Summary
The molecular mechanism by which mutations in the cytoskeleton-organizing protein PSTPIP1 cause the autoinflammatory PAPA syndrome is still elusive. Here, we demonstrate that PSTPIP1 requires the Familial Mediterranean Fever protein pyrin to assemble the ASC pyroptosome, a molecular platform that recruits and activates caspase-1. We provide evidence that pyrin is a cytosolic receptor for PSTPIP1. Pyrin exists as a homotrimer in an autoinhibited state due to intramolecular interactions between its pyrin domain (PYD) and B-box. Ligation by PSTPIP1, which is also a homotrimer, activates pyrin by unmasking its PYD, thereby allowing it to interact with ASC and facilitate ASC oligomerization into an active ASC pyroptosome. Because of their high binding affinity to pyrin's B-box, PAPA-associated PSTPIP1 mutants were found to be more effective than WT PSTPIP1 in inducing pyrin activation. Therefore, constitutive ligation and activation of pyrin by mutant PSTPIP1 proteins explain the autoinflammatory phenotype seen in PAPA syndrome.
Introduction
Caspase-1 plays a pivotal role in innate immunity and host defense against pathogenic infections. Caspase-1 is activated by intracellular assemblies, called inflammasomes, in response to diverse pathogenic infections and cellular stresses (Mariathasan and Monack, 2007). The activated caspase-1 cleaves the inactive pro-IL-1β and pro-IL-18 to produce the active proinflammatory cytokines IL-1β and IL-18, respectively, which are potent pro-inflammatory cytokines. Caspase-1 also plays an important role in an inflammatory form of cell death called pyroptosis (Fernandes-Alnemri et al., 2007; Fink and Cookson, 2005). During pyroptosis, caspase-1 is activated by a unique supramolecular platform called the pyroptosome, which is composed of oligomerized dimers of the adaptor protein ASC (Fernandes-Alnemri et al., 2007).
Deregulated activation of caspase-1 is responsible for a number of systemic autoinflammatory diseases in humans (Ting et al., 2006). These diseases represent a group of inherited disorders characterized by recurrent episodes of inflammation and fever without an apparent stimulus and of a major involvement of autoantibodies or autoreactive T cells (Galeazzi et al., 2006; Hull et al., 2003). Mutations in basic components of the inflammasomes appear to be responsible for some of these autoinflammatory diseases. For example, mutations in the CIAS1 gene which encodes cryopyrin cause three autoinflammatory diseases in humans (Feldmann et al., 2002; Hoffman et al., 2001). Our recent studies suggest that some of these mutations increase self-self interactions and oligomerization of the mutant cryopyrin proteins thereby causing more oligomerization of ASC and activation of caspase-1 (Yu et al., 2006). Likewise, mutations in the MEFV gene which encodes pyrin are associated with the most common autoinflammatory disease, Familial Mediterranean Fever (FMF) in humans (Consortium, 1997a; Consortium, 1997b). Pyrin interacts with ASC, and like cryopyrin, triggers ASC oligomerization, activation of procaspase-1 and IL-1β processing (Yu et al., 2006). How the FMF-associated mutations lead to increased inflammation is not yet clear, but it is likely that these mutations alter the activity of pyrin leading to increased ASC oligomerization in FMF patients.
Some human auto-inflammatory diseases are caused by mutations in upstream regulatory components of the inflammasomes (Galeazzi et al., 2006; Hull et al., 2003; Ting et al., 2006). In particular, two missense mutations in a protein called proline serine threonine phosphatase-interacting protein 1 (PSTPIP1) (also known as CD2-binding protein 1, CD2BP1) have been reported in patients with the dominantly-inherited auto-inflammatory syndrome of pyogenic arthritis, pyoderma gangrenosum, acne (PAPA) (Wise et al., 2002). PSTPIP1 interacts with pyrin (Shoham et al., 2003), and also associates with actin and plays an important role in the organization of the cytoskeleton (Badour et al., 2003; Cote et al., 2002; Spencer et al., 1997). The two PAPA-associated mutations appear to diminish the interaction of PSTPIP1 with PEST-type protein tyrosine phosphatase PTP-PEST (Wise et al., 2002), but markedly increase the binding of PSTPIP1 to pyrin (Shoham et al., 2003). Based on these observations, it was suggested that these PSTPIP1 mutants exert a dominant negative effect on pyrin and inhibit its anti-inflammatory activity leading to increased production of IL-1β in PAPA patients (Shoham et al., 2003). However, based on our recent observations that pyrin could assemble an inflammasome and could promote ASC oligomerization and caspase-1 activation (Yu et al., 2006), we propose that binding of the PSTPIP1 mutants to pyrin increases its ability to assemble the ASC pyroptosome, rather than inhibits its anti-inflammatory activity.
In this report, we investigated the role of pyrin in the mechanism of activation of caspase-1 by the PAPA-associated PSTPIP1 mutants in THP-1 monocytes and a HEK-293T cell-based reconstitution system. In support of our hypothesis, we found that pyrin is required for formation of the ASC pyroptosome and caspase-1 activation by the autoinflammatory PSTPIP1 mutants. We have also elucidated the mechanism of activation of pyrin by PSTPIP1. Our results show for the first time that both pyrin and PSTPIP1 are homotrimers and that pyrin is a cytosolic receptor for PSTPIP1. The pyrin homotrimer is not fully active due to the masking of its PYD by its B-box. Binding of PSTPIP1 to the B-box of pyrin unmasks the PYD, which then interacts with ASC, thereby causing oligomerization of ASC and formation of the ASC pyroptosome that recruits and activates procaspase-1. Our findings therefore provide the biochemical basis for understanding how pyrin activity is regulated, and shed light on pyrin as a direct activator of the ASC pyroptosome in innate immunity.
Results
Expression of PAPA-associated PSTPIP1 mutants in THP-1 induces caspase-1 activation
PAPA syndrome, like FMF, is associated with increased generation of IL-1β and is responsive to treatment with the IL-1 receptor antagonist anakinra (Chae et al., 2006; Dierselhuis et al., 2005), suggesting that the underlying cause of this disease is likely attributed to an excessive activation of caspase-1 by the mutant PSTPIP1 proteins. To test this hypothesis, we examined the effect of retrovirus-mediated transient expression of PAPA-associated PSTPIP1 mutants in THP-1 monocytes on caspase-1 activation and IL-1β generation. Indeed, expression of the A230T or E250Q PSTPIP1 mutants in THP-1 cells resulted in substantially more caspase-1 activation and IL-1β secretion compared with expression of WT PSTPIP1 or an empty vector control (Fig. 1A and B).
Figure 1. PAPA-associated PSTPIP1 mutants induce caspase-1 activation in THP-1 cells.
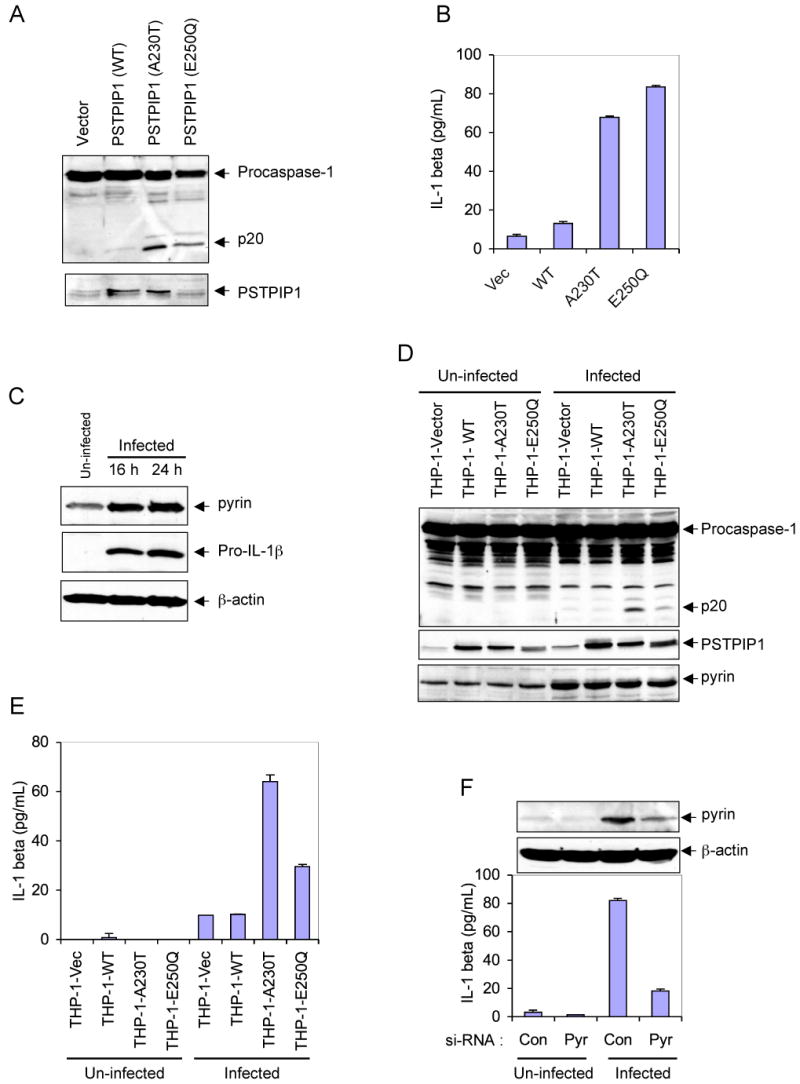
(A) Western blot analyses showing caspase-1 and PSTPIP1 in THP-1 cells 24h post-infection with an empty MSCV retroviral vector, or MSCV retroviruses encoding the indicated WT or mutant PSTPIP1 proteins.
(B) IL-1β in the culture media of the cells described in A (mean ± SD; n = 3).
(C) Western blot analyses showing pyrin, pro-IL-1β and β-actin in un-infected or empty MSCV retrovirus-infected THP-1 cells at 16 and 24h post-infection.
(D) Western blot analyses showing caspase-1, PSTPIP1 and pyrin in the indicated stable THP-1 cells, which were left without infection (un-infected) or infected with empty MSCV retrovirus for 24h.
(E) IL-1β in the culture media of the cells described in D (mean ± SD; n = 3).
(F) Stable THP-1-A230T cells were transfected with control non-specific (Con) or pyrin-specific (Pyr) siRNAs as indicated. The cells were then left untreated (Un-infected) or infected with an empty MSCV retrovirus for 24h. The secreted IL-1β in the culture media of these cells is shown (mean ± SD; n = 3). The upper panels show western blots of Pyrin and β-actin in these cells.
Retroviral infection of THP-1 induces pyrin and enhances caspase-1 activation by the mutant PSTPIP1 proteins
While studying the effect of retrovirus-mediated expression of PSTPIP1 variants in THP-1 cells, we noticed that retroviral infection, even with an empty retroviral vector, causes a dramatic increase in the expression of endogenous pyrin and pro-IL-1β proteins in the infected cells 16-24 h post-infection (Fig. 1C). Therefore, we became intrigued by the possibility that pyrin might play an important role in the robust caspase-1 activation and IL-1β generation by the autoinflammatory PSTPIP1 mutants, especially since pyrin has been shown to interact with PSTPIP1 (Shoham et al., 2003), and also to induce caspase-1 activation (Yu et al., 2006). To test this possibility, we examined the effect of retroviral infection on caspase-1 activation in THP-1 cells that stably express WT PSTPIP1 (THP-1-WT) or the two PSTPIP1 mutants A230T (THP-1-A230T) or E250Q (THP-1-E250Q). As shown in Fig. 1D, expression of endogenous pyrin was substantially increased in the retrovirus-infected cells (5th to 8th lanes) compared with the un-infected cells (1st to 4th lanes). There was also noticeable caspase-1 activation (Fig. 1D, 5th to 8th lanes) and IL-1β generation (Fig. 1E, 5th to 8th columns) in the infected cells compared to un-infected cells. Significantly, retroviral infection caused more caspase-1 activation (Fig. 1D, 7th and 8th lanes) and IL-1β generation (Fig. 1E, 7th and 8th columns) in the mutant PSTPIP1-expressing cells compared to the empty vector or the WT PSTPIP1-expressing cells (Fig. 1D, 5th and 6th lanes; Fig. 1E, 5th and 6th columns). Combined, these results reveal that pyrin is induced by retroviral infection, and its induction is associated with increased caspase-1 activation and IL-1β generation in cells expressing the PAPA-associated PSTPIP1 mutants.
Pyrin is important for IL-1β generation by the mutant-PSTPIP1 proteins in THP-1 cells
After establishing that retroviral infection causes induction of pyrin and more activation of caspase-1 in the mutant PSTPIP1-expressing cells, we next examined the effect of siRNA-mediated knockdown of pyrin on retrovirus-induced IL-1β generation in the stable THP-1-A230T cells. These cells were transiently transfected with control or pyrin-specific siRNAs, and 48 h after transfection the cells were infected with a GFP-encoding retrovirus for an additional 24 h. As shown in Fig. 1F, knocking-down pyrin significantly reduced retrovirus-induced IL-1β secretion from these cells. Taken together, our results indicate that pyrin plays an important role in the activation of caspase-1 by the auto-inflammatory PSTPIP1 mutants, since its induction increases caspase-1 activation and IL-1β generation and its knockdown has an opposite effect.
Pyrin is necessary for PSTPIP1-induced caspase-1 activation
To investigate in more detail the role of pyrin in the mechanism of caspase-1 activation by the auto-inflammatory PSTPIP1 mutants, we used a HEK-293 cell-based reconstitution system. HEK293 cells do not normally express PSTPIP1, pyrin, caspase-1 or the adaptor protein ASC (Yu et al., 2006), which makes it an ideal system to reconstitute the PSTPIP1-pyrin complex to study how PSTPIP1 interacts with pyrin to induce caspase-1 activation. Therefore, we generated stable HEK293T cell lines (293-C1AP) that express physiological levels of procaspase-1, ASC and pyrin. Other cell lines that stably express procaspase-1, ASC and cryopyrin (293-C1AC), procaspase-1 and pyrin (293-casp1-pyrin) or procaspase-1 and ASC (293-casp1-ASC) were also produced to use as controls. Next, we transfected these cell lines with WT PSTPIP1 or PAPA-associated PSTPIP1 mutants and assayed the activation of caspase-1 by western blot analysis and IL-1β processing. As shown in Fig. 2A right panels, expression of WT PSTPIP1 or PAPA-associated mutants in 293-C1AP cell line, which expresses procaspase-1, ASC and pyrin, resulted in caspase-1 activation and IL-1β processing. As observed in THP-1 cells, the PAPA-associated mutants induced more caspase-1 activation and IL-1β processing than the WT PSTPIP1 protein (3rd and 4th lanes). In contrast, expression of these PSTPIP1 proteins in the 293-casp1-ASC cells that express procaspase-1 and ASC without pyrin, did not induce caspase-1 activation (Fig. 2A, left panels), indicating that pyrin is required for PSTPIP1-induced caspase-1 activation. Similarly, expression of the PSTPIP1 proteins in the 293-casp1-pyrin cell line that expresses pyrin and procaspase-1 without ASC also did not induce caspase-1 activation (Fig. 2A, middle panels), indicating that ASC is also required in addition to pyrin for PSTPIP1 to induce caspase-1 activation.
Figure 2. Pyrin is required for activation of caspase-1 by PSTPIP1.
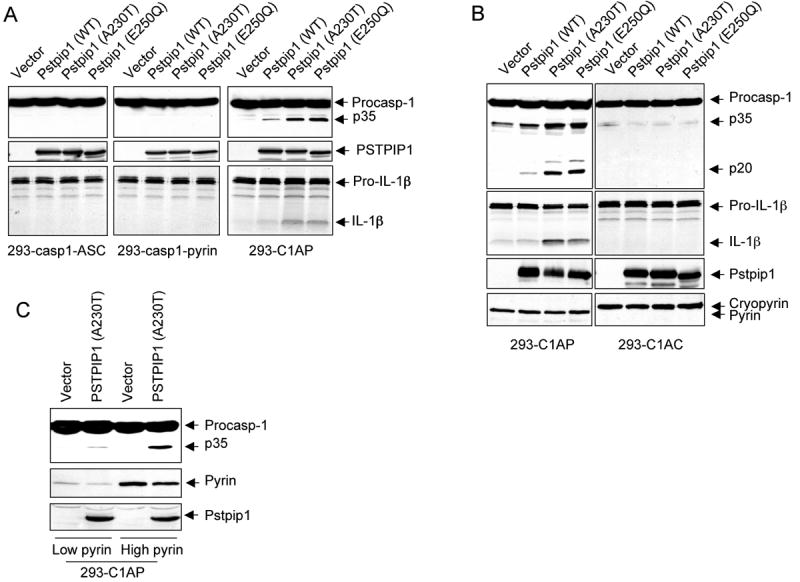
(A-C) The indicated stable 293T cell lines were transfected with the indicated plasmids. Caspase-1 and IL-1β processing were determined as in “Experimental Procedures”.
To further demonstrate that pyrin is specifically required for PSTPIP1-induced caspase-1 activation, we compared the effect of expression of PSTPIP1 variants on caspase-1 activation in the pyrin-expressing 293-C1AP and the cryopyrin-expressing 293-C1AC cell lines. In contrast to 293-C1AP, ectopic expression of WT or the PAPA-associated PSTPIP1 mutants in the cryopyrin-expressing 293-C1AC cells did not induce caspase-1 activation or IL-1β processing (Fig. 2B). These results indicate that pyrin, but not cryopyrin, is required for PSTPIP1 to induce caspase-1 activation and that the disease-associated PSTPIP1 mutants are more potent than the WT PSTPIP1 in inducing pyrin-dependent caspase-1 activation. Consistent with the above results, the ability of PSTPIP1 to activate caspase-1 was dependent on the level of pyrin in the cell. When the PSTPIP1 A230T mutant protein was expressed in two stable 293-C1AP cell lines having different levels of pyrin, it activated more caspase-1 in the higher pyrin-containing cells, than in the lower pyrin-containing cells (Fig. 2C). Taken together, our results demonstrate that pyrin is absolutely required for caspase-1 activation by PSTPIP1 and that the ability of PSTPIP1 to activate caspase-1 is enhanced at higher pyrin concentrations.
PSTPIP1 induces formation of the ASC pyroptosome in a pyrin-dependent manner
Diverse pro-inflammatory stimuli trigger the assembly of an ASC pyroptosome in monocytes and macrophages by inducing ASC dimerization (Fernandes-Alnemri et al., 2007). ASC pyroptosome assembly can be observed in live cells using a THP-1 cell line (THP-1-ASC-GFP cells) that stably expresses an ASC-GFP fusion protein (see supplementary movies 1 and 2). Considering that ASC is also important for PSTPIP1-induced caspase-1 activation, we examined the effect of retrovirus-mediated ectopic expression of the A230T PSTPIP1 mutant on ASC-GFP in the THP-1-ASC-GFP cells. As shown in Fig. 3A, infection with an empty retroviral vector induced small amount of pyroptosome formation in these cells. In contrast, infection with a retrovirus encoding the A230T PSTPIP1 mutant induced substantially more ASC pyroptosome formation compared to the empty vector control. These results indicate that PSTPIP1 induces caspase-1 activation by triggering the formation of the ASC pyroptosome in THP-1 monocytes.
Figure 3. PSTPIP1 induces pyrin-dependent ASC oligomerization.
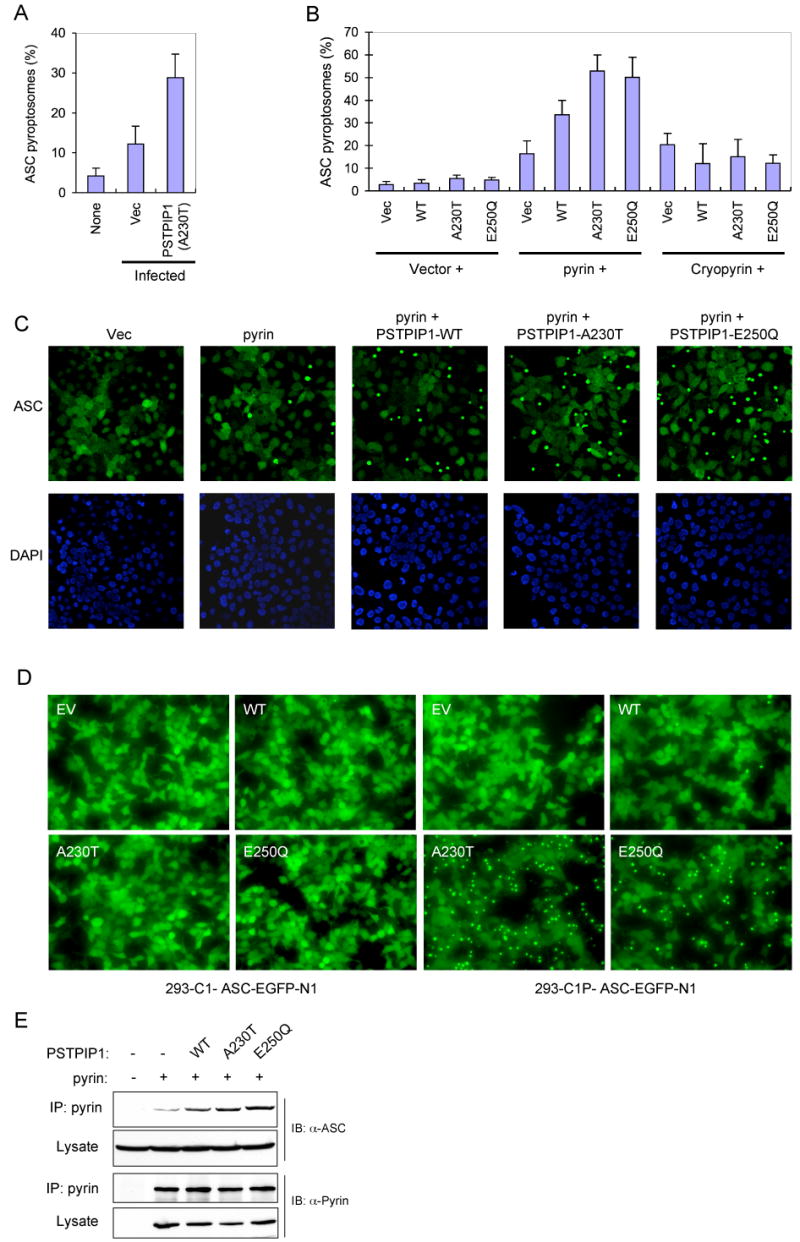
(A) Percentages of ASC pyroptosomes in THP-1-ASC-GFP cells, which were left without infection (none) or infected with an empty MSCV (Vec) or PSTPIP1-A230T-encoding (A230T) retroviral vectors (mean ± SD; n = 5).
(B) Percentages of ASC pyroptosomes in 293-ASC-EGFP-N1 cells which were co-transfected with an empty vector (1st to 4th columns) or plasmids encoding pyrin (5th to 8th columns) or cryopyrin (9th to 12th columns) together with an empty vector (Vec), or the indicated PSTPIP1 plasmids (WT, A230T, E250Q) (mean ± SD; n = 5).
(C) Fluorescence confocal micrographs showing ASC-GFP (green) or DAPI-stained nuclei (Blue) in 293-ASC-EGFP-N1 cells 24h after transfection with empty vector or the indicated plasmids.
(D) Fluorescence confocal micrographs showing ASC-GFP in 293-caspase-1-ASC-EGFP-N1 cells or 293-C1P-ASC-EGFP-N1 cells 24h after transfection with empty vector (EV) or the indicated PSTPIP1 expression plasmids (WT, A230T, and E250Q).
(E) Pyrin was immunoprecipitated (IP) from 293-ASC cell lysates 24h after co-transfection with pcDNA-pyrin-myc-His plasmid (+) together with constructs for the indicated WT or mutant PSTPIP1 proteins. The lysates and IPs were fractionated by SDS-PAGE and immunoblotted (IB) with ASC or pyrin antibodies.
To reconstitute the signaling pathway that leads to the formation of the ASC pyroptosome in response to PSTPIP1, we used a 293T-based ASC pyroptosome assembly assay similar to the THP-1-based assay. As shown in Fig. 3B and C, expression of the WT PSTPIP1 or the PAPA-associated PSTPIP1 mutants with pyrin in a stable 293-ASC-EGFP-N1 cell line, which expresses an ASC-GFP fusion protein, induced substantially more ASC pyroptosome formation than expression of pyrin alone. Consistent with the caspase-1 activation results (Fig. 2), the PSTPIP1 mutants induced more ASC pyroptosomes than WT PSTPIP1 in these cells (Fig. 3B and C). No significant change in ASC pyroptosome formation was observed when PSTPIP1 proteins were expressed without pyrin, or when co-expressed with cryopyrin, indicating that pyrin is specifically required for PSTPIP1-induced ASC pyroptosome formation (Fig. 3B).
To provide additional evidence on the critical role of pyrin in PSTPIP1-induced ASC pyroptosome formation, we examined the effect of ectopic expression of WT or mutant PSTPIP1 proteins on ASC pyroptosome formation in a HEK293 cell line that expresses caspase-1, pyrin and an ASC-GFP fusion protein (designated 293-C1P-ASC-EGFP-N1 cells) and a control HEK293 cell line that expresses only caspase-1 and ASC-GFP without pyrin (designated 293-C1-ASC-EGFP-N1 cells). As in the 293-ASC-EGFP-N1 cells, the ASC-GFP in the 293-C1P-ASC-EGFP-N1 cells was evenly distributed in the entire cytoplasm and nucleus, indicating that stable co-expression of pyrin and caspase-1 together with ASC-GFP does not affect its distribution. Consistent with the above results, the PAPA-associated PSTPIP1 mutants induced dramatic ASC pyroptosome formation in the pyrin-expressing 293-C1P-ASC-EGFP-N1 cells (Fig. 3D, right panels). In contrast, these PSTPIP1 mutants were not able to induce ASC pyroptosome formation in the control HEK293-C1-ASC-EGFP-N1 cells, which do not express pyrin (Fig. 3D, left panels). These results underscore the essential role of pyrin in mutant PSTPIP1-induced ASC oligomerization. Our results also suggest that the engagement of pyrin by the mutant PSTPIP1 proteins generates the molecular signal necessary for ASC oligomerization.
To determine how engagement of pyrin by mutant PSTPIP1 induces more ASC oligomerization, we measured the interaction of pyrin with ASC in the presence or absence of PSTPIP1. As shown in Fig. 3E, the interaction between pyrin and ASC was enhanced by co-expression of pyrin with WT PSTPIP1 and further enhanced by co-expression with the disease-associated PSTPIP1 mutants. These results indicate that PSTPIP1 induces ASC oligomerization by increasing the interaction of pyrin with ASC.
Pyrin is a homotrimer
The ability of pyrin to induce ASC oligomerization suggests that pyrin itself is an oligomer or it oligomerizes before it engages ASC. To examine the first possibility, we performed chemical cross-linking analyses with ethylene glycol bis (succinimidylsuccinate) (EGS) to determine the oligomeric state of full length pyrin. As shown in Fig. 4A, treatment of full-length pyrin from pyrin-transfected 293T cells or THP-1 cells, or purified from bacteria with low concentrations of EGS produced a major cross-linked species with apparent molecular mass of ∼300 kDa. Since monomeric pyrin has an apparent molecular mass of ∼100 kDa in SDS-PAGE, this indicates that native pyrin is a homotrimer. These results were confirmed by gel-filtration on Superdex 200, which also revealed that the native form of pyrin is indeed a homotrimer (Supplementary Fig. 1E).
Figure 4. Pyrin is a homotrimer.
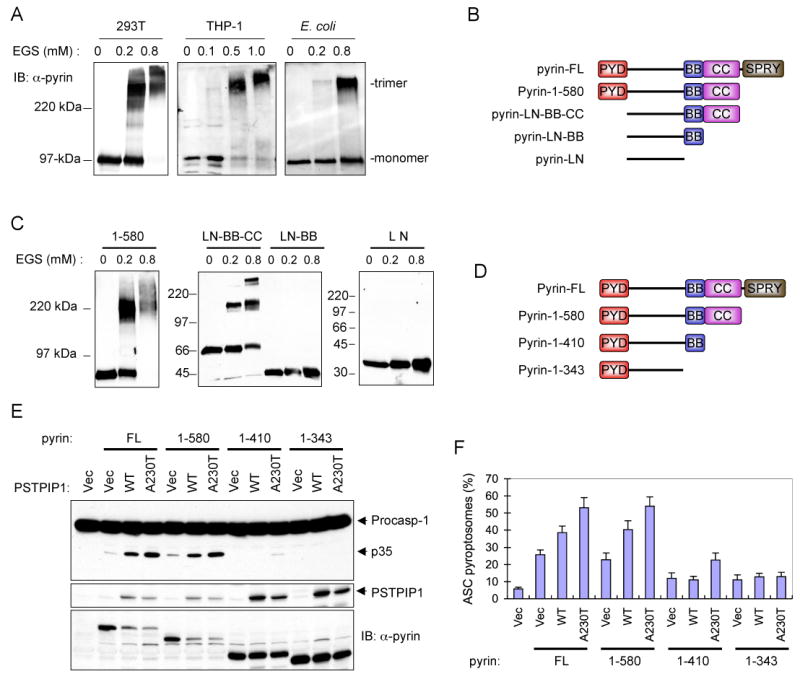
(A) Western blots showing pyrin from 293T, THP-1 or E. coli after cross-linking with the indicated concentrations of EGS.
(B) Schematic representations of the domain structure of the full-length pyrin (FL) and the truncated pyrin mutants used in C below. PYD, pyrin domain; BB, B-Box; CC, coiled-coil; SPRY, domain in SPIa and Ryanodine receptor.
(C) Western blots showing the indicated bacterially-expressed truncated pyrin mutants after cross-linking with the indicated concentrations of EGS.
(D) Schematic representations of the three C-terminal-truncated pyrin mutants (1-580, 1-410 and 1-343) used in E below.
(E) Western blots showing caspase-1, PSTPIP1 and pyrin in 293-caspase-1-ASC cells 28h after transfection with an empty vector (1st lane), or co-transfection with the indicated pyrin expression plasmids together with an empty vector (Vec), or the indicated PSTPIP1 plasmids (WT, A230T, E250Q). The decrease in pyrin expression (FL and 1-580) in the presence of PSTPIP1 is due to cell death and cleavage of pyrin by the activated caspase-1. Our data show that caspase-1 cleaves pyrin into smaller fragments (see supplementary Fig. 5)
(F) Percentages of ASC pyroptosomes in 293-ASC-EGFP-N1 cells similarly transfected as in E (mean ± SD; n = 5).
The coiled-coil domain of pyrin mediates its homotrimerization
Human pyrin contains four distinct domains; the N-terminal PYD (residues 1-92) followed by the B-box (BB) domain (residues 370-412), the coiled-coil (CC) domain (residues 420-582) and the SPRY domain (residues 597-781) (Fig. 4B, and supplementary Fig. 2). Between the PYD and B-box, pyrin contains a 278 amino acid long linker region with no homology to any known domains. The PYD of pyrin is required for pyrin-induced ASC oligomerization since PYD mutations that abolish its interaction with ASC or deletion of the PYD of pyrin inhibit pyrin-induced ASC oligomerization ((Yu et al., 2006) and data not shown).
To identify the exact region in pyrin that mediates its homotrimerization, we first determined the oligomeric state of a truncated pyrin mutant lacking the SPRY domain (pyrin-1-580). As shown in Fig. 4C, left panel, treatment of pyrin-1-580, which has an apparent molecular mass of ∼75 kDa in SDS-PAGE, with low concentrations of EGS yielded a major cross-linked species with apparent molecular mass of ∼220 kDa corresponding to a trimeric form of pyrin. This indicates that deletion of the SPRY domain of pyrin does not affect the trimeric state of pyrin. Next, we deleted the N-terminal PYD and the C-terminal SPRY domain and determine the oligomeric state of this truncated pyrin mutant (designated pyrin-LN-BB-CC) (Fig. 4B). Treatment of this truncated pyrin mutant with low concentrations of EGS also yielded a distinct trimeric species (Fig. 4C, middle panel, 2nd and 3rd lanes). This indicates that the remaining linker region, B-box or coiled-coil domain mediates pyrin homotrimerization. To test this possibility, we generated two additional truncated pyrin mutants; one containing the linker region and the B-box (pyrin-LN-BB) and the other containing only the linker region (pyrin-LN). In contrast to the pyrin-LN-BB-CC, the pyrin-LN-BB and pyrin-LN mutants, which lack the coiled-coil domain were no longer able to form any higher molecular mass cross-linked species and migrated in SDS-PAGE as monomers (Fig. 4C, middle panel, 4th to 6th lanes; right panel 1st to 3rd lanes). Collectively, these results indicate that pyrin is a homotrimer and that its trimeric state is maintained by self-association of its coiled-coil domain.
Homotrimerization of pyrin is important for its ability to induce ASC oligomerization and caspase-1 activation
To determine if homotrimerization of pyrin is critical for its activity, we examined the effect of different deletions that remove the SPRY domain, SPRY plus coiled-coil domains, or SPRY, coiled-coil plus B-box domains (Fig. 4D) on the ability of pyrin to induce ASC pyroptosome formation and caspase-1 activation in the 293-ASC-EGFP-N1 and 293-casp1-ASC cells, respectively. As shown in Fig. 4E and F, deletion of the SPRY domain of pyrin did not affect the basal or PSTPIP1-induced activities of pyrin, as similar amounts of ASC pyroptosome formation and caspase-1 activation was observed with pyrin-1-580 compared with the full-length protein. In contrast, further deletion of the coiled-coil or the coiled-coil plus B-box domains impaired both the basal and PSTPIP1-induced activities of pyrin. These results indicate that the coiled-coil domain of pyrin is critical for its basal and PSTPIP1-induced activities, whereas the SPRY domain is not essential for either activity.
Considering that deletion of the coiled-coil domain inactivates pyrin by abrogating its homotrimerization, we asked whether restoring homotrimerization with a homologous domain from the related family member Trim5α (Javanbakht et al., 2006; Mische et al., 2005) could restore pyrin activity. To this end we deleted the coiled-coil and SPRY domains of pyrin and replaced them with the homologous domains from Trim5α (Fig. 5A, 3rd diagram from top). The resulting PT-CC-SPRY chimeric protein, which contains the first 410 residues of pyrin followed by the coiled-coil and SPRY domains of Trim5α exhibited similar low basal activity as the WT pyrin protein, and induced similar amounts of ASC pyroptosome formation and caspase-1 activation (Fig. 5B and C). Furthermore, like the WT pyrin, the activity of the chimeric protein was also enhanced by co-expression with the A230T mutant PSTPIP1, indicating that the first 410 amino acids of pyrin contains all the necessary elements required for regulation by PSTPIP1. Together, these results indicate that coiled-coil-mediated trimerization of pyrin is critical for its activity.
Figure 5. The B-box regulates the activity of pyrin.
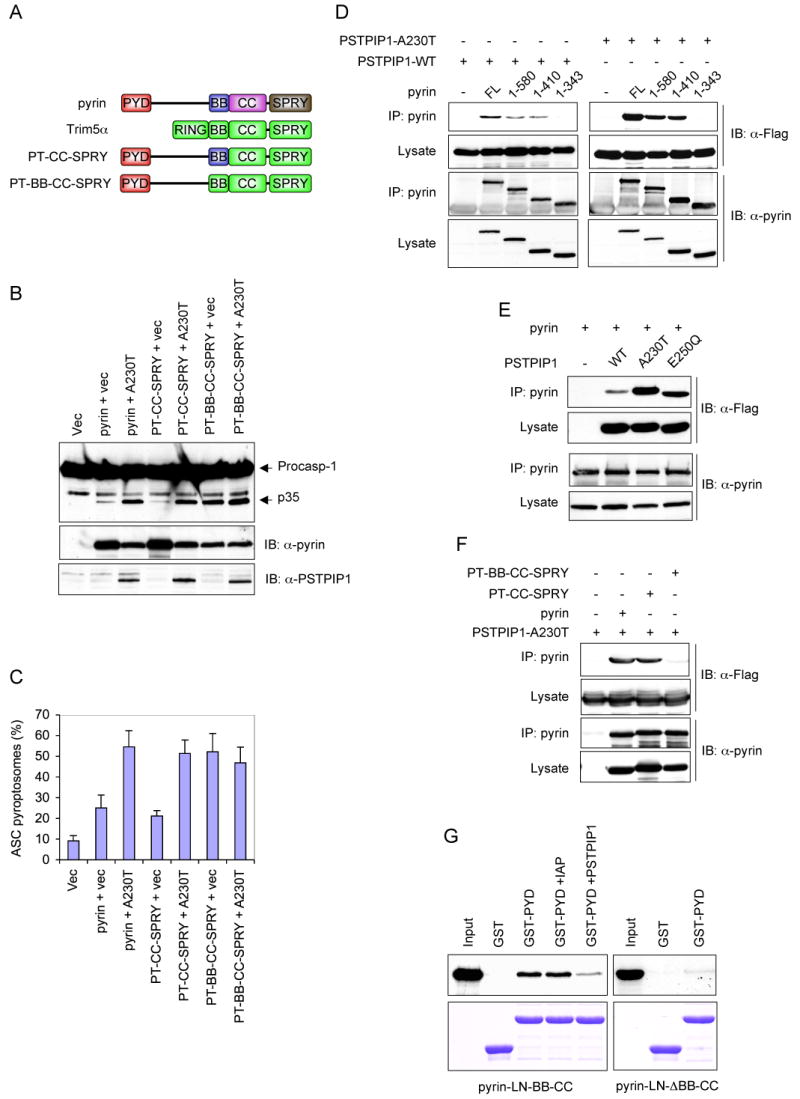
(A) Schematic representations of the domain structure of pyrin and Trim5α, and the chimeric pyrin-Trim5α mutants used in B, C and F below.
(B) Western blots showing caspase-1, PSTPIP1 and pyrin or chimeric pyrin mutants in 293-caspase-1-ASC cells 28h after transfection with an empty vector (1st lane), or co-transfection with the indicated pyrin or chimeric pyrin-Trim5α mutants plus an empty vector (vec) or PSTPIP1-A230T mutant plasmid (A230).
(C) Percentages of ASC pyroptosomes in 293-ASC-EGFP-N1 cells similarly transfected as in B (mean ± SD; n = 5).
(D-F) Pyrin or pyrin mutants were immunoprecipitated (IP) from HEK293 cell lysates 24h after transfection with the indicated plasmids for full-length (FL) pyrin or pyrin mutants plus the indicated Flag-tagged PSTPIP1 plasmids. The lysates and IPs were fractionated by SDS-PAGE and immunoblotted (IB) with anti-pyrin or anti-Flag (detects PSTPIP1) antibodies.
(G) The PYD of pyrin interacts with its B-box. GST or GST-PYD fusion protein (pyrin PYD residues 1-100) were incubated with 35S-labeled pyrin-LN-BB-CC (left panels) or pyrin-LN-ΔBB-CC (right panels) in the absence (2nd and 3rd lanes) or the presence of dIAP as a non-specific control (4th lane) or PSTPIP1 A230T (5th lane). The bound proteins were fractionated by SDS-PAGE and detected by autoradiography (top panels). The corresponding immobilized GST and GST-PYD proteins are shown in the lower panels.
PSTPIP1 activates pyrin by binding to its B-box
The ability of PSTPIP1 to enhance the activity of pyrin suggests that PSTPIP1 might interact with a regulatory domain in pyrin to modulate its activity. To map the PSTPIP1-interaction domain in pyrin, we performed co-immunoprecipitation experiments with WT PSTPIP1 or the A230T PSTPIP1 mutant, and full-length pyrin or the truncated pyrin mutants described in Fig. 4D. Both WT and the A230T mutant PSTPIP1 interacted with full length, pyrin 1-580 and pyrin 1-410, but not with the B-box-truncated pyrin 1-343 mutant (Fig. 5D), indicating that the B-box is required for this interaction. Interestingly, the disease-associated PSTPIP1 mutants, A230T and E250Q, exhibited substantially more binding to pyrin compared with the WT PSTPIP1 (Fig. 5E). These results indicate that PSTPIP1 interacts with the B-box of pyrin and that the disease-associated PSTPIP1 mutants exhibit more binding to pyrin than the WT PSTPIP1. These results also explain why the disease-associated PSTPIP1 mutants induce more robust activation of pyrin compared with the WT PSTPIP1 protein.
The B-box of pyrin is an inhibitory domain
The above results demonstrate that WT pyrin and the pyrin-Trim5α chimera PTCC-SPRY are not fully active without binding of PSTPIP1 to their B-box. This suggests that in the unoccupied state the B-box might exert an inhibitory effect on the PYD of pyrin thereby preventing it from engaging ASC. To examine these possibilities, we substituted the pyrin B-box in the PT-CC-SPRY chimera with the homologous B-box from Trim5α (Fig. 5A, 4th diagram from top). Unlike WT pyrin or the PT-CC-SPRY chimera, the new chimera (PT-BB-CC-SPRY), which contains the first 363 residues of pyrin followed by the B-box, coiled-coil and SPRY domains of Trim5α could not bind to PSTPIP1 (Fig. 5F), indicating that the B-box of Trim5α does not interact with PSTPIP1. Significantly, the basal activity of the new PT-BB-CC-SPRY chimera was substantially higher than that of the WT pyrin or the PT-CC-SPRY chimera (Figure 5B, 6th lane; Figure 5C, 6th column). The basal activity of the new chimera was comparable to the PSTPIP1-induced activity of WT pyrin and the PT-CC-SPRY chimera, and was not enhanced by co-expression with PSTPIP1. These results indicate that the B-box of pyrin indeed functions as an inhibitory domain to maintain pyrin in an inactive conformation, and that binding of PSTPIP1 to the B-box or substituting it with the B-box of Trim5α relieves its inhibitory activity resulting in activation of pyrin. Consistent with this, deletion of the B-box of pyrin generates a constitutively active pyrin that does not require PSTPIP1 to induce robust ASC oligomerization and caspase-1 activation (supplementary Fig. 3). However, the B-box-deleted pyrin mutant was slightly less active than the PSTPIP1-activated WT pyrin, perhaps because the B-box is important for proper folding and trimerization of pyrin. Indeed, it has been shown previously that an intact B-box is required for efficient oligomerization of the ret finger protein, which is also a member of the Trim family (Cao et al., 1997).
The B-box might inhibit the activity of pyrin by binding and sequestering the PYD thereby preventing it from engaging ASC. To test this possibility, we measured the interaction of the isolated PYD of pyrin with truncated pyrin-LN-BB-CC mutant, which lacks the PYD and SPRY domain. As shown in Fig. 5G, left panel, the isolated bacterially expressed PYD was able to interact with this mutant (3rd lane). This interaction was substantially reduced by PSTPIP1 (5th lane) suggesting that it is mediated by the B-box, since PSTPIP1 binds to the B-box of pyrin. Indeed deletion of the B-box from this mutant (pyrin-LN-ΔBB-CC) substantially reduced this interaction (Fig. 5G, right panel). Collectively, these results indicate that the B-box inhibits the activity of pyrin by sequestering the PYD and that binding of PSTPIP1 to the B-box or deletion of the B-box unmasks the PYD resulting in activation of pyrin.
Colchicine inhibits pyrin activity
FMF is highly responsive to treatment with the microtubule-disrupting agent colchicine (Dinarello et al., 1974; Margolis and Wilson, 1977; Zemer et al., 1986; Zemer et al., 1974) and the colchicine's responsiveness is an important diagnostic tool for FMF. Furthermore, low doses of colchicine have been shown to be effective in the treatment of pyoderma gangrenosum (Kontochristopoulos et al., 2004), a condition similar to PAPA syndrome. These observations indicate that the cytoskeleton is an important element in the pyrin inflammatory pathway. Considering these observations and that PSTPIP1 and pyrin are associated with the cytoskeleton we asked whether disruption of the cytoskeleton by colchicine could inhibit activation of caspase-1 by pyrin. Consistent with the therapeutic benefit of colchicine in FMF, colchicine completely inhibited processing of caspase-1 in response to ectopic expression of PSTPIP1 (Fig. 6A). Similar results were obtained with nocodazol, another microtubule-disrupting agent (Fig. 6B). These results thus provide further support for the critical role of pyrin as a pro-inflammatory molecule, and show for the first time that the pyrin-dependent caspase-1 activation process is a target for the microtubule-disrupting agents like colchicine and nocodazol.
Figure 6. Microtubule disrupting agents inhibit pyrin-induced caspase-1 activation.
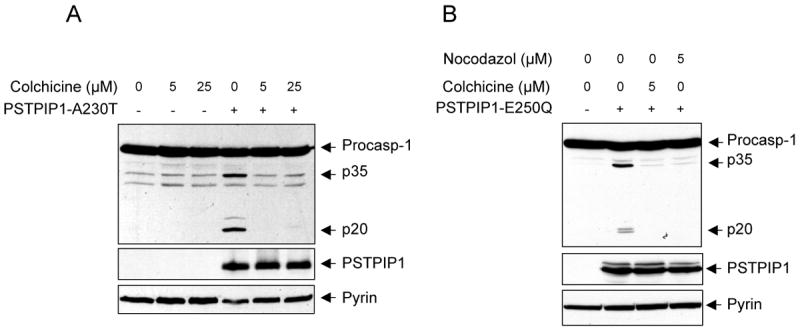
(A and B) Western blots showing caspase-1, PSTPIP1 and pyrin in 293-C1AP cells 24h after transfection with an empty vector or the indicated PSTPIP1 plasmids in the presence of the indicated concentrations of colchicine or nocodazol.
Discussion
Two missense mutations in the coiled-coil domain of the cytoskeleton binding protein PSTPIP1 have been identified in patients with the auto-inflammatory PAPA syndrome (Wise et al., 2002). Macrophages from these patients secrete excessive amounts of IL-1β compared to macrophages from normal subjects in response to LPS stimulation (Shoham et al., 2003), suggesting that the disease-associated PSTPIP1 mutants some how increase the activity of caspase-1, which is responsible for processing of the inactive pro-IL-1β to the mature biologically active IL-1β cytokine. In this report we studied the mechanism by which the disease-associated PSTPIP1 mutants activate caspase-1 and characterized the role of pyrin in this process. We demonstrate that the disease-associated PSTPIP1 mutants require pyrin to induce formation of the ASC pyroptosome (Fernandes-Alnemri et al., 2007), a molecular platform that recruits and activates caspase-1.
The exact role of pyrin in innate immunity and inflammation is still controversial. One model suggests that pyrin inhibits pro-inflammatory signaling pathways by sequestration of ASC or inhibition of the enzymatic activity of caspase-1 via its SPRY (also known as B30.2) domain (Chae et al., 2003; Chae et al., 2006), and its loss of function could lead to development of an auto-inflammatory disease. However, this model does not address previous observations that human pyrin is induced as an immediate-early response gene by pro-inflammatory stimuli like interferon alpha and gamma (Centola et al., 2000). Even more significantly mouse pyrin does not contain the SPRY domain (Chae et al., 2000), which indicates that the reported inhibition of caspase-1 by the SPRY domain of pyrin is not a conserved function of pyrin. This model also does not explain why mice that express a practically inactive C-terminal truncated pyrin do not develop an auto-inflammatory disease similar to FMF, but are only slightly hyper-responsive to LPS-induced endotoxic shock (Chae et al., 2003). An alternative model presented here suggests that pyrin functions as a pro-inflammatory mediator particularly in the case of the auto-inflammatory PAPA syndrome. This model is supported by our recently published biochemical data, which clearly showed that the interaction between pyrin and ASC results in ASC oligomerization and activation of caspase-1 (Yu et al., 2006). This model is also supported by several lines of evidence presented here, which demonstrate that pyrin is dramatically induced by retroviral infection, and its induction is associated with increased caspase-1 activation and IL-1β generation in THP-1 cells expressing the PAPA-associated PSTPIP1 mutants. In agreement with this model, siRNA-mediated knockdown of pyrin in THP-1 cells that stably express the PSTPIP1 A230T mutant reduced IL-1β secretion in response to retroviral infection. Furthermore, ectopic expression of the PAPA-associated PSTPIP1 mutants in 293 cells containing caspase-1 and ASC but lacking pyrin did not induce caspase-1 activation. In contrast, expression of these mutants in 293 cells containing caspase-1, ASC and pyrin induced caspase-1 activation, which was directly proportional to the expression level of pyrin. Altogether, the above evidence indicates that pyrin plays a critical role as a mediator of caspase-1 activation in response to expression of the disease-associated PSTPIP1 mutants.
Initiator caspases, like caspase-1, require dimerization for activation. This process is performed by oligomeric molecular platforms such as the Nalp1 inflammasome (Faustin et al., 2007), and the ASC pyroptosome that we described recently (Fernandes-Alnemri et al., 2007). These molecular platforms recruit procaspase-1 thereby increasing its local concentration on the surface of the platform to a critical level that prompts its dimerization and activation. In this study, we demonstrated that engagement of pyrin by PAPA-associated PSTPIP1 mutants induces potent ASC pyroptosome assembly. Additionally, we elucidated the mechanism by which the PSTPIP1 mutants activate the ASC pyroptosome. Our model is illustrated in Fig. 7. As demonstrated here by chemical cross-linking and gel filtration analyses, both pyrin and PSTPIP1 preexist as homotrimers (Fig. 4 and supplementary Fig. 1). In the unbound conformation, the PYD of pyrin is masked by direct interactions with its B-box, preventing recruitment of ASC to the PYD of pyrin. PSTPIP1 homotrimer binds to the pyrin homotrimer via a direct interaction with the B-box. Binding of PSTPIP1 to pyrin results in unmasking of the PYD of pyrin, which now becomes free to interact with the PYD of ASC. The close proximity of ASC monomers on the surface of the pyrin homotrimer induces ASC oligomerization, which we believe is an important initial step in the nucleation and subsequent assembly of the ASC pyroptosome. The PYD of pyrin is critical for ASC oligomerization, since point mutations in the PYD of pyrin that abrogate its interaction with the PYD of ASC also abrogate ASC oligomerization and caspase-1 activation by pyrin (Yu et al., 2006).
Figure 7. Mechanism of activation of pyrin by PSTPIP1.
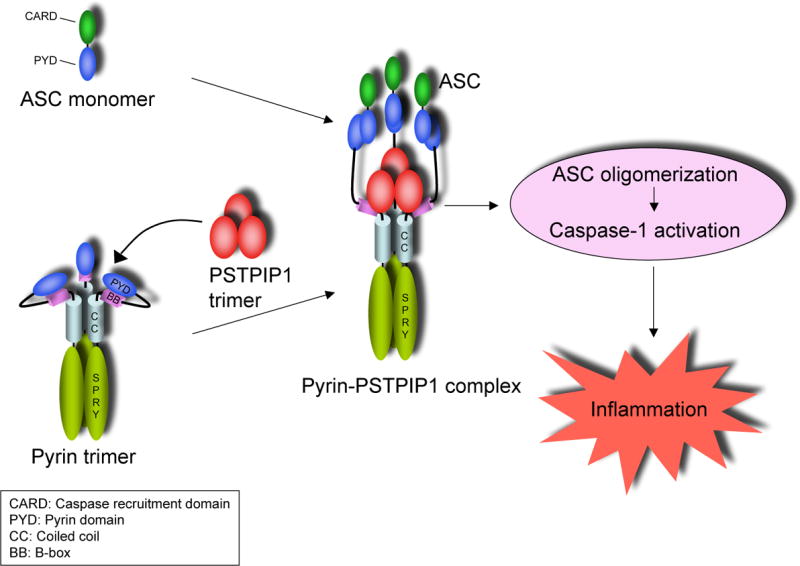
See discussion for details.
We show that the region required for optimal binding to PSTPIP1 is present within the B-box of pyrin. This region is not only important for PSTPIP1 binding but also important for auto-inhibition of pyrin, since substitution with a homologous region from Trim5α or deletion of this region resulted in constitutive activation of pyrin. These important observations explain why pyrin has a low basal activity, although it is a homotrimer. It is well established that activation of initiator caspases in mammalian cells is performed by oligomeric platforms that are assembled from monomers in response to specific stimuli. For instance, the Apaf-1 apoptosome is assembled into a heptameric molecular platform after binding of monomeric Apaf-1 to cytochrome c and ATP (Bao and Shi, 2007). Similarly, the Nalp1 inflammasome is assembled into an oligomeric platform after binding of monomeric Nalp1 to MDP and ribonucleoside triphosphates (Faustin et al., 2007). In contrast to these examples, pyrin preexists in an autoinhibited homotrimeric state, and binding of PSTPIP1 to its B-box transforms it into the active conformation. Homotrimerization of pyrin is mediated by its coiled-coil domain. This domain is a protein-protein interaction domain that has been shown to mediate oligomerization and formation of high molecular weight oligomeric complexes in the pyrin-related Trim family of proteins (Javanbakht et al., 2006; Meroni and Diez-Roux, 2005; Mische et al., 2005; Peng et al., 2000; Reymond et al., 2001). It is not yet obvious whether activation by PSTPIP1 induces further oligomerization of the pyrin homotrimer, but our cross-linking and gel filtration experiments revealed that both pyrin and PSTPIP1 form oligomeric species larger than homotrimers. Like pyrin, PSTPIP1 contains a coiled-coil domain, which likely mediates its homotrimerization (supplementary Fig. 1A). However, it is not clear whether trimerization of PSTPIP1 is required or whether a monomeric PSTPIP1 could also activate pyrin. To test this possibility, monomeric PSTPIP1 mutants that can still interact with pyrin are necessary. Generation of these monomeric PSTPIP1 mutants might be difficult because this requires deleting or mutating critical regions of the coiled-coil domain of PSTPIP1, which is necessary for its association with pyrin (Shoham et al., 2003), and further harbors the two PAPA-associated mutations.
Albeit minor differences, it appears that the mechanism of activation of pyrin resembles that of the Caenorhabditis elegans Ced-4 protein. Ced-4 preexists as an inhibited dimer in a complex with the anti-apoptotic protein Ced-9 (Yan et al., 2005). In this complex, Ced-9 acts like the B-box of pyrin to prevent Ced-4 from activating the Ced-3 procaspase. Binding of the pro-apoptotic Egl-1 protein to Ced-9 induces significant conformational changes in Ced-9 prompting the dissociation of Ced-9 from the Ced-4 dimer. The freed Ced-4 dimer further dimerizes to form a tetramer that recruits and induce activation of Ced-3. In a sense, the roles of Egl-1 in Ced-4 activation and PSTPIP1 in pyrin activation are very similar, namely to relieve the inhibitory effect of Ced-9 on Ced-4 and the B-box on pyrin, respectively.
The trimeric nature of pyrin could explain in part why the majority of FMF-associated mutations are seemingly recessive mutations, and why they predispose some heterozygous carriers to other inflammatory diseases (Grimaldi et al., 2006; Ozen et al., 2003; Rabinovich et al., 2007). In heterozygous subjects the probability of having a pyrin trimer containing three mutated pyrin monomers is 12.5 % of the total pyrin trimers. This might not be sufficient to show a dominant phenotype, but might predispose subjects to other inflammatory diseases. The remaining pyrin trimers are either made entirely of WT monomers (12.5 %) or of a mixture of WT and mutant pyrin monomers (75 %). The WT-mutant pyrin heterotrimers may not be able to show a total gain of function phenotype, but they might have a low level of activity, because the three monomers in these pyrin heterotrimers must be all activated at the same time to optimally recruit and oligomerize ASC.
The disease-associated PSTPIP1 mutations are clearly gain-of-function mutations because PAPA syndrome is a dominantly inherited disease. Indeed co-immunoprecipitation and in vitro pull-down experiments, demonstrated that the mutant PSTPIP1 proteins exhibit more binding to pyrin than the WT protein. According to our model this enhanced association of PSTPIP1 mutants with pyrin B-box could induce constitutive activation of pyrin thereby leading to more ASC oligomerization, and subsequently more caspase-1 activation. This is indeed supported by immunoprecipitation experiments, which revealed that pyrin exhibits enhanced association with ASC in the presence of the disease-associated PSTPIP1 mutants. Moreover, pyrin induced more ASC pyroptosome assembly in the presence of the PSTPIP1 mutants compared to WT PSTPIP1. As to why mutations of PSTPIP1, also a functional trimer, behave dominantly unlike the pyrin mutations, it is likely that one or two mutant subunit(s) of the PSTPIP1 trimer might be sufficient for binding to pyrin. Once bound, the remaining WT subunit(s) of PSTPIP1 might be able to disrupt the PYD-B box association by a steric hindrance mechanism or by a cooperative binding mechanism (i, e., binding of the mutant subunit might facilitate binding of the WT subunit to the B-boxes of the pyrin homotrimer). In support of this, we have observed that a heterotrimer of WT and A230T mutant PSTPIP1 exhibit strong binding to pyrin comparable to binding of a homotrimer of mutant A230T to pyrin (supplementary Fig. 4A). The WT PSTPIP1 subunit(s) in the heterotrimer exhibited significantly more binding than the WT subunits in the WT homotrimer (supplementary Fig. 4B, compare 4th and 6th lanes). These results explain why mutations of PSTPIP1 behave dominantly.
Although there is no direct evidence as yet to support a role for pyrin in viral restriction, the evidence presented here showing induction of endogenous pyrin in response to retroviral infection of THP-1 cells, and previous observations that interferon alpha (Centola et al., 2000) induces pyrin expression in monocytes suggest that pyrin might indeed play an important role as anti-viral pro-inflammatory mediator. Significantly, the domain architecture of pyrin is present in a family of proteins designated the tripartite motif (Trim) family with many members (Trim5α, Trim19, Trim22, Trim32) that function to restrict viral replication in mammalian cells (Meroni and Diez-Roux, 2005; Nisole et al., 2005). The best characterized among these is Trim5α which, like human pyrin, contains a central B-box and coiled-coil domain and a C-terminal SPRY domain, but differs only by the presence of an N-terminal RING domain in place of the PYD in pyrin. It has been recently shown that the SPRY domain of Trim5α is involved in the recognition of retroviral capsid proteins and viral restriction (Nisole et al., 2005; Sebastian and Luban, 2005; Yap et al., 2004; Yap et al., 2005). Interestingly, a single amino acid substitution in SPRY domain of human Trim5α changes its specificity and confers on it the ability to recognize HIV capsids and restrict HIV-1 infection (Yap et al., 2005). Therefore, it is reasonable to speculate that the SPRY domain of pyrin might also function similarly as in Trim5α to recognize retroviral capsid proteins and restrict viral infection via activation of the pyrin/ASC pyroptosome. The SPRY domain might interact with the B-box after binding to retroviral proteins in a manner similar to that of PSTPIP1, resulting in activation of pyrin. It remains to be seen if that is the case, and whether the FMF-associated amino acid substitutions in the SPRY domain of pyrin were selected for during human evolution to cope up with evolving strains of retroviruses and to expand the spectrum of viruses that could be recognized by pyrin's SPRY domain.
Further evidence for the possible involvement of the SPRY domain in the regulation of the inflammasome activity came during sequence analysis of the zebrafish genome which surprisingly revealed that zebrafish does not contain a pyrin gene but actually contains a hybrid gene that encodes a protein homologous to both human pyrin and cryopyrin proteins, with a C-terminal SPRY domain (Genbank accession # XP_69186, supplementary Fig. 2). It is well established now that cryopyrin is a major sensor of pathogens and stress stimuli and a central activator of the caspase-1 inflammasome in response to these stimuli. The domain structure of the zebrafish XP_69186 protein (zebrafish cryopyrin) is similar to that of human cryopyrin but it contains an additional SPRY domain at the C-terminus. It appears that the C-terminal half of the LRR domain in cryopyrin is replaced with a SPRY domain in the zebrafish cryopyrin. This suggests that the functions of human pyrin and cryopyrin proteins are combined in one molecule in the zebrafish. Furthermore, the physical association of the SPRY domain with zebrafish cryopyrin together with the observations that the majority of the FMF-disease associated mutations are present in the SPRY domain of human pyrin more than underscores the importance of this domain in pathogen recognition and in regulation of the inflammasome activity. Further sequence analysis of the zebrafish genome revealed the presence of the SPRY domain at the C-termini of other zebrafish cryopyrin related genes (Genbank accession #s XP_690650, XP_699873).
The cytoskeleton plays an important role in the pyrin/PSTPIP1 inflammatory pathway. Both pyrin and PSTPIP1 were found associated with the actin cytoskeleton (Mansfield et al., 2001; Spencer et al., 1997), and in the case of pyrin it was also found in association with microtubules (Mansfield et al., 2001), indicating that the two proteins may participate in the regulation of the cytoskeleton during inflammation. Consistent with the importance of the cytoskeleton organization for pyrin function, the pyrin inflammatory pathway is uniquely sensitive to the microtubule disruptive agent colchicine, and colchicine's responsiveness is an important diagnostic element for FMF (Dinarello et al., 1974; Margolis and Wilson, 1977; Zemer et al., 1986; Zemer et al., 1974). In support of these observations our biochemical data clearly demonstrates that the ability of pyrin to activate caspase-1 is inhibited by colchicine and nocodazol, thus establishing that the therapeutic benefit of colchicine is attributed to its ability to target pyrin, preventing it from activating caspase-1.
Experimental Procedures
Generation of Stable THP-1
Stable THP-1 cells expressing WT (THP-1-WT) or mutant PSTPIP1 proteins (THP-1-A230T and THP-1-E250Q), or ASC-GFP fusion protein (THP-1-vector) were generated by retroviral gene transfer with recombinant MSCV expression vectors as described in supplementary information.
Retrovirus Infection of THP-1 and Assay of IL-1β Secretion and Caspase-1 Activation
THP-1 cells were infected with culture supernatants containing retroviral particles produced in Phoenix cells as described in the supplementary information. 24 h after infection, the culture supernatants were collected and assayed for IL-1β by enzyme-linked immunosorbent assay (ELISA) (R&D systems, Minneapolis, MN, USA). In some experiments, THP-1 cells were transfected with the pyrin specific HS_MEFV_2_HP siRNA (Qiagen) using Amaxa Nucleofector™ (Amaxa, Cologne, Germany) method according to the manufacturer's protocol. 48h after transfection, the cells were infected with MSCVgfp retrovirus for 24h and then the culture supernatants were collected and assayed for IL-1β by ELISA. In some experiments the cell pellets were collected, lysed and analyzed by western blotting with anti-human caspase-1, pyrin, IL-1β, or PSTPIP1 antibodies.
Caspase-1 Processing and IL-1β Cleavage Assays in HEK293 Cells
These experiments were performed as described in (Yu et al., 2006) and supplemental materials.
Additional details on cells, plasmids and antibodies, and immunoprecipitation and pull-down assays are given in the Supplementary Information.
Supplementary Material
Merged green-gray time-lapse movie of LPS-treated THP-1-ASC-GFP cells. This one color movie shows merged ASC-GFP and gray phase contrast images. THP-1-ASC-GFP cells were seeded in 35 mM cover glass bottom culture dishes and then primed with PMA (0.5 μM) for 3h and allowed to attach for 24 h. Time-lapse imaging was performed on an LSM 510 META Confocal Microscope System (Carl Zeiss) equipped with a temperature and CO2-controlled sample chamber for live-cell imaging. The GFP protein was excited with the 488 nm Argon laser. Ten minutes after crude LPS (5 μg/ml) stimulation, images from the GFP signal were recorded every 17.5 seconds for an additional 30 minutes.
Notice how cells explode after formation of the ASC pyroptosome (green cluster) to release their contents in the extracellular space, and then the plasma membrane reseals and swells to form a balloon around the condensed nucleus.
This movie shows the ASC-GFP channel without phase contrast images of supplementary Movie 1.
Acknowledgments
We thank Douglas Miller (Merck) for the anti-caspase-1 antibody and Dr. Junji Sagara for the ASC antibody. We also thank Wojciech Jankowski for help with the confocal and fluorescence microscopy. This work was supported by NIH grants AG14357 and CA78890 to ESA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, Field D, Siminovitch KA. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18:141–154. doi: 10.1016/s1074-7613(02)00516-2. [DOI] [PubMed] [Google Scholar]
- Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- Cao T, Borden KL, Freemont PS, Etkin LD. Involvement of the rfp tripartite motif in protein–protein interactions and subcellular distribution. J Cell Sci. 1997;110:1563–1571. doi: 10.1242/jcs.110.14.1563. [DOI] [PubMed] [Google Scholar]
- Centola M, Wood G, Frucht DM, Galon J, Aringer M, Farrell C, Kingma DW, Horwitz ME, Mansfield E, Holland SM, et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood. 2000;95:3223–3231. [PubMed] [Google Scholar]
- Chae JJ, Centola M, Aksentijevich I, Dutra A, Tran M, Wood G, Nagaraju K, Kingma DW, Liu PP, Kastner DL. Isolation, genomic organization, and expression analysis of the mouse and rat homologs of MEFV, the gene for familial mediterranean fever. Mamm Genome. 2000;11:428–435. doi: 10.1007/s003350010082. [DOI] [PubMed] [Google Scholar]
- Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, Kastner DL. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A. 2006;103:9982–9987. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, T F F. A candidate gene for familial Mediterranean fever. The French FMF Consortium. Nat Genet. 1997a;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- Consortium, T I F. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997b;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- Cote JF, Chung PL, Theberge JF, Halle M, Spencer S, Lasky LA, Tremblay ML. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J Biol Chem. 2002;277:2973–2986. doi: 10.1074/jbc.M106428200. [DOI] [PubMed] [Google Scholar]
- Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 2005;44:406–408. doi: 10.1093/rheumatology/keh479. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Wolff SM, Goldfinger SE, Dale DC, Alling DW. Colchicine therapy for familial mediterranean fever. A double-blind trial. N Engl J Med. 1974;291:934–937. doi: 10.1056/NEJM197410312911804. [DOI] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. Epub 2002 May 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007 Jun 29; doi: 10.1038/sj.cdd.4402194. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeazzi M, Gasbarrini G, Ghirardello A, Grandemange S, Hoffman HM, Manna R, Podswiadek M, Punzi L, Sebastiani GD, Touitou I, Doria A. Autoinflammatory syndromes. Clin Exp Rheumatol. 2006;24:S79–85. [PubMed] [Google Scholar]
- Grimaldi MP, Candore G, Vasto S, Caruso M, Caimi G, Hoffmann E, Colonna-Romano G, Lio D, Shinar Y, Franceschi C, Caruso C. Role of the pyrin M694V (A2080G) allele in acute myocardial infarction and longevity: a study in the Sicilian population. J Leukoc Biol. 2006;79:611–615. doi: 10.1189/jlb.0705416. [DOI] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull KM, Shoham N, Chae JJ, Aksentijevich I, Kastner DL. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61–69. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Javanbakht H, Yuan W, Yeung DF, Song B, Diaz-Griffero F, Li Y, Li X, Stremlau M, Sodroski J. Characterization of TRIM5alpha trimerization and its contribution to human immunodeficiency virus capsid binding. Virology. 2006;353:234–246. doi: 10.1016/j.virol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kontochristopoulos GJ, Stavropoulos PG, Gregoriou S, Zakopoulou N. Treatment of Pyoderma gangrenosum with Low-Dose Colchicine. Dermatology. 2004;209:233–236. doi: 10.1159/000079897. [DOI] [PubMed] [Google Scholar]
- Mansfield E, Chae JJ, Komarow HD, Brotz TM, Frucht DM, Aksentijevich I, Kastner DL. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood. 2001;98:851–859. doi: 10.1182/blood.v98.3.851. [DOI] [PubMed] [Google Scholar]
- Margolis RL, Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977;74:3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27:1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- Mische CC, Javanbakht H, Song B, Diaz-Griffero F, Stremlau M, Strack B, Si Z, Sodroski J. Retroviral restriction factor TRIM5alpha is a trimer. J Virol. 2005;79:14446–14450. doi: 10.1128/JVI.79.22.14446-14450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- Ozen S, Bakkaloglu A, Yilmaz E, Duzova A, Balci B, Topaloglu R, Besbas N. Mutations in the gene for familial Mediterranean fever: do they predispose to inflammation? J Rheumatol. 2003;30:2014–2018. [PubMed] [Google Scholar]
- Peng H, Begg GE, Schultz DC, Friedman JR, Jensen DE, Speicher DW, Rauscher FJ., 3rd Reconstitution of the KRAB-KAP-1 repressor complex: a model system for defining the molecular anatomy of RING-B box-coiled-coil domain-mediated protein-protein interactions. J Mol Biol. 2000;295:1139–1162. doi: 10.1006/jmbi.1999.3402. [DOI] [PubMed] [Google Scholar]
- Rabinovich E, Shinar Y, Leiba M, Ehrenfeld M, Langevitz P, Livneh A. Common FMF alleles may predispose to development of Behcet's disease with increased risk for venous thrombosis. Scand J Rheumatol. 2007;36:48–52. doi: 10.1080/03009740600759639. [DOI] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, et al. The tripartite motif family identifies cell compartments. Embo J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian S, Luban J. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, Kastner DL. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, Simanis V, Lasky LA. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, Lovett M. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- Yan N, Chai J, Lee ES, Gu L, Liu Q, He J, Wu JW, Kokel D, Li H, Hao Q, et al. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature. 2005;437:831–837. doi: 10.1038/nature04002. [DOI] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Lynch C, Stoye JP. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci U S A. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15:73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med. 1986;314:1001–1005. doi: 10.1056/NEJM198604173141601. [DOI] [PubMed] [Google Scholar]
- Zemer D, Revach M, Pras M, Modan B, Schor S, Sohar E, Gafni J. A controlled trial of colchicine in preventing attacks of familial mediterranean fever. N Engl J Med. 1974;291:932–934. doi: 10.1056/NEJM197410312911803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Merged green-gray time-lapse movie of LPS-treated THP-1-ASC-GFP cells. This one color movie shows merged ASC-GFP and gray phase contrast images. THP-1-ASC-GFP cells were seeded in 35 mM cover glass bottom culture dishes and then primed with PMA (0.5 μM) for 3h and allowed to attach for 24 h. Time-lapse imaging was performed on an LSM 510 META Confocal Microscope System (Carl Zeiss) equipped with a temperature and CO2-controlled sample chamber for live-cell imaging. The GFP protein was excited with the 488 nm Argon laser. Ten minutes after crude LPS (5 μg/ml) stimulation, images from the GFP signal were recorded every 17.5 seconds for an additional 30 minutes.
Notice how cells explode after formation of the ASC pyroptosome (green cluster) to release their contents in the extracellular space, and then the plasma membrane reseals and swells to form a balloon around the condensed nucleus.
This movie shows the ASC-GFP channel without phase contrast images of supplementary Movie 1.


