the decreased bladder sensation observed in older subjects during bladder filling (Pfisterer et al., 2006).
Decreased response might reflect changes in the aging brain, especially since there are changes in cerebral connectivity also (see Table 5). Alternatively however it might be of peripheral origin, signifying reduced bladder afferent input. As explained in the Supplementary Material, such a reduction could lead automatically to changes in connectivity, without any fundamental change in brain function. Consistent with this suggestion, according to Table 5 the effects of increased age and decreased bladder volume are similar, perhaps because both reduce afferent input. Thus peripheral changes could be a contributing factor to the age-associated decrease in response.
Brain changes cannot be ruled out however, and an interesting question is whether the age-associated changes are a result of undetected brain pathology in the elderly. In the right insula (for example) the responses in all five older subjects (• 67 y) were weaker than those in the five younger subjects (• 46 y). Thus, if pathology was responsible it affected all five volunteers aged 67 y or older, suggesting either that it was very common (e.g. white matter hyperintensities) or that the changes were part of normal aging.
From the discussion in Supplementary Material, a significant PhPI connectivity coefficient indicates effective connection from the seed regions to the target. The sign of the coefficient most plausibly is positive if the target is activated and negative if it is deactivated. If these conditions are fulfilled then it is likely that there is connectivity with the seed regions. However, the connectivity coefficient is a measure of the strength of the interaction, not the strength of the connectivity; in particular a non-significant connectivity coefficient does not rule out connectivity from seed regions to target. The results shown Table 5 can be interpreted on this basis. In the seed regions RI and dACC, bladder infusion leads to activation which becomes weaker with age and stronger with increasing bladder volume. In other non-frontal regions (i.e. excluding seed regions and medial prefrontal cortex) there is similar activation and, as predicted, the coefficient of connectivity (interaction) with RI and dACC is positive. The connectivity coefficient tends to increase with age and to decrease with increasing bladder volume. Thus if the activation of RI or dACC diminishes (whether through increased age or diminished volume) the connectivity coefficient tends to become larger. This behavior may be accounted for by the automatic mechanism discussed in the Supplementary Material. It is tempting to view it as an example of geriatric compensation (the counterbalancing of an age-related deterioration of function in one part by greater activity or development in other parts). Such changes may predispose older people with normal bladder function to later loss of bladder control (urge incontinence).
The “other non-frontal regions” in Table 5 include some reported by others but not extensively discussed: putamen/globus pallidus (Matsuura et al., 2002; Seseke et al., 2006; Zhang et al., 2005) and parahippocampal gyrus (Di Gangi Herms et al., 2006; Matsuura et al., 2002). There is also a trend to effective connectivity from RI and dACC to the pontine micturition center (PMC). This suggests that the PMC – usually supposed to be activated only for voiding (Blok et al., 1997; Fowler et al., 2008) – receives signals from RI and dACC during bladder filling also. Presumably these signals are inhibitory, thus maintaining continence.
The evidence cited in the Introduction (Andrew et al., 1964)(Griffiths et al., 1994)(Griffiths et al., 2008a) suggests that a different area of the brain – the medial prefrontal cortex (mPFC) – is particularly important for continence. Age-associated changes in this area would therefore be expected to have a pronounced effect on bladder control. Suggestively, mPFC response to bladder infusion appears to have unique characteristics illustrated in Figure 3 and Table 5, including a trend to deactivation rather than activation. One interpretation would be that mPFC is part of a “default mode network”, active during resting conditions (Raichle et al., 2007). Deactivation would be a sign that default mode activity is suspended while the brain processes an event that requires attention, such as bladder filling. Possibly consistent with this conjecture, deactivation was observed in the left prefrontal lobe (although not the mPFC) when healthy young women directed their attention to bladder filling sensations (Kuhtz-Buschbeck et al., 2005).
If there is deactivation, the connectivity coefficient is expected to be negative. Table 5 indeed shows a trend to negative interaction in medial frontal regions; the existence of connectivity is confirmed by a significant association between connectivity coefficient and age. mPFC response to bladder infusion also depends on age (Table 5 and Figure 3). The association is negative, implying that mPFC deactivation becomes more prominent with increasing age. A natural interpretation is that mPFC deactivation is part of a mechanism that inhibits the voiding reflex, of which the elderly make more use because age-associated changes predispose to weakened bladder control. Such changes might include alterations in the function of the mPFC and its connecting pathways, or an overall decrease in brain activation initiated by weakened bladder afferents.
This study has outlined age-dependent patterns of activation and connectivity evoked by bladder infusion (filling) in continent female subjects. It represents a first step towards a functional model of the bladder control network. A limitation is that deactivation of mPFC, although apparently critical for continence, is not particularly strong among these continent subjects, so that significant results are difficult to achieve. Deactivation is more pronounced among urge-incontinent subjects (Griffiths et al., 2008b), whose behavior may yield further insight. Another limitation is that this is a cross-sectional, not a longitudinal study. Therefore the observed age-associated changes may not be representative of the aging process in any one individual, nor may this process necessarily tend toward urge incontinence.
To summarize: (1) in old age, the signals in the network that controls brain responses to bladder filling become generally weaker, perhaps predisposing to eventual loss of bladder control; (2) in mPFC, bladder-related responses and connectivity differ from those elsewhere in the brain, consistent with a special role for this region in maintaining continence; (3) deactivation of mPFC appears to be part of a mechanism, relied on especially by the elderly, to maintain inhibition of the voiding reflex; changes in its function or connecting pathways may be responsible for the development of urge incontinence.
Supplementary Material
Acknowledgments
Mary Alyce Riley BSN provided indispensable help with urodynamics and scanning sessions. We are grateful to Mary Jo Sychak and the staff of UPMC Magnetic Resonance Research Center for their dedication and enthusiasm and to the anonymous reviewers for their insightful and stimulating comments. This work was supported by US Public Health Service grants R03AG25166, R01AG020629, P01AG04390 and 5T32AG0218, and by University of Pittsburgh Competitive Medical Research Fund, John A. Hartford Center of Excellence in Geriatric Medicine, and Institute of Clinical Research and Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Andrew J, Nathan PW. Lesions of the anterior frontal lobes and disturbances of micturition and defaecation. Brain. 1964;87:233–262. doi: 10.1093/brain/87.2.233. [DOI] [PubMed] [Google Scholar]
- Blok BF, Holstege G. The central nervous system control of micturition in cats and humans. Behav Brain Res. 1998;92:119–125. doi: 10.1016/s0166-4328(97)00184-8. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Di Gangi Herms AMR, Veit R, Reisenauer C, Herms A, Grodd W, Enck P, Stenzl A, Birbaumer N. Functional imaging of stress urinary incontinence. NeuroImage. 2006;29:267–275. doi: 10.1016/j.neuroimage.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, De Groat WC. The neural control of micturition. Nature Reviews Neuroscience. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Fowler CJ. Neural control. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. 4th International Consultation on Incontinence. Paris: Health Sciences Publications; 2008a. in press. [Google Scholar]
- Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol Urodyn. 2008b;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths DJ, McCracken PN, Harrison GM, Moore KN. Urinary incontinence in the elderly: the brain factor. Scand J Urol Nephrol. 1994;157:83–88. [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- INRIAlign. Toolbox for fMRI realignment in SPM99. 2000 http://www-sop.inria.fr/epidaure/software/INRIAlign/
- Kuhtz-Buschbeck JP, Van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Juenemann KP. Cortical representation of the urge to void: A functional magnetic resonance imaging study. J Urol. 2005;174:1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Pfisterer M, Griffiths D, Schaefer W, Resnick N. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc. 2006;54:405–412. doi: 10.1111/j.1532-5415.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, R-H R, Seseke F, Dechent P. Voluntary pelvic floor muscle control - an fMRI study. Neuroimage. 2006;31:1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage. 2008;39:1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Department of Imaging Neuroscience. Statistical parametric mapping: SPM2. 2003 http://www.fil.ion.ucl.ac.uk/spm/spm2.html.
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, III, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Research: Neuroimaging. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24:174–180. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


