Abstract
Ciclosporin A (CsA) is widely utilized for the treatment of inflammatory skin diseases such as psoriasis. The therapeutic effects of CsA are thought to be mediated via its immunosuppressive action on infiltrating lymphocytes in skin lesions. CsA and tacrolimus block T cell activation by inhibiting the phosphatase calcineurin and preventing translocation from the cytoplasm to the nucleus of the transcription factor Nuclear Factor of Activated T cells (NFAT). As calcineurin and NFAT 1 have been shown to be functionally active in cultured human keratocytes, expression of other NFAT family members such as NFAT-2 and possible functional activation was investigated in human keratocytes. RT-PCR and Western Analysis were used to investigate the presence of NFAT-2 mRNA and protein in human keratocytes. Tissue culture of human keratocytes and immunostaining of cells on coverslips and confocal microscopy were used to assess the degree of nuclear localisation of NFAT-2 in cultured cells. Keratome biopsies were taken from patients with psoriasis (lesional and non-lesional skin) and normal skin and immunohistochemistry was used to assess the NFAT-2 localisation in these biopsies using a well characterized anti-NFAT-2 antibody. The NFAT-2 mRNA and protein expression was demonstrated using RT-PCR and Western blotting. Moreover, the expression of NFAT-2 in normal skin, non-lesional and lesional psoriasis showed a striking basal staining suggesting a role for NFAT-2 in keratocytes proliferation. A range of cell types in the skin express NFAT-2. The expression of NFAT-2 in human keratocytes and response to different agonists provides perhaps a unique opportunity to examine the regulation, subcellular localization and kinetics of translocation of different NFATs in primary cultured human cells. In these experiments the author assessed the expression, localization of NFAT-2 in cultured human keratocytes and measured the degree of nuclear localisaion of NFAT-2 using immunofluorescence and confocal microscopy and whether CsA and tacrolimus inhibit NFAT-2 nuclear translocation. As with NFAT 1, differentiation-promoting agents that increase intracellular calcium concentration induced nuclear translocation of NFAT-2 in cultured keratocytes but with different kinetics. These data provide the first evidence of that NFAT-2 is expressed in normal skin, psoriasis and that NFAT-2 functionally active in human keratocytes and that nuclear translocation of NFAT-2 in human skin cells has different kinetics than NFAT 1 suggesting that NFAT-2 may play an important role in regulation of keratocytes proliferation and differentiation at a different stage. Inhibition of this pathway in human epidermal keratocytes many account, in part for the therapeutic effects of CsA and tacrolimus in skin disorders such as psoriasis. Thus, supporting our previous work data that calcineurin/NFAT is functionally active not only in T cells, but in skin cells.
Keywords: Ciclosporin A (CsA), keratocytes differentiation, calcineurin, NFAT-2, intracellular calcium, psoriasis, Tacrolimus
Introduction
Psoriasis is a chronic, disabling skin disorder, affecting approximately 2% of the Western population. Psoriasis is characterized by epidermal hyperplasia due to high keratocytes proliferation rate, abnormal keratocytes differentiation, infiltration of the skin by activated lymphocytes and neutrophils.
Ciclosporin A (CsA) is widely utilized for the treatment of inflammatory skin diseases such as psoriasis vulgaris and atopic dermatitis. The therapeutic effects of CsA are thought to be mediated via its immunosuppressive action on infiltrating lymphocytes in skin lesions. CsA and tacrolimus block T cell activation by inhibiting the phosphatase calcineurin and preventing translocation from the cytoplasm to the nucleus of the transcription factor Nuclear Factor of Activated T cells (NFAT). The role of NFAT 1, NFAT-2 and NFAT 4 in the immune response has been well characterised [1-8]. NFAT 1, NFAT-2 and NFAT 4 are known to be expressed in cells of the immune system as well as in a specific number of cell types, whereas NFAT 3 was shown to be expressed mainly outside the immune system [9].
For NFAT 1 to 4 proteins, NFAT dependent gene transcription requires calcium mobilisation typically a sustained increase in the intracellular free calcium levels [10]. These NFAT proteins are characteristically activated by stimulation of receptors coupled to calcium mobilisation. They are all cytoplasmic and highly phosphorylated in resting cells. After cell stimulation, four consecutive steps lead to the activation of NFAT: 1. Dephosphorylation by the calcium-dependent phosphatase calcineurin, a process inhibited by immune-suppressive drugs CsA and tacrolimus [11-16]. 2. Translocation into the nucleus [14, 17, 18]. 3. Binding to specific DNA elements in the regulatory regions of the target genes, which occurs in functional or physical co-operation with other transcription factors including AP-1 (Fos/Jun), c-Maf, and GATA family proteins [9, 15, 19-22]. 4. interaction with known or putative coactivator proteins such as p300, CBP, and NIP-45 [1, 23, 24] (Figure 1).
Figure 1.
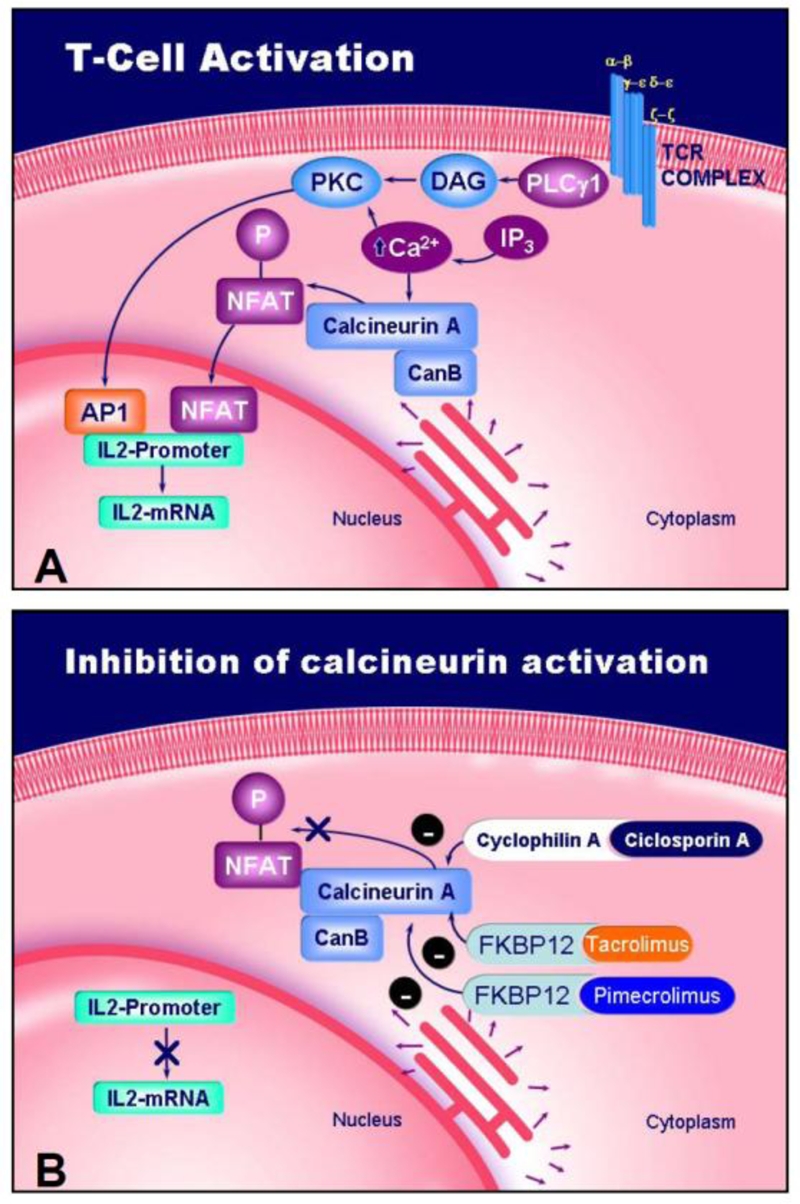
Schematic representation of T cell activation (A) and mechanism of action of ciclosporin A (CsA), tacrolimus and pimecrolimus (B). Inhibition of the phosphatase calcineurin blocks nuclear translocation of NFAT.
NFAT 1-4 show 60-70% homology within the DNA binding region and about 40% homology within the NFAT homology region that accounts for the functional similarities. An in vitro DNA-binding assay and the data obtained from a reporter gene assay conducted in transfection systems suggested an overlapping DNA-binding and transcriptional activities among NFAT family members [9, 25]. However, the C and N terminal regions of NFAT 1-4 show relatively little sequence conservation [9, 26]. The function of these domains remains to be fully elucidated, but they are likely to account for the functional differences between family members.
In lymphocytes, calcium entry regulates calcineurin activity that dephosphorylates NFAT family member unmasking the nuclear localisation sequence resulting in nuclear translocation of NFAT [15, 27]. Lymphocytes express NFAT 1, NFAT-2 and NFAT 4 [28] and each NFAT member translocates to the nucleus with the same kinetics in response to TPA plus ionomycin [25]. Together with the relatively mild phenotypes of mutant mice lacking single NFAT, suggested that NFAT family members might be functionally redundant [25, 29]. Three members of NFAT (NFAT 1, NFAT-2 and NFAT 4) were shown to be present in the cytoplasm of human muscle cells at all stages of myogenesis. However, in cultured human skeletal muscle cells each NFAT undergoes nuclear translocation at a different stage of myogenesis, suggesting that each NFAT may regulate different subsets of genes necessary for muscle cell physiology [30]. In T cells and mast cells NFAT activation is mediated by calcium signals emerging from their respective antigen receptors, TCR [31] and FcɛRI respectively [32]. The relevant receptors and signaling pathways that activate NFAT in other cell types have not been well studied. Calcineurin and NFAT 1 have been implicated in differentiation of a preadipocyte cell line to adipocytes in culture 33. NFAT 1 was also shown to inhibit cartilage cell growth and differentiation [34]. In addition, the pattern of tissue expression and phenotypes affecting non-immune tissue in knockout models indicates a specific role for individual NFAT members.
T cells from mice lacking NFAT-2 hyper-proliferate and have impaired IL-4 production [4, 5, 7]. These results are consistent with the function of NFAT-2 as a direct transcriptional activator of the IL-4 gene [4, 5, 7]. Kinoshita et al, (1997) reported interaction between NFAT-2 and NFκB and Tat resulting in enhancement of HIV-1 replication in T cell. These results suggested that NFAT-2 regulates HIV-1 replication in T cells [35].
The knockout phenotype of mice lacking NFAT-2 revealed a critical role for this protein in cardiac morphogenesis. These animals die from cardiac failure in utero due to defect in the formation of cardiac valves and ventricular septa [36, 37]. This process was calcium sensitive and is characterised by epithelial/mesenchymal transformation of the endo-cardial cushion [36, 37].
Materials and methods
Materials
Ciclosporin A (CsA) and tacrolimus were provided by Novartis Pharma AG (Basle, Switzerland) and Fujisawa Pharmaceutical Co (Osaka, Japan), respectively. Tacrolimus was also obtained from Affinity Research Products Ltd (Exeter, UK). Keratocytes growth medium (MCDB 153) and trypsin/ethylenediamine-tetraacetic acid (EDTA) were purchased from Sigma laboratories (Poole, UK). NFAT-2 primers were synthesised by MWG-Biotech AG (Ebersberg, Germany).
Keratocytes differentiation agents and growth factors including TPA, ionomycin, Transforming growth factor β TGF-β, Retinoic Acid (RA) and Dimethyl Sulphoxide (DMSO) (vehicle control) were obtained from Sigma (Poole, UK).
Precast polyacrylamide gels were purchased from Invitrogen (Paisley, UK). Hybond enhanced chemiluminescence (ECL) nitrocellulose membranes, ECL molecular weight markers were obtained from Amersham (Buckinghamshire, UK). Prestained protein standards were provided by Bio-Rad Laboratories Ltd (Herts, UK). Anti-NFAT-2 dilution was 1:7500.
Anti-NFATc1 (NFAT-2) was obtained from (BD Pharminagen, CA, USA) and Anti-NFAT-2 (801) was kindly made available by Dr Nancy Rice, NCI-Frederick Cancer Research and Developmental Centre, Maryland, USA. This antibody has been previously characterised [28].
Tissue culture
The general tissue culture methods used followed those described by Freshney [38]. Keratocytes were isolated from normal human skin obtained from plastic and paediatric surgical procedures. Keratocytes were cultured in T75 flasks in MCDB-13 (Sigma, Poole, UK) as described [39].
Imunofluorescence staining of cultured cells for immunofluorescence microscopy
Cells were trypsinised from flasks and seeded onto sterile coverslips placed in twelve well plates, so that there were 3×104 cells on each coverslip. Coverslips were incubated in an incubator at 37°C in 5% CO2. Coverslips were prepared as described [39, 40].
Keratocytes or fibroblasts were treated with specific agents, DMSO (1:1000) (vehicle control), or switched to medium containing raised extracellular calcium (1.5 mM CaCl2) 15 min and 18 h. Some coverslip cultures were pre-treated with CsA or tacrolimus for 1 h. After the time of incubations, the medium was aspirated and the cells were washed three times in Ca2+ and Mg2+ -free ice cold PBS before being fixed.
Non-specific binding was blocked by incubating coverslips in blocking serum (diluted 1:60 in PBS) by using serum from the species in which the secondary antibody was raised [41, 42] for 10 min. 100 μl of primary antibodies against NFAT 1was added to each cover slip and incubated at room temperature for 45 min. Cells were washed three times in PBS. Cells were then incubated with 100 μl of FITC-conjugated anti-rabbit and FITC-conjugated anti-goat secondary antibody for 45 min at room temperature. Cells were washed three times with Ca2+ and Mg2+-free PBS. Cells were then incubated with 50 μg/ml propidium iodide (PI) (Sigma Laboratories; Poole, UK) for 1 h at room temperature. Finally, cells were washed three times with Ca2+ and Mg2+-free PBS. Coverslips were mounted onto slides using vectorshield fluorescence mounting medium (Vector Laboratories Ltd; Peterborough, UK) and the edges sealed with clear nail varnish.
Counting of cells showing nuclear positivity
To assess the sub cellular location quantitatively, counting was done in 4 fields of each cover slip using conventional fluorescence microscopy (Carl Zeiss, Germany) using a X60 objective lens. The numbers of cells showing positive nuclear staining were counted. In practice, at least 50 cells in 3 independent experiments (150 cells in total) were assessed at each time point.
Confocal microscopy
Cells were analysed using a Bio-Rad MRC 600 confocal laser scanning microscope (BioRad; Herts, UK), mounted on a Nikon Optiphot II (Nikon UK Ltd; Surrey, UK) upright stand with a Krypton/argon laser giving 448 nm, 568 nm, and 647 nm excitation lines. Suitable areas on the slide were located with X20 na 0.4 lens, and then imaged with a X60 na 1.4 oil immersion lens. Cells were imaged utilising 488 nm lines (FITC, Oregon Green) and 568 nm lines (Alexa 568, PI) into Photo Multiplier Tube (PMT) channel 2 and 1 respectively. Excitation using the 488 and 568 laser lines independently was necessary to reduce some effects of ‘cross-talk’ between the fluoro-chromes due to the overlap of emission spectra and was gathered stack by stack. Z-series of approximately 10 to 15 optical sections (using 1 μm Z step) were then acquired and stored on a Panasonic optical drive (1GB), later transferred to a compact disc for analysis and archival using COMOS software (Bio-Rad, version 7.0). Independent Z series images were projected and composite images merged using Confocal Assistant software (version 4.2, Todd Clark Brelje). The later processed using Adobe PhotoShop (San Jose, CA, USA). In summary, cells were fixed in 4% paraformaldehyde, permeabilised with 0.2% Triton X-100, incubated sequentially with rabbit-polyclonal anti-NFAT-2 antibody (1:400), goat anti-rabbit FITC (Sigma laboratories; Poole), UK, PI (50 μg/ml) and visualized using a Biorad confocal microscope.
Non-immune rabbit serum (Vector laboratories; Peterborough, UK) was included at equivalent concentrations as the primary antibodies in immunofluorescence studies as negative controls. In addition, equal dilution of secondary antibody was used with both the primary antibody and the negative control. Negative controls were scanned using the same settings (gain, black level and confocal aperture) as the positive control cover slips, thus ensuring that the pixel brightness values were due to antibody labeling rather than other factors such as autofluorescence or nonspecific binding. Pixel brightness data were analysed using COMOS software.
Reverse transcription-polymerase chain reaction (RT-PCR)
NFAT-2 cDNA sequences were obtained from GenBank at http://www.ncbi.nlm.nih and complementary primers were designed to amplify target sequence specific for NFAT-2. Primers sequences were confirmed using the blast analysis at http://www.ncbi.nlm.nih.gov/blast. Coding sequence for NFAT-2 was aligned using Lasergene software (DNA Star Inc., Madison; USA) and primers were designed for each calcineurin subtype or NFAT isoform in areas of low homology. Primer set for for human NFAT-2 was forward: 5′CTACTTCCTCTCCTCCGGCC 3′ and Backward: 5′GTCTCTGTAGGCCTCCAGGC 3′, resulting in amplify-cation 224 bp. To prevent ribonuclease (RNases) contamination, a number of precautions were taken to avoid RNase contamination as described [43].
Total RNA was isolated using RNeasy Mini Kit (QIAGEN; West Sussex, UK) according to manufacturer's instruction. Polymerase Chain Reaction (PCR) was performed as described [43]. Briefly, 3-5 μl of cDNA was amplified in 50μl PCR reaction which consisted of 1.5 μl of 50 mM MgCl2 (Bioline; London, UK), 5 μl 10× NH4 buffer (Bioline; London, UK), 5 μl DMSO, 1.25 μl of 25 pmol forward primer, 1.25 μl of 25 pmol reverse primer and 4 μl of dNTP's (2.5 mM each dNTP). Distilled water was added to make the total reaction volume equal 50 μl. Negative controls were included in each reaction by replacing the cDNA with water. 0.2 μl of 0.625 U BioTaq™, DNA polymerase (Bioline; London, UK) was added to the reaction after heating to 94°C for 5 min, followed by 34 cycles of denaturation at 94°C for 1 min, re-annealing at 55-57°C for 1 min and elongation at 72°C for 2 min. A final cycle of 72°C for 15 min was used. Similar cycle conditions were used for each set of primers.
PCR products agarose gel electrophoresis and DNA sequencing
PCR products were electrophoresed through 1.5% agarose gels to determine product size. Loaded samples were visualised on a UVP transilluminator and photographed (Mitsubishi camera/ Polaroid black and white film type 667).
PCR products were gel purified using a QIAGEN kit (QIAGEN; West Sussex, UK) to obtain single fragments for sequencing. DNA was separated using agarose gel electrophoresis. The appropriate band was excised, weighed and sent for sequencing. Automated sequencing was carried out by MWG-Biotech AG (Ebersberg, Germany).
Western blotting
Cells were lyzed in 2 × Sodium Dodecyl Sulphate (SDS), sample buffer (125 mM Tris-HCl, pH 6.8, 0.05% bromophenyl blue, 4% SDS, 20% glycerol and 10% β-mercapto-ethanol). Equal amounts of samples and enhanced chemiluminescence molecular weight markers (Amersham, Bucks, UK) were electophoresed through 10% polyacrylamide gels, and Western Blotting were performed using anti-NFAT-2 antibody (1:7500).
Immunohistochemical analysis of skin biopsies
Skin biopsies were obtained from normal volunteers and patients with psoriasis, following local ethical committee approval. Patients with psoriasis were excluded if they had received systemic anti-psoriatic, ultraviolet B (UVB), Psoralen and UVA (PUVA) or anti-inflammatory therapy during the last 3 months. Patients discontinued topical anti-psoriatic medication apart from emollients for two weeks prior to study. Following informed consent, paired 6 mm punch biopsies were obtained from the edge of psoriatic plaques (lesional) and non-lesional (uninvolved) skin on the lower back/buttock, under local anesthesia and embedded in optimal cutting temperature (OCT) compound, frozen and stored at −70°C until required for study. The author thanks Prof N J Reynolds (Dermatology Department, University of Newcastle upon Tyne) for providing these biopsies. Four normal volunteers (3 males, 1 female, mean age 35 years) and five patients with stable plaque psoriasis (4 males, 1 female, mean age 51.5 years) were recruited for NFAT-2 studies.
Five μm sections were cut on a cryostat (Bright; Huntingdon, England), placed on to APES-coated slides and fixed in ice-cold acetone for 15 min. Non-specific binding was blocked by incubating skin sections in blocking serum (diluted 1:60 in PBS). This was done by using serum from the species in which the secondary antibody was raised [41, 42] for 20 min at room temperature. The sections were stained with anti-NFAT-2 rabbit polyclonal antibodies (1:800) in 0.1% BSA and in Ca2+ Mg2-free PBS for 1 h at the room temperature. Sections were developed using an avidin-biotin immunoperoxidase kit (Vector Laboratories, Peterborough, UK) using Ni2+ plus 3,3′-diaminobenzidine as the chromagen and counterstained with methyl green as described [44].
The degree of staining was assessed on a semi-quantitative scale by the author but blinding was not possible due to characteristic morphological features of lesional psoriatic biopsies. The intensity of immunostaining was evaluated by using an ordinal 0-4 scale, where 0=no staining; 1= minimal; 2=minimal-moderate; 3=moderate and 4=maximal staining. Furthermore, localisation (cytoplasmic versus nuclear) of each staining was examined in basal, suprabasal and high suprabasal layers in all stained sections as described [45].
Statistical analysis
To compare the effects of specified treatment on the number of cells showing nuclear immunostaining, Chi square analysis was used. Data were analysed using Arcus Quick-stat software (Biomedical version 1.0).
Results
Expression of NFAT-2 mRNA in cultured keratocytes and cultured fibroblasts
RT-PCR of keratocyte and fibroblast cDNA, using NFAT-2 specific primers, produced the appropriate fragment size as predicted, demonstrating the presence of NFAT-2 mRNA in human epidermal keratocytes and cultured dermal fibroblasts (Figure 2). Sequencing of RT-PCR products followed by BLAST analysis confirmed the identity of the products that show 100% homology with both the predicted NFAT-2 (Accession# NM_006162), cDNA from Jurkat T cell mRNA was amplified as a positive control in these experiments.
Figure 2.
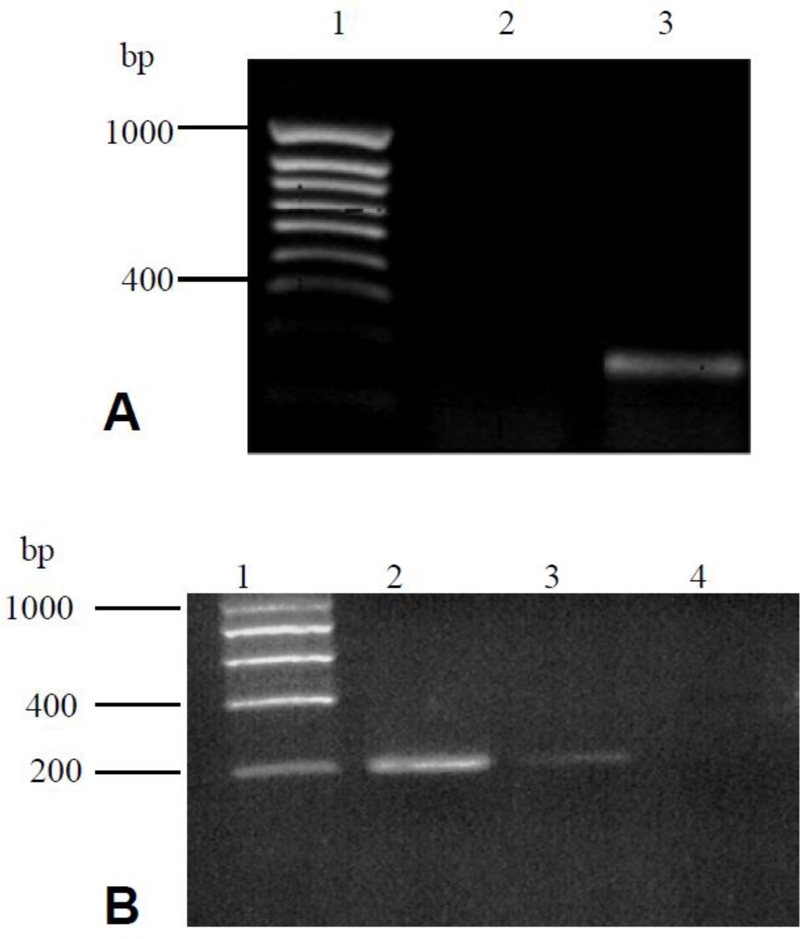
NFAT-2 mRNA expression in cultured human keratocytes, Jurkat T cells and dermal fibroblasts. Total RNA was extracted from cultured cells; reverse transcribed and PCR performed with NFAT-2-specific primers. Reaction products were separated by electro-phoresis in 1.5% agarose gels. (A), lane 1: hyperladder IV; lane 2, negative control (water); lane 3, cultured keratocytes (NFAT-2). (B) Lane 1; hyperladder I, Lane 2; Jurkat T cells (NFAT-2), lane 3; cultured dermal fibroblasts (NFAT-2), lane 4; negative control (water). Sequencing studies confirmed the expression of NFAT-2 mRNA in cultured keratocytes, dermal fibroblasts. The predicted size of the product is 224 bp for NFAT-2.
Confirmation of expression of NFAT-2, in cultured keratocytes and dermal fibroblasts by Western analysis
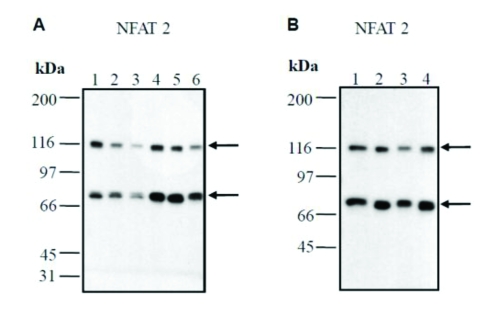
Western blotting showed that cultured human keratocytes and dermal fibroblasts co-express NFAT-2 at the protein level (Figure 3). These experiments also demonstrated that the antibodies used in immunostaining experiments detected the appropriate molecular weight of two different NFAT-2 isoforms (∼ 86 kDa and ∼ 116 kDa). In addition, another monoclonal anti-NFATc 1 (NFAT-2) antibody also detected the two isoforms of ∼ 86 kDa and ∼ 116 kDa confirming previous results. The anti-NFAT-2 antibody used did not cross react with other proteins in lysates prepared from human keratocytes or dermal fibroblasts.
Figure 3.
Western analysis confirms the expression of NFAT-2 in cultured epidermal keratocytes and dermal fibroblasts. Cell lysates were prepared from cultured keratocytes and dermal fibroblasts, separated by SDS-PAGE and immunoblotted with anti-NFAT-2 (801) antibody. This experiment confirmed that antibodies used in immunostaining detects the appropriate molecular weight of two NFAT-2 isoforms (86 kDa and 116 kDa) (A and B). (A), lane 1, medium control (keratocytes) (donor 1); lane 2, medium control (keratocytes) (donor 2); lane 3, DMSO 4 h (keratocytes) (vehicle) (donor 2); lane 4, DMSO 4 h (vehicle) (keratocytes) (donor 1); lane 5, TPA/ionomycin 4 h (donor 2); lane 6, TPA/ionomycin 4 h (donor 1). (B), lane 1, medium control (fibroblasts) (donor 1); lane 2, medium control (fibroblasts) (donor 2); lane 3, DMSO 4 h (vehicle) (fibroblasts) (donor 2); lane 4, DMSO 4 h (fibroblasts) (vehicle) (donor 1).
NFAT-2 is co-expressed in normal and psoriatic skin in vivo
Immunohistochemical studies of normal human and psoriatic skin showed prominent expression of NFAT-2 (Figure 4), in epidermal keratocytes together with expression in dermal fibroblasts. NFAT-2 immunostaining was also observed in skin appendages in normal and psoriatic skin (Figure 4E) and within the dermal inflammatory cell infiltrate in psoriasis. High levels of NFAT-2 expression were observed within the cytoplasm and the nucleus of basal epidermal keratocytes, with reduced levels within the more differentiated, suprabasal cells (Figure 4 and Table 1). No consistent differences were observed between normal skin and lesional or non-lesional psoriatic skin with respect to NFAT-2 localisation (Table 1). However, increased nuclear localisation of NFAT-2 was moderately increased in suprabasal and high suprabasal layers of psoriatic skin (Table 1).
Figure 4.
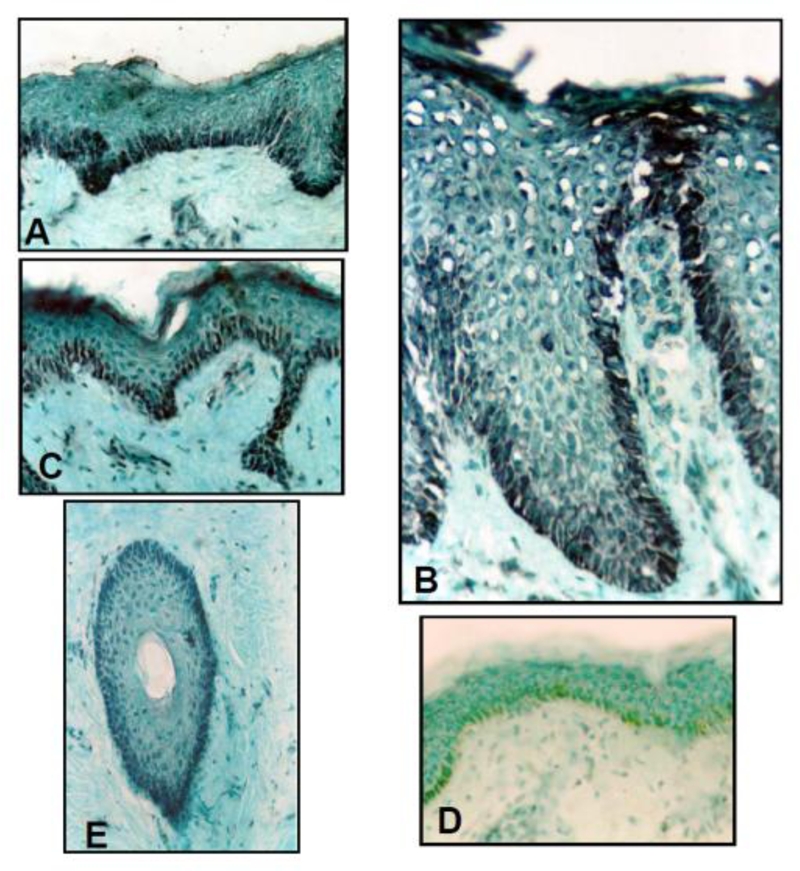
Localisation of NFAT-2 in normal human skin, lesional psoriatic skin and non-lesional psoriatic skin. Frozen sections of normal human skin (A), lesional (plaque) (B) psoriatic skin and non-lesional (uninvolved) (C) psoriatic skin were immunostained with antibody against NFAT-2 (801). NFAT-2 shows predominantly cytoplasmic and nuclear localisation in the basal layer normal and psoriatic skin (Original magnification X25). For negative control (D), equivalent dilutions of non-immune serum (normal rabbit serum) were used. (E), NFAT-2 shows predominantly nuclear and cytoplasmic localisation in the outer root sheath of hair follicles in normal skin (Original magnification X25).
Table 1.
Distribution of NFAT-2 in normal (A) and psoriatic skin (B)
| A: Normal human skin | |||
|---|---|---|---|
| Basal | Suprabasal | High suprabasal | |
| Subject 1 | 4C | 0 | 0 |
| Subject 2 | 4C | 0 | 0 |
| Subject 3 | 4N/C | 0 | 0 |
| Subject 4 | 4C | 0 | 1C |
| B. Psoriatic skin | |||
|---|---|---|---|
| Skin type | Basal | Suprabasal | High suprabasal |
| Subject 1 | |||
| Lesional | 3N/C | 0 | 1C |
| Non-lesional | 4N/C | 1C | 0 |
| Subject 2 | |||
| Lesional | 4N/C | 0 | 1N/C |
| Non-lesional | 4N | 2N | 0 |
| Subject 3 | |||
| Lesional | 4C | IN | 2N/C |
| Non-lesional | 4N/C | 0 | IN |
| Subject 4 | |||
| Lesional | 3N/C | 2N | 1C |
| Non-lesional | 4N | IN | 0 |
| Subject 5 | |||
| Lesional | 4N/C | IN | IN |
| Non-lesional | 3N/C | 1N/C | 1N/C |
The intensity of immunostaining was evaluated by using an ordinal 0-4 scale, where 0= no staining; 1= minimal; 2=minimal-moderate; 3= moderate and 4=maximal staining. N indicates that the staining was predominantly nuclear, C indicates predominantly cytoplasmic and N/C indicates an equal distribution between the two compartments.
Nuclear translocation of NFAT-2 in human keratocytes is inhibited by tacrolimus
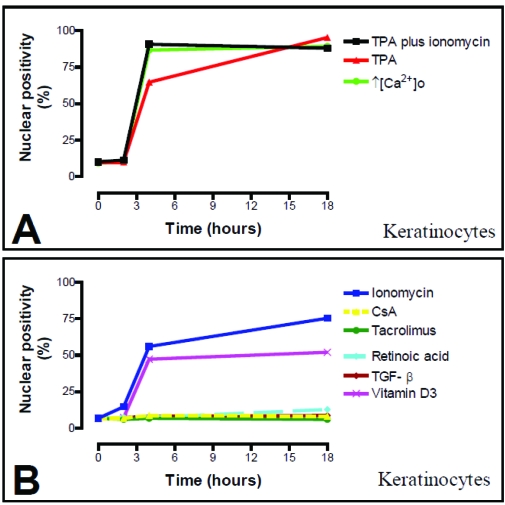
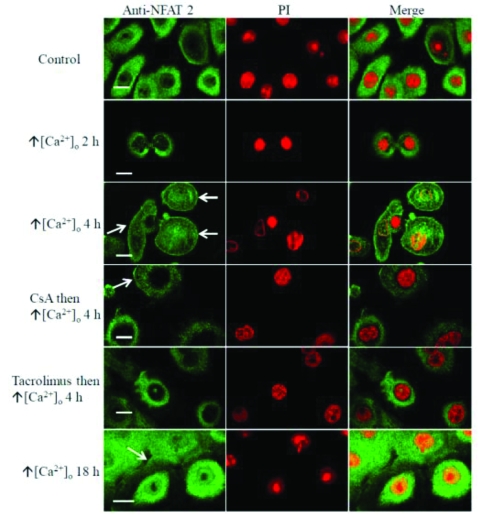
As calcineurin activation in T cells results in translocation of NFAT and calcineurin to the nucleus [9], the intracellular distribution of the NFAT-2 in cultured keratocytes was studied to determine whether this pathway is functionally active in these cells. In untreated keratocytes or cells treated with vehicle, NFAT-2 was found predominately in the cytoplasm (9.3% and 10.0% nuclear positivity respectively, n=150 cells) (Table 2). Because activation of calcineurin requires a sustained rise in intracellular calcium [9] and CsA inhibits TPA-induced changes in mouse skin [46], initially cultured human keratocytes were treated with TPA (50 nM) plus ionomycin (1 μM), agonists that induce keratocyte differentiation [47]. Increased nuclear localisation of NFAT-2 was found by 4 h (90.7 % nuclear positivity, P<0.0001), which persisted to 18 h (88.0 % nuclear positivity, P<0.0001) (Figure 5, Figure 9A and Table 2). TPA (50 nM) alone (Figure 6, Figure 9B and Table 2) or increasing extracellular calcium from 70 μM to 1.5 mM (Figure 7) also cause a rise in intracellular calcium and induce keratocyte differentiation and both these agonists resulted in increased nuclear localisation of NFAT-2 with similar kinetics to TPA plus ionomycin (Figure 1, 5 and Figure 9A).
Table 2.
Nuclear translocation of NFAT-2 in human keratocytes
| Treatment | NFAT-2 | |
|---|---|---|
| % cells showing nuclear positivity | Number of cells counted | |
| Medium control* | 9.3 | 150 |
| DMSO control† | 10.0 | 150 |
| TPA/ionomycin 2h‡ | 11.3 | 150 |
| TPA/ionomycin 4h‡ | 90.7*** | 150 |
| CsA§ 1μM then TPA/ionomycin 4h | 92.7 | 150 |
| Tacrolimus∥ 1μM then TPA/ionomycin 4h | 55.3‡‡ | 150 |
| TPA/ionomycin 18h‡ | 88.0*** | 150 |
| TPA 2h¶ | 10.0 | 150 |
| TPA 4 h¶ | 64.7¶¶ | 150 |
| CsA§ 1μM then TPA (4h) | 60.7 | 150 |
| Tacrolimus∥ 1μM then TPA (4h) | 16.0‡‡‡ | 150 |
| TPA 18h¶ | 95.3¶¶ | 150 |
| Increased extracellular calcium 2h** | 10.0 | 150 |
| Increased extracellular calcium 4h** | 86.7¶¶¶ | 150 |
| CsA§ 1μM then increased extracellular calcium 4h | 43.3§§ | 150 |
| Tacrolimus∥ 1μM then increased extracellular calcium 4h | 12.7§§ | 150 |
| Increased extracellular calcium 18h** | 89.3¶¶¶ | 150 |
Low calcium (70 μM) medium,
Vehicle control,
TPA (50 nM) plus ionomycin (1 μM),
Pre-treatment with CsA for 1 h,
TPA (50 nM),
Pre-treatment with tacrolimus for 1 h,
Raised extracellular calcium (1.5 mM),
P<0.0001 compared to DMSO control,
P<0.0001 compared to DMSO control,
P<0.0001 compared to medium control,
P<0.0001 compared to increased extracellular calcium 4 h,
P<0.0001 compared to TPA 4 h,
P<0.0001 compared to TPA plus ionomycin 4 h.
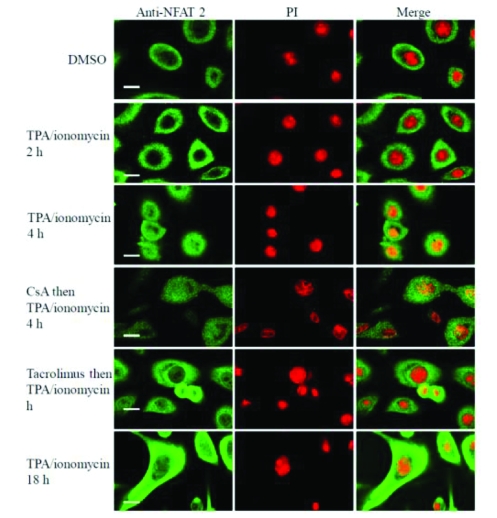
Figure 5.
Tacrolimus inhibits nuclear translocation of NFAT-2 induced by TPA plus ionomycin in human keratocytes. These results are representative of 3 experi-ments on keratocytes derived from 3 independent donors. Scale bar 25 μM.
Figure 9.
Time course of NFAT-2 subcellular localisation in response to different agonists in cultured keratocytes (A and B). Human keratocytes were cultured on coverslips and immunostained as described in Materials and Methods. The number of cells showing positive nuclear staining was counted using epifluorescence microscopy. At least 150 cells from 3 independent experiments were assessed at each time point.
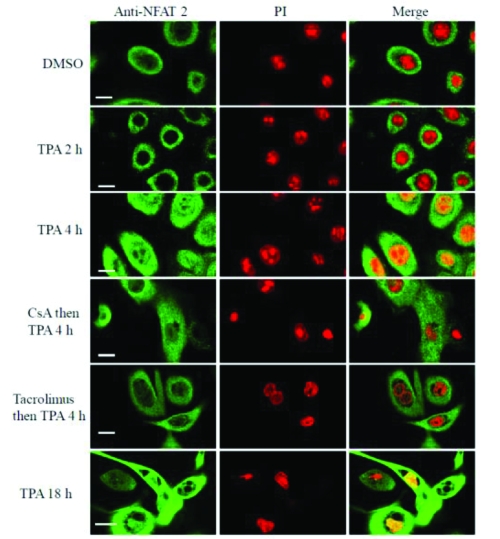
Figure 6.
Tacrolimus inhibits TPA induced nuclear translocation of NFAT-2 in human keratocytes. These results are representative of 3 experiments on keratocytes derived from 3 independent donors. Scale bar 25 μM.
Figure 7.
Nuclear and plasma membrane translocation of NFAT-2 induced by raised extracellular calcium in human keratocytes. These results are representative of 3 experiments on keratocytes derived from 3 independent donors. Scale bar, 25μM.
Whether CsA and tacrolimus inhibited translocation of endogenous NFAT-2 in human keratocytes was next investigated. CsA (1 μM) and tacrolimus (1 μM) significantly inhibited nuclear translocation of NFAT-2 induced by increased extracellular calcium at 4 h (Table 2). However, caution should be taken in interpreting analysis of this data (Table 2) as blockade by CsA was observed only in 2 out of 3 experiments, which is not consistent with a binomial distribution. Interestingly, tacrolimus (1 μM), but not CsA (1 μM), significantly inhibited nuclear translocation of NFAT-2 induced by TPA plus ionomycin or TPA alone. The comparisons of the data presented in Table 2 reflect the structure of the data and the hypotheses being tested.
In addition, increasing extracellular calcium from 70 μM to 1.5 mM resulted in marked plasma membrane translocation of NFAT-2 after 4 h and to lesser extent after 18 h (Figure 7). Tacrolimus (1 μM), but not CsA (1 μM), inhibited plasma membrane translocation of NFAT-2, that was induced by increasing extracellular calcium concentration (Figure 7). NFAT-2 plasma membrane translocation was not as marked after 18 h of raising extracellular calcium as it was after 4 h.
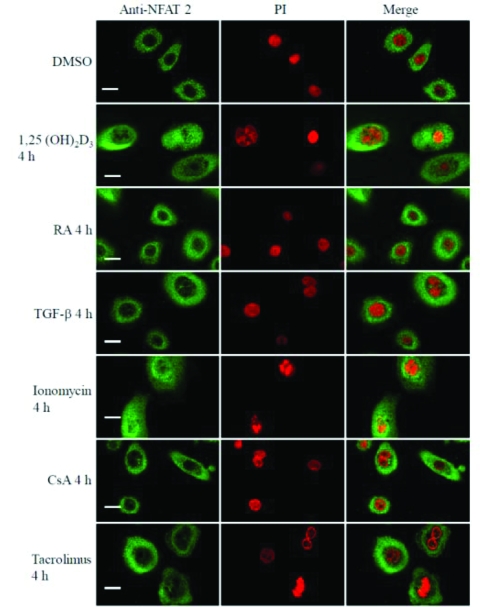
In further experiments, ionomycin (1 μM) effect on NFAT-2 activation was also examined in cultured keratocytes. As NFAT 1, ionomycin resulted in nuclear translocation of NFAT-2 in epidermal keratocytes by 2 h (14.7% nuclear positivity, P=0.04) which was maximal at 18 h (75.3% nuclear positivity, P<0.0001) compared to 6.7% nuclear positivity in cells treated with vehicle control (Figure 8, Figure 9B and Table 3). Inhibition by CsA and tacrolimus of nuclear translocation of NFAT-2 induced by ionomycin was not examined.
Figure 8.
Nuclear translocation of NFAT-2 in human keratocytes. These results are representative of 3 experiments on keratocytes derived from 3 independent donors. Scale bar, 25μM.
Table 3.
Nuclear translocation of NFAT-2 in human keratocytes
| Treatment | NFAT-2 | |
|---|---|---|
| % cells showing nuclear positivity | Number of cells counted | |
| Medium control* | 6.7 | 150 |
| DMSO control† | 6.7 | 150 |
| Ionomycin 2h‡ | 14.7§§ | 150 |
| Ionomycin 4h‡ | 56.0§§§ | 150 |
| Ionomycin 18 h‡ | 75.3§§§ | 150 |
| CsA 2h¶ | 6.7 | 150 |
| CsA 4 h¶ | 8.7 | 150 |
| CsA 18 h¶ | 8.0 | 150 |
| Tacrolimus 2 h∥ | 6.0 | 150 |
| Tacrolimus 4 h∥ | 6.7 | 150 |
| Tacrolimus 18 h∥ | 6.0 | 150 |
| Retinoic acid 2 h** | 7.3 | 150 |
| Retinoic acid 4 h** | 7.3 | 150 |
| Retinoic acid 18 h** | 12.7 | 150 |
| TGF-β 2 h§ | 7.3 | 150 |
| TGF-β 4 h§ | 8.0 | 150 |
| TGF-β 18 h§ | 8.7 | 150 |
| 1,25(OH)2D3 2 h# | 7.3 | 150 |
| 1,25(OH)2D3 4 h# | 47.3## | 150 |
| 1,25(OH)2D3 18 h# | 52.0## | 150 |
Low calcium (70 μM) medium,
Vehicle control,
Ionomycin (1 μM),
CsA (1 μM),
Tacrolimus (1 μM),
Retinoic acid (100 nM),
TGF-β (1ng/ml),
1, 25 (OH)2D3 (100nM),
P=0.04 compared to DMSO control,
P<0.0001 compared to DMSO control,
P<0.0001 compared to DMSO control.
1α, 25 dihydroxycholecalciferol vitamin D3 induces nuclear translocation of NFAT-2 in cultured keratocytes
As TPA and raised extracellular calcium induce keratocyte growth arrest and differentiation, the effects of 1, 25 (OH)2D3, a differentiation promoting agent was investigated. Addition of 100 nM of 1,25 (OH)2D3 to cultured keratocytes is known to induce a gradual increase in intracellular calcium concentration after 4 h [49]. In untreated keratocytes or cells treated with vehicle, NFAT-2 was found predominately in the cytoplasm (6.7% and 6.7% nuclear positivity respectively, n=150 cells in three independent experiments). 1, 25 (OH)2D3 (100 nM) resulted in increased nuclear localisation of NFAT-2 by 4 h (47.3 % nuclear positivity, P<0.0001) that persisted to 18 h (52.0 % nuclear positivity, P<0.0001) (Figure 8, Figure 9B and Table 3).
Retinoic acid and TGF-β do not induce nuclear translocation of NFAT-2 in cultured keratocytes
To determine whether NFAT-2 nuclear translocation was associated with growth arrest or differentiation, the effects of both RA and TGF-β were investigated. RA and TGF-β do not increase intracellular calcium and do not promote differentiation of cultured keratocytes [48, 50, 51]. RA (100 nM) or TGF-β (1ng/ml) did not significantly result in increased nuclear localisation of NFAT-2 in keratocytes between 2 h and 18 h compared to untreated cells or cells treated with vehicle control (Figure 8, Figure 9B and Table 3).
Discussion
These studies show for the first time that NFAT-2 mRNA and proteins are expressed in both cultured epidermal keratocytes and cultured dermal fibroblasts by using RT-PCR and Western blotting techniques.
The NFAT-2, NFAT 3 and NFAT 4 antibodies have been previously characterized [28] but their specificity in skin cells is supported by the single band observed on Western blot analysis. Compared to NFAT 1, the various staining patterns in skin, the subcellular localisation for NFAT-2 observed by immunofluorescence studies and the kinetics and patterns of translocation observed in response to a variety of stimuli varied considerably.
While basal epidermal cells mainly expressed NFAT-2, all dermal fibroblasts appeared to express NFAT-2 protein. The different pattern of expression of various NFAT proteins in normal and psoriatic epidermis suggests diverse functions of each NFAT member in keratocytes [39].
The epidermis is a continuously renewing, keratinising squamous epithelium. A basal layer of relatively undifferentiated, proliferative keratocytes give rise to daughter cells that are moved upward to form the supra basal differentiated layers. During this migration, the daughter cells withdraw from the cell cycle and undergo changes that are consequence of differential gene expression [52]. Finally, these events transform the keratocyte into a terminal corneocyte, which consists of a network of disulfide cross-linked keratin surrounded by a covalently cross-linked envelope of protein [53-56]. The striking localisation of NFAT-2 in basal layers suggests that NFAT-2 might play a role in keratocytes proliferation. Therefore, treating keratocytes with growth factors such as TGF α and EGF might identify a possible role for NFAT in regulation of keratocytes proliferation. However, EGF (10ng/ml) did not induce NFAT transactivation in mouse epidermal JB6 cells [57].
Multiple additional NFAT-2 isoforms arise because of alternative splicing, and some studies suggest that these isoforms can differentially regulate transcription [31, 58, 59]. In human tissues, NFAT-2 is expressed as two mRNA of 4.7 kb and 2.7 kb [60-62]. The 2.7 kb mRNA was restricted to muscle, leukocytes and thymus. On the other hand, the 4.7 kb mRNA showed a broader tissue distribution. It was highly expressed in skeletal muscle but low levels were seen in the thymus, colon, ovary, small intestine, testis, lung, heart, and prostate [60-62]. At the protein level anti-sera specific against NFAT-2 immunoprecipitated mainly an isoform of 86 kDa from T cells. Two additional isoforms 110-120 kDa and 140 kDa were also immunoprecipitated and may correspond to splice variants [28]. The expression of the three different NFAT-2 isoforms was also observed by Western analysis using anti-NFAT-2 (clone 7A6) antibody [25]. Two isoforms (86 kDa and 110-120 kDa) were detected by Western blotting in both cultured keratocytes and cultured fibroblasts. However, the 140 kDa isoform was not detected in skin cells. This appears to reflect ongoing transcription of the genes encoding NFAT-2 in cultured keratocytes and dermal fibroblasts because these cells constitutively express NFAT-2 mRNA. These results were obtained using two different NFAT-2 specific antibodies.
Keratocytes are the main cell type in the epidermis. Although there are some differences between cell culture and human skin in vivo, cultured keratocytes have proved useful in investigating keratocyte growth and differentiation [63, 64]. Therefore, cultured keratocytes were used to study the role of calcineurin/NFAT pathway in keratocyte growth and differentiation.
NFAT 1 and NFAT-2 all appeared predominantly cytoplasmic in untreated subconfluent keratocytes. As with NFAT 1, differentiation-promoting agents that increase intracellular calcium concentration induced nuclear translocation of NFAT-2. For example, nuclear translocation of NFAT-2 in response to raised extracellular calcium concentrations, TPA alone and TPA plus ionomycin appeared to happen later compared to NFAT 1.
Previous studies have shown that NFAT-dependent transactivation requires a second signal provided by protein kinase C [26, 65, 66]. However, ionomycin alone induced nuclear translocation of NFAT-2 in B cells [10] and in HeLa cells [67]. Furthermore, luciferase expression was stimulated when pNFAT-luc-transfected HeLa cells were treated with ionomycin alone [67]. As with NFAT 1, ionomycin alone did induce nuclear translocation of NFAT-2 in cultured keratocytes.
The relatively wide distribution of NFAT proteins, coupled with the known function of GSK-3β in developmental signalling pathways in Xenopus and Drosophila, also suggested that NFAT proteins might be important in cellular differentiation programs outside the immune system [68-70]. This is because GSK-3β is required for ventral differentiation, while dorsal differentiation involves suppression of GSK-3β activity in Drosophila [68]. In addition, a potential role of NFAT in the process of adipocyte differentiation has been documented [33]. Indeed, nuclear translocation of endogenous NFAT-2 in normal human keratocytes in response to differentiation promoting agents provides extra evidence that the calcineurin/NFAT pathway is functionally active in these cells. Together with the differentiation dependent pattern of expression of NFAT family members, suggest an essential role for these proteins in specifying the temporal and spatial pattern of gene expression during keratocyte differentiation. Further studies utilising, for example, GFP-tagged proteins are required to confirm immunostaining studies as described. However studies of NFAT 4-GFP in cultured mouse keratocytes showed nuclear translocation of NFAT 4-GFP in response to increasing extracellular calcium from 70 μM to 2 mM [71], supporting our immunofluorescence data with NFAT 4.
The steroid hormone 1, 25 (OH)2D3 regulates keratocytes proliferation and differentiation. 1, 25 (OH)2D3 inhibits growth of keratocytes in monolayer culture and promotes differentiation [72-74]. 1,25 (OH)2D3 (100 nM), which is known to increase intracellular calcium concentration in cultured keratocytes [49], resulted in nuclear translocation of NFAT-2 in keratocytes. This further supports a role of NFAT-2 in regulating keratocyte differentiation.
TGF-β exerts a wide range of biological effects on keratocytes. In keratocytes, TGF-β has anti-proliferative effects and is known to increase the production of extracellular matrix and synthesis of plasminogen activator [75-77]. TGF-β induces keratocyte growth arrest and does not alter intracellular calcium concentration [48]. As expected, TGF-β did not induce nuclear translocation of NFAT-2 in epidermal keratocytes. Thus, agents that increase intracellular calcium concentration, but not these inducing growth arrests alone, appear to be important regulators of both keratocytes differentiation and NFAT-2 activation. A number of keratocyte differentiation markers such as loricrin, filaggrin and transglutaminase are inhibited by RA [78]. A decrease in intracellular free calcium levels has been shown using the calcium-sensitive dye, Indo-1, in human epidermal keratocytes [50]. Similarly, RA that is known to induce growth arrest and do not promote keratocyte differentiation, did not result in nuclear translocation of NFAT-2 in cultured keratocytes.
The different kinetics of translocation of each NFAT might be important in keratocyte differentiation since other studies indicated that multiple types of NFAT proteins can be expressed in certain tissue, but only during specific stages of development do individual NFAT proteins undergo nuclear translocation in response to increase intracellular calcium concentrations. Examples of such developmental specificity were observed in skeletal muscle cell [30, 79], and during thymocyte differentiation [80]. Furthermore, although all four known NFAT family members are present in cartilage cells, only NFAT 1 regulates the process of chondrogenesis [34]. In accordance with these studies, our results may imply that different NFAT proteins may regulate specific sets of genes required during different stages of keratocyte differentiation.
In contrast to NFAT 1, tacrolimus (1 μM), but not CsA (1 μM), inhibited nuclear translocation of NFAT-2. At subnanomolar concentrations, it was shown that tacrolimus inhibits the proliferation of human T cells stimulated by specific antigens [81-84]. It was clear in these studies that tacrolimus exerts its effect in a manner similar to CsA but with 10-100-fold higher potency [81-84]. Tacrolimus appeared to be more potent in inhibiting NFAT-2 nuclear translocation in cultured keratocytes than it does for NFAT 1. Further studies using lower concentrations of tacrolimus and higher CsA concentrations are required to further investigate this issue in skin cells. Inhibition of this pathway in epidermal keratocytes may account, in part, for therapeutic effects of CsA and tacrolimus in skin diseases such as psoriasis.
This study according to my knowledge is one of the first observations of plasma membrane translocation of NFAT-2 proteins. Similar to Calcineurin B [39], plasma membrane translocation of NFAT-2 in keratocytes was induced by increasing extracellular calcium from 70 μM to 1.5 mM and was also most marked at 4 h. In contrast to CaNB, CsA did not inhibit plasma membrane translocation of NFAT-2. In addition, CsA induced plasma membrane translocation of NFAT-2 in human dermal fibroblasts. Furthermore, TPA plus ionomycin also induced plasma membrane translocation of NFAT 4 in dermal fibroblasts. It was reported that Bcl-2 (an apoptosis suppresser) forms a tight complex with calcineurin, resulting in targeting of calcineurin to Bcl-2 sites on the plasma membrane [85]. Recently, it was demonstrated that the potential to regulate NFAT is a conserved property of Nef, a 27-34 kDa myristoylated protein unique to primate lentivirus [86], and that Nef residues involved in membrane targeting and SH3 binding are essential for Nef function [87]. Nef genes are important for the development of AIDS in humans [86]. LCK, a Src non-receptor protein tyrosine kinase is essential for signal transduction via TCR and must be attached to the plasma membrane via dual acylation of its N-terminus for proper TCR signalling [88]. Expression of specific mutants of these acylated sites in LCK negative cell line activated NFAT [89].
Hypertrichosis is a well-known side effect of systemic CsA. This side effects of CsA is probably independent of T cells as they occur in T cell-deficient nude mice 90, 91. The calcium signal is transmitted into an intracellular response by different calcium-binding proteins. Some of these proteins such as annexin-1, caldesmon and calmodulin have been studied by immunohistochemistry in hair follicles [92-94]. Calmodulin was reported to be expressed with a potential significance in hyper-proliferative skin disorders [94]. In addition, the protein kinase C isoform nPKC eta, which can be stimulated by activation of calmodulin was localised only to the outer root sheath [95]. Interestingly, all the calcineurin/NFAT pathway components were localised to the outer root sheath of hair follicles, suggesting a role for this pathway in increasing hair growth in CsA treated patients.
We have previously shown that treatment of cultured human keratocytes with agents that induce a sustained rise in intracellular calcium, including elevation of extracellular calcium leads to nuclear translocation of endogenous NFAT1, which was inhibited by pre-treatment with CsA, tacrolimus39, 96 and recently with nifedipine [40]. These data provide the first evidence of that NFAT-2 is functionally active in human keratocytes and dermal fibroblasts and that nuclear translocation of NFAT-2 in human skin cell may play an important role in regulation of keratocytes differentiation as demonstrated by response to differentiation signal and sustained mobilization of calcium, as in T cells, is required for NFAT activation. Inhibition of this pathway in human epidermal keratocytes many account, in part for the therapeutic effects of CsA and tacrolimus in skin disorders such as psoriasis. Ultimately, the identification of NFAT-regulated genes in skin cells will be crucial for developing an understanding of the physiological role that these transcription factors play in skin differentiation. However, the functional significance of each NFAT member in skin remains to be fully explored.
Acknowledgments
The first author thanks Dr. N. J. Reynolds for his support as this work has been carried in his laboratory. However, all experimental design, actual work, funding analysis and writing were performed by Dr Wael Al-Daraji, as part of his Doctor of Medicine degree thesis, University of Newcastle, 2002.
References
- 1.Hodge MR, Chun HJ, Rengarajan J, Alt A, Lieberson R, Glimcher LH. NF-AT-Driven interleukin-4 transcription potentiated by NIP45. Science. 1996;274:1903–5. doi: 10.1126/science.274.5294.1903. [DOI] [PubMed] [Google Scholar]
- 2.Kiani A, Viola JP, Lichtman AH, Rao A. Down-regulation of IL-4 gene transcription and control of Th2 cell differentiation by a mechanism involving NFAT1. Immunity. 1997;7:849–60. doi: 10.1016/s1074-7613(00)80403-3. [DOI] [PubMed] [Google Scholar]
- 3.Oukka M, Ho IC, de la Brousse FC, Hoey T, Grusby MJ, Glimcher LH. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9:295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 4.Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–35. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- 5.Ranger AM, Hodge MR, Gravallese EM, et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998;8:125–34. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 6.Viola JP, Kiani A, Bozza PT, Rao A. Regulation of allergic inflammation and eosinophil recruitment in mice lacking the transcription factor NFAT1: role of interleukin-4 (IL-4) and IL-5. Blood. 1998;91:2223–30. [PubMed] [Google Scholar]
- 7.Yoshida H, Nishina H, Takimoto H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–24. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 8.Xanthoudakis S, Viola JP, Shaw KT, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–5. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 9.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 10.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 11.Masuda ES, Liu J, Imamura R, Imai SI, Arai KI, Arai N. Control of NFATxl nuclear translocation by a calcineurin-regulated inhibitory domain. Mol Cell Biol. 1997;17:2066–75. doi: 10.1128/mcb.17.4.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo C, Burgeon E, Rao A. Mechanisms of transactivation by nuclear factor of activated T cells-1. J Exp Med. 1996;184:141–7. doi: 10.1084/jem.184.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Shaw KT, Raghavan A, et al. Interaction of calcineurin with a domain of the transcription factor NFAT1 that controls nuclear import. Proc Natl Acad Sci U S A. 1996;93:8907–12. doi: 10.1073/pnas.93.17.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibasaki F, Price ER, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–3. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 15.Jain J, McCaffrey PG, Miner Z, et al. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–5. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 16.Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–37. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–9. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–40. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 19.Molkentin JD, Lu JR, Antos CL, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho N, Gullberg M, Chatila T. Activation protein 1-dependent transcriptional activation of interleukin 2 gene by Ca2+/calmodulin kinase type IV/Gr. J Exp Med. 1996;184:101–12. doi: 10.1084/jem.184.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature. 1998;392:42–8. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- 22.Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–4. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 23.Avots A, Buttmann M, Chuvpilo S, et al. CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity. 1999;10:515–24. doi: 10.1016/s1074-7613(00)80051-5. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rodriguez C, Rao A. Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP) J Exp Med. 1998;187:2031–6. doi: 10.1084/jem.187.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmerman LA, Healy JI, Ho SN, Chen L, Goodnow CC, Crabtree GR. Redundant expression but selective utilization of nuclear factor of activated T cells family members. J Immunol. 1997;159:2735–40. [PubMed] [Google Scholar]
- 26.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–81. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 27.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–7. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 28.Lyakh L, Ghosh P, Rice NR. Expression of NFAT-family proteins in normal human T cells. Mol Cell Biol. 1997;17:2475–84. doi: 10.1128/mcb.17.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–72. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- 30.Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905–16. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh C, Carew JA, Kim J, Hogan PG, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–54. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner H, Cantrell DA. Distinct Ras effector pathways are involved in Fc epsilon RI regulation of the transcriptional activity of Elk-1 and NFAT in mast cells. J Exp Med. 1997;185:43–53. doi: 10.1084/jem.185.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho IC, Kim JH, Rooney JW, Spiegelman BM, Glimcher LH. A potential role for the nuclear factor of activated T cells family of transcriptional regulatory proteins in adipogenesis. Proc Natl Acad Sci U S A. 1998;95:15537–41. doi: 10.1073/pnas.95.26.15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranger AM, Gerstenfeld LC, Wang J, et al. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med. 2000;191:9–22. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–44. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 36.Ranger AM, Grusby MJ, Hodge MR, et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–90. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 37.de la Pompa JL, Timmerman LA, Takimoto H, et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–6. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 38.Freshney RI. Culture of animal cells: a manual of basic techniques. New York: John Wiley and Sons Inc.; 2000. [Google Scholar]
- 39.Al-Daraji WI, Grant KR, Ryan K, Saxton A, Reynolds NJ. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–88. doi: 10.1046/j.1523-1747.2002.01709.x. [DOI] [PubMed] [Google Scholar]
- 40.Al-Daraji WI, Reynolds NJ. Nifedipine inhibits nuclear translocation of NFAT1 in human keratocytes. Clin Exp Dermatol. 2005 In press. [Google Scholar]
- 41.Zhou FC, Lumeng L, Li TK. Quantitative immunocytochemical evaluation of serotonergic innervation in alcoholic rat brain. Neurochem Int. 1995;26:135–43. doi: 10.1016/0197-0186(94)00108-7. [DOI] [PubMed] [Google Scholar]
- 42.Vente JD, Garssen J, Tilders FJ, Steinbusch HW, Schipper J. Single cell quantitative immunocytochemistry of cyclic GMP in the superior cervical ganglion of the rat. Brain Res. 1987;411:120–8. doi: 10.1016/0006-8993(87)90688-3. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Russel D. Molecular cloning: a laboratory maual. Vol. One. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 44.Reynolds NJ, Todd C, Angus B. Overexpression of protein kinase C-alpha and -beta isozymes by stromal dendritic cells in basal and squamous cell carcinoma. Br J Dermatol. 1997;136:666–73. [PubMed] [Google Scholar]
- 45.Reynolds NJ, Yi JY, Fisher GJ, Cooper KD, Voorhees JJ, Griffiths CE. Down-regulation of Langerhans cell protein kinase C-beta isoenzyme expression in inflammatory and hyperplastic dermatoses. Br J Dermatol. 1995;133:157–67. doi: 10.1111/j.1365-2133.1995.tb02611.x. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds NJ, Voorhees JJ, Fisher GJ. Cyclosporin A inhibits 12-O-tetradecanoyl-phorbol-13-acetate-induced cutaneous inflammation in severe combined immunodeficient mice that lack functional lymphocytes. Br J Dermatol. 1998;139:16–22. doi: 10.1046/j.1365-2133.1998.02307.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Sudol M, Hanafusa H, Krueger J. Increased tyrosine kinase activity of c-Src during calcium-induced keratocyte differentiation. Proc Natl Acad Sci U S A. 1992;89:8298–302. doi: 10.1073/pnas.89.17.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpe GR, Fisher C, Gillespie JI, Greenwell JR. Growth and differentiation stimuli induce different and distinct increases in intracellular free calcium in human keratocytes. Arch Dermatol Res. 1993;284:445–50. doi: 10.1007/BF00373354. [DOI] [PubMed] [Google Scholar]
- 49.Jones KT, Sharpe GR. Intracellular free calcium and growth changes in single human keratocytes in response to vitamin D and five 20-epi-analogues. Arch Dermatol Res. 1994;286:123–9. doi: 10.1007/BF00370738. [DOI] [PubMed] [Google Scholar]
- 50.Varani J, Inman DR, Gibbs DF, Fligiel SE, Voorhees JJ. Modulation of Ca2+ levels in keratocytes by all-trans retinoic acid. Pathobiology. 1992;60:93–9. doi: 10.1159/000163705. [DOI] [PubMed] [Google Scholar]
- 51.Varani J, Burmeister B, Perone P, Bleavins M, Johnson KJ. All-trans retinoic acid inhibits fluctuations in intracellular Ca2+ resulting from changes in extracellular Ca2+ Am J Pathol. 1995;147:718–27. [PMC free article] [PubMed] [Google Scholar]
- 52.Eckert RL, Yaffe MB, Crish JF, Murthy S, Rorke EA, Welter JF. Involucrin–structure and role in envelope assembly. J Invest Dermatol. 1993;100:613–7. doi: 10.1111/1523-1747.ep12472288. [DOI] [PubMed] [Google Scholar]
- 53.Rice RH, Green H. The cornified envelope of terminally differentiated human epidermal keratocytes consists of cross-linked protein. Cell. 1977;11:417–22. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- 54.Rice RH, Green H. Relation of protein synthesis and transglutaminase activity to formation of the cross-linked envelope during terminal differentiation of the cultured human epidermal keratocyte. J Cell Biol. 1978;76:705–11. doi: 10.1083/jcb.76.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979;18:681–94. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 56.Yaffe MB, Murthy S, Eckert RL. Evidence that involucrin is a covalently linked constituent of highly purified cultured keratocyte cornified envelopes. J Invest Dermatol. 1993;100:3–9. doi: 10.1111/1523-1747.ep12349857. [DOI] [PubMed] [Google Scholar]
- 57.Huang C, Mattjus P, Ma WY, et al. Involvement of nuclear factor of activated T cells activation in UV response. Evidence from cell culture and transgenic mice. J Biol Chem. 2000;275:9143–9. doi: 10.1074/jbc.275.13.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chuvpilo S, Avots A, Berberich-Siebelt F, et al. Multiple NF-ATc isoforms with individual transcriptional properties are synthesized in T lymphocytes. J Immunol. 1999;162:7294–301. [PubMed] [Google Scholar]
- 59.Chuvpilo S, Zimmer M, Kerstan A, et al. Alternative polyadenylation events contribute to the induction of NF-ATc in effector T cells. Immunity. 1999;10:261–9. doi: 10.1016/s1074-7613(00)80026-6. [DOI] [PubMed] [Google Scholar]
- 60.Masuda ES, Naito Y, Tokumitsu H, et al. NFATx, a novel member of the nuclear factor of activated T cells family that is expressed predominantly in the thymus. Mol Cell Biol. 1995;15:2697–706. doi: 10.1128/mcb.15.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–72. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 62.Park J, Takeuchi A, Sharma S. Characterization of a new isoform of the NFAT (nuclear factor of activated T cells) gene family member NFATc. J Biol Chem. 1996;271:20914–21. doi: 10.1074/jbc.271.34.20914. [DOI] [PubMed] [Google Scholar]
- 63.Watt FM. Keratocyte cultures: an experimental model for studying how proliferation and terminal differentiation are co-ordinated in the epidermis. J Cell Sci. 1988;90:525–9. doi: 10.1242/jcs.90.4.525. [DOI] [PubMed] [Google Scholar]
- 64.Todd C, Reynolds NJ. Up-regulation of p21WAF1 by phorbol ester and calcium in human keratocytes through a protein kinase independent pathway. Am J Pathol. 1998;153:39–45. doi: 10.1016/S0002-9440(10)65543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rincon M, Flavell RA. Regulation of the activity of the transcription factors AP-1 and NFAT during differentiation of precursor CD4+ T-cells into effector cells. Biochem Soc Trans. 1997;25:347–54. doi: 10.1042/bst0250347. [DOI] [PubMed] [Google Scholar]
- 66.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–83. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 67.Scott ES, Malcomber S, O'Hare P. Nuclear translocation and activation of the transcription factor nfat is blocked by herpes simplex virus infection. J Virol. 2001;75:9955–65. doi: 10.1128/JVI.75.20.9955-9965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–22. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 69.Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. Embo J. 1996;15:4526–36. [PMC free article] [PubMed] [Google Scholar]
- 70.Bourouis M, Moore P, Ruel L, Grau Y, Heitzler P, Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. Embo J. 1990;9:2877–84. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–80. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frampton RJ, Omond SA, Eisman JA. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. 1983;43:4443–7. [PubMed] [Google Scholar]
- 73.Bikle DD, Ng D, Tu C, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratocyte differentiation. Mol Cell Endocrinol. 2001;177:161–71. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 74.Abe J, Kondo S, Nishii Y, Kuroki T. Resistance to 1,25-dihydroxyvitamin D3 of cultured psoriatic epidermal keratocytes isolated from involved and uninvolved skin. J Clin Endocrinol Metab. 1989;68:851–4. doi: 10.1210/jcem-68-4-851. [DOI] [PubMed] [Google Scholar]
- 75.Shipley GD, Pittelkow MR, Wille JJ, Jr., Scott RE, Moses HL. Reversible inhibition of normal human prokeratocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986;46:2068–71. [PubMed] [Google Scholar]
- 76.Keski-Oja J, Koli K. Enhanced production of plasminogen activator activity in human and murine keratocytes by transforming growth factor-beta 1. J Invest Dermatol. 1992;99:193–200. doi: 10.1111/1523-1747.ep12616826. [DOI] [PubMed] [Google Scholar]
- 77.Hashiro M, Matsumoto K, Hashimoto K, Yoshikawa K. Stimulation of fibronectin secretion in cultured human keratocytes by transforming growth factor-beta not by other growth inhibitory substances. J Dermatol. 1991;18:252–7. doi: 10.1111/j.1346-8138.1991.tb03078.x. [DOI] [PubMed] [Google Scholar]
- 78.Tomic M, Jiang CK, Epstein HS, Freedberg IM, Samuels HH, Blumenberg M. Nuclear receptors for retinoic acid and thyroid hormone regulate transcription of keratin genes. Cell Regul. 1990;1:965–73. doi: 10.1091/mbc.1.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delling U, Tureckova J, Lim HW, De Windt U, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol. 2000;20:6600–11. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adachi S, Amasaki Y, Miyatake S, Arai N, Iwata M. Successive expression and activation of NFAT family members during thymocyte differentiation. J Biol Chem. 2000;275:14708–16. doi: 10.1074/jbc.275.19.14708. [DOI] [PubMed] [Google Scholar]
- 81.Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718–26. [PubMed] [Google Scholar]
- 82.Lin CS, Boltz RC, Siekierka JJ, Sigal NH. FK-506 and cyclosporin A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol. 1991;133:269–84. doi: 10.1016/0008-8749(91)90103-i. [DOI] [PubMed] [Google Scholar]
- 83.Dumont FJ, Melino MR, Staruch MJ, Koprak SL, Fischer PA, Sigal NH. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990;144:1418–24. [PubMed] [Google Scholar]
- 84.Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251–8. [PubMed] [Google Scholar]
- 85.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–31. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 86.Remkema GH, Saksela K. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front Biosci. 2000;5:D268–83. doi: 10.2741/renkema. [DOI] [PubMed] [Google Scholar]
- 87.Manninen A, Huotari P, Hiipakka M, Renkema GH, Saksela K. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J Virol. 2001;75:3034–7. doi: 10.1128/JVI.75.6.3034-3037.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. Embo J. 1997;16:4983–98. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kosugi A, Hayashi F, Liddicoat DR, Yasuda K, Saitoh S, Hamaoka T. A pivotal role of cysteine 3 of Lck tyrosine kinase for localization to glycolipid-enriched microdomains and T cell activation. Immunol Lett. 2001;76:133–8. doi: 10.1016/s0165-2478(01)00174-2. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto S, Kato R. Hair growth-stimulating effects of cyclosporin A and FK506, potent immunosuppressants. J Dermatol Sci. 1994;7:S47–54. doi: 10.1016/0923-1811(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 91.Gilhar A, Etzioni A, Krueger GG. Hair growth in human split-thickness skin grafts transplanted onto nude rats: the role of cyclosporin. Dermatologica. 1990;181:117–21. doi: 10.1159/000247898. [DOI] [PubMed] [Google Scholar]
- 92.Fava RA, Nanney LB, Wilson D, King LE., Jr Annexin-1 localization in human skpossible association with cytoskeletal elements in keratocytes of the stratum spinosum. J Invest Dermatol. 1993;101:732–7. doi: 10.1111/1523-1747.ep12371684. [DOI] [PubMed] [Google Scholar]
- 93.Matsumura F, Yamashiro S. Caldesmon. Curr Opin Cell Biol. 1993;5:70–6. doi: 10.1016/s0955-0674(05)80010-9. [DOI] [PubMed] [Google Scholar]
- 94.van de Kerkhof PC, van Erp PE. Epidermal calmodulin and skin disease. Int J Dermatol. 1985;24:507–8. doi: 10.1111/j.1365-4362.1985.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 95.Koizumi H, Kohno Y, Osada S, Ohno S, Ohkawara A, Kuroki T. Differentiation-associated localization of nPKC eta, a Ca(++)-independent protein kinase C, in normal human skin and skin diseases. J Invest Dermatol. 1993;101:858–63. doi: 10.1111/1523-1747.ep12371707. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds NJ, Al-Daraji WI. Calcineurin inhibitors and sirolimus: mechanisms of action and applications in dermatology. Clin Exp Dermatol. 2002;27:555–61. doi: 10.1046/j.1365-2230.2002.01148.x. [DOI] [PubMed] [Google Scholar]