Abstract
Aims
Serum cystatin C, a novel marker of kidney function, is reported to be superior to serum creatinine as a risk factor for atherosclerotic disease, but associations may vary across vascular beds.
Methods and results
A cross-sectional study of chronic kidney disease (CKD) and peripheral arterial disease (PAD) in 3089 adult participants aged 40+ from the 1999–2002 National Health and Nutrition Examination Survey (NHANES). Kidney function, assessed by estimated glomerular filtration rate (eGFR), was determined from serum creatinine and cystatin C using established equations. Peripheral arterial disease defined by an ankle brachial index <0.90. Glomerular filtration rate estimated using cystatin C was more strongly associated with PAD compared with eGFR using serum creatinine before and after multivariable adjustment. Further, after adjustment for cystatin C, kidney function based on serum creatinine was no longer significantly associated with PAD. However, cystatin C remained significantly associated with PAD even after adjustment for GFR estimated by serum creatinine. Compared with optimal kidney function (eGFRserum creatinine ≥60, eGFRcystatin C >90), the odds ratio for PAD was 3.11 (95% confidence interval 1.26–7.64) for preclinical CKD (eGFRserum creatinine ≥60, eGFRcystatin C <76.7) and 5.07 (3.01–8.52) for ‘confirmed’ CKD (eGFRserum creatinine <60, eGFRcystatin C <60).
Conclusion
Chronic kidney disease was strongly and independently associated with PAD. Cystatin C was a more potent marker of lower extremity PAD when compared with the serum creatinine equation currently used in clinical practice. Our results suggest that cystatin C may have clinical utility when combined with serum creatinine in evaluation of individuals who may have PAD.
Keywords: Peripheral arterial disease, Chronic kidney disease, glomerular filtration rate, Cystatin C, Epidemiology, NHANES
Introduction
There is growing recognition that chronic kidney disease (CKD) is independently associated with atherosclerotic disease. Previous studies have documented the high risk of cardiovascular disease among individuals with CKD.1,2 However, less is known of the relationship of CKD to lower extremity peripheral arterial disease (PAD), a common condition caused by the development of atherosclerosis in the lower extremities. Limb amputation, a frequent clinical complication of PAD, is common among individuals with kidney disease3,4 and diabetes,5 but the association of subclinical PAD and moderately decreased kidney function is not well characterized. Serum creatinine is the most common clinical marker of kidney function and is used in conjunction with mathematical equations to estimate the glomerular filtration rate (GFR) and to diagnose and define the stages of CKD.6 Several recent studies have shown an independent association between CKD—as defined by serum creatinine-based estimating equations—and PAD in the general population.7–9 Serum cystatin C, a novel marker of kidney function, is thought to be less affected by age, gender, and body composition than serum creatinine and has been reported to be superior for the estimation of kidney function, particularly in the presence of mild to moderate kidney disease and in older individuals.10–14 An important clinical question is whether cystatin C or GFR estimated from cystatin C is a marker of atherosclerotic disease and if the magnitude of any association differs from that for GFR estimated using creatinine.
The objective of this study was to investigate the association of kidney function—as estimated from serum creatinine and cystatin C—and PAD in a nationally representative sample of the US adult population. We hypothesized that: (i) kidney function would be associated with PAD independently of known cardiovascular risk factors; (ii) GFR estimated using cystatin C would be more strongly associated with PAD when compared with that estimated from serum creatinine; and (iii) cystatin C would add additional risk stratification information within currently defined categories of kidney function using serum creatinine.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing cross-sectional, multistage, stratified, clustered probability sample of the US civilian non-institutionalized population. Detailed in-person interviews, physical examinations, and blood samples were obtained from ∼10 000 participants aged 12 or older in the 1999–2002 survey.15 A subset (n = 5718) of these participants including all persons aged 60 or older and a 25% random sample of participants aged <60 years were selected for measurement of serum cystatin C.16 Serum was available for the measurement of cystatin C on 98.6% of individuals selected for the substudy (n = 5638). For the present analyses, we further limited our study sample to individuals non-missing information on lower extremity PAD (only assessed in participants aged 40 and older), for a final study sample size of 3089 adults (83% of eligible participants).
Assessment of lower extremity peripheral arterial disease
Peripheral arterial disease can be determined with high sensitivity and specificity using the ratio of the systolic blood pressure in the ankle to that in the arm (ankle brachial index, ABI).17,18 We defined PAD on the basis of ABI measurements obtained from NHANES 1999–2002 in participants aged 40 and over during the examination component of the survey. In NHANES, the established ABI technique employed measurement of systolic blood pressure at the right brachial artery and at both posterior tibial arteries using Doppler ultrasound. If the participant had a medical condition that did not permit the use of right arm measurement, the left arm was used for brachial pressure measurement. Systolic blood pressure was measured twice at each limb site for participants aged 40–59 and once at each site for participants aged 60 and over. The left and right ABI measurements were obtained by dividing the mean systolic blood pressure in the left and right ankle, respectively, by the mean blood pressure in the arm. Peripheral arterial disease was defined as an ABI <0.90 in either leg. We excluded individuals with ABI >1.5, values usually related to non-compressible vessels in the legs.
Measures of kidney function
Blood samples were collected by trained personnel. Serum creatinine was measured using a kinetic rate Jaffe method.19–21 To appropriately estimate GFR, all serum creatinine measurements were re-calibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH, USA) as detailed recently.21 A re-calibration equation was applied to serum creatinine in the 1999–2000 survey only (standard creatinine = 0.147 + 1.013 × NHANES 1999–2000 uncalibrated serum creatinine). No correction to the creatinine values in the 2001–2002 survey was needed.21
Serum from the original blood collection in 1999–2002 was stored at −70°C until 2006, when we measured cystatin C at the Cleveland Clinical Research Laboratory. Samples were assayed for cystatin C with a particle-enhanced immuno-nephelometric assay (N Latex Cystatin C, Dade Behring, IL, USA).22
The estimation of kidney function, accounting for age, sex, and race in serum creatinine-based estimating equations, is crucial as these factors influence serum creatinine production. Serum creatinine-based GFR was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) Study formula re-expressed to use standard creatinine.23,24
Serum cystatin C concentrations are less related to age, sex, and race, and we therefore estimated GFR from cystatin C using a single variable equation consisting of a mathematical transformation of cystatin C: eGFR = 76.7 × CysC−1.19.25 This single variable equation is equivalent to modelling raw cystatin C levels but with a transformation to achieve commensurate units of estimated GFR; the ranking of individuals is unchanged. We also evaluated a GFR equation, including cystatin C in combination with age, sex, and race (cystatin C multivariable equation): eGFR = 127.7 × CysC−1.17 × age−0.13 × (0.91 if female) × (1.06 if black).
In all equations, estimated GFR is reported in mL/min/1.73 m2, race/ethnicity was either black or not, and values that exceeded 200 mL/min/1.73 m2 were truncated at that level. Kidney function was categorized based on the classification system established by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative.26 Individuals with eGFR <15 mL/min/1.73 m2 were excluded from analyses of kidney function categories because estimates of this stage of kidney disease are likely to be unreliable due to the small number of individuals and the likelihood of being ill or receiving dialysis would depress the response rate.
We also conducted analyses combining serum creatinine and cystatin C eGFR categories, utilizing cystatin C as a ‘confirmatory’ test. We divided persons with normal kidney function (eGFR ≥60 by MDRD) into categories according to cystatin C level, using a cystatin C cut point of 1.0 mg/L (equivalent to eGFR 76.7 by serum cystatin C using the single variable equation) to define ‘pre-clinical CKD’,27 generating the following categories: eGFR ≥60 by MDRD and ≥90 by cystatin C (‘optimal’ kidney function), eGFR ≥60 by MDRD and 76.7–89 by cystatin C (‘low normal’ kidney function), and eGFR ≥60 by MDRD and <76.7 by cystatin C (‘pre-clinical’ kidney disease). In those persons defined as having CKD (eGFR 30–59 by MDRD), we used cystatin C to ‘confirm’ the diagnosis, classifying these individuals into the following categories: eGFR <30–59 by MDRD and ≥60 by cystatin C (‘unconfirmed’ CKD) and eGFR <30–59 by MDRD and <60 by cystatin C (‘confirmed’ CKD). Individuals with eGFR 15–29 by MDRD were defined as having severe CKD.6
Other study variables
The NHANES examination included measurement of height, weight, and blood pressure. Hypertension was defined as a mean systolic blood pressure of 140 mmHg or greater, a mean diastolic blood pressure of 90 mmHg or greater, or hypertension medication use. Total cholesterol was measured enzymatically.20 Hypercholesterolaemia was defined as a total cholesterol level 240 mg/dL or higher, or medication use. Diabetes was defined by a self-reported physician diagnosis. Persons reporting ‘borderline diabetes’ or solely reporting a diabetes diagnosis during pregnancy were considered non-diabetic. Information on age, sex, race/ethnicity, and smoking was based on self-report during the questionnaire portion of the survey. A history of cardiovascular disease was defined on the basis of a self-reported history of coronary heart disease, angina, previous heart attack, or stroke. Smoking status was determined using answers to the questions, ‘Have you smoked at least 100 cigarettes in your life?’ and ‘Do you now smoke cigarettes?’ We also examined cotinine levels, a major metabolite of nicotine, and a commonly used marker for both active and second-hand cigarette smoke exposure. Serum cotinine was measured by an HPLC/atmospheric-pressure ionization tandem mass spectrometry method. Detailed information regarding the collection of data in NHANES is available elsewhere.15
Statistical analysis
As cystatin C was measured on a subset of NHANES 1999–2002 participants (25% stratified random sample of individuals with valid serum creatinine levels), the original examination sampling weights require modification to provide nationally representative estimates and address potential bias. Modification of the original sampling weights to account for both missing serum creatinine and cystatin C was performed by the investigators (E.S. and J.C.) according to standard methods,28 and protocols were reviewed and approved by the National Center for Health Statistics (NCHS). The modified sampling weights were applied in all analyses and standard errors for estimates were obtained using the Taylor series (linearization) method.29 Adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated using logistic regression models. All statistical analyses were performed using Stata v10.0 (Stata Corp, College Station, TX, USA) svy commands.
Protocols for conduct of NHANES were approved by the NCHS institutional review board. Informed consent was obtained from all participants. Both the NCHS and the Johns Hopkins Bloomberg School of Public Health institutional review boards approved the protocols for measurement of cystatin C from stored serum samples.
Results
The characteristics of the study population are shown in Table 1. Compared with individuals without PAD, those with PAD were older, more likely to be non-Hispanic black, current or former smokers, and more likely to have major cardiovascular risk factors. Levels of serum creatinine, cystatin C, GFR estimated using the serum creatinine equation (2005 MDRD formula), the single variable cystatin C equation, and the multivariable cystatin C equation all indicated lower average kidney function among individuals with PAD compared with those without PAD in this unadjusted comparison.
Table 1.
Population characteristics of US adults aged 40 years or older by peripheral arterial disease status (n = 333 cases), NHANES 1999–2002
| Unweighted (n) | Overall % (SE) | PAD % (SE) | No PAD % (SE) | |
|---|---|---|---|---|
| Age, mean | 3089 | 56.3 (0.44) | 68.1 (1.51) | 55.6 (0.40) |
| Age group | ||||
| 40–60 | 671 | 64.6 (1.5) | 22.6 (5.6) | 67.1 (1.5) |
| 60–70 | 1169 | 17.7 (1.1) | 23.3 (3.2) | 17.3 (1.1) |
| 70+ | 1249 | 17.7 (0.8) | 54.2 (4.4) | 15.6 (0.7) |
| Sex | ||||
| Female | 1490 | 52.2 (1.5) | 49.9 (3.9) | 52.3 (1.6) |
| Male | 1599 | 47.8 (1.5) | 50.1 (3.9) | 47.7 (1.6) |
| Race/ethnicitya | ||||
| Non-Hispanic White | 1720 | 77.7 (1.5) | 76.8 (3.1) | 77.7 (1.5) |
| Non-Hispanic Black | 533 | 9.5 (1.3) | 15.3 (3.3) | 9.1 (1.2) |
| Mexican American | 650 | 4.4 (0.7) | 3.7 (1.1) | 4.4 (0.8) |
| Diabetes, self-report | 460 | 8.9 (0.5) | 19.7 (3.3) | 8.3 (0.5) |
| Hypertension | 1798 | 41.2 (1.9) | 74.4 (3.7) | 39.9 (1.9) |
| Hypercholesterolaemia | 1108 | 32.2 (1.8) | 42.5 (4.3) | 31.5 (1.8) |
| Body mass index (kg/m2), mean | 3026 | 28.2 (0.3) | 28.2 (0.7) | 28.3 (0.3) |
| Body mass index category (kg/m2) | ||||
| <25 | 858 | 31.9 (2.0) | 31.7 (4.1) | 32.1 (2.1) |
| 25–29.99 | 1221 | 37.0 (1.7) | 34.6 (3.4) | 37.0 (1.7) |
| ≥30 | 947 | 31.0 (1.9) | 33.8 (4.5) | 30.8 (1.9) |
| Smoking status | ||||
| Current | 474 | 19.8 (1.4) | 27.2 (3.2) | 19.3 (1.3) |
| Former | 1176 | 33.6 (1.7) | 40.1 (3.7) | 33.7 (1.8) |
| Never | 1433 | 46.6 (2.0) | 32.7 (4.6) | 47.0 (2.1) |
| Measures of kidney function | ||||
| Standard serum creatinine (mg/dL), mean | 3089 | 0.923 (0.010) | 1.09 (0.045) | 0.913 (0.011) |
| Standard serum creatinine (mg/dL), median (IQR) | 3089 | 0.90 (0.75, 1.00) | 0.96 (0.68, 1.16) | 0.90 (0.75, 1.0) |
| Serum cystatin C (mg/L), mean | 3089 | 0.937 (0.010) | 1.22 (0.044) | 0.92 (0.010) |
| Serum cystatin C (mg/L), median (IQR) | 3089 | 0.88 (0.77, 1.01) | 1.13 (0.92, 1.34) | 0.87 (0.76, 0.99) |
| Glomerular filtration rate from estimating equations | ||||
| eGFR serum creatinine (mL/min/1.73 m2), 2005 MDRD equation re-expressed for standard creatinine | 3089 | 80.4 (0.7) | 69.1 (2.4) | 81.1 (0.8) |
| eGFR serum cystatin C (mL/min/1.73 m2), single variable equation | 3089 | 89.7 (0.9) | 69.1 (1.8) | 90.93 (0.8) |
| eGFR serum cystatin C (mL/min/1.73 m2), multiple variable equation | 3089 | 85.0 (0.9) | 64.6 (1.9 | 86.2 (0.8) |
PAD, peripheral arterial disease; eGFR, estimated glomerular filtration rate; IQR, inter-quartile range; NHANES, National Health and Nutrition Examination Survey. SI conversion factor: To convert creatinine to μmol/L, multiple values by 88.4. Estimates are % (standard error) unless otherwise indicated.
a‘Other’ race/ethnicity category not shown.
The crude prevalence of CKD was much higher among individuals with PAD compared with those without PAD or the overall population. The prevalence estimates of CKD (eGFR <60) using the serum creatinine equation, cystatin C only, and the multivariable cystatin C equation were 37.0% (95% CI 28.1–45.9), 35.2% (26.2–44.2), and 42.9% (34.6–51.3), respectively. In contrast, the corresponding prevalence of CKD in the total population aged 40 and older by each of these equations was: 11.9% (10.0–13.8), 8.9% (7.3–10.5), and 12.2% (10.2–14.3).
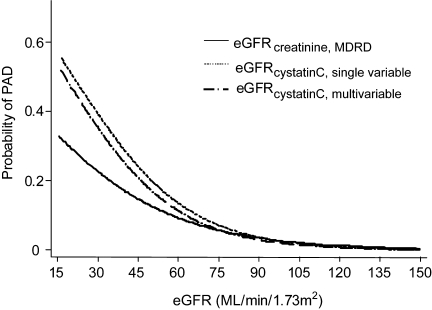
Figure 1 displays the continuous unadjusted association between eGFR and PAD for each GFR estimating equation. Lower kidney function (lower estimated GFR) was strongly associated with an increased probability of PAD across the spectrum of kidney function in this general population. Figure 1 illustrates the stronger association of GFR estimated using cystatin C compared with serum creatinine, particularly at lower levels of kidney function. Indeed, cystatin C appears much more strongly associated with PAD at estimated GFR levels below 60 when compared with estimated GFR using serum creatinine.
Figure 1.
Probability of peripheral arterial disease by level of kidney function comparing glomerular filtration rate estimating equations (mL/min/1.732) using the Modification of Diet in Renal Disease serum creatinine equation, a single variable cystatin C equation, and a multivariable cystatin C equation, US adults 40+, 1999–2002.
Before conducting multivariable models, we examined the distribution of risk factors across definitions of CKD using serum creatinine and cystatin C (Table 2). These results show relative differences in the strength of potentially important confounders in the CKD–PAD association using different definitions of CKD. Most factors were similar across the CKD definitions (Table 2) with the exception of hypertension, history of cardiovascular disease, and smoking status. In particular, the prevalence of current smoking and serum cotinine level were substantially higher in cystatin C-defined CKD compared with creatinine-defined CKD. In multivariable models of CKD adjusted for all variables listed in Table 2, hypertension, history of cardiovascular disease, and smoking all remained significantly associated with cystatin C-defined CKD but not creatinine-defined CKD (data not show). We adjusted for these risk factors in subsequent multivariable models of kidney function and PAD prevalence. Body mass index and waist circumference were also more strongly associated with GFR equations using cystatin C when compared with that using serum creatinine; however, measures of adiposity were not found to be important risk factors for PAD in this study.
Table 2.
Population characteristics of US adults aged 40 years or older by chronic kidney disease status defined by estimating equations for glomerular filtration rate using serum creatinine or serum cystatin C, NHANES 1999–2002
| Chronic kidney disease, eGFR<60 mL/min/1.73 m2 |
||||
|---|---|---|---|---|
| Serum creatinine, MDRD formula eGFR <60 | Cystatin C, single variable equation eGFR <60 | Cystatin C, multiple variable equation eGFR <60 | ||
| Age, mean (SE) | 68.8 (0.9) | 69.9 (1.4) | 70.3 (1.2) | |
| Sex | ||||
| Female | 60.4 (1.9) | 58.6 (3.6) | 63.2 (3.0) | |
| Male | 39.6 (1.9) | 41.4 (3.6) | 35.8 (3.0) | |
| Race/ethnicitya | ||||
| Non-Hispanic White | 86.1 (2.0) | 84.9 (2.6) | 86.1 (2.6) | |
| Non-Hispanic Black | 6.3 (1.1) | 7.4 (1.8) | 6.2 (1.4) | |
| Mexican American | 1.6 (0.4) | 1.8 (0.5) | 1.8 (0.5) | |
| Diabetes, self-report | 13.9 (2.4) | 15.3 (1.9) | 14.7 (1.8) | |
| Hypertension | 67.7 (3.1) | 74.4 (2.5) | 72.7 (2.4) | |
| Hypercholesterolaemia | 43.8 (2.2) | 43.4 (4.0) | 43.6 (3.1) | |
| History of cardiovascular disease, self-report | 28.3 (2.0) | 34.9 (2.5) | 29.2 (2.0) | |
| Body mass index (kg/m2), mean (SE) | 28.3 (0.6) | 29.6 (0.7) | 29.3 (0.5) | |
| Smoking status | ||||
| Current | 13.1 (1.8) | 16.3 (3.2) | 15.8 (3.2) | |
| Former | 36.2 (3.2) | 38.6 (4.0) | 36.4 (3.3) | |
| Never | 50.7 (3.0) | 45.0 (4.9) | 47.8 (4.2) | |
| Serum cotinine (ng/mL), mean (SE) | 38.7 (4.4) | 55.9 (12.8) | 49.9 (10.7) | |
| Standard serum creatinine (mg/dL), median (IQR) | 1.20 (1.00, 1.36) | 1.10 (0.96, 1.36) | 1.06 (0.90, 1.30) | |
| Serum cystatin C (mg/L), median (IQR) | 1.21 (1.01, 1.43) | 1.39 (1.29, 1.56) | 1.30 (1.22, 1.48) | |
PAD, peripheral arterial disease; eGFR, estimated glomerular filtration rate; IQR, inter-quartile range; NHANES, National Health and Nutrition Examination Survey. SI conversion factor: To convert creatinine to μmol/L, multiple values by 88.4. Estimates are % (standard error) unless otherwise indicated.
a‘Other’ race/ethnicity category not shown.
The adjusted ORs for PAD by categories of kidney function are presented in Table 3. Model 1 (adjusted for age only) shows a strong, graded association between level of kidney function and PAD across all kidney function equations. The association persisted even after multivariable adjustment for important PAD risk factors (age, sex, race/ethnicity, diabetes, hypertension, hypercholesterolemia, history of cardiovascular disease, and smoking status) (Model 2). The association of kidney function with PAD was consistently stronger using GFR estimated from cystatin C compared with serum creatinine-based GFR. Models 3 and 4 are the same as Model 2, but further simultaneously adjusted for the other measure of kidney function. In Model 3, the logistic models of eGFR categories defined by serum creatinine are adjusted for all variables in Model 2 plus additional adjustment for categories of eGFR defined by the single variable cystatin C equation. Similarly, the logistic models of eGFR categories and CKD defined by cystatin C are adjusted for eGFR defined by the serum creatinine eGFR equation. Model 4 is similar to Model 3 except that the simultaneous adjustment for kidney function is conducted with the respective eGFR kidney function variable modelled continuously (instead of clinical categories). Models 3 and 4 demonstrate that after adjustment for cystatin C (using the single variable equation), kidney function categories defined by serum creatinine are no longer significantly associated with PAD. However, all kidney function categories defined by cystatin C using either the single or the multivariable equations remain significantly associated with PAD even after adjustment for eGFR estimated by serum creatinine. Sensitivity analyses (data not shown) using models with additional adjustment for serum cotinine and measures of adiposity (body mass index and waist circumference), stratification by smoking status, and exclusion of individuals with a history of cardiovascular disease did not appreciably alter any of these results.
Table 3.
Adjusted odds ratios (95% confidence intervals) of peripheral arterial disease by categories of kidney function comparing serum creatinine- and cystatin C-based glomerular filtration rate equations, US adults 40 and older, NHANES 1999–2002
| Adjustment for cardiovascular risk factors |
Additional simultaneous adjustment for measures kidney function |
|||
|---|---|---|---|---|
| Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 3, OR (95% CI) | Model 4, OR (95% CI) | |
| Categories of kidney function by estimated glomerular filtration rate, mL/min/1.73 m2 | ||||
| Estimated GFR by standard serum creatinine | ||||
| 90+ | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 60–89 | 0.79 (0.39–1.61) | 0.88 (0.38–2.06) | 0.62 (0.23–1.66) | 0.63 (0.24–1.68) |
| 30–59 | 1.65 (0.82–3.33) | 1.73 (0.76–3.93) | 0.81 (0.26–2.50) | 0.74 (0.19–2.88) |
| 15–29 | 5.46 (1.92–15.52) | 3.60 (1.15–11.31) | 1.46 (0.30–7.12) | 0.97 (0.95–0.99) |
| Estimated GFR by serum cystatin C, single variable equation | ||||
| 90+ | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 60–89 | 2.75 (1.39–5.45) | 2.55 (1.18–5.52) | 2.88 (1.07–7.72) | 2.74 (1.08–6.98) |
| 30–59 | 6.23 (3.43–11.32) | 5.37 (2.98–9.68) | 5.46 (2.10–14.14) | 6.23 (2.25–17.24) |
| 15–29 | 11.25 (3.71–34.10) | 6.28 (2.11–18.68) | 4.32 (1.01–18.52) | 8.44 (1.35–52.68) |
| Estimated GFR by serum cystatin C, multiple variable equations | ||||
| 90+ | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 60–89 | 2.53 (1.21–5.27) | 2.71 (1.15–6.39) | 3.12 (1.04–9.33) | 2.92 (1.05–8.14) |
| 30–59 | 5.64 (2.85–11.16) | 5.87 (2.80–12.34) | 6.19 (2.05–18.72) | 6.87 (2.13–22.12) |
| 15–29 | 15.86 (6.23–40.35) | 11.09 (4.18–29.43) | 9.80 (2.32–41.29) | 15.13 (2.59–88.25) |
Model 1: Adjusted for age only. Model 2: Adjusted for age, sex, race, diabetes, hypertension, hypercholesterolaemia, history of cardiovascular disease, and smoking status. Model 3: Adjusted for all variables in Model 2 plus mutual adjustment for serum creatinine- or serum cystatin C-based eGFR categories using the single variable equations. Model 4: Adjusted for all variables in Model 2 plus mutual adjustment for serum creatinine- or serum cystatin C-based eGFR (continuous) using the single variable equations.
The adjusted ORs for PAD according to categories of kidney function defined by both serum creatinine and cystatin C are presented in Table 4. A dose–response relation was present across all six kidney function categories defined using a combination of serum creatinine and cystatin C levels. Indeed, among persons with normal kidney function defined by serum creatinine (eGFR ≥60 by MDRD), cystatin C remained significantly associated with prevalent PAD. The OR of PAD for ‘pre-clinical CKD’ was 3.11 (1.26–7.64). Among persons with CKD defined by serum creatinine (eGFR <60 by MDRD), cystatin C also appeared to further refine risk categories. The ORs of PAD for CKD ‘unconfirmed’ and ‘confirmed’ by cystatin C were 3.34 (1.61–6.91) and 5.07 (3.01–8.52), respectively. Severe CKD (defined by eGFR 15–29 by MDRD) was strongly associated with PAD (OR 9.11, 2.65–31.26).
Table 4.
Adjusted odds ratios (95% confidence intervals) of peripheral arterial disease by categories of kidney function combining serum creatinine- and cystatin C-based glomerular filtration rate estimating equations, US adults 40 and older, NHANES 1999–2002
| Combined categories of chronic kidney disease | Categories of estimated GFR by serum creatinine, mL/min/1.73 m2 | Categories of estimated GFR by cystatin C (single variable eq.), mL/min/1.73 m2 | Adjustment for cardiovascular risk factors |
|
|---|---|---|---|---|
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | |||
| Optimal kidney function | ≥60 | ≥90 | 1.0 (ref) | 1.0 (ref) |
| Low normal kidney function | ≥60 | 76.7a–89 | 2.16 (0.94–4.97) | 2.08 (0.84–5.13) |
| Pre-clinical kidney disease | ≥60 | <76.7a | 3.56 (1.65–7.68) | 3.11 (1.26–7.64) |
| Unconfirmed chronic kidney disease | 30–59 | ≥60 | 3.50 (1.91–6.40) | 3.34 (1.61–6.91) |
| Confirmed chronic kidney disease | 30–59 | <60 | 5.93 (3.66–9.59) | 5.07 (3.01–8.52) |
| Severe chronic kidney disease | 15–29 | — | 15.45 (4.94–48.27) | 9.11 (2.65–31.26) |
Model 1: Adjusted for age only. Model 2: Adjusted for age, sex, race, diabetes, hypertension, hypercholesterolaemia, history of cardiovascular disease, and smoking status.
aAn estimated GFR of 76.7 mL/min/1.73 m2 from the cystatin C single variable equation is equivalent to a serum cystatin C value of 1.0 mg/L.
Discussion
We found a high prevalence of CKD, ranging from 35 to 43%, among individuals with PAD defined by an ABI <0.90. Because the presence of PAD is known to confer a high risk for cardiovascular outcomes, the condition is considered a coronary heart disease ‘risk equivalent’.30,31 This is of particular importance in the setting of low kidney function as CKD is also independently associated with an elevated risk of cardiovascular events.1,2 The high prevalence of CKD in persons with PAD suggests that screening for CKD might be considered in individuals with a low ABI and/or symptomatic PAD.
This study demonstrates a robust, graded, and independent association between kidney function—assessed using serum creatinine and/or serum cystatin C—and PAD. The association was observed across the normal range of kidney function in a nationally representative sample of US adults 40 and older. Cystatin C and GFR estimated using cystatin C were more strongly associated with PAD than GFR estimated using serum creatinine, particularly at low GFR levels. Indeed, our models with simultaneous adjustment for both serum creatinine and cystatin C suggest that cystatin C captures most of the information related to the association of kidney function with PAD in this population. We also found that using cystatin C in combination with serum creatinine further refined the association of kidney function with PAD. ‘Preclinical kidney disease’ (elevated cystatin in the context of ‘normal’ kidney function defined by eGFR MDRD)27 was significantly associated with PAD (OR 3.11, 1.26–7.64) even after multivariable adjustment. In persons with CKD (eGFR <60 by MDRD), cystatin C also further refined risk, suggesting the possible clinical utility of using cystatin C as a ‘confirmatory’ test for identifying individuals at high risk for clinical outcomes. The advantage of the confirmatory approach is that serum creatinine is already routinely measured in nearly all PAD patients.
To our knowledge, these are the first nationally representative data to demonstrate an association between kidney function estimated by cystatin C in the normal range and lower extremity PAD. Previous studies have not directly compared serum creatinine and cystatin C GFR estimating equations in their association with clinical outcomes.
Recently, a case–control study demonstrated higher levels of cystatin C in PAD patients compared with controls.32 A large cohort study in elderly adults found that cystatin C was associated with incident severe PAD (defined by surgical procedures, related hospitalizations, and amputation), whereas creatinine level and serum creatinine-based eGFR were not associated with future PAD events.33 The authors found the risk of PAD associated with cystatin C did not increase in a stepwise fashion; the observed increase was present only at the highest quintile of cystatin C. However, the overall number of severe PAD events observed in this elderly cohort was small. In contrast, our data suggest that kidney function is associated with PAD across the normal range, with no evidence for a threshold effect.
Our results contribute to the debate regarding the clinical utility of serum cystatin C. This study adds to the accumulating evidence that moderately impaired kidney function may play a role in the development of cardiovascular disease.34,35 Recent data suggest that GFR estimating equations based on cystatin C may provide similar but less confounded (by muscle mass and co-morbidities) estimates of kidney function than equations using serum creatinine,25 and previous studies have shown that cystatin C is more highly correlated with measured GFR than is serum creatinine.12,14,34 Our results suggest that GFR equations using cystatin C are also more strongly associated with PAD when compared with the currently used serum creatinine MDRD formula.
Our findings also raise the possibility that cystatin C may be a risk marker for atherosclerotic disease through mechanisms other than kidney function. We found an independent association of cystatin C with PAD even after adjustment for kidney function estimated from serum creatinine. Nonetheless, it is unclear from these data whether this independent association is a result of incomplete adjustment for kidney function—particularly when muscle mass is reduced—or whether this finding represents a true independent (non-renal) association of cystatin C with lower extremity disease.
Important limitations of this study include reliance on estimated GFR rather than measured GFR, a more precise but also more burdensome measure of kidney function not feasible to obtain in routine clinical or research settings. Because this is a cross-sectional study, we also cannot determine the directionality of the observed associations nor draw firm conclusions regarding causality.
Some strengths of this study include the large sample size, the rigorous measurement of risk factors and serum markers of kidney function by trained personnel according to standardized protocols, and the nationally representative study design. Kidney function and ABI were assessed in all participants regardless of the participant's or their physician's awareness of the condition. Finally, the cross-section design is similar to the clinical situation where a physician is faced with a PAD patient with or without CKD.
In conclusion, we found that CKD was strongly and independently associated with PAD, suggesting that kidney function may be related to the development of atherosclerosis in the lower extremities. We also found that cystatin C was a much more potent marker of lower extremity PAD when compared with the serum creatinine equation currently used in clinical practice to estimate kidney function. Indeed, the association of cystatin C with PAD persisted even after adjustment for kidney function estimated from serum creatinine. Ultimately, our results suggest that cystatin C may be an important marker of atherosclerotic vascular disease in the lower extremities and may have clinical utility when combined with serum creatinine in the evaluation of individuals who may have PAD.
Funding
This research was supported by grants UO1 DK 053869, UO1 DK 067651, and UO1 DK 35073. Dr E.S. was supported by NIH/NIDDK grant K01 DK076595. Dr A.K. was supported by a Fellowship from the German Research Foundation. Dr E.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: none declared.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Weiner DE, Tighiouart H, Amin MG, Stark PC, Macleod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 3.Landray MJ, Thambyrajah J, McGlynn FJ, Jones HJ, Baigent C, Kendall MJ, Townend JN, Wheeler DC. Epidemiological evaluation of known and suspected cardiovascular risk factors in chronic renal impairment. Am J Kidney Dis. 2001;38:537–546. doi: 10.1053/ajkd.2001.26850. [DOI] [PubMed] [Google Scholar]
- 4.O'Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol. 2001;12:2838–2847. doi: 10.1681/ASN.V12122838. [DOI] [PubMed] [Google Scholar]
- 5.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 6.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 7.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 8.O'Hare AM, Glidden DV, Fox CS, Hsu Cy. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109:320–323. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 10.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 11.Newman DJ. Cystatin C: what more do we need to know? Nephron Clin Pract. 2003;93:c122–c123. doi: 10.1159/000070230. [DOI] [PubMed] [Google Scholar]
- 12.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 14.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006;15:610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 15.Survey Operations Manuals, Brochures, and Consent Documents: 1999-current NHANES. National Center for Health Statistics, Centers for Disease Control; 2007. http://www.cdc.gov/nchs/about/major/nhanes/currentnhanes.htm . [Google Scholar]
- 16.Köttgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the United States: The Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 18.Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA. Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol. 1994;140:526–534. doi: 10.1093/oxfordjournals.aje.a117279. [DOI] [PubMed] [Google Scholar]
- 19.NHANES 2001-2002 Public Release Data File: Laboratory 40—Standard Biochemistry Profile. National Center for Health Statistics, Centers for Disease Control; 2007. http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l40_b_doc.pdf . [Google Scholar]
- 20.NHANES 1999–2000 Laboratory Procedure Manual: Creatinine. National Center for Health Statistics, Centers for Disease Control; 2007. http://www.cdc.gov/nchs/data/nhanes/frequency/lab18doc.pdf . [Google Scholar]
- 21.Selvin E, Manzi J, Stevens LA, Van LF, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39:89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D for the Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F for the Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, III, Zhang Y, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Lohr SL. Sampling Design and Analysis. Pacific Grove, CA: Duxbury Press; 1999. [Google Scholar]
- 29.National Center for Health Statistics: National Health and Nutrition Examination Survey (NHANES) Analytic Guidelines. http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/analytical_guidelines.htm. (9 May 2007)
- 30.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 32.Arpegard J, Ostergren J, de Faire U, Hansson LO, Svensson P. Cystatin C—a marker of peripheral atherosclerotic disease? Atherosclerosis. 2008;199:397–401. doi: 10.1016/j.atherosclerosis.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 33.O'Hare AM, Newman AB, Katz R, Fried LF, Stehman-Breen CO, Seliger SL, Siscovick DS, Shlipak MG. Cystatin C and incident peripheral arterial disease events in the elderly: results from the Cardiovascular Health Study. Arch Intern Med. 2005;165:2666–2670. doi: 10.1001/archinte.165.22.2666. [DOI] [PubMed] [Google Scholar]
- 34.Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147:19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 35.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the Risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]