Abstract
Arylcycloalkylamines, such as phenyl piperidines and piperazines and their arylalkyl substituents, constitute pharmacophoric groups exemplified in several antipsychotic agents. A review of previous reports indicates that arylalkyl substituents can improve the potency and selectivity of the binding affinity at D2-like receptors. In this paper, we explored the contributions of two key pharmacophoric groups, i.e., 4′-fluorobutyrophenones and 3-methyl-7-azaindole groups, to the potency and selectivity of synthesized agents at D2-like receptors. Preliminary observation of binding affinities indicates that there is little predictability of specific effects of the arylalkyl moieties but the composite structure is responsible for selectivity and potency at these receptors.
Keywords: Haloperidol analog, antipsychotics, butyrophenone, dopamine receptor ligands, D2-like receptor ligands, diazepane, bicyclic analogs
It is now widely accepted based on gene cloning and recombinant DNA techniques that there are least five major dopamine (DA) receptor subtypes classified as D1, D2, D3, D4 and D5. Originally, these receptors were classified into only two groups, D1-like and D2-like receptors with D1 and D falling into the first and D2, D3 and D4 making up the later group.1 Of the two groups, the D2- receptors have been the subject of great therapeutic interest because of their involvement in several psychiatric disorders.2 The D2 subtype receptor has been identified as the primary site of action antipsychotic agents.3 In addition, they are also implicated in the reinforcing and dependency producing drugs of abuse.4 The D4 receptor subtype mediates functions that include motor activity, initiation and inhibition of behavior and working memory.5–7 More recently, the D4 receptor subtype has attracted attention because of its association with the induction of penile erection.8–10 While D2 and D4 subtypes have become potential targets for drug development for several therapeutic indications, the functions of the D3 subtype have remained largely uncertain.2
Thousands of DA ligands have appeared in the literature over the years.2 However, a cursory evaluation of the common structural features in D2 and D4 receptor subtype ligands reveals consistent presence of arylcycloalkylamines in the form of alkylated arylpiperidines such haloperidol (Chart 1) and piperazines such as clozapine. The nature of the alkylated moieties varies and there is little evidence to suggest the role of these alkyl moieties in the selectivity of the ligands for each receptor subtype. In an attempt to understand the structural contributions of pharmacophoric elements at D2-like receptors, we have compared the haloperidol analog, 1 with Merck compound, L745,870 (Chart 1).11,12 In addition, several other publications have evaluated 3 methyl-7-azaindole and 3-methylindole moieties for D4 receptor selectivities.13
Chart 1.
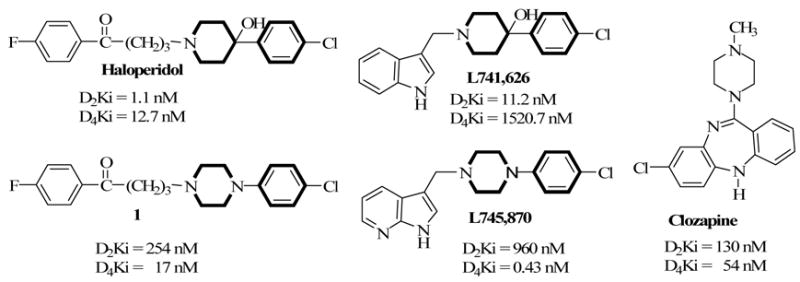
The comparison of the binding affinity data at cloned human D2-like receptors suggests that the presence of the butyrophenone and the 3-methyl-7-azaindole moieties significantly affects binding affinity and selectivity of these compounds at the D2-like receptors.11 In particular, a comparison of compound 1 and L745,870 suggests that the presence of the 3-methyl-7-azaindole moiety on 4- chlorophenyl piperazine confers ~40-fold D4 potency on L745,870 while the butyrophenone confers less than a 4-fold D2 potency. In addition, the 3-methyl-7-azaindole moiety appears to have increased D4 selectively from 15-fold to over 2200-fold. On the other hand, the arylpiperidine and arylpiperazine groups common among CNS drugs appear to have preferences for the D2 and D4 subtype receptors, respectively. The aim of this study was to further explore the role of the two alkyl moieties and their impact on D2/D4 selectivity and potency (Charts 2 and 3).
Chart 2.
Chart 3.
CHEMISTRY
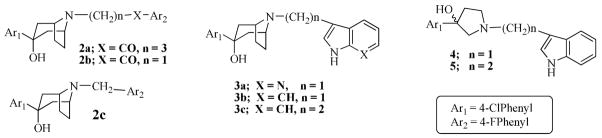
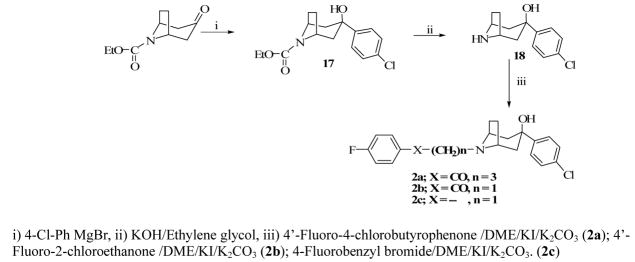
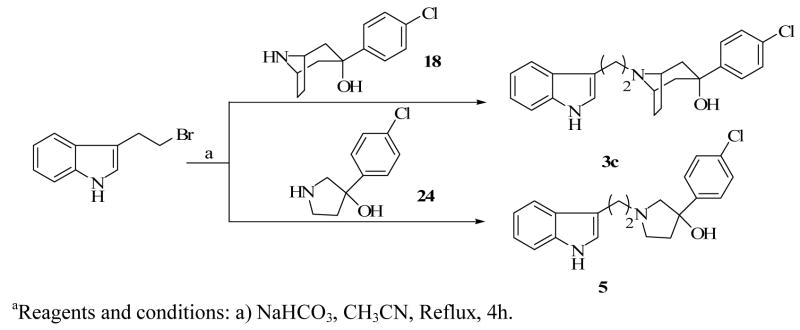
The binding affinities of compounds 1, 2a–c, 6, 8, 10, 12 and 14 were previously reported.11,14 However, the full details of the synthetic procedures for several of them were not provided nor discussed. The key intermediate for the synthesis of compounds 2a–c and 3a–c, 3-(4-chlorophenyl)-8-azabicyclo[3.2.1]octan-3-ol, was obtained by treating commercially available carbamate protected tropinone with 4-chlorophenyl magnesium bromide under Grignard reaction conditions to form a carbamate protected aminoalcohol (17) which was decarbamylated to the aminoalcohol 18.
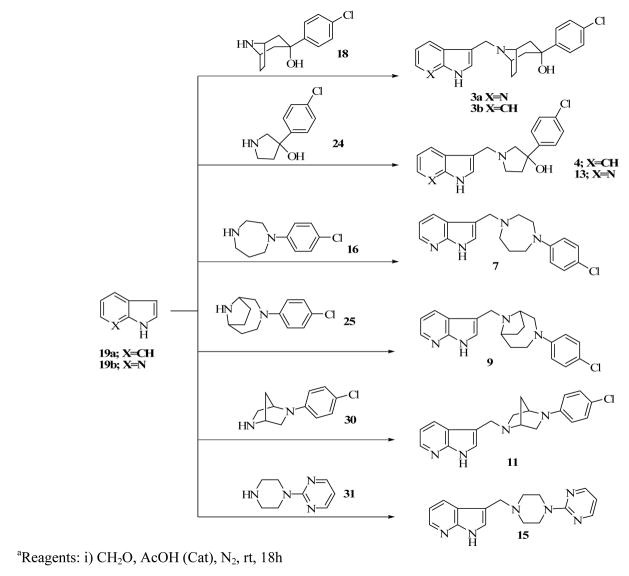
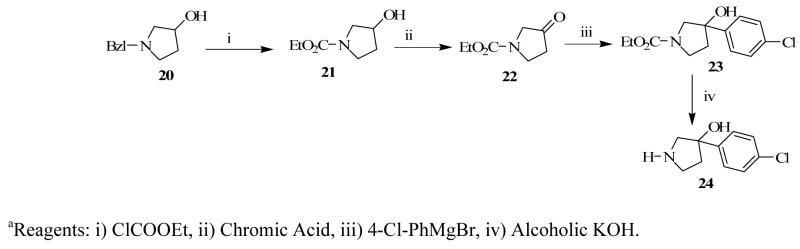
Compounds 2a–c were obtained by simple alkylation of compound 18 with the appropriate alkylating groups (Scheme 1). Compound 18 was also subjected to treatment with 7-azaindole and formaldehyde under Mannich reaction conditions, to give the desired product, 3a (Scheme 3). Compound 3b was similarly prepared using indole instead of 7-azaindole and the synthesis of compound 3c was accomplished by alkylating compound 18 with 3-(2-bromoethylindole (Scheme 4). Compound 24 (Scheme 2), which was previously reported by our laboratory,15 served as the key intermediate for the synthesis of compounds 4 and 5. The first step was to convert the commercially available benzyl-protected pyrrolidinol, 20 to the carbamate-protected pyrrolidinol (21) in order to avoid the anticipated dechlorination that often accompanied debenzylation under hydrogenolysis conditions. Oxidation of compound 21 to form ketone 22 and subsequent Grignard reaction with 4-chlorophenyl magnesium bromide produced the carbamate-protected pyrrolidinol 23. Deprotection with potassium hydroxide produced 24 and subsequent alkylation with the appropriate alkylating agents yielded the desired compounds 4 and 5 (Schemes 3 and 4).
Scheme 1.
Scheme 3.
Scheme 4.
Scheme 2.
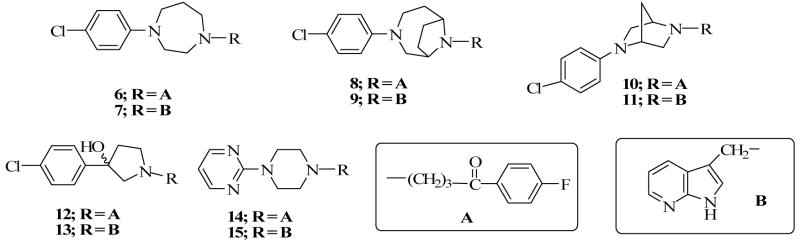
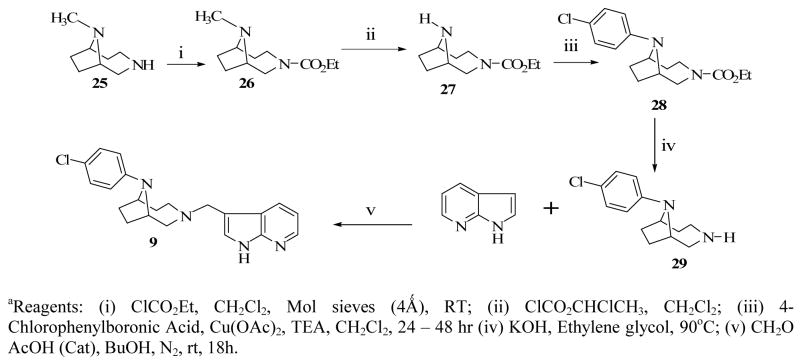
We have previously reported the detailed synthetic procedures for compound 6 including the synthesis of 1-(4-chlorophenyl)-1,4-diazepane, using CuI-catalyzed coupling of 4-chlorophenyl iodide with 1,4-diazepane.14 Mannich reaction involving 1-(4-chlorophenyl)-1,4-diazepane, formaldehyde and 7-azaindole produced compound 7 in good yield (Scheme 3). The synthesis of compounds 8 and 9 required the previously synthesized 9-methyl-3,9-diazabicyclo[4.2.1]nonane (25) as a key intermediate.14 To obtain compound 8, intermediate 25 was carbamylated using ethyl chloroformate (26) and then demethylated to yield the secondary amine of the bridged nitrogen. N-arylation of 27 with 4-chlorophenylboronic acid produced compound 28 which underwent deprotection to now produce the secondary amine of the other nitrogen (29) (Scheme 5). Alkylation of compound 29 with 4-chloro-4′-fluorobutyrophenone delivered the target compound 8 as desired. Compound 9 was obtained by reacting 9-(4-chlorophenyl)-3,9-diazabicyclo[4.2.1]nonane (29) with 7-azindole under Mannich reaction condition (Method A) (Scheme 3). The synthesis of compound 10 was previously reported 14 while compound 11 was synthesized using the commercially available 5-(4-chlorophenyl)-2,5-diazabicyclo[2.2.1]heptane (30) as the starting material. Compound 30 was treated with 7-azaindole and formaldehyde under Mannich reaction condition as before to form compound 11 (Scheme 3). The synthesis of compounds 12 and 14 were also previously reported14 and compounds 13 and 15 were synthesized using the same procedure for the synthesis of compound 11 (Scheme 3). 3-(4-chlorophenyl)-3-hydroxypyrrolidine 24, previously reported,15 (Scheme 2) the commercially available 1-(2-pyrimidyl)piperazine 31 served as starting materials for Mannich reaction mediated conversion to target compounds 13 and 15, respectively (Scheme 3).
Scheme 5.
Results and Discussion
We have previously shown that replacing the piperidine ring in haloperidol with a tropane moiety enhanced binding affinity to dopamine D2 receptors.15–16 Later, we compared the effect of a 4′-fluorobutyrophenone and 3-methyl-7-azaindole moieties on 4-(4-chlorophenyl)piperazine and observed that 3-methyl-7-azaindole moiety conferred both potency and D4 selectivity.11 At the time, we were drawn to the possibility that the distance between the aromatic ring and the N atom in the piperazine ring might be important. That hypothesis was tested using the potent tropane analog of haloperidol (2a) and the shorter arylalkyl groups (2b and 2c) but we failed to achieve either increased potency or D4 selectivity.11 To further explore other physicochemical aspects of the azaindole ring, we synthesized compounds 3a–c and evaluated their binding affinities for the D2-like receptors. Compounds 3a [Ki (nM), D2 = 588; D4 = 7873] and 3b [Ki (nM), D2 = 160; D4 = 3007] are analogs of L745,870 and L741,626 with the piperazine and piperidinol moieties replaced with the tropanol moiety respectively. Surprisingly, both compounds had significantly reduced affinity for the D2 receptor. In addition, one would have expected 3b to at least retain its binding affinity at the D2 receptor (Ki of L741,626 at D2 = 11.2 nM) since replacement of the piperidine ring with tropane (2a) in haloperidol enhanced potency at the D2 subtype. Addition of a methylene group to extend the chain of 3b to 3c [Ki (nM) at D2 = 53.0; D4 = 277.5] improved affinity somewhat at all DA subtypes. In particular, affinity was significantly improved at the D3 subtype with moderate selectivity when compared to the D2 and D4. Comparison of compounds 3a and 3b also suggests that the presence of the pyridine nitrogen in the azaindole analog detracts somewhat from binding affinity to the D2-like receptors. Further attempts to improve binding affinity by synthesizing pyrrolidinol analogs of 3b and 3c (compounds 4 and 5, respectively) were also unsuccessful. These surprising observations led us to synthesize and evaluate the 3-methyl-7-azaindole and 4′-fluorobutyrophenone moieties on several aryl cycloalkylamine structures shown in Chart 3.
We recently reported that compound 6, a diazepane analog of haloperidol, has a favorable atypical antipsychotic profile.14 Replacement of the butyrophenone moiety with the 3-methyl-7-azaindole moiety led to the formation of compound 7. Evaluation of the binding affinities of the two compounds shows 7 has a 7- and 3-fold lower affinity for the D2 and D4 receptors, respectively when determined using the same assay procedure in the same laboratory (Table 2). The Ki values in parenthesis for compound 6 are obtained in a different laboratory. This observation demonstrates that the 3-methyl-7-azaindole moiety does not necessarily confer D4 receptor potency on its own but is dependent on the amine to which it is attached. A similar observation was made when the bridged analog of compounds 6 and 7, i.e., 8 [Ki (nM); D2 = 178.4; D4 = 41.8) and 9 [Ki (nM); D2 > 10,000; D4 = 583.7] were evaluated. Compound 8 did meet our initial criterion for further evaluation. At 5-HT receptors, 8 has weak binding affinity for 5-HT1A and 5-HT2C receptors but a moderate affinity for 5-HT2A receptors [Ki (nM); 5-HT1A= 2332; 5-HT2A = 194.8; 5-HT2C = 3513)]. Compared to its un-bridged counterpart, compound 6, 8 has an 8-fold lower affinity for the 5-HT2A receptor. These differences can be exploited to investigate the correlation of D2/D4 affinity ligands without substantial 5-HT binding affinity and the absence of extrapyramidal activity associated with typical antipsychotic agents. Compound 10 is the boat-constrained analog of 1 and 11 is an analog of 10 with the butyrophenone replaced with the 3-methyl-7-azaindole moiety. Compound 10 has moderate affinity for the D2 and D3 receptors and a weak affinity for the D4 receptor. However, unlike the previous two pairs of compounds, 11 has a significantly higher affinity for all the D2-like receptors. Indeed, compound 11 has the highest D3 receptor affinity of all the compounds tested [Ki (nM); D2 = 62.0; D3 = 11.0; D4 = 69.0] in this paper.
Table 2.
Binding Affinity Constants of Synthetic Compounds to D2-like Receptors.
| Compounds | a Binding Data of Synthetic Compounds, Ki ± SEM (n) in nM | |||
|---|---|---|---|---|
| D2 | D3 | D4 | D2/D4 | |
| Clozapineb | 130 | 240 | 54 | 2.4 |
| 6b | 43.3±13.3 (130) | 158.8±35.1 (567) | 6.6±0.6 (56) | 6.6 (2.3) |
| 7 c | 970 (n = 2) | 370 (219 – 631) | 18.6 (14.5 – 24.0) | 5.1 |
| 8b | 178.4±29.2 | 548.1±246.0 | 41.8±9.0 | 4.3 |
| 9 | >10,000 | 335.5±178.0 | 583.7±114.9 | >17 |
| 10c | 170.0 (123 – 234) | 220.0 (148 – 339) | 513.0 (447 – 589) | 0.33 |
| 11 c | 62.0 (38.0 – 100) | 11.0 (7.94 – 15.1) | 69.0 (56.2 – 85.1) | 0.90 |
| 12 c | 33.0 (21.9 – 50.1) | 200.0 (144.5 – 275.4) | 11.0 (8.9 – 12.3) | 3.0 |
| 13 | MPA | MPA | 1213±260 | |
| 14b | 98.0±15.3 | 244.1±106.0 | 6.5±0.8 | 15 |
| 15c | 1170 (n = 2) | 1500 (912 – 2399) | 56.0 (45.7 – 69.2) | 21 |
= Data obtained from the NIMH-PDSP. Data for compounds 6 (parenthesis) 7, 10, 11, 12, and 15 were provided by A. W. Schmidt, at Pfizer laboratories as described in ref 15. Ki is the mean value obtained on triplicate or quadruplicate determinations unless otherwise indicated. MPA = Missed primary assay threshold of 50% inhibition.
Binding data were previously reported (ref 14).
The data in brackets is the range of the Mean relative to the SEM.
We have also previously shown that replacement of the piperidine in haloperidol with the pyrrolidine ring (12) results in an analog with reduced binding affinity at the D2 subtype.15 Separation and evaluation of the enantiomers indicated that the (+)-enantiomer is the eutomer and its behavioral profile was desirable.17 Thus, we opined that replacement of the 4′-fluorobutyrophenone moiety in 12 with the 3-methyl-7-azaindole moiety might be useful. The results indicate that there was little or no affinity for both D2 and D3 receptor subtypes and over 100-fold lower affinity for the D4 receptor subtype. Synthesis and evaluation of compound 15 [Ki (nM); D2 = 1170; D3 = 1500; D4 = 56.0), a 7-azaindole counterpart of the previously reported compound 14, also resulted in diminished binding affinity for the D2-like receptors. It is important to note however that the binding affinities were determined in different laboratories and hence inter-laboratory differences (see the results for the determination of haloperidol, compound 2a and 6) may play a role as well.
Overall, these results suggest that the N-arylalkyl substituents on aryl cycloalkylamines can modify significantly the binding affinities of the resulting compounds at the dopamine receptor subtypes. There does not appear to be a specific and predictable effect of the nature of the arylalkyl moiety and the binding affinity appears to be due to a combination of effects involving the two component parts. These observations are consistent with the fact that the pharmacophoric elements of both typical and atypical antipsychotic drugs are found in both the cycloalkylamine and the arylalkyl moieties of these compounds.
Experimental
Melting points were determined on a Gallenkamp (UK) apparatus and are uncorrected. NMR spectra were obtained on a Varian 300 MHz Mercury Spectrometer. Elemental analyses were carried out by Atlantic Microlab, Inc., Norcross, GA and are within 0.4% of theory unless otherwise noted. Flash chromatography was performed with Davisil grade 634 silica gel. N,N-Dimethylformamide was distilled from CaSO4 and stored over 4Ǻ molecular sieves. 4-Chloro-4′-fluorobutyrophenone was obtained from Sigma-Aldrich but was purified by distillation under reduced pressure to a colorless liquid prior to use. Other starting materials were used without further purification.
Preparation of 3-(4-chlorophenyl)-8-azabicyclo[3.2.1]octan-3-ol (18)
A Grignard reagent, p-chlorophenyl magnesium bromide,18 was generated in situ by reacting 4-bromochlorobenzene (5.82 g, 30.40 mmol), Mg (0.8 g, 32.90 mmol) and I2 (ca 1 mg) in anhydrous Et2O (20 ml), and refluxing the mixture for 4 h. A solution of N-carbethoxy tropinone (16) (2 g or 10.1 mmol) in anhydrous THF (10 mL) was slowly added to the reaction mixture and further refluxing continued for 18 hours. The resulting mixture was allowed to cool to room temperature, saturated NH4Cl solution (50 mL) was added, and the mixture was extracted with EtOAc (3×50 mL). The combined organic phase was washed with H2O (50 mL) followed by brine (50 mL), dried over Na2SO4, filtered, and the filtrate was concentrated in vacuo. Column chromatography (gradient solvent of 8:2 to 7:3 hexane/EtAOc) on silica gel afforded ethyl-3-(4-chlorophenyl)-3-hydroxy-8-azabicyclo[3.2.1]octane-8-carboxylate (17) as a yellowish oil which solidified on standing at room temperature for one day, 1.3 g, 41.5 %. 1HNMR (300 MHz, CDCl3): δ 1.26 (3H, t, J = 7.1 Hz), 1.55 (2H, d, J = 9.2 Hz), 1.81 (2H, d, J = 14.5 Hz), 1.96 (2H, m), 2.27 (4H, d, J = 6.8 Hz), 4.15 (2H, q, J = 7.1, J = 7.3 Hz), 7.28 (4H, d, J = 4.0 Hz). A mixture of KOH (3.2 g, 56.5 mmol) in ethylene glycol (20 mL) was added to a solution of 17 (2.5 g or 8.1 mmol) in MeOH (10 mL) and the resulting mixture was heated at 150 °C, with constant stirring, for 4 hours and then allowed to cool to room temperature. Water (200 mL) was added, and the mixture was extracted with EtOAc (2×100 mL) followed by CH2Cl2 (3×100 mL). The organic phases were combined, washed with H2O (400 mL), brine (100 ml) and dried (Na2SO4). The organic phase was filtered, and the filtrate was concentrated in vacuo and the residue was column chromatographed on silica gel (4:2 CH2Cl2/MeOH) to give white yellowish crystals of 3-(4-chlorophenyl)-8-azabicyclo[3.2.1]octan-3-ol (18) (1.40 g, 73%). 1HNMR (300 MHz, CDCl3): δ 1.82 (4H, m), 2.17 (2H, dd, J = 3.7, J = 3.7 Hz), 2.34 (2H d, J = 7.5 Hz), 3.56 (2H, brs), 7.27 (2H, d, J = 8.7 Hz), 7.48 (2H, d, J = 8.7 Hz).
4-[3-(4-chlorophenyl)-3-hydroxy-8-azabicyclo[3.2.1]oct-8-yl]-1-(4-fluorophenyl)butan-1-one (2a)
A mixture of 18 (0.46 g, 1.94 mmol), 4-chloro-4′-fluorobutyrophenone (0.60 g, 3 mmol), KI (0.5 g, 3 mmol), K2CO3 (0.70 g, 5.1 mmol) in DME (10 mL) was refluxed under N2 for 18 h. The reaction mixture was allowed to cool to room temperature, H2O (20 mL) was added, and the mixture was extracted with EtOAc (4 × 50 mL). The combined organic phase was washed with brine, dried over Na2SO4 and concentrated in vacuo. Column chromatography was carried out using CH2Cl2 followed by 2:3 EtOAc/MeOH to afford a yellowish oil of 2a (0.18 g, 23%) which was then converted to its HCl salt, mp 237.2 –238.3 °C. 1HNMR (300 MHz, CD3OD): δ 2.24 (m, 6H), 2.57 (m, 2H,) 2.72 (d, J = 9.0 Hz, 2H), 3.17 (m, 2H), 3.27 (t, J = 6.6 Hz, 2H), 4.19 (brs, 2H), 7.26 (dd, JH- F = 8.7 Hz, JH-H = 8.8 Hz, 2H), 7.37 (d, J = 8.7 Hz, 2H), 7.56 (d, J = 8.7, 2H), 8.11 (dd, JH-F = 5.4 Hz, JH-H = 8.8 Hz, 2H). Anal. (C23H26Cl2FN2O.0.5H2O): C 61.75, H 6.08, N 3.13. Found: C 61.93, H 6.03, N 3.20.
2-[3-(4-chlorophenyl)-3-hydroxy-8-azabicyclo[3.2.1]oct-8-yl]-1-(4-fluorophenyl)ethanone (2b)
A mixture of 18 (0.700 g, 2.94 mmol), 2-chloro-4′-fluoroacetophenone (0.83 g, 3.83 mmol), KI (0.800 g, 2.94 mmol), and K2CO3 (2.44 g, 17.6 mmol) in DME (10 mL) was refluxed for 18 hours. The reaction was cooled to rt., H2O (20 mL) was added, and the mixture was extracted with EtAOc (3×50 mL). The combined organic phase was washed with brine (50 mL), dried over Na2SO4, filtered, and the filtrate was concentrated in vacuo. Column chromatography (7:3 hexane/EtAOc) resulted in a yellowish oil of 2b (0.480 g, 44 %) which was converted into an HCl salt; mp 256.8– 257.5 °C. 1HNMR (300 MHz, CD3OD): δ 2.28 (7H, m), 2.71 (4H, m), 4.19 (2H, brs), 7.34 (2H, t, JH- F = 8.8, JH-H = 8.8 Hz), 7.38 (2H, d, J = 8.8, JH-H = 8.8 Hz) 7.59 (2H, d, J = 8.8), 8.18 (2H, dd, JH-F = 5.5, JH-H = 8.8 Hz). Calcd for C21H22Cl2FNO2·0.2H2O: C 60.94, H 5.45, N 3.38. Found: C 60.96, H 5.34, N 3.40.
3-(4-chlorophenyl)-8-(4-fluorobenzyl)-8-azabicyclo[3.2.1]octan-3-ol (2c)
A mixture of18 (0.50 g, 2.10 mmol), 4-fluorobenzyl bromide (0.517 g, 2.70 mmol), KI (0.350 g, 2.10 mmol), and K2CO3 (1.74 g, 12.6 mmol) was refluxed in DME (6 ml) for 18 h. After cooling to room temperature, H2O (20 mL) was added and the mixture was extracted with EtAOc (4×50 ml). Organic phases were combined, washed with brine (30 mL), dried over Na2SO4, filtered, and the filtrate was concentrated in vacuo. Column chromatography on silica gel (starting with 100 % hexane and then 7:3 hexane/EtAOc) yielded a yellowish oil of 2c (0.425 g, 59 %), which was then converted into an HCl salt, mp 110.4–111.1 °C. 1HNMR (300 MHz, CDCl3): δ 2.09 (2H, d, J = 14 Hz), 2.18 (2H, m), 2.80 (2H, m), 3.10 (2H, d, J = 14 Hz), 3.70 (2H, brs), 4.07 (2H, d, J = 6.0 Hz), 7.12 (2H, t, JH-F = 8.5, JH-H = 8.5 Hz), 7.26 (2H, d, J = 8.5), 7.78 (2H, d, J = 8.6 Hz), 7.87 (2H, dd, JH-F = 5.3, JH-H = 8.5 Hz). Calcd for C20H22Cl2FNO·0.25H2O: C 62.10, H 5.86, N 3.62. Found: C 62.05, H 6.01, N 3.60.
3-(4-chlorophenyl)-8-(1H-pyrrolo[2,3-b]pyridin-3-ylmethyl)-8-azabicyclo[3.2.1]octan-3-ol (3a)
Method A: A mixture of 18 (0.60 g, 2.50 mmol), 7-azaindole (0.400 g, 3.40 mmol), AcOH (6 drops, 17 M), and CH2O (0.203 g, 2.50 mmol) in CH2Cl2 (5 mL) was stirred at room temperature for 18 hrs. The reaction mixture was basified with NaOH (10 % aqueous solution) and extracted with CH2Cl2 (4×25 mL). The combined organic phase was washed with brine (20 mL), dried over Na2SO4, filtered, and the filtrate was concentrated in vacuo. Purification by preparatory TLC (4:2 CH2Cl2/MeOH) yielded flaky white crystals of 3a (0.46 g, 50 %), mp 94.2–94.5 °C. 1HNMR (300 MHz, CD3OD): δ 1.84 (2H, d, J = 14 Hz), 2.30 (6H, m), 3.45 (2H, brs), 3.84 (2H, brs), 7.14 (1H, dd, J = 5.0, J = 7.8 Hz), 7.27 (2H, d, J = 8.6 Hz), 7.46 (1H, s) 7.50 (2H, d, J = 8.6 Hz), 8.20 (2H, m). Calcd for C21H22ClN3O·0.75H2O: C 66.13, H 6.21, N 11.02; Found: C 66.12, H 6.04, N 10.87.
3-(4-chlorophenyl)-8-(1H-indol-3-ylmethyl)-8-azabicyclo[3.2.1]octan-3-ol (3b)
A mixture of 18 (0.50 g, 2.10 mmol), indole (0.250 g, 2.1 mmol), AcOH (6 drops, 17 M), and CH2O (0.065 g, 2.10 mmol) in CH2Cl2 (5 mL) was stirred at room temperature for 18 h. The reaction mixture was basified (10 % aq. NaOH solution), extracted with CH2Cl2 (4×25 mL), the pooled organic layers were combined, washed with brine (20 mL), dried over Na2SO4 and the filtrate was concentrated in vacuo. Purification by preparatory TLC (4.5:0.5 CH2Cl2/2 M NH3 in MeOH) yielded yellowish crystals of 3b (0.39 g, 51 %). Mp 72.4–73.1 C, 1HNMR (300 MHz, CD3OD): δ 1.83 (2H, d, J = 14 Hz), 2.26 (5H, m), 2.37 (2H, d, J = 6.1 Hz), 2.40 (1H, d, J = 7.2 Hz), 3.35 (1H, s), 3.84 (2H d, J = 11.0 Hz), 7.04 (1H, m), 7.11 (1H, m), 7.26 (2H, d, J = 8.6 Hz), 7.36 (1H, m), 7.52 (2H, d, J = 8.6 Hz), 7.70 (1H, m). Calcd for C22H23ClN2O.1.0H2O: C 68.65, H 6.55, N 7.28. Found: C 68.43, H 6.29, N 7.04.
3-(4-chlorophenyl)-8-(1H-pyrrolo[2,3-b]pyridin-3-ylethyl)-8-azabicyclo[3.2.1]octan-3-ol (3c)
A mixture of 18 (0.200 g, 0.84 mmol), 3-(2-bromoethyl)indole (0.094 g, 0.42 mmol), and NaHCO3 (0.14 g, 1.68 mmol) in anhydrous CH3CN (5 mL) was refluxed for 4 h under N2. The reaction mixture was allowed to cool to rt and H2O (15 mL) was added. The mixture was extracted with CH2Cl2 (4×25 ml) and the combined organic layers was washed with brine (20 mL), dried over Na2SO4 and the filtrate was concentrated in vacuo. White crystals of 3c (0.18 g, quantitative) were obtained after preparatory TLC purification (4:1 CH2Cl2/MeOH), mp 186.5–187.1 °C. 1HNMR (300 MHz, CD3OD): δ 1.83 (2H, d, J = 14 Hz), 2.00 (2H, m), 2.32 (5H, m), 2.82 (2H, m), 3.03 (2H, m), 3.50 (2H, brs), 7.08 (1H, s), 7.10 (1H, dt, J = 1.2, J = 8.0 Hz), 7.28 (2H, d, J = 8.7 Hz), 7.32 (2H, m), 7.54 (2H, d, J = 8.7Hz), 7.56 (2H, m). Calcd for C23H25ClN2O·0.75H2O: C 70.04, H 6.77, N 7.10. Found: C 69.86, H 6.56, N 7.00.
3-(4-chlorophenyl)-1-(1H-indol-3-ylmethyl)pyrrolidin-3-ol (4)
A solution of 3-(4- chlorophenyl)pyrrolidin-3-ol (0.60 g, 3.04 mmol), indole (0.46 g, 3.95 mmol), AcOH (6 drops, 17 M), and CH2O (0.08 ml, 3.04 mmol) in CH2Cl2 (5 mL) was stirred at room temperature for 18 h. The mixture was basified (10 % NaOH solution), extracted with CH2Cl2 (4×25 mL), the combined organic layers was washed with brine (20 mL), dried over Na2SO4 and the filtrate was concentrated in vacuo. Purification using preparatory TLC (4.5:0.5: CH2Cl2/2M NH3 in MeOH) gave yellowish crystals of 4 (0.49 g, 49 %), mp 56.9–57.2 °C, 1HNMR (300 MHz, CD3OD): δ 2.13 (1H, m), 2.25 (1H, m), 2.94 (1H, m), 2.98 (2H, m), 3.08 (1H, m), 3.96 (2H, s), 7.03 (1H, m), 7.10 (1H, m), 7.24 (1H, s), 7.28 (2 H, d, J = 8.7 Hz), 7.35 (1H, m), 7.45 (2H, d, J = 8.7 Hz), 7.66 (1H, m). Calcd for C19H19ClN2O·0.4H2O: C 68.32, H 5.97, N 8.39. Found: C 68.40, H 5.86, N 8.28.
3-(4-Chlorophenyl)-1-[2-(1H-indol-3-yl)ethyl]pyrrolidin-3-ol (5)
A mixture of 3-(4- chlorophenyl)pyrrolidin-3-ol (0.40 g, 2.02 mmol), 3-(2-bromoethyl)indole (0.23 g, 1.01 mmol), and NaHCO3 (0.68 g, 8.08 mmol) in anhydrous CH3CN (5 mL) was refluxed for 4 h under N2. The reaction mixture was allowed to cool to room temperature and H2O (15 mL) was added. The mixture was extracted with CH2Cl2 (4×25 mL), the pooled organic layers was washed with brine (20 mL), dried over Na2SO4 and the filtrate was concentrated in vacuo. Purification by column chromatography (4:1 CH2Cl2/MeOH) afforded white crystals of 5 (0.15 g, 22 %), mp 54.1–55.7 °C. 1HNMR (300 MHz, CDCl3): δ 2.15 (1H, m), 2.28 (1H, m), 2.60 (2H, m), 2.92 (4H, m), 3.06 (1H, d, J = 9.7 Hz), 3.25 (1H, m), 6.98 (1H, s), 7.09 (2H, m), 7.26 (2H, m), 7.38 (2H, d, J = 8.6 Hz), 7.55 (1H, d, J = 7.8 Hz), 7.93 (1H, brs). Calcd for C20H21ClN2O·0.5MeOH: C 68.99, H 6.50, N 7.85. Found: C 68.80, H 6.27, N 7.49.
3-{[4-(4-chlorophenyl)-1,4-diazepan-1-yl]methyl}-1H-pyrrolo[2,3-b]pyridine, HCl (7)
Using Method A and 1-(4-chlorophenyl)-1,4-diazepane and 7-azaindole as starting materials, compound 7 was obtained as the HCl salt, mp 158.0 –159.0 °C. 1H NMR (300 MHz, CDCl3): δ 9.30 (1H, brs), 8.28 (1H, d, J = 4.5 Hz), 7.96 (1H, dd, J = 1.5, 7.5 Hz), 7.19 (1H, s), 7.12 (2H, d, J = 9.0 Hz), 7.03(1H, dd, J = 4.8, 8.1 Hz), 6.58 (2H, d, J = 9.0 Hz), 3.79 (2H, s), 3.51 (2H, t, J = 4.8 Hz), 3.47 (2H, t, J = 6.3 Hz), 2.78 (2H, t, J = 4.8 Hz), 2.65 (2H, t, J = 4.8 Hz), 1.90 (2H, m). Calcd for C19H21ClN4·HCl·0.35 H2O: C 59.49, H 5.78, N 14.60; Found: C 59.40, H 5.54, N 14.59.
3-[(1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-9-(4-chlorophenyl)-3,9-diazabicyclo[4.2.1]nonane (9)
The method of Michaels and Zaugg was followed.19 Ethyl chloroformate (4 mL) was added in a drop-wise manner to compound 2514 (2.78 g, 19.8 mmol) dissolved in dry CH2Cl2 (50 mL) in the presence of molecular sieves (1 g). The reaction mixture was stirred for 16 h at rt, the solvent was evaporated and the resulting crude product subjected to column chromatography on silica gel (4:2 CH2Cl2:MeOH) to give 3,9-diazabicyclo[4.2.1]nonane-3-carboxylic acid ethyl ester, compound 26 as an oil; 1H NMR (300MHz, CDCl3): δ 1.23 (t, 3H, J = 6.9 Hz), 1.78–2.00 (m, 3H), 2.15–2.29 (m, 2H), 2.3–2.58 (m, 2H), 2.87 (s, 3H), 3.65–4.24 (m, 7H). Compound 26 (1.9 g, 8.95 mmol) was dissolved in ClCH2CH2Cl (10 mL) and stirred well at 0 °C. At 0 °C, α-chloroethyl chloroformate (1.9 g, 13.5 mmol) was added in a drop wise manner and the reaction was allowed to cool to room temperature. The reaction mixture was then refluxed for 3 h, MeOH (10 mL) was added and refluxing continued for an additional 1 h, solvent was evaporated and the crude reaction mixture was subjected to column chromatography (silica gel, 9:1 CH2Cl2:MeOH) to yield 3,9-diazabicyclo[4.2.1]nonane-3-carboxylic acid ethyl ester, 27 (0.55 g, 31 %). Without characterization, a mixture of compound 27 (0.5g, 2.5 mmol), 4-chlorophenylboronic acid (0.78g, 5 mmol), copper acetate (0.82 g, 4.56 mmol), Et3N (0.5 mL) in CH2Cl2 (50 mL) with molecular sieves (0.8 g) was stirred in open air for 48 h. The reaction mixture was monitored by TLC until the formation of the product was observed before quenching with methanolic NH3 solution. The resulting mixture was filtered over celite, extracted with CH2Cl2 (3 × 35 ml) dried and solvent evaporated under vacuum. The crude reaction mixture was subjected to column chromatography (silica gel, 4:1 CH2Cl2:MeOH ) to yield a yellowish syrup of 9-(4-chlorophenyl)-3,9-diazabicyclo[4.2.1]nonane-3-carboxylic acid ethyl ester, 28 (0.21g, 27 %). Compound 28 (0.2 g, 0.65 mmol), KOH (0.2 g in 0.2 ml H2O) in ethylene glycol (0.2 mL) was heated at 90 °C overnight. The reaction mixture was extracted with CH2Cl2 (2 × 80 mL), dried over Na2SO4 and evaporated under vacuum. The crude product was subjected to column chromatography (silica gel, 4:2 CH2Cl2:MeOH) to yield compound 29 (0.09 g, 58 %). A mixture of 9-(4-chlorophenyl)-3,9-diazabicyclo[4.2.1]nonane, 29 (0.130g, 0.55 mmol), 7- azaindole (0.130g, 1.1mmol), AcOH (6 drops, 17M) CH2O (0.016g, 0.53mmol) in butanol (8ml) was refluxed overnight under N2. The excess butanol was removed in vacuo and residue was extracted with methylene chloride (3×60ml). The organic phase was dried (Na2SO4) and removed. The crude product was purified on silica gel column with 1:1 CH2Cl2 and EtOAc to yield compound 9 (80 mg, 62.2%) as a white hygroscopic crystalline solid. MP: 194–196 °C. 1H NMR (300MHz, CDCl3): δ 8.30 (m, 1H), 8.05 (m, 1H), 7.04 (m, 4H), 6.56 (d, J=6.5Hz, 2H), 3.58 (d, J=6.5Hz, 2H), 3.50 (m, 6H), 3.12 (m, 1H), 2.20-1.10 (m, 6H). Anal. Calcd. for C21H23N4Cl·0.125H2O : C, 68.33; H, 6.35; N, 15.18 Found C, 68.17; H, 6.30, N, 14.92.
2-{(1H-pyrrolo[2,3-b]pyridin-3-yl)methyl}-5-(4-chlorophenyl)-2,5-diazabicyclo[2.2.1]heptane (11)
Using method A and 5-(4-chlorophenyl)-2,5-diazabicyclo[2.2.1]heptane and 7-azaindole as starting materials, compound 11 was obtained as the free base, mp 176–178 °C. 1H NMR (CDCl3): δ 10.03 (1H, brs), 8.29 (1H, d, J = 4.5 Hz), 8.01 (1H, d, J = 8.1 Hz), 7.22 (1H, s), 7.13 (2H, d, J = 9.0 Hz), 7.05 (1H, dd, J = 4.8, 7.8 Hz), 6.47 (2H, d, J = 9.0 Hz), 4.18 (1H, s), 3.85 (2H, s), 3.60 (1H, s), 3.36 (1H, d, J = 9.0 Hz), 3.29 (1H, d, J = 9.0 Hz), 2.93 (1H, d, J = 9.6 Hz), 2.73 (1H, d, J = 9.6 Hz), 2.00 (1H, d, J = 9.3 Hz), 1.86 (1H, d, J = 9.3 Hz). Calcd for C19H19ClN4: C 67.35, H 5.65, N 16.54; Found: C 67.10, H 5.74, N 16.25.
3-(4-chlorophenyl)-1-(1H-pyrrolo[2,3-b]pyridin-3-ylmethyl)pyrrolidin-3-ol (13)
Using Method A and 3-(4-chlorophenyl)pyrrolidin-3-ol, 24 and 7-azaindole as starting materials, compound 13 was obtained in 37 % yield, mp 62.9–63.2 °C. 1HNMR (300 MHz, CD3OD): δ 2.15 (1H, m), 2.90 (1H, m), 2.95 (2H, m), 3.05 (1H, m), 3.93 (2H, s), 7.12 (1H, dd, J = 5.0, J = 7.8 Hz), 7.29 (2H, d, J = 8.6 Hz), 7.39 (1H, s), 7.47 (2H, d, J = 8.6 Hz), 8.16 (2H, m). Calcd for C18H18ClN3O·0.4H2O: C 64.53, H 5.66, N 12.54. Found: C 64.48, H 5.57, N 12.39.
3-{(4-(pyrimidin-2-yl)piperazin-1-yl)methyl}-1H-pyrrolo[2,3-b]pyridine (15)
Using method A and 1-(2-pyrimidyl)piperazine and 7-azaindole, produced compound 15 as a solid, mp 185–187 °C. 1H NMR (CDCl3): 9.00 (1H, brs), 8.31 (1H, dd, J = 1.5, 5.1 Hz), 8.28 (2H, d, J = 4.8 Hz), 8.09 (1H, dd, J = 1.5, 7.8 Hz), 7.24 (1H, s), 7.08 (1H, dd, J = 4.8, 8.4 Hz), 6.45 (1H, t, J = 4.8 Hz), 3.83 (4H, t, J = 5.4Hz), 3.73 (2H, s), 2.54 (4H, t, J = 5.4 Hz). Calcd for C16H18N6: C 65.29, H 6.16, N 28.55; Found: C 65.02, H 6.20, N 28.26.
Biology
Receptor Binding Studies
Binding affinities reported in Tables 1 – 3 were conducted by the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP) unless otherwise stated. Details of the methods and radioligands used for the binding assays were previously reported.20
Table 1.
Binding Affinity Constants of Synthetic Compounds to D2-like Receptors.
| Compounds | a Binding Data of Compounds, Ki ± SEM (nM) | |||
|---|---|---|---|---|
| D2 | D3 | D4 | D2/D4 | |
| Haloperidol | 1.1±0.07 [ 0.89] | 5.5±3.0[ 4.6] | 12.7±7.2 [10.0] | 0.10 [0.09] |
| 2a | 1.6 ±0.14 [0.31] | 5.1±3.0 [0.71] | 5.3± 0.99 [12.1] | 0.30 [0.03] |
| 2b | 1231±145 | >10,000 | 789±363 | 1.60 |
| 2c | 1050±209 | 172±33 | 1015±179 | 1.03 |
| 3a | 588±57.6 | 128±13 | 7873±1437 | 0.07 |
| 3b | 160.3±11.8 | 25.0±1.7 | 3007±561 | 0.05 |
| 3c | 53.0±6.4 | 18.0±1.6 | 277.5±26.5 | 0.19 |
| 4 | MPA | 2874±584 | 816.5±194.4 | --- |
| 5 | MPA | 3074±553 | MPA | --- |
= Data obtained from the NIMH-PDSP and those in square brackets are from ref 15. Ki is the mean value obtained on triplicate or quadruplicate determinations unless otherwise indicated. MPA = Missed primary assay threshold of 50% inhibition.
Acknowledgments
We gratefully acknowledge the financial support of the National Institute of General Medical Studies (NIGMS) for MBRS Grant No. GM 08111, NIMH Psychoactive Drug Screening Program, RCMI Grant No. G12 RR 03020 from NCRR, and a Title III Grant to Florida A&M University. The authors also acknowledge Dr Abdul Khan in the synthesis of compounds 8 and 9 and Dr. A. W. Schmidt at Pfizer Global Research for conducting the original binding studies for several of the reported compounds. This work was supported in part by the Pharmaceutical Research Center NIH/NCRR 1 C06-RR12512-01 Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Zhang A, Neumeyer JL, Baldessarini RJ. Recent progress in the development of dopamine receptor subtype-selective agents; Potential therapeutics for neurological and psychiatric disorders. Chem Rev. 2007;107:274–302. doi: 10.1021/cr050263h. [DOI] [PubMed] [Google Scholar]
- 3.a) Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]; b) Wilson JM, Sanyal S, Van Tol HHM. Dopamine D2 and D4 receptor ligands: relation to antipsychotic action. Eur J Pharmacol. 1998;351:273–286. doi: 10.1016/s0014-2999(98)00312-4. [DOI] [PubMed] [Google Scholar]; c) Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]; d) Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 4.a) Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]; b) Heidbreder CA, Andreoli M, Marcon C, Thanos PK, Ashby CR, Gardner EL. Role of dopamine D-3 receptors in the addictive properties of ethanol. Drugs Today. 2004;40:355–365. doi: 10.1358/dot.2004.40.4.820081. [DOI] [PubMed] [Google Scholar]; c) Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]; d) Joyce JN, Milian MJ. Dopamine D-3 receptor antagonists as therapeutic agents. Drug Discovery Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]; e) Le Foll B, Goldberg SR, Sokoloff P. The dopamine D-3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:718–726. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- 6.Nayak S, Cassaday HJ. The novel dopamine D4 receptor agonist (PD 168,077 maleate): doses with different effects on locomotor activity are without effect in classical conditioning. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:441–449. doi: 10.1016/S0278-5846(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 7.Browman KE, Curzon P, Pan JB, Molesky AL, Komater VA, Decker MW, Brioni JD, Moreland RB, Fox GB. A-412997, a selective dopamine D4 agonist, improves cognitive performance in rats. Pharmacol Biochem Behav. 2005;82:148–155. doi: 10.1016/j.pbb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Brioni JD, Moreland RB, Cowart M, Hsieh GC, Stewart AO, Hedlund P, Donnelly-Roberts DL, Nakane M, Lynch JJ, 3rd, Kolasa T, Polakowski JS, Osinski MA, Marsh K, Andersson KE, Sullivan JP. Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats. Proc Natl Acad Sci USA. 2004;101:6758–6763. doi: 10.1073/pnas.0308292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh GC, Hollingsworth PR, Martino B, Chang R, Terranova MA, O’Neill AB, Lynch JJ, Moreland RB, Donnelly-Roberts D, Kolasa T, Mikusa JP, McVey JM, Marsh KC, Sullivan JP, Brioni JD. Central Mechanisms Regulating Penile Erection in Conscious Rats: The Dopaminergic Systems Related to the Pro-erectile Effect of Apomorphine. J Pharmacol Exp Ther. 2004;308:330–338. doi: 10.1124/jpet.103.057455. [DOI] [PubMed] [Google Scholar]
- 10.Cowart M, Latshaw SP, Bhatia P, Daanen JF, Rohde J, Nelson SL, Patel M, Kolasa T, Nakane M, Uchic ME, Miller LN, Terranova MA, Chang R, Donnelly-Roberts DL, Namovic MT, Hollingsworth PR, Martino BR, Lynch JJ, III, Sullivan JP, Hsieh GC, Moreland RB, Brioni JD, Stewart AO. Discovery of 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole (ABT-724), A Dopaminergic Agent with a Novel Mode of Action for the Treatment of Erectile Dysfunction. J Med Chem. 2004;47:3853–64. doi: 10.1021/jm030505a. [DOI] [PubMed] [Google Scholar]
- 11.Sikazwe DMN, Li S, Mardenborough L, Cody V, Roth BL, Ablordeppey SY. Haloperidol: Towards further understanding of the structural contributions of its pharmacophoric elements at D2-like receptors. Bioorg Med Chem Lett. 2004;14:5739–5742. doi: 10.1016/j.bmcl.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 12.a) Curtis NR, Kulagowski JJ, Leeson PD, Ridgill MP, Emms F, Freedman SB, Patel S, Patel S. Synthesis and SAR of 2- and 3-substituted 7-azaindoles as potential dopamine D4 ligands. Bioorg Med Chem Lett. 1999;9:585–588. doi: 10.1016/s0960-894x(99)00025-6. [DOI] [PubMed] [Google Scholar]; b) Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Patel S, Ragan CI, Leeson PD. 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- 13.a) Grundt P, Husband SL, Luedtke RR, Taylor M, Newman AH. Analogues of the dopamine D2 receptor antagonist L741,626: Binding, function, and SAR. Bioorg Med Chem Lett. 2007;17:745–749. doi: 10.1016/j.bmcl.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stewart AO, Cowart MD, Moreland RB, Latshaw SP, Matulenko MA, Bhatia PA, Wang XM, Sullivan JP, Brioni JD, Daanen JF, Nelson SL, Terranova MA, Namovic MT, Donnelly-Roberts DL, Miller LN, Nakane M, Sullivan JP, Brioni JD. Dopamine D4 ligands and models of receptor activation: 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole and related heteroarylmethylarylpiperazines exhibit a substituent effect responsible for additional efficacy tuning. J Med Chem. 2004;47:2348–2355. doi: 10.1021/jm0305669. [DOI] [PubMed] [Google Scholar]
- 14.Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Suresh Kumar EVK, Jackson T, Khan A, Roth BL. Identification of A Butyrophenone Analog as a Potential Atypical Antipsychotic Agent: 4-[4-(4-Chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008;16:7291–7301. doi: 10.1016/j.bmc.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyles-Eggleston M, Altundas R, Xia J, Sikazwe DMN, Fan P, Yang Q, Li S, Zhang W, Zhu X, Schmidt AW, Vanase-Frawley M, Shrihkande A, Villalobos A, Ablordeppey SY. Design, Synthesis, and Evaluation of Metabolism-Based Analogues of Haloperidol Incapable of Forming MPP+-like Species. J Med Chem. 2004;47:497–508. doi: 10.1021/jm0301033. [DOI] [PubMed] [Google Scholar]
- 16.Sikazwe DMN, Li S, Lyles-Eggleston M, Ablordeppey SY. The Acute EPS of Haloperidol May be Unrelated to Its Metabolic Transformation to BCPP+ Bioorg Med Chem Lett. 2003;13:3779–3782. doi: 10.1016/j.bmcl.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Ablordeppey SY, Lyles-Eggleston M, Bricker B, Zhang W, Zhu XY, Goodman C, Roth BL. Evaluation of the Eutomer of 4-{3-(4-chlorophenyl)-3-hydroxypyrrolidin-1-yl}-1-(4-fluorophenyl) butan-1-one, {(+)-SYA 09}, a Pyrrolidine Analog of Haloperidol. Bioorg Med Chem Lett. 2006;16:3219– 3223. doi: 10.1016/j.bmcl.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 18.Ablordeppey SY, Borne RF. Design and Synthesis of Novel analogues of Haloperidol incapable of forming MPP+-like metabolites. Med Chem Res. 1993;3:459–467. doi: 10.1021/jm0301033. [DOI] [PubMed] [Google Scholar]
- 19.Michaels RJ, Zaugg HE. Synthesis of 9-Methyl-3,9-diazabicyclo[4.2.1]-nonane. J Org Chem. 1960;25:637–638. [Google Scholar]
- 20.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacol. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]