Abstract
Aims
Current guidelines recommend stopping oral anticoagulation (OAC) and starting heparin infusion before implanting/replacing a pacemaker/implantable cardioverter-defibrillator (ICD) in patients with high risk for thrombo-embolic events. The aim of this study was to demonstrate that the maintenance of OAC during device implantation/replacement is as safe as bridging to intravenous heparin and shortens in-hospital stay.
Methods and results
A cohort of 101 consecutive patients with high risk for embolic events and indication for implant/replacement of a pacemaker/ICD were randomized to two anticoagulant strategies: bridging from OAC to heparin infusion (n = 51) vs. maintenance of OAC to reach an INR = 2 ± 0.3 at the day of the procedure (n = 50). Haemorrhagic and thrombo-embolic complications were evaluated at discharge, 15 and 45 days after the procedure. A total of 4/51 patients (7.8%) from heparin group and 4/50 (8.0%) from the OAC group developed pocket haematoma following the implant (P = 1.00). One haematoma in each group required evacuation (1.9 vs. 2%, P = 1.00). No other haemorrhagic events or embolic complications developed during the follow-up. Duration of the hospital stay was longer in the heparin group [median of 5 (4–7) vs. 2 (1–4) days; P < 0.001].
Conclusion
Implant of devices maintaining OAC is as safe as bridging to heparin infusion and allows a significant reduction of in-hospital stay.
Keywords: Pacemaker, Mechanical prosthetic valve, Atrial fibrillation, Pocket haematoma, Thrombo-embolism
Introduction
Oral anticoagulation (OAC) is commonly used for the prevention of thrombo-embolic events. Peri-operative management of patients with high risk of thrombo-embolic events is a relevant and common clinical problem. In these patients, interrupting anticoagulation might promote embolic events such as cerebral stroke or prosthetic thrombosis. Conversely, peri-operative maintenance of OAC may increase the risk of post-operative bleeding. Kearon and Hirsh1 suggested the discontinuation of OAC and the initiation of intravenous (i.v.) heparin or low-molecular-weight heparin as a ‘bridging’ therapy. Other studies have shown little or no increased risk of bleeding with the maintenance of OAC in low-risk procedures such as dental work, arthrocentesis, cataract surgery, upper endoscopy, or colonoscopy.2 Larson et al.3 reported that a moderate-intensity anticoagulant therapy with OAC targeting a goal INR 1.5–2.0 appears to be a safe and feasible method for preventing thrombo-embolic complications in these patients.
The use of i.v. heparin that current guidelines recommend in these patients4 has been associated with an increased risk of haematoma,5,6 and the maintenance of OAC did not increase the rate of pocket haematoma in two previous small series.7,8 The aim of this study was to demonstrate that the maintenance of OAC during device implantation or replacement is as safe as bridging to i.v. heparin in patients with high risk of thrombo-embolic events. The primary comparison was the incidence of pocket haematomas and/or thrombo-embolic events during a 45-day period following the procedure. The secondary endpoint was to compare the duration of the hospital stay between both groups.
Methods
This is a prospective and randomized single-centre study approved by the Institutional Human Research Committee.
Inclusion criteria
Patients on chronic OAC who required implantation/replacement of a pacemaker or an implantable cardiac defibrillator (ICD) were included in the study if they fulfilled any of the following conditions that imply a high risk for embolic events:9 (i) mechanical prosthetic valve; (ii) atrial fibrillation (AF) with previous stroke, transient ischaemic attack (TIA), systemic embolic event, mitral stenosis, or prosthetic heart valve; or (iii) AF with at least three criteria of intermediate risk of embolic events: hypertension, diabetes, and left ventricular ejection fraction <35% or age >75 years; (iv) intracavitary thrombi protruding into the cardiac chambers; and (v) recent deep venous thrombosis (<3 months). Patients were randomized in two groups: the first switched from OAC to i.v. heparin (heparin group) and the other maintained OAC with acenocumarol, a vitamin K antagonist (OAC group).
In the heparin group, OAC was discontinued 4 days before implant/replacement; i.v. heparin was started at INR ≤ 2 and stopped 6 h before the implant. After the procedure, OAC was reinstituted at night and i.v. heparin was started 24 h after the implant with i.v. bolus of 60 U/kg (maximum dosage of 4000 U) and an infusion rate that maintained activated partial thromboplastin time between 55 and 70 s. Heparin perfusion was stopped at INR ≥ 2. In the OAC group, INR was tested 15 days before implant to adjust acenocumarol dosage to achieve an INR 2 ± 0.3 at the day of the implant. After the procedure, testing continued until an adequate INR was achieved.
All patients were evaluated at discharge, 15 and 45 days after the procedure to assess pocket haematoma, thrombo-embolic events, or other bleeding.
Definitions
Pocket haematoma was defined as palpable mass that protruded >2 cm anterior to pulse generator. Pocket haematoma was evacuated if it caused tense swelling with poor capillary perfusion, progressive enlargement, or severe pain to the patient.5
Any thrombo-embolic event that took place between OAC dosage modification and 45 days after the procedure was recorded. Arterial thrombo-embolic events were defined as ischaemic stroke, TIAs, or peripheral arterial thrombo-embolism. Venous thrombo-embolic events were defined as acute symptomatic pulmonary embolism or acute symptomatic deep venous thrombosis. Prosthetic valve thrombosis was only ruled out based on clinical symptoms. Echocardiograms were not systematically obtained before and after the implant.
Surgical techniques
All procedures were performed in our centre by two experienced electrophysiologists and one fellow in electrophysiology (L.M., A.B., and J.M.T.). More than 500 devices are implanted each year, and each of the three physicians implants approximately one-third of them. The number of years of experience in implanting devices when the study started was as follows: L.M., 10 years; A.B., 6 years; and J.M.T., 1 year.
New implant
Implants were done according to our institutional protocol. After administration of prophylactic antibiotic and local anaesthesia, leads were implanted through subclavian vein (one puncture per lead) under fluoroscopic guidance. Most ventricular leads had passive fixation except in the cases of severe tricuspid regurgitation or pulmonary hypertension. Atrial leads on pacemakers had passive fixation except in patients with previous cardiac surgery. In patients treated with cardiac resynchronization (CRT), all left ventricular leads were inserted though the coronary sinus. As a general rule, in our department, we follow the recommendation of current guidelines and abandon the implant of the CRT after 4 h or 60 min of X-ray, if the procedure is unsuccessful. Devices were placed in a subcutaneous pocket localized in the pre-pectoral region. After the procedure, a pressure dressing was applied to the wound for 6 h.
Replacement
After administration of the prophylactic antibiotic, an incision was made, the generator was exteriorized and disconnected, and then the leads were tested to confirm the correct sensing, pacing thresholds, and impedance parameters. The new generator was connected and inserted into the existing pocket, the wound was closed in layers, and a pressure dressing was applied for at least 6 h, or until bleeding stopped.
Statistical methods
Based on our own experience, an incidence of 10% of pocket haematoma and/or thrombo-embolic complications was expected in the heparin group. A sample size of 50 patients per arm had 80% of power to detect an expected absolute difference of 24% in the incidence of the primary endpoint in the OAC group, using the two-sided Fisher's exact test and with a significance level of 0.05. No loss to follow-up was expected.
Patients were randomly assigned to one study group by a computer-generated randomization scheme in permuted blocks of 4 in equal proportions. The random sequence was performed and kept by one statistician; patient treatment was entirely performed by personnel unrelated to the study; patient enrolment, implant procedure, and follow-up tasks were performed by the investigators. Once the informed consent was obtained, one of the investigators notified the inclusion of the patient and the statistician revealed the group allocation only to the physician responsible for the pre-implant treatment. Patient group was blinded to the investigators. Data were analysed on an intention-to-treat basis.
Results were reported as mean ± SD or using median (Q1–Q3) as appropriate. Comparisons between continuous variables were performed using Student's t-test or the Mann–Whitney U-test. For categorical variables, the χ2 test or the exact method was used. For the comparison of the number of leads implanted, the Goodman and Kruskal Tau test was used. A two-sided P-value of ≤0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Inc.) 12.0.
Results
From September 2005 to October 2007, 101 consecutive patients were included. No patient was lost during the follow-up or crossed over between groups.
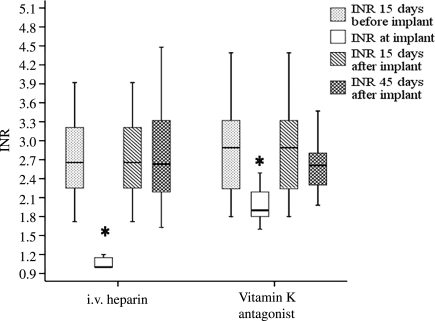
Baseline characteristics are shown in Table 1. No statistically significant baseline differences were observed between the groups in this series. In both groups, most procedures were new implants: 41/51 (80.4%) in the heparin group vs. 38/50 (76%) in the OAC group. There was no statistically significant difference in the number of leads implanted in each group (Table 2). Most of the devices implanted/replaced in both groups were pacemakers: 36/51 (70.6%) in the heparin group vs. 34/50 (68%) in the OAC group. Mean INR at the time of implant was 1.1 ± 0.2 vs. 2 ± 0.3 (P < 0.001) and the INR at 15 and 45 days of implant was 2.8 ± 0.8 vs. 2.9 ± 1 (P = 0.69) and 2.6 ± 0.8 vs.2.7 ± 0.5, respectively (P = 0.72), as shown in Figure 1.
Table 1.
Baseline characteristics of the patients included in the study
| Heparin group (n = 51) | OAC group (n = 50) | P-value | |
|---|---|---|---|
| Age (years) | 66 ± 11 | 68 ± 10 | 0.28 |
| Male sex | 33 (65%) | 30 (60%) | 0.45 |
| BMI (kg/m2) | 25 ± 5 | 27 ± 5 | 0.62 |
| New implant | 41 (80%) | 38 (76%) | 0.36 |
| ICD | 15 (29.4%) | 16 (32%) | 0.88 |
| Mechanical prosthetic valve | 26 (51%) | 28 (56%) | 0.77 |
| Mitral mechanical prosthetic valve | 20 (39%) | 19 (38%) | 0.77 |
| Mitro-aortic prosthetic valve | 6 (12%) | 9 (18%) | 0.42 |
| AF and prosthetic valve | 17 (37%) | 19 (38%) | 0.73 |
| Prosthetic valve + ictus | 10 (19%) | 5 (10%) | 0.15 |
| AF and previous stroke | 11 (21%) | 14 (28%) | 0.52 |
| AF and ≥3 moderate risk factors | 7 (14%) | 5 (10%) | 0.55 |
| AF + non-mechanical prosthetic valve | 4 (8%) | 2 (4%) | 0.43 |
| Protruding intracavitary thrombi | 1 (2%) | 1 (2%) | 1.00 |
| DVT or PTE | 2 (4%) | 0 (0%) | 0.24 |
| LVEF (%) | 42.2 ± 17 | 39.9 ± 17 | 0.55 |
OAC, oral anticoagulants; LVEF, left ventricular ejection fraction; ICD, internal cardioverter defibrillator; CRT, resynchronization device; AF, permanent, persistent or paroxysmal atrial fibrillation; HTA, hypertension; DVT, deep venous thrombosis; PTE, pulmonary thrombo-embolism; moderate risk factors: age ≥75, LVEF ≤35%, HTA, diabetes.
Table 2.
Number of leads implanted (patients with a new implant n = 79)
| Heparin group (n = 41) | OAC group (n = 38) | P-value | |
|---|---|---|---|
| One lead | 17/41 (41.5%) | 16/38 (42.1%) | 0.650 |
| Two leads | 12/41 (29.3%) | 8/38 (21.1%) | |
| Three leads | 12/41 (29.3%) | 14/38 (36.8%) |
OAC, oral anticoagulants; one puncture per lead implanted.
Figure 1.
Comparison of mean INR levels registered at 15 days pre-implant, day of implant, and 15 and 45 days post-implant, between i.v. heparin and vitamin K antagonist groups. *P < 0.05.
Five implants in the OAC group had to be postponed because the INR was too high; three of them were implanted the next day and two were rescheduled until the INR was adequate. All devices were successfully implanted. The mean duration of the procedure was 120 ± 40 min for three-lead devices, 65 ± 40 min for two-lead devices, and 44 ± 30 min for one-lead devices.
Haematoma and embolic events
Pocket haematoma and/or thrombo-embolic events occurred in 4/51 (7.8%) and in 4/50 (8.0%) patients of the heparin and OAC groups up to 45 days after the procedure, respectively (OR: 1.022; 95% CI: 0.263–3.971; P = 1.00). Pocket haematoma occurred in four patients of each group at pre-discharge. Among them, only one patient in each group required drainage, with no need for blood transfusion. No new haematoma occurred after discharge. In patients with haematoma, the mean drop in haemoglobin was −6.8 ± 3.7 g/L. No thrombo-embolic events occurred in any patient of this series (Table 3).
Table 3.
Complications registered up to 45 days after device implant/replacement
| Heparin group (n = 51) | OAC group (n = 50) | P-value | |
|---|---|---|---|
| Pocket haematoma | 4/51 (7.8%) | 4/50 (8%) | 1.00 |
| Drainage haematoma | 1/51 (1.9%) | 1/50 (2%) | 1.00 |
| Thrombo-embolic events | 0/51 (0%) | 0/50 (0%) | 1.00 |
| Active endocarditis | 1/51 (1.9%) | 1/50 (2%) | 1.00 |
| Pneumothorax | 1/51 (1.9%) | 0/50 (0%) | 0.49 |
| Lead displacement | 0/51 (0%) | 1/50 (2%) | 0.50 |
OAC, oral anticoagulants.
Other complications
One patient from each group suffered a fatal endocarditis on the prosthetic valve. In the heparin group patient, this was due to Staphylococcus aureus and attributed to left-arm phlebitis. The patient from the OAC group underwent a pacemaker implant after a complete atrioventricular block following a mitral valve replacement and subsequently developed a pocket infection and prosthetic valve endocarditis. None of these two patients developed any haematoma at discharge.
One patient in the heparin group (1.9%) had a pneumothorax that required evacuation and one patient in the OAC group (2%) had a left ventricular lead displacement 1 day after implantation that required a new intervention (Table 3).
In-hospital stay
Duration of the hospital stay had a median of 5 (Q1–Q3: 4–7 days) and 2 days (Q1–Q3: 1–4 days) in the heparin and AOC groups, respectively (P < 0.001). The presence of pocket haematoma prior to discharge increased the mean in-hospital stay in three patients.
Discussion
To our knowledge, this is the first randomized study to show that OAC maintenance is as safe as bridging with i.v. heparin in the implantation/replacement of a pacemaker or ICD in patients at very high risk for thrombo-embolic events (e.g. >50% had a mechanical prosthetic valve in the mitral position). Moreover, the maintenance of OAC in these patients reduced the hospital stay by a mean of 2.5 ± 0.7 days. This is an important finding, since shortening hospital stay will reduce admission costs importantly. Moreover, one may consider implanting pacemakers as an outpatient procedure, as is currently done in patients without OAC treatment.
A recent prospective observational study10 has analysed the risk of complete interruption of warfarin therapy in a large series of patients who underwent minor interventions. A brief periprocedural interruption of OAC was associated with a low risk of thrombo-embolism and haemorrhage. However, the population included in this study was a heterogeneous group of patients with widely differing thrombo-embolic risk among them. Our study included only patients with high thrombo-embolic risk; >50% of the population included in our study had a mechanical prosthetic valve and ∼40% had a previous embolic event. Current guidelines4 recommend peri-operative bridging therapy with heparin in this group of patients.
Safety of oral anticoagulation maintenance
Other studies7,8 have described the safety of implanting or replacing a pacemaker or ICD while maintaining OAC, but these were observational and non-randomized trials. In our study, the incidence of pocket haematomas was higher. Goldstein et al.8 was a retrospective study and most of the procedures were replacements (62%); in the Al-Kadra7 study, 9/47 patients (19%) had a normal INR at the time of the implant. Furthermore, the percentage of ICD and CRT devices implanted in both studies was lower than in our cohort of patients. The fact that the procedures in our series were more complex may account for the difference in the incidence of pocket haematomas. Giudici et al.11 postulated the safety of implanting a pacemaker or ICD without stopping OAC and reported on the biggest series of patients studied to date who received a pacemaker or ICD without reversing OAC. The authors of this study retrospectively analysed the data from a registry, comparing 470 patients with an INR > 1.5 at the moment of the implant with 555 patients with an INR < 1.5 and found no significant difference in the incidence of pocket haematoma between the two groups (2.5 vs. 1.6%, respectively). In this analysis, the low incidence of pocket haematomas was similar between those taking anticoagulants and those who were not. However, these data were obtained in a retrospective analysis of a registry that probably was not designed to evaluate bleeding and thrombo-embolic events in high-risk patients. The patients were evaluated by different physicians and there was no pre-established definition of pocket haematoma. As a result, the study may underestimate the incidence of pocket haematomas. Furthermore, it was not clear how many patients included in the anticoagulation group had a high risk for thrombo-embolic events; therefore, it was impossible to properly evaluate the incidence of embolic events among these patients. Nevertheless, this study suggested that implantation of these devices without stopping OAC is safe in centres with experienced personnel. Milic et al.12 demonstrated a reduction in the percentage of pocket haematoma by applying a fibrin sealant prior to wound closure, independent of maintaining OAC or bridging to i.v. heparin. The number of patients included in the study was small (20 patients in each group) and the percentage of haematomas in the control group was high (∼25%). In our study, the percentage of haematomas is ∼7.5% and only 2% of the patients required evacuation. Although the use of pocket sealant may be an option to minimize the number of pocket haematomas, these products are expensive and may lead to other complications, such as viral transmission, allergic reactions to bovine proteins, accidental intravascular application of the product, or infections. Other randomized studies with higher numbers of patients are needed to demonstrate the safety and feasibility of these products.
Restarting i.v. heparin
In our study, 7.8% of the patients randomized to the i.v. heparin arm developed pocket haematoma. There were no embolic events. Although the study was not designed to determine the best moment to restart i.v. heparin after the procedure, our data suggest that restarting 24 h after the procedure decreases the incidence of haematoma. In contrast with our findings, Michaud et al.5 found a pocket haematoma in ∼20% of the patients, independent of restarting heparin 6 or 24 h after the implantation.
Endocarditis
Two patients developed a fatal endocarditis, both with pre-existing risk factors as described by Klug et al.13 (pre-operatory fever due to left-arm phlebitis secondary to intravenous line, and previous mitral valve replacement during the same hospital stay, with temporary pacing). The 2% infection is higher than the one observed in a previous analysis published in our institution (∼0.6%).14 However, the present study has been performed in a high-risk population with a high percentage of prosthetic mitral valves.
Limitations
All OAC patients were treated with acenocumarol since this is the main vitamin K antagonist used in Spain. The results of our study could be extrapolated to other vitamin K antagonists, although pre- and post-implant intervals might be different from other drugs due to differences in half-life and metabolism.
Because of the small number of adverse events, and the small sample size of our study, large and probably multicentre studies with many more events are needed to get a definitive answer for the management of these patients.
Conclusion
In centres with experienced operators, maintaining OAC at INR levels of 2 ± 0.3 during the implant or replacement of a pacemaker or ICD in patients at high risk for thrombo-embolic events can be as safe as bridging to i.v. heparin, with the benefit of reducing both the in-hospital stay and the cost of the procedure.
Funding
D.T. was supported by a grant from Institut de Investigació Biomèdica August Pi i Sunyer (IDIBAPS). Funding to pay the Open Access publication charges for this article was provided by ‘Fundació Privada Clinic per a la Recerca Biomèdica’.
Conflict of interest: none declared.
Acknowledgements
This work was part of Clinical and Preclinical Heart Failure Research Network (REDINSCOR RET0308) and (HERACLES RD/06/0009) Cardiovascular Network, Spain.
References
- 1.Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1506–1511. doi: 10.1056/NEJM199705223362107. [DOI] [PubMed] [Google Scholar]
- 2.Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants. Arch Intern Med. 2003;163:901–908. doi: 10.1001/archinte.163.8.901. [DOI] [PubMed] [Google Scholar]
- 3.Larson BJ, Zumberg MS, Kitchens CS. A feasibility study of continuing dose-reduced warfarin for invasive procedures in patients with high thromboembolic risk. Chest. 2005;127:992–997. doi: 10.1378/chest.127.3.922. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lutle BW, Nishimura RA, O'Gana PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography Interventions; Society of Thoracic Surgeons. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a Report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation. 2006;114:84–231. [Google Scholar]
- 5.Michaud G, Pelosi F, Noble MD, Knight BP, Morady F, Strickberger SA. A randomized trial comparing heparin initiation 6 h or 24 h after pacemaker or defibrillator implantation. J Am Coll Cardiol. 2000;35:1915–1918. doi: 10.1016/s0735-1097(00)00633-1. [DOI] [PubMed] [Google Scholar]
- 6.Wiegand UK, LeJeune D, Boguschewski F, Bonnemeier H, Eberhardt F, Schunkert H, Bode F. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy and perioperative antiplatelet/anticoagulation therapy. Chest. 2004;126:1177–1186. doi: 10.1378/chest.126.4.1177. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kadra AS. Implantantion of pacemakers and implantable cardioverter defibrillators in orally anticoagulated patients. PACE. 2003;26:511–514. doi: 10.1046/j.1460-9592.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein D, Losquadro W, Spotnitz H. Outpatient pacemaker procedures in orally anticoagulated patients. PACE. 1998;21:1730–1734. doi: 10.1111/j.1540-8159.1998.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 9.Fuster V, Ryden LE, Cannom DS, Crihns HJ, Curtis AB, Ellembogen KA, Halperin JL, Le Heuzey J, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S. Task Force on Practice Guidelines, American College of Cardiology/American Heart Association: Committee for Practice Guidelines; European Society of Cardiology; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC2006 guidelines for the management of patients with atrial fibrillation. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice guidelines. Eur Heart J. 2006;27:1979–2030. doi: 10.1093/eurheartj/ehl176. [DOI] [PubMed] [Google Scholar]
- 10.Garcia DA, Regan S, Henault LE, Upadhya A, Baker J, Othman M, Hylek EM. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168:63–69. doi: 10.1001/archinternmed.2007.23. [DOI] [PubMed] [Google Scholar]
- 11.Giudici M, Barold S, Paul D, Bontu P. Pacemaker and implantable cardioverter defibrillator implantation without reversal of warfarin therapy. PACE. 2004;27:358–360. doi: 10.1111/j.1540-8159.2004.00441.x. [DOI] [PubMed] [Google Scholar]
- 12.Milic DJ, Perisic Z, Zivic SS, Stanojkovic ZA, Stojkovic AM, Karanovic ND, Krstic NH, Salinger SS. Prevention of pocket related complications with fibrin sealant in patients undergoing pacemaker implantation who are receiving anticoagulant treatment. Europace. 2005;7:374–379. doi: 10.1016/j.eupc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Klug D, Balde M, Pavin D, Hidden-Luce F, Clemnty J, Saoul N, Rey JL, Land G, Lazarus A, Victor J, Barnya C, Grandbastien B, Kacet S for the PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators. Results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 14.Del Río A, Anguera I, Miro JM, Mont L, Fowler VG, Azqueta M, Mestres CA and the Hospital Clínic Endocarditis Study Group. Surgical treatment of pacemaker and defibrillator lead endocarditis. The Impact of electrode lead extraction on outcome. Chest. 2003;124:1451–1459. doi: 10.1378/chest.124.4.1451. [DOI] [PubMed] [Google Scholar]