Abstract
Aims
The aim of this study was to assess whether perioperative N-acetylcysteine (NAC), an antioxidant, prevents acute renal injury (ARI) after cardiac surgery.
Methods and results
We performed a systematic review of randomized controlled trials (RCTs) of NAC in adult cardiac surgery patients. The RCTs were identified by searching MEDLINE (1960–2008), clinicaltrials.gov website, and hand-searching references of relevant publications. Primary outcome was ARI (absolute increase >0.5 mg/dL or relative increase >25%, in serum creatinine from baseline within 5 days after surgery). Random effects model was used to perform a meta-analysis. Forest plots and I2 test were used to assess heterogeneity among studies. Ten RCTs (n = 1163 patients) were included. Mean age was 70 ± 7.4 years, 71% were male, and 66% underwent coronary artery bypass surgery. N-Acetylcysteine did not reduce ARI incidence [35% NAC vs. 37% placebo; relative risk (RR) 0.91, 95% CI 0.79–1.06, P = 0.24]. Overall, 3.3% of patients required haemodialysis (NAC vs. placebo; RR = 1.13, 95% CI 0.59–2.17) and 3% died (RR = 1.10, 95% CI 0.56–2.16). There was a trend towards reduced ARI incidence among patients with baseline chronic kidney disease assigned to intravenous NAC (RR = 0.80, 95% CI 0.64–1.01, P = 0.06).
Conclusion
This meta-analysis of RCTs showed that prophylactic perioperative NAC in cardiac surgery does not reduce ARI, haemodialysis, or death.
Keywords: Cardiac surgery, Kidney, Antioxidants, Meta-analysis, Mortality
Introduction
Acute renal injury (ARI) is a common complication following cardiac surgery, associated with infections, prolonged hospitalization, and death.1–11 The mortality risk after cardiac surgery increases with even minor elevations in creatinine from baseline,4 exceeding 50% in the most severe cases where haemodialysis is required.11 The risk of post-operative ARI is particularly increased (∼30–40%), among patients with high-risk features such as advanced age, chronic kidney disease (CKD), diabetes mellitus, heart failure,8–10 and in those undergoing complex surgical procedures with prolonged cardiopulmonary bypass time. Temporal trends in cardiac surgery show that patients with co-morbidities and complex surgical procedures are increasing.12 Thus, ARI after cardiac surgery is an important health problem that is expected to increase in incidence.
Although there are isolated reports suggesting that perioperative administration of fenoldopam, clonidine, natriuretic peptides, sodium nitroprusside, or elective pre-operative haemodialysis may prevent ARI, none of these interventions has demonstrated clear efficacy13 and exploration of promising new strategies continues. The pathophysiology of ARI in the setting of cardiac surgery consists of numerous adverse factors including exogenous toxins, metabolic factors, ischaemia, inflammation, oxidative stress, and neurohormonal activation that occur during the perioperative period.1,14,15
N-Acetylcysteine (NAC), a thiol compound with antioxidant and vasodilatory properties, reduces oxygen free radical production,16 pump-related ischaemia–reperfusion injury,17 and proinflammatory cytokine levels.18 N-Acetylcysteine ameliorates kidney injury in rats undergoing cardiopulmonary bypass19,20 and appears to reduce the risk of contrast nephropathy in humans.21,22 Whether perioperative NAC administration reduces the risk of ARI after cardiac surgery remains unclear. The few studies addressing this question have been single-centre studies with relatively small sample size.23–32 Despite potential effectiveness and increasing interest, there have been no systematic reviews that comprehensively assess the potential efficacy and adverse effects of perioperative NAC administration in adults undergoing cardiac surgery. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to fill this knowledge gap.
Methods
Data sources and study search strategy
We identified RCTs published in MEDLINE from 1960 through January 2008 using the following keywords: [acetylcysteine and all subheadings or antioxidants or sulphhydril compounds or thiol derivatives] and [thoracic surgery and all subheadings or coronary artery bypass surgery or valve surgery]. We limited our search criteria to include studies published in English language and those involving humans. We identified additional studies by searching RCTs recorded at clinicaltrials.gov website and by hand-searching the references cited in relevant publications.
Study selection
We included RCTs of adults undergoing cardiac surgery, in which at least one of the treatment groups received NAC, administered orally or intravenously, immediately before, during, or immediately after cardiac surgery at any dose, for any length of time. We required that individual studies reported pre-operative (baseline) and post-operative (within 5 days after surgery) creatinine levels or the incidence of ARI after heart surgery in each treatment group. When data were missing or incomplete, we contacted the authors of the original studies to provide the necessary information.
Data extraction
Two reviewers (A.S.A. and A.K.N.) examined the titles, abstracts, and full-length articles identified by the described search strategy to determine whether the study met the inclusion and exclusion criteria. Both reviewers separately abstracted full-length articles that met the systematic review inclusion criteria using standardized data collection forms. Discrepancies between the two reviewers regarding study eligibility and data extraction were infrequent and resolved by discussion. Results were reviewed with all members of the study team. Abstracted information included study characteristics (design, enrolment criteria, comparison group, blinding, intention-to-treat analysis, methods of allocation concealment, and length of follow-up), patient characteristics (age, sex, baseline creatinine, hypertension, diabetes mellitus, peripheral arterial disease, New York Heart Association functional class, and body mass index), details of cardiac surgery (type of surgery, cardiopulmonary bypass time, ischaemic time, and proportion of re-do and urgent surgery), dosage and timing of NAC, adverse effects, dropouts, and relevant outcomes.
Outcomes
The primary outcome variable was the incidence of ARI, defined as an absolute increase of >0.5 mg/dL or a relative increase of >25%, in serum creatinine from the pre-operative value (baseline) to within 5 days following surgery. Secondary outcomes included maximum change in serum creatinine from baseline within 5 days following surgery, need for post-operative haemodialysis, all-cause mortality, and lengths of stay in the intensive care unit (ICU) and the hospital.
Methodological quality
Factors considered in methodological quality included concealment of treatment allocation, similarity of study groups at baseline, blinding of the patient, clinician, and outcome assessor, point estimates and measures of variability for the primary outcome variable, and application of intention-to-treat analysis.33 We also explored potential heterogeneity in estimates of treatment efficacy attributable to each quality criteria.
Assessment of heterogeneity
We used Forest plots to visualize the extent of heterogeneity among studies.22 We examined I2, a standard test for heterogeneity that measures the degree of inconsistencies across studies.22 I2 values, which range from 0% to 100%, describe the proportion of variation in treatment effect estimates that is due to true variation rather than sampling error.34 A value of 0% indicates no observed heterogeneity. Higgins et al.34 suggest describing I2 values of 25%, 50%, and 75% as low, moderate, and high, respectively. We obtained the group-specific and overall I2 as a standard output of the metan program. We limited publication bias by using a comprehensive electronic and hand-search strategy as well as contacting authors of identified studies.
Data synthesis and statistical analysis
We assessed outcomes for individual studies. When judged to be both clinically appropriate and statistically feasible, we used a random effects model to combine data. For continuous variables, weighted mean differences and 95% confidence interval (CI), and for categorical variables, weighted risk ratios and 95% CI were calculated. We performed a predetermined subgroup evaluation to assess if the subjects' risk profile or the route of administration of NAC influenced the primary outcome variable. Studies in which enrolment was predicated upon risk factors for the development of ARI, such as baseline CKD, age ≥70 years, diabetes mellitus, left ventricular ejection fraction <35%, New York Heart Association functional class III/IV, and valve, re-do, or urgent surgery, were considered to have high-risk subjects. All comparisons were two-sided and a P-value <0.05 was considered statistically significant. Review Manager (version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003) was used in all analyses.
Results
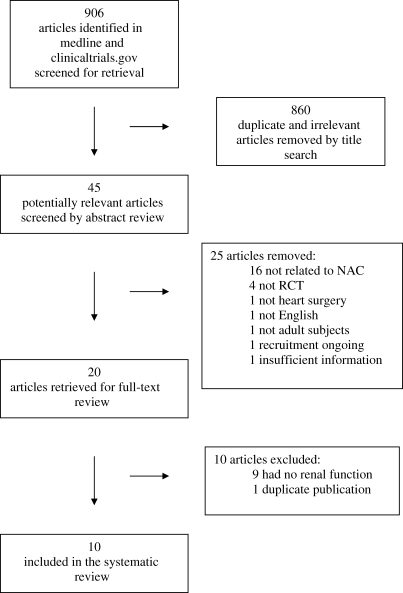
Our initial search yielded 906 citations (Figure 1). We excluded 860 of these by title search due to irrelevant content or duplicate publications. The abstracts of the remaining 45 articles were reviewed and an additional 25 were excluded because the intervention was not NAC (n = 16), study was not an RCT (n = 4), procedure was not cardiac surgery (n = 1), study was not reported in English (n = 1), study subjects were neonates (n = 1), study recruitment was continuing (n = 1), and insufficient information in a study that was only published as an abstract (n = 1). After full-text review, 10 of the remaining 20 articles were excluded because 9 did not report renal function and 1 was a duplicate publication. The remaining 10 articles were included in the systematic review (Figure 1).23–32
Figure 1.
Selection of the studies included in the systematic review.
Study and patient characteristics
The characteristics of the 10 studies that met eligibility criteria are displayed in Table 1. All studies were published between 2005 and 2008. Three were performed in Canada,25,26,32 two in the United States,23,24 and the others in Australia,28 Finland,30 Germany,27 Italy,31 and Turkey.29 Placebo control was used in all but one study, where the control group consisted of patients under usual care.29 One study had a 2 × 2 factorial design, in which participants were randomized to NAC, fenoldopam, both, or neither.24 Only the NAC and placebo arms of this study were considered in the meta-analysis. In 6 of the 10 studies, participants were considered to have a high-risk profile for developing ARI,23–25,28,31,32 although the criteria for high risk varied across studies. In four studies, baseline CKD was a compulsory inclusion criterion.23,24,31,32 In eight studies, NAC was administered intravenously,25–32 although there was considerable variation in the dose, duration, and route of administration. Three studies involved patients undergoing coronary artery bypass graft (CABG) surgery alone,26,27,29 where the other seven included CABG or valve surgery (Table 1). Two studies reported follow-up creatinine at 14 and 30 days after surgery.23,24 The mean duration of follow-up for creatinine was 8.2 days and ranged from 3 to 30 days. Study quality varied, with eight studies23–25,27,28,30–32 reporting adequate allocation concealment and five clearly describing an intention-to-treat analysis.23–25,28,32 Withdrawals were reported in five studies23,28,30–32 and adverse events in three.23,25,32
Table 1.
Description of randomized clinical trials of perioperative N-acetylcysteine after cardiac surgery
| Author | Participants | Surgery | Intervention | Study quality |
|---|---|---|---|---|
| Adabag et al.23 | NAC: 50; placebo: 52; total: 102 | CABG only: 66 (65%) | 600 mg p.o. b.i.d. × 14 | Allocation concealment: adequate |
| High-risk patients with GFR <60 mL/min/1.73 m2 | CABG + valve: 17 (17%) | Intention-to-treat: yes | ||
| Valve only: 19 (19%) | Dropouts reported: yes | |||
| Adverse effects reported: yes | ||||
| Barr and Kolodner24 | NAC: 20; placebo: 19; total: 79 | CABG only: 33 (85%) | 600 mg p.o. b.i.d. × 4 | Allocation concealment: adequate |
| High-risk patients with GFR ≤40 mL/min | Valve only: 6 (15%) | Intention-to-treat: yes | ||
| Dropouts reported: no | ||||
| Adverse effects reported: no | ||||
| Burns et al.25 | NAC: 148; placebo: 147; total: 295 | CABG only: 250 (88%) | 600 mg i.v. b.i.d. × 4 | Allocation concealment: adequate |
| High-risk patients with ≥1 criteria below: creatinine >1.4 mg/dL, age ≥70 years, diabetes mellitus, EF <35%, complex surgery or re-do | CABG + valve: 42 (14%) | Intention-to-treat: yes | ||
| Valve only: 2 (1%) | Dropouts reported: no | |||
| Adverse effects reported: yes | ||||
| El-Hamamsy et al.26 | NAC: 50; placebo: 50; total: 100 | CABG only: 100 (100%) | 600 mg p.o. × 1 | Allocation concealment: unclear |
| Low-risk patients CABG or CPB | 150 mg/kg i.v. × 1 | Intention-to-treat: unclear | ||
| 12.5 mg/kg/h i.v. × 24 h | Dropouts reported: no | |||
| Adverse effects reported: no | ||||
| Fischer et al.27 | NAC: 20; placebo:20; total: 40 | CABG only: 40 (100%) | 100 mg/kg i.v. × 1 | Allocation concealment: adequate |
| Low-risk patients with CABG or CPB | 20 mg/kg/h × 75 min | Intention-to-treat: unclear | ||
| Dropouts reported: no | ||||
| Adverse effects reported: no | ||||
| Haase et al.28 | NAC: 31; placebo: 30; total: 61 | CABG only: 18 (30%) | 150 mg/kg i.v. × 1 | Allocation concealment: adequate |
| High-risk patients with ≥1 criteria below: creatinine >1.36 mg/dL, age >70 years, NYHA class III/IV or EF <50%, diabetes mellitus, valve or complex or re-do surgery | CABG + valve: 19 (31%) | 50 mg/kg i.v. × 1 | Intention-to-treat: yes | |
| Valve only: 24 (39%) | 100 mg/kg × 1 | Dropouts reported: yes | ||
| Adverse effects reported: no | ||||
| Orhan et al.29 | NAC: 10; usual care: 10; total: 20 | CABG only: 20 (100%) | 50 mg/kg i.v. × 1 | Allocation concealment: unclear |
| Low-risk patients with elective CABG with CPB | Intention-to-treat: unclear | |||
| Dropouts reported: no | ||||
| Adverse effects reported: no | ||||
| Ristikankare et al.30 | NAC: 40; placebo: 40; total: 80 | CABG only: 38 (49%) | 150 mg/kg × 1 i.v. | Allocation concealment: adequate |
| Low-risk patients with creatinine >1.14 mg/dL and cardiac surgery CPB | CABG + valve: 25 (32%) | 50 mg/kg × 1 i.v. | Intention-to-treat: no | |
| Valve only: 14 (18%) | 100 mg/kg × 1 i.v. | Dropouts reported: yes | ||
| Adverse effects reported: no | ||||
| Sisillo et al.31 | NAC: 129; placebo: 125; total: 256 | CABG only: 105 (41%) | 1200 mg × 4 i.v. | Allocation concealment: adequate |
| High-risk patients with GFR <60 mL/min | CABG + valve: 32 (13%) | Intention-to-treat: unclear | ||
| Valve only: 117 (46%) | Dropouts reported: yes | |||
| Adverse effects reported: no | ||||
| Wijeysundera et al.32 | NAC: 88; placebo: 87; total: 175 | CABG only: 93 (53%) | 100 mg/kg × 1 i.v. | Allocation concealment: adequate |
| High-risk patients with GFR <60 mL/min with elective CABG with CPB | CABG + valve: 48 (27%) | 20 mg/kg/h i.v. | Intention-to-treat: yes | |
| Valve only: 34 (19%) | Dropouts reported: yes | |||
| Adverse effects reported: yes |
GFR, glomeruler filtration rate; NAC, N-acetylcysteine; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; EF, ejection fraction.
The 10 RCTs included a total of 1163 patients (range 20–295), randomly assigned to NAC (n = 584) vs. control (n = 579) groups. The pooled baseline clinical and surgical characteristics of the study patients are displayed in Table 2. Patients were 70 ± 7.4 years in age and 71% were male. Co-morbid conditions such as diabetes mellitus, heart failure, and prior myocardial infarction were common. Overall, 66% of the patients underwent CABG alone or combined CABG and valve surgery (Table 2). The baseline characteristics were well-balanced in each individual trial (data not shown).
Table 2.
Baseline clinical and surgical characteristics (pooled) of the study populations
| Variable | Weighted mean or frequency among study participants | No. of studies reporting the variable | References of the studies reporting the variable |
|---|---|---|---|
| Clinical characteristics | |||
| Age (mean + SD)a | 70 ± 7.4 | 10 | 23–32 |
| Male, n (%) | 831/1163 (71%) | 10 | 23–32 |
| Baseline creatinine, mg/dL (mean + SD) | 1.29 ± 0.4 | 9 | 23,25–32 |
| BMI, kg/m2 (mean + SD) | 29.4 ± 3.8 | 5 | 23,26,28–30 |
| NYHA class III/IV, n (%) | 186/789 (24%) | 7 | 23,26,28–32 |
| Diabetes mellitus, n (%) | 402/1163 (34%) | 10 | 23–32 |
| LV dysfunction (EF <50%), n (%) | 307/1026 (30%) | 7 | 23–26,28,31,32 |
| Hypertension, n (%) | 832/1104 (75%) | 8 | 23,25–28,30–32 |
| Peripheral arterial disease, n (%) | 152/749 (20%) | 6 | 23–25,28,30,32 |
| Prior myocardial infarction, n (%) | 172/618 (28%) | 6 | 23,25–29 |
| Surgical characteristics | |||
| CABG only, n (%) | 763/1163 (66%) | 10 | 23–32 |
| CABG and valve, n (%) | 183/1124 (16%) | 9 | 23,25–32 |
| Valve only, n (%) | 210/1124 (18%) | 9 | 23,25–32 |
| CPB time, min (mean + SD) | 105 ± 38 | 9 | 23,25–32 |
| Ischaemic time, min (mean + SD) | 70 ± 26 | 9 | 23–30,32 |
| Off-pump surgery, n (%) | 50/1163 (4%) | 10 | 23–32 |
| Prior cardiac surgery, n (%) | 55/1046 (5%) | 8 | 23–26,28,29,31,32 |
| Urgent/emergent surgery, n (%) | 4/907 (2%) | 6 | 23,25,28,29,31,32 |
BMI, body mass index; NYHA, New York Heart Association; LV, left ventricle; CABG, coronary artery bypass graft surgery; CPB, cardiopulmonary bypass; EF, ejection fraction.
aWeighted mean + SD.
Incidence of outcomes and efficacy of N-acetylcysteine
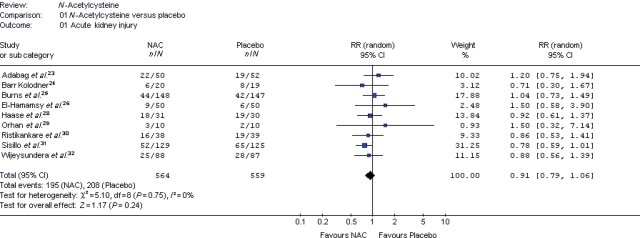
An overview of the kidney-related outcomes reported in each clinical trial is presented in Table 3. Compared with placebo, NAC did not provide a statistically significant reduction in any of the assessed outcomes. There was no difference in the incidence of ARI [35% NAC vs. 37% placebo; relative risk (RR) 0.91, 95% CI 0.79–1.06, P = 0.24] (Figure 2) or maximum change in serum creatinine from baseline (0.32 mg/dL ± 0.51 vs. 0.32 mg/dL ± 0.47; P = 0.95) (Table 4). The individual studies were inadequately powered to assess mortality and haemodialysis, but in the pooled analysis, these outcomes were not different between NAC and placebo groups (Table 4). Overall, 3.3% of patients required haemodialysis (NAC vs. placebo; RR 1.13, 95% CI 0.59–2.17) and 3% of patients died (RR 1.10, 95% CI 0.56–2.16). N-Acetylcysteine did not statistically reduce the length of ICU or hospital stay, although the lengths of stay varied widely between studies (Table 4).
Table 3.
Outcomes reported (X) in randomized clinical trials of perioperative N-acetylcysteine after cardiac surgery
| Author | Acute kidney injury | Maximum change in creatinine | Peak post-operative creatinine | Long-term kidney function | Haemodialysis | Deatha | Length of stay (ICU) | Length of stay (hospital) |
|---|---|---|---|---|---|---|---|---|
| Adabag et al.23 | Xb | Xb | Xb | Xc | X | X | X | X |
| Barr, 200824 | Xd | Xe | X | X | X | X | ||
| Burns, 200525 | Xb | X | X | X | X | |||
| El-Hamamsy et al.26 | Xb | Xb | Xb | X | X | X | X | |
| Fischer et al.27 | Xb | Xb | X | X | ||||
| Haase et al.28 | Xb | Xb | Xb | X | X | X | X | |
| Orhan et al.29 | Xb | Xb | Xb | X | X | X | X | |
| Ristikankare et al.30 | Xb | Xb | Xb | X | X | X | ||
| Sisillo et al.31 | Xd | Xd | Xd | X | X | X | ||
| Wijeysundera et al.32 | Xd | Xd | Xd | X | X | X | X |
ICU, intensive care unit.
aIn-hospital or 30-day.
bBy post-operative Day #5.
cPost-operative Day #30.
dBy post-operative Day #3.
ePost-operative Day #14.
Figure 2.
Forest plot describing the relative risk of acute kidney injury among cardiac surgery patients randomized to NAC vs. placebo.
Table 4.
Pooled relative risk or weighted mean difference of the outcome variables in patients randomized to NAC vs. placebo/usual care
| NAC | Control | Relative risk (95% confidence limits) | Weighted mean difference (95% confidence limits) | P-value | |
|---|---|---|---|---|---|
| Acute kidney injury, n (%)23–26,28–32 | 195/564 (35%) | 208/559 (37%) | 0.91 (0.79–1.06) | 0.24 | |
| Operative mortality, n (%)23–32 | 19/584 (3.3%) | 16/579 (2.8%) | 1.10 (0.56–2.16) | 0.78 | |
| Haemodialysis, n (%)23–32 | 20/584 (3.4%) | 18/579 (3.1%) | 1.13 (0.59–2.17) | 0.71 | |
| Maximum change in creatininea (mg/dL)23,26–32 | 0.32 ± 0.51 | 0.32 ± 0.47 | 0.00 (−0.11–0.10) | 0.95 | |
| ICU length of staya (days)23,24,26,28–32 | 3.6 ± 2.5 | 3.5 ± 2.9 | 0.07 (−0.92–1.06) | 0.89 | |
| Hospital length of staya (days)23,24,26,28,29,32 | 8.2 ± 2.1 | 8.2 ± 3.4 | 0.04 (−1.00–1.07) | 0.95 |
NAC, N-acetylcysteine; ICU, intensive care unit.
aMean ± SD.
In subgroup analysis, the patient risk profile and the NAC route of administration were not associated with ARI. However, there was a trend towards reduced ARI incidence among patients with baseline CKD randomized to NAC (RR 0.86, 95% CI 0.70–1.05, P = 0.14), particularly if NAC preparations were administered intravenously (RR 0.80, 95% CI 0.64–1.01, P = 0.06).
Post-hospitalization renal function
In two RCTs, kidney function was evaluated 14 and 30 days after cardiac surgery.23,24 In both studies, the trends in kidney function were similar in the NAC and control groups at 14 and 30 days after surgery. Although kidney function improved in both groups, it did not return to baseline in many patients. Mean creatinine clearance was ∼5 mL/min lower than baseline 14 days after surgery24 and 14% of the patients still had ARI 30 days after cardiac surgery (compared with 40% within the first five post-operative days).23
Adverse events
Adverse events were reported in only 3 of the 10 studies.23,25,32 Among 436 patients, nausea and vomiting occurred in 26 patients (9 NAC vs. 17 placebo), and hypotension and diarrhoea were reported in 1 patient each. There was no difference in the need for intravenous inotrope or pressor medications.24
Assessment of within-group heterogeneity
We found no evidence of statistical heterogeneity in the effects of NAC on ARI (I2 = 0; P = 0.75), haemodialysis (I2 = 0; P = 0.58), or death (I2 = 0; P = 0.94) among pooled studies suggesting that results were consistent across studies. On the other hand, we found moderate to high level of heterogeneity on the effects of NAC on maximum change in creatinine (I2 = 50%; P = 0.05), ICU length of stay (I2 = 94%; P < 0.00001), and total hospital length of stay (I2 = 55%; P = 0.05).
Discussion
In this systematic review of 10 RCTs involving more than 1100 patients who underwent cardiac surgery, we found that prophylactic administration of NAC in the perioperative period did not reduce any of the preplanned clinical outcomes including the incidence of ARI, haemodialysis, death, or length of stay in the ICU and in the hospital. In an exploratory subgroup analysis, there was a trend towards reduced ARI among patients with baseline CKD who were administered intravenous NAC. We also found that NAC was, in general, well tolerated and not associated with adverse events in the few studies that reported this information.
Post-operative ARI is a common event following cardiac surgery.1–11 When defined as a 50% increase in serum creatinine, ARI complicates between 7% and 39% of all operative procedures.35–38 Small increases in serum creatinine following cardiac surgery have been associated with increased duration of mechanical ventilation,39 length and cost of hospitalization, and the risk of short-term mortality.4,40,41 Recently, a number of studies have evaluated the long-term complications of small changes in serum creatinine during the hospitalization.42,43 Increases in serum creatinine following myocardial infarction are associated with an increased long-term risk of end-stage kidney disease42 and mortality.43 While it currently remains unclear whether ARI itself is pathological or simply a marker of poor outcomes, current epidemiological data suggest that preventing increases in serum creatinine following cardiac surgery may reduce adverse health outcomes. N-Acetylcysteine has been demonstrated to ameliorate ARI in rats following cardiac surgery19,20 and appears to decrease the rise in creatinine associated with contrast administration.21,22 However, the results from the present systematic review do not suggest that prophylactic NAC administration prior to cardiac surgery reduces either the incidence of ARI or the maximal increase in serum creatinine following surgery.
Although there is no consensus in the definition of post-cardiac surgery ARI in the medical literature, an absolute increase in serum creatinine >0.5 mg/dL or a relative increase >25% from baseline after cardiac surgery has been widely used in previous studies. The risk of complications and death related to kidney injury are highest among patients who require haemodialysis following cardiac surgery.12 Haemodialysis and death are also hard endpoints that a recent consensus statement has encouraged investigators to use as outcome variables in studies of ARI after cardiac surgery.13 However, these are also relatively rare outcomes. In our investigation, these outcomes occurred in 3% of the study participants, many of whom had high-risk features at baseline. N-Acetylcysteine did not alter the risk of either haemodialysis or death compared with placebo. Given that our observed incidence for haemodialysis and death is similar to previous cohort studies44–46 of cardiac surgery patients, our results are likely generalizable to larger populations. Furthermore, a recent meta-analysis of RCTs of NAC administered perioperatively to patients undergoing cardiac or abdominal surgeries has also reported no difference in mortality, haemodialysis, ARI, and ICU length of stay, supporting our results.47
Six of the 10 RCTs in this systematic review included only those patients perceived to be at high risk for developing ARI after surgery.23–25,28,31,32 However, baseline CKD, a very important predictor of post-operative ARI, was a compulsory entry criterion in only four studies.23,24,31,32 In the other two studies, patients with serum creatinine >1.4 mg/dL comprised fewer than 25% of their study population and led to a lower incidence of haemodialysis.25,28 In subgroup analyses, a trend towards reduced ARI with NAC was observed among patients with baseline CKD, particularly if NAC was administered intravenously. These observations suggest that it may be more prudent to make baseline CKD a compulsory inclusion criterion in future studies evaluating interventions to reduce renal injury after heart surgery.
The strengths of this systematic review include the methodology used in identifying and analysing the individual studies and its ability in garnering a sufficiently large study population in this area dominated by relatively small studies. The limitations, on the other hand, include the following. Acute renal injury has been used as a surrogate measure to assess intervention efficacy. However, this is less desirable than hard clinical outcomes such as haemodialysis or death. Although, there was not a trend towards benefit (or harm) with NAC in haemodialysis or death, none of the individual studies nor our pooled analysis of these trials was of sufficient size and/or duration to detect (or exclude) a statistically or clinically significant difference in these relatively rare, but important outcomes. Also, in the individual studies, the diagnosis of ARI was primarily based on an increase in serum creatinine, which may not accurately reflect true changes in glomeruler filtration rate (GFR).48 Further, it has recently been suggested that NAC may decrease serum creatinine concentration without affecting renal function.49 This observation, although subsequently contradicted by data demonstrating that NAC improves both GFR and creatinine concentration, suggests that newer urinary biomarkers such as cystatin C or NGAL may be more sensitive to identify kidney damage.50,51 However, at present, serum creatinine is the cheapest and most broadly accepted marker of kidney function.52 Also, the regimen of NAC administration varied between studies. We performed subgroup analyses to account for the differences in route of NAC administration (e.g. oral vs. intravenous). Finally, although we did not test for publication bias, which is difficult to assess with a small number of studies, we took steps to minimize it by including broad search and inclusion criteria, hand-searching of references, and contacting the authors of the primary studies.53
In conclusion, randomized trial evidence demonstrates that prophylactic administration of NAC in the perioperative period to patients undergoing cardiac surgery does not reduce the incidence of ARI, haemodialysis, death, or lengths of stay. Future trials are needed to determine if intravenous NAC improves these clinical outcomes in patients with baseline CKD. Until then, the use of NAC in patients undergoing cardiac surgery is not supported by evidence.
Funding
This work was supported by the Department of Veterans Affairs Health Services Research and Development Program, USA, and Minneapolis/VISN-23 Center for Chronic Diseases Outcomes Research (CCDOR), USA. Dr Adabag is supported in part by Veterans Administration Clinical Science Research and Development Service (Grant no. 04S-CRCOE 001). Part of Dr Wilt's effort was supported by NIDDK RO1 Grant no. DK063300-01A2.
Conflict of interest: none declared.
Acknowledgements
We are indebted to Mr Roderick McDonald and Indy Rutks for their assistance in the preparation of the manuscript. We also thank the investigators of the original studies for generously sharing their data with us.
References
- 1.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 2.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 3.Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 4.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 5.Antunes PE, Prieto D, Ferrão de Oliveira J, Antunes MJ. Renal dysfunction after myocardial revascularization. Eur J Cardiothorac Surg. 2004;25:597–604. doi: 10.1016/j.ejcts.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 7.Ronco C, Kellum JA, Bellomo R. Cardiac surgery-associated acute kidney injury. Int J Artif Organs. 2008;31:156–157. doi: 10.1177/039139880803100208. [DOI] [PubMed] [Google Scholar]
- 8.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Zanardo G, Michielon P, Paccagnella A, Rosi P, Caló M, Salandin V, Da Ros A, Michieletto F, Simini G. Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg. 1994;107:1489–1495. [PubMed] [Google Scholar]
- 10.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;10:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 11.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson TB, Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL STS National Database Committee. Decade of change—risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Ann Thorac Surg. 2002;73:480–489. doi: 10.1016/s0003-4975(01)03339-2. [DOI] [PubMed] [Google Scholar]
- 13.Schetz M, Bove T, Morelli A, Mankad S, Ronco C, Kellum JA. Prevention of cardiac surgery-associated acute kidney injury. Int J Artif Organs. 2008;31:179–189. doi: 10.1177/039139880803100211. [DOI] [PubMed] [Google Scholar]
- 14.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997;29:465–477. doi: 10.1016/s0272-6386(97)90212-2. [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Auriemma S, Fabbri A, D'Onofrio A, Katz N, McCullough PA, Ricci Z, Shaw A, Ronco C. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–178. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 16.Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, Suedkamp M, Kasper SM, Hellmich M, Mehlhorn U. N-Acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513–1520. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 17.Sucu N, Cinel I, Unlu A, Aytacoglu B, Tamer L, Kocak Z, Karaca K, Gul A, Dikmengil M, Atik U, Oral U. N-Acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg Today. 2004;34:237–242. doi: 10.1007/s00595-003-2699-8. [DOI] [PubMed] [Google Scholar]
- 18.Bakker J, Zhang H, Depierreux M, van Asbeck S, Vincent JL. Effects of N-acetylcysteine in endotoxic shock. J Crit Care. 1994;9:236–243. doi: 10.1016/0883-9441(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Yin R, Shao H, Dong G, Luo L, Jing H. N-Acetylcysteine to ameliorate acute renal injury in a rat cardiopulmonary bypass model. J Thorac Cardiovasc Surg. 2007;133:696–703. doi: 10.1016/j.jtcvs.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 20.DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-Acetylcysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:F292–F298. doi: 10.1152/ajprenal.1997.272.3.F292. [DOI] [PubMed] [Google Scholar]
- 21.Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy. Arch Intern Med. 2006;166:161–166. doi: 10.1001/archinte.166.2.161. [DOI] [PubMed] [Google Scholar]
- 22.Kelly A, Dwamena B, Cronin P, Bernstein S, Carlos R. Meta analysis: effectiveness of drugs for preventing contrast-induced neuropathy. Ann Intern Med. 2008;148:284–294. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 23.Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, McFalls EO, Bloomfield HE, Chandrashekhar Y. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized control trial. Am Heart J. 2008;155:1143–1149. doi: 10.1016/j.ahj.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Barr LF, Kolodner K. N-Acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit Care Med. 2008;36:1427–1435. doi: 10.1097/CCM.0b013e31816f48ba. [DOI] [PubMed] [Google Scholar]
- 25.Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, Stitt LW, Heidenheim AP, Myers ML, Moist L. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing CABG surgery: a randomized controlled trial. JAMA. 2005;294:342–350. doi: 10.1001/jama.294.3.342. [DOI] [PubMed] [Google Scholar]
- 26.El-Hamamsy I, Stevens LM, Carrier M, Pellerin M, Bouchard D, Demers P, Cartier R, Page P, Perrault LP. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2007;133:7–12. doi: 10.1016/j.jtcvs.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 27.Fischer UM, Tossios P, Mehlhorn U. Renal protection by radical scavenging in cardiac surgery patients. Curr Med Res Opin. 2005;21:1161–1164. doi: 10.1185/030079905X53289. [DOI] [PubMed] [Google Scholar]
- 28.Haase M, Haase-Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, Matalanis G, Buxton B, Doolan L, Bellomo R. Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med. 2007;35:1324–1331. doi: 10.1097/01.CCM.0000261887.69976.12. [DOI] [PubMed] [Google Scholar]
- 29.Orhan G, Yapici N, Yuksel M, Sargin M, Senay S, Yalçin AS, Aykaç Z, Aka SA. Effects of N-acetylcysteine on myocardial ischemia-reperfusion injury in bypass surgery. Heart Vessels. 2006;21:42–47. doi: 10.1007/s00380-005-0873-1. [DOI] [PubMed] [Google Scholar]
- 30.Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta-Ylinen R, Salmenperä M, Pöyhiä R. Lack of renoprotective effect of i. v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. 2006;97:611–616. doi: 10.1093/bja/ael224. [DOI] [PubMed] [Google Scholar]
- 31.Sisillo E, Ceriani R, Bortone F, Juliano G, Salvi L, Veglia F, Fiorentini C, Marenzi G. N-Acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med. 2008;36:338–340. doi: 10.1097/01.CCM.0000295305.22281.1D. [DOI] [PubMed] [Google Scholar]
- 32.Wijeysundera D, Beattie W, Rao V, Granton J, Chan C. N-Acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anaesth. 2007;54:872–881. doi: 10.1007/BF03026790. [DOI] [PubMed] [Google Scholar]
- 33.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers N, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi concensus. J Clin Epidemiol. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abel RM, Buckley MJ, Austen WG, Barnett GO, Beck CH, Jr, Fischer JE. Etiology, incidence, and prognosis of renal failure following cardiac operations. Results of a prospective analysis of 500 consecutive patients. J Thorac Cardiovasc Surg. 1976;71:323–333. [PubMed] [Google Scholar]
- 36.O'Hare AM, Feinglass J, Sidawy AN, Bacchetti P, Rodriguez RA, Daley J, Khuri S, Henderson WG, Johansen KL. Impact of renal insufficiency on short-term morbidity and mortality after lower extremity revascularization: data from the Department of Veterans Affairs’ National Surgical Quality Improvement Program. J Am Soc Nephrol. 2003;14:1287–1295. doi: 10.1097/01.asn.0000061776.60146.02. [DOI] [PubMed] [Google Scholar]
- 37.Ryckwaert F, Alric P, Picot MC, Djoufelkit K, Colson P. Incidence and circumstances of serum creatinine increase after abdominal aortic surgery. Intensive Care Med. 2003;29:1821–1824. doi: 10.1007/s00134-003-1958-x. [DOI] [PubMed] [Google Scholar]
- 38.Ryckwaert F, Boccara G, Frappier JM, Colson PH. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med. 2002;30:1495–1498. doi: 10.1097/00003246-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Vieira JM, Jr, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore L, Jr, Imanishe MH, Abdulkader RC, Deheinzelin D. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35:184–191. doi: 10.1097/01.CCM.0000249828.81705.65. [DOI] [PubMed] [Google Scholar]
- 40.Brown JR, Cochran RP, Dacey LJ, Ross CS, Kunzelman KS, Dunton RF, Braxton JH, Charlesworth DC, Clough RA, Helm RE, Leavitt BJ, Mackenzie TA, O'Connor GT Northern New England Cardiovascular Disease Study Group. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413. doi: 10.1161/CIRCULATIONAHA.105.000596. [DOI] [PubMed] [Google Scholar]
- 41.Falvo A, Horst HM, Rubinfeld I, Blyden D, Brandt MM, Jordan J, Faber MD, Silverman N. Acute renal failure in cardiothoracic surgery patients: what is the best definition of this common and potent predictor of increased morbidity and mortality. Am J Surg. 2008;196:379–383. doi: 10.1016/j.amjsurg.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 42.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 43.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987–995. doi: 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 44.Swaminathan M, Shaw AD, Phillips-Bute BG, McGugan-Clark PL, Archer LE, Talbert S, Milano CA, Patel UD, Stafford-Smith M. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med. 2007;35:2286–2291. doi: 10.1097/01.ccm.0000282079.05994.57. [DOI] [PubMed] [Google Scholar]
- 45.Fortescue EB, Bates DW, Chertow GM. Predicting acute renal failure after coronary bypass surgery: cross-validation of two risk-stratification algorithms. Kidney Int. 2000;57:2594–2602. doi: 10.1046/j.1523-1755.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 46.Geraci JM, Johnson ML, Gordon HS, Petersen NJ, Shroyer AL, Grover FL, Wray NP. Mortality after cardiac bypass surgery: prediction from administrative versus clinical data. Med Care. 2005;43:149–158. doi: 10.1097/00005650-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Ho KM, Morgan DJR. Meta-analysis of N-acetylcysteine to prevent acute renal failure after major surgery. Am J Kidney Dis. 2009;53:33–40. doi: 10.1053/j.ajkd.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Schultz MJ. N-Acetylcysteine as a preventive measure for acute renal failure: a plea for more accurate detection of renal function in critically ill patients. Crit Care Med. 2007;35:1633–1634. doi: 10.1097/01.CCM.0000266798.38920.BA. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–410. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- 50.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 51.Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. Curr Opin Crit Care. 2007;13:638–644. doi: 10.1097/MCC.0b013e3282f07570. [DOI] [PubMed] [Google Scholar]
- 52.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Br Med J. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]