Abstract
The reconsolidation hypothesis posits that memories that have been reactivated can be either enhanced or disrupted by pharmacological manipulation. Synaptic plasticity is presumed to underlie the reconsolidation process. Matrix metalloproteinases are proteins that regulate the extracellular matrix involved in plasticity events, and these proteins have recently been shown to influence learning and memory. However, all studies on the role of matrix metalloproteinases in learning and memory have employed tasks that rely on contextual cues. The goal of this study was to determine the extent to which FN-439 would disrupt the consolidation and/or reconsolidation of a fear memory associated with a conditioned stimulus that signaled tone-shock pairings and that was independent of contextual cues. Male Sprague-Dawley rats were given infusions of FN-439 (35 μg intracerebroventricular) 30 min prior to conditioning (tone-shock paired association) or 30 min prior to a single reactivation session given 24 hr after conditioning. Administration of FN-439 did not disrupt consolidation of the freezing response when the tone (conditioned stimulus) was presented. In contrast, FN-439 infusion disrupted reconsolidation of the fear memory in a reactivation-dependent manner. The reduced freezing behavior was not due to a decrease in general anxiety levels, since FN-439 had no effect on the percent of open-arm time or open-arm entries in an elevated-plus maze task. Thus, we demonstrated for the first time that matrix metalloproteinase inhibition in the brain is capable of disrupting the reconsolidation of a tone-shock association memory that does not depend on contextual cues. The finding that a fear response to a previously paired conditioned stimulus can be disrupted by treatment with an MMP inhibitor during a single reactivation session suggests that this class of compounds may have therapeutic potential for posttraumatic stress disorder and/or simple phobias.
Keywords: Consolidation, Fear conditioning, Matrix metalloproteinase, Memory, Reconsolidation
1. Introduction
An intriguing aspect of memory research is the recent realization that specific memories may be targeted for disruption by pharmacotherapies, indicating that the unwanted and persistent memories present in individuals with posttraumatic stress disorder (PTSD) may be diminished. Posttraumatic stress disorder is a psychological disorder affecting 8% of United States citizens (Iribarren, Prolo, Neagos, & Chiappelli, 2005; Guess, 2006), and can develop when an individual experiences or witnesses a life-threatening or traumatic episode such as a natural disaster, terrorist attack, violent personal attack or military combat (Iribarren et al., 2005). Symptoms of an individual diagnosed with PTSD include intense fear, persistent increased arousal, persistent re-experiencing of the trauma, and avoidance of the stimuli associated with the trauma (Tiller, Kyrios, & Bennett, 1996). These disturbances cause clinically significant distress in the individual, making it difficult to function in social or occupational settings.
The traditional consolidation hypothesis postulated that memory consolidation is initially labile after acquisition but becomes strengthened over time and as a result is less susceptible to amnesic treatment (Sara, 2000; Alberini, 2005). However, several studies have shown that upon reactivation (recall of the memory), a well-consolidated memory may return to a labile state, during which time it can be modified by pharmacological agents (Misanin, Miller, & Lewis, 1968; Przybyslawski and Sara, 1997; Przybyslawski, Roullet, & Sara, 1999; Nader, Schafe, & LeDoux 2000a). Several pharmacological manipulations that disrupt consolidation also disrupt reconsolidation of the same task (Przybyslawski et al., 1999; Nader et al., 2000a; Sangha, Scheibenstock, & Lukowiak, 2003). However, numerous studies have also shown that consolidation and reconsolidation have dissociable component processes (see Alberini, 2005 and Nader, 2007 for review). For instance, infusion of an antisense oligodeoxynucleotide for brain-derived neurotrophic factor (BDNF) into the basolateral amygdala blocks consolidation but not reconsolidation of a fear memory, and administration of antisense for Zif268 blocks reconsolidation but not consolidation (Lee, Everitt, & Thomas, 2004). This divergence in consolidation and reconsolidation disruption has been observed for several different tasks: inhibition of protein synthesis in the hippocampus disrupts the consolidation but not reconsolidation of an inhibitory avoidance memory (Taubenfeld, Milekic, Monti, & Alberini, 2001), and inhibition of protein synthesis in the amygdala disrupts consolidation but not reconsolidation of taste aversion memory (Bahar, Dorfman, & Dudai, 2004). The studies described above suggest that different behavioral paradigms recruit different molecular machinery when undergoing memory consolidation compared with memory reconsolidation.
The focus of the present study is on the role of matrix metalloproteinases (MMPs) in fear conditioning. These proteins regulate the extracellular matrix (ECM), a highly dynamic meshwork that influences tissue remodeling in response to changing environments. Activity-dependent changes in the organization of the ECM are believed to alter synaptic architecture and physiology in a way that changes the efficiency of synaptic transmission (Rauvala and Peng, 1997; Hoffman, Martinez, & Lynch, 1998a; Hoffman, Pinkstaff, Gall, & Lynch, 1998b; Lynch, 1998; Lauri, Kaukinen, Kinnunen, Ylinen, Imai, Kaila, Taira, & Rauvala, 1999). The ECM proteins include structural proteins like collagens and adhesive proteins that interact with cell adhesion molecules (CAMs) (Wright, Reichert, Davis, & Harding, 2002). The MMPs are a large family of enzymes with more than 20 members that are characterized by different specificities for ECM proteins. MMPs are zinc metalloendopeptidases that are produced as zymogens and secreted by cells. They have been implicated in neural plasticity via degradation of the ECM and thus restructuring the ECM (Dzwonek, Rylski, & Kaczmarek, 2004; Wright and Harding, 2004). Most recently, MMPs have been shown to directly impact dendritic spines, thus enhancing or hampering synaptic transmission (Tian, Stefanidakis, Ning, Van Lint, Nyman-Huttunen, Libert, Itohara, Mishina, Rauvala, & Gahmberg, 2007; Gawlak, Gorkiewicz, Gorlewicz, Konopacki, Kaczmarek, & Wilczynski, 2008).
Previous studies in our laboratory and others have shown that intracerebroventricular (i.c.v.) infusion of FN-439, a broad spectrum MMP inhibitor, disrupts the consolidation of cocaine-conditioned place preference (CPP) (Brown, Forquer, Cocking, Jansen, Harding, & Sorg, 2007), the consolidation of spatial memory tasks (Meighan, Meighan, Choudhury, Davis, Olson, Zornes, Wright, & Harding, 2006; Wright, Brown, & Harding, 2007), and the induction and duration of LTP, a molecular correlate of learning (Meighan, Meighan, Davis, Wright, & Harding, 2007; Okulski, Jay, Jaworski, Duniec, Dzwonek, Konopacki, Wilczynski, Sánchez-Capelo, Mallet, & Kaczmarek, 2007). We have also shown that FN-439 infused i.c.v. 30 min before or just after reactivation sessions attenuated cocaine-primed reinstatement of cocaine CPP. The attenuation in cocaine-primed reinstatement was not observed in animals unless they were reactivated in the CPP context, suggesting that reconsolidation of a cocaine-CPP memory could be disrupted by inhibition of MMP activity (Brown et al., 2007).
All of the previous learning and memory studies utilizing FN-439 or other MMP inhibitors have used behavioral tasks that rely on spatial recognition or the use of contextual cues. Therefore, our goal was to determine the extent to which FN-439 would disrupt the consolidation and/or reconsolidation of a fear memory associated with a CS that signaled tone-shock pairings and that was independent of spatial or contextual cues. We tested the hypothesis that MMP inhibition with FN-439 would impair the consolidation and/or reconsolidation of a conditioned fear memory.
2. Materials and Methods
2.1 Animal Housing and Drugs
Male Sprague-Dawley rats weighing 260–300 g were obtained from Simonsen Laboratories (Gilroy, CA). Experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and experimental protocols were approved by the University Animal Care and Use Committee. Animals were housed in pairs with free access to food and water in a temperature- and humidity- controlled room with a 12-hour light/dark cycle (lights on at 06:00h). After cannulae implantation, animals were housed individually.
FN-439 was obtained from Calbiochem (La Jolla, CA; FN-439 = MMP inhibitor I) and dissolved at a concentration of 35 μg in 5 μL (= 14.3 mM) in artificial cerebral spinal fluid (aCSF; 5 mM d-glucose, 2.7 mM KCl, 140 mM NaCl, 1.2 mM MgCl2, 1.4 mM CaCl2, 0.15% PBS). This dose was chosen based on our previous study that FN-439 given i.c.v. impaired consolidation and reconsolidation of a cocaine-CPP task (Brown et al., 2007) and on previous work demonstrating the effects of FN-439 on a spatial task (Meighan et al. 2006).
2.2 Animal surgery and microinjection
2.2.1 Surgery
Rats were anesthetized with zyket (ketamine 87 mg/kg + xylazine 13 mg/kg) and placed into a stereotaxic apparatus. For i.c.v. microinjection, a unilateral 26 gauge stainless steel cannulae (Plastics One, Roanoke, VA) was fixed with dental acrylic cement over the lateral ventricle (−1.0 from bregma, ± 1.5 mm from midline and −4.0 mm from skull. Obturators (33 g) measuring the length of the guide cannulae were inserted into the cannulae at all times other than during microinjection. Rats recovered one week prior to experimentation. At the end of the experiment, all cannulae placements were verified with dye injection into the lateral ventricle.
2.2.2 Microinjection
Intracranial injections were done using a 33 ga stainless steel needle connected to PE-20 tubing leading to a 5.0 μL Hamilton syringe. The 33 ga needles were lowered 1 mm below the guide cannulae, and a volume of 5.0 μL was delivered over a period of 2 min using an infusion pump. The needles were allowed to remain in place for 30 sec following the injection. A microinjection of saline was always given prior to the first day of test microinjections to adapt the animals to the procedure.
2.3 Behavioral Procedures
2.3.1 Conditioned Fear Task
The fear-conditioned apparatus was the same as that previously described (Cloutier, Forquer, & Sorg, 2006). For the consolidation experiment, animals were habituated to the shock chamber for 10 min for two consecutive days prior to conditioning. Either aCSF or FN-439 was infused i.c.v. 30 min prior to conditioning, which consisted of a single 10-sec tone presentation (conditioned stimulus, CS) that coterminated with a 1 sec, 1.0 mA footshock (unconditioned stimulus, US). Freezing behavior was assessed 24 hr later in a separate chamber (test chamber) as described below. For the reconsolidation experiments, animals were habituated for 2 days in alternating fashion to the shock chamber and test chamber. Conditioning consisted of four tone-shock pairings given with a 10-sec tone presentation, which co-terminated with a 1sec, 1.0 mA footshock. Each presentation was separated by a 4-min intertrial interval. Twenty-four hr following conditioning, rats were given an i.c.v. infusion of aCSF or FN-439 (same dose as for consolidation experiments) 30 min prior to reactivation. The reactivation session consisted of 3 min of no CS followed by 3 min of tone CS presentation in the test chamber. Some rats (no CS reactivation group) were given aCSF or FN-439, and 30 min later were simply placed in the test chamber for 6 min total. Subsequent freeze monitoring was conducted, which is described below.
The reason for using one tone-shock pairing for consolidation experiments and four tone-shock pairings for the reconsolidation experiments is that we first conducted some of the reconsolidation experiments and chose to use multiple pairings, as we had done previously (Cloutier et al., 2006). We observed profound effects of FN-439 treatment in this reconsolidation experiment (see Results). We therefore chose to attempt to disrupt the consolidation of a weaker memory produced with a single tone-shock pairing. Given that we did not find an effect of FN-439 on consolidation, we did not conduct a further test to determine the effects of this drug using additional pairings.
2.3.2 Freeze monitoring
Freezing behavior was monitored using the VersaMax® Freeze Monitoring System (version 2.80F software; Accuscan Instruments, Columbus, OH) as described previously (Cloutier et al., 2006). Animals were monitored in a 6-min test during which there was no CS presentation for the first 3 min followed by the tone CS presentation for the second 3 min. For the consolidation experiment, rats were tested 24 hr and 7 days after conditioning. For reconsolidation experiments, rats were tested 3 hr, 24 hr, and 7 days after reactivation.
2.3.3 Elevated-plus maze
The elevated plus-maze was as previously described (Cloutier et al., 2006) and consisted of a “plus”-shaped platform made of black opaque Plexiglas that was 10 cm in width and 50 cm in length, creating a 10 × 10 cm neutral zone in the center. The plus-maze was elevated 50 cm from the floor. Two of the arms were enclosed with black Plexiglas walls 40 cm high, with no ceiling.
The elevated plus-maze relies on the animal’s natural fear of open spaces, and the percent time spent on the open arms and percent of open arm entries are believed to be measures of general anxiety level. For this task, aCSF or FN-439 (same dose as for the consolidation and reconsolidation experiments) was infused i.c.v. either 30 min, 3 hr or 7 days prior to rats being placed individually into the center (neutral) zone of the maze, facing an open arm. Rats were allowed to explore the maze for a 5 min period, and the number of open and closed arm entries and time spent on the open and closed arms were recorded. Animals were considered to be in the open or closed arms only when all four paws crossed out of the neutral zone. Percent open arm time was calculated by taking the time spent in the open arms and dividing it by the sum of the time spent in the open arms plus time spent in the closed arms. The percent of open arm entries was calculated by taking the number of open arm entries and dividing it by the sum of the number of open arm plus closed arm entries.
2.4 Statistical analysis
For analysis of freezing behavior shown in Figures 1B, 2C and 2E, a three-way, repeated measures ANOVA was conducted (aCSF vs. FN-439 as the between-subjects measure and stimulus condition (Pre-CS vs. CS) and Day as the within-subjects measures). In the case of a significant three-way interaction, a 2-way ANOVA was conducted separately for each day of testing (aCSF vs. FN-439 as the between-subjects measure and stimulus condition (Pre-CS vs. CS) as the within-subjects measure). For analysis of freezing behavior shown in Figure 2B, data were analyzed using a two-way, repeated measures ANOVA (aCSF vs. FN-439 as the between-subjects measure and stimulus condition (Pre-CS vs. CS) as the within-subjects measure). Elevated plus-maze data were analyzed with separate, two-tailed t-tests for each day and endpoint measured. All group sizes are reported in the figure legends.
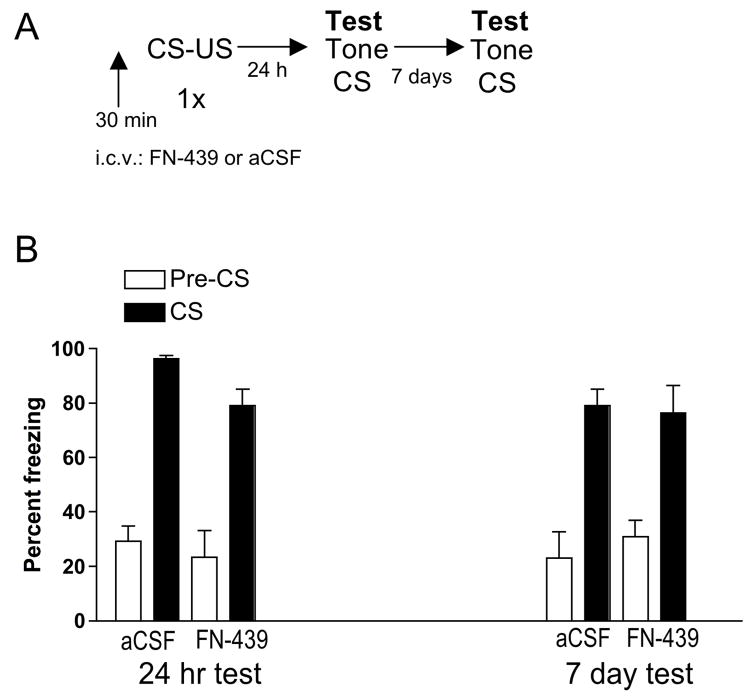
Figure 1. MMP inhibition with FN-439 treatment prior to conditioning in the shock chamber does not block subsequent freezing in response to the tone CS.
A. Experimental timeline. B. Data are mean ± SEM of percent freezing. Percent freezing = percent freezing in the absence of tone presentation (Pre-CS) followed by percent freezing in the presence of tone presentation (CS). Tests were conducted 24 hr and 7 days after conditioning. I.C.V. infusion of aCSF or FN-439 was done 30 min prior to the conditioning session in the shock box. N = 6 for aCSF group; N = 8 for FN-439 group.
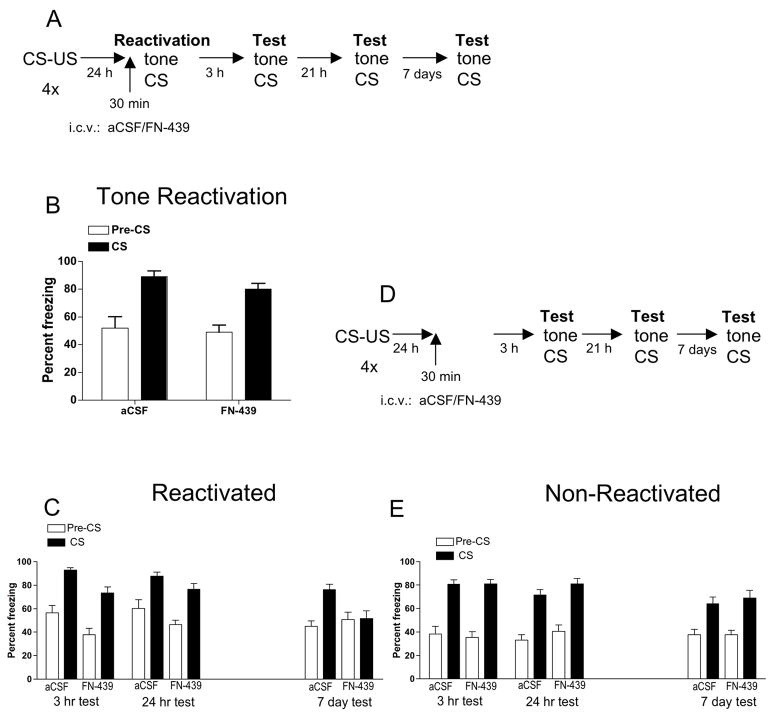
Figure 2. MMP inhibition with FN-439 treatment prior to tone reactivation in the freeze monitoring chamber blocks freezing in response to the tone CS 7 days later.
(A) Experimental timeline for rats given the tone CS reactivation session. (B) Tone reactivation session. Data are mean ± SEM of percent freezing. Percent freezing = percent freezing in the absence of tone presentation (Pre-CS) followed by percent freezing in the presence of tone presentation (CS). Injection of aCSF or FN-439 was given 30 min prior to the tone reactivation session in the freeze monitoring chamber (test chamber). Both the aCSF group and FN-439 group showed a significant elevation in freezing when the tone CS was present compared to the pre-CS period, with no difference in freezing between the two treatment groups. N = 11 for aCSF group; N = 10 for FN-439 group. (C) Test in reactivated animals shown in 2B when assessed 3 hr, 24 hr and 7 day later for freezing response to the tone CS. Rats given FN-439 during the reactivation session did not demonstrate increased freezing to the CS on the 7 day test. (D) Experimental timeline for non-reactivation control groups. Rats were given identical treatment as shown in the Figure 2A protocol, but on the day of reactivation, animals were placed into the test chamber for 6 min with no tone CS presentation. (E) Non-reactivation control groups. Data are mean ± SEM of percent freezing. Rats were tested 3 hr, 24 hr and 7 day later. There were no differences in freezing between treatment groups at any of the time points. N = 16 for aCSF group; N = 15 for FN-439 group.
3. Results
Figure 1 shows the results from Experiment 1 testing whether FN-439 disrupted consolidation of the tone-shock association, which would be indicated by a reduction in freezing behavior in response to the tone CS. There was a significant effect of stimulus state (F1,55 = 79.5, p < 0.0001). However, there was no difference in freezing behavior between the aCSF and FN-439 treated animals when tested 24 hr or 7 days later, indicating that FN-439 did not disrupt the consolidation of conditioned freezing behavior.
Experiment 2 tested whether FN-439 could disrupt reconsolidation of the tone-shock association, and the results are shown in Figure 2. The protocol for animals given the reactivation session is shown in Figure 2A. Rats were microinjected 30 min prior to tone reactivation in the test cage, and freezing behavior was recorded. During the reactivation session, Figure 2B shows that both treatment groups demonstrated a significant increase in freezing behavior after the CS presentation compared with the pre-CS portion of the session, since there was a significant effect of stimulus state (F1,19 = 34.24, p < 0.0001) but no other effects. Figure 2C shows the response to the tone CS 3 hr, 24 hr and 7 days later. There was a significant effect of treatment (F1,3 = 9.88, p < 0.005), day (F3,57 = 4.29, p < 0.008), stimulus state (Pre-CS vs. CS; F1,57 = 102, p < 0.0001) and a significant interaction among treatment, day, and stimulus state (F3,57 = 2.89, p < 0.04). Subsequent two-way ANOVAs revealed that, at the 3 hr test, there was a significant treatment effect (F1,19 = 12.0, p < 0.003), and a significant stimulus effect (F1,19 = 66.6, p < 0.0001). At the 24 hr test, there was a significant stimulus effect (F1,19 = 46.9, p < 0.0001). At 7 days, there was a significant stimulus effect (F1,19 = 14.2, p < 0.001) and a significant treatment × stimulus interaction (F1,19 = 11.7, p = 0.003). These results indicate that when rats were tested 7 days later, prior FN-439 treatment significantly decreased freezing to the tone CS compared with aCSF treatment. In the FN-439 group, there was no longer a difference between pre-and post-CS freezing at 7 days (Figure 2C).
To determine whether FN-439 disrupted the tone-shock-associated memory rather than produced a non-specific amnesic effect, we tested the effect of FN-439 treatment prior to placement of rats in the test chamber in the absence of the tone CS to determine whether this compound would interfere with freezing to the tone CS in subsequent tests given 3 hr, 24 hr or 7 days later. Figure 2D shows the treatment protocol for this experiment, which is identical to that shown in Figure 2A except for the omission of the reactivation session in which the tone CS was presented. Figure 2E shows that there was no difference in freezing response to the CS between aCSF and FN-439 treated rats 3 hr, 24 hr or 7 days after reactivation. There was a significant effect of day (F2,58 = 3.92, p < 0.03) and stimulus (F1,58 = 92.8, p < 0.0001). Taken together, these results indicate that disruption of the conditioned fear response was dependent on reactivation of the memory using the tone CS while FN-439 was present, and suggest that FN-439 disrupted reconsolidation of the tone-shock-associated memory.
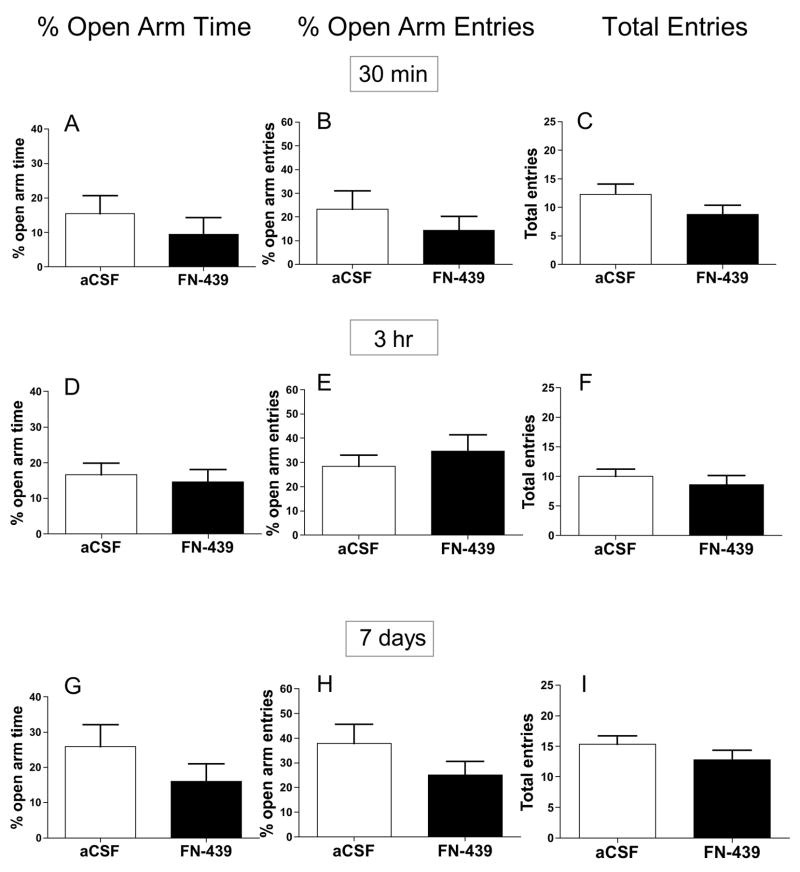
It is possible that FN-439 altered general anxiety-like behavior that did not involve a conditioning component, producing longer term effects on freezing when tested 7 days later. We also tested whether FN-439 infusion would alter anxiety levels either 30 min or 3 hr later to assess whether there may have been any short-term effects on anxiety-like behavior. To address these issues, FN-439 was infused 30 min, 3 hr, or 7 days prior to testing rats on an elevated-plus maze (Figure 3). There was no difference in any of the parameters analyzed (percent open arm time, percent open arm entries and total entries) at any of the three time points examined, suggesting that the results observed in Figure 2C could not be explained by a reduction in general anxiety-like behavior or by an alteration in general exploratory motor activity.
Figure 3. MMP inhibition with FN-439 treatment does not alter anxiety-like behavior in the elevated-plus maze.
Data are mean ± SEM of percent open arm time (in sec), percent open arm entries, and total arm entries at 30 min (A – C), 3 hr (D – F), or 7 days (G – I ) after i.c.v. FN-439 infusion. (A – C) N = 4 for each group. (D – F) N = 13 for aCSF group; N = 14 for FN-439 group. (G – I) N = 10 for each group. There were no significant differences between treatment groups for any of the measures at any of the three times tested.
4. Discussion
This study sought to extend previous observations in our lab and others that FN-439, a broad-spectrum MMP inhibitor, could impair learning, consolidation and/or the reconsolidation of a memory. All studies to date have tested the impact of FN-439 or other MMP inhibitors on memory that relies upon spatial or contextual cues. Therefore, our goal was to determine the extent to which FN-439 would disrupt the consolidation and/or reconsolidation of a memory associated with a CS that signaled tone-shock pairings and that was independent of spatial or contextual cues. The main findings from this study are: 1) FN-439 administration did not suppress the freezing response to the tone CS when presented 24 hr or 7 days later, suggesting that it did not interfere with consolidation; 2) FN-439 administration during a reactivation session in which the tone CS was presented suppressed the freezing response to the CS 7 days after reactivation, but not at 3 hr or 24 hr after reactivation; 3) FN-439 treatment in rats placed in the test chamber in the absence of the tone CS did not alter the freezing response to the CS 3 hr, 24 hr, or 7 days later; and 4) The decrease in freezing behavior in reactivated FN-439 treated rats 7 days later was not due to a general reduction in anxiety behavior. Thus, FN-439 did not disrupt the consolidation of a tone-shock memory but did disrupt the reconsolidation of a tone-shock memory.
Memories that are formed after initial experiences are thought to become consolidated, or strengthened, over time (Sara, 2000). An initial memory that is formed is susceptible to interference early after its formation (Nader, Schafe, & LeDoux, 2000b; Wright et al., 2002; Eisenberg, Kobilo, Berman, & Dudai, 2003; Riccio, Millin, & Bogart, 2006). This memory can be strengthened over time, and it was believed for years that it became less susceptible to interference as time passed. The idea that consolidated memories are stable and not susceptible to interference was first challenged in the late 1960’s (Misanin et al., 1968), but this notion lay largely dormant until a study by Nader et al. (2000a) demonstrated that a consolidated tone-shock association was disrupted with a protein synthesis inhibitor when that tone was given to reactivate the original memory (Nader et al., 2000b). Since that time, numerous studies have shown that stable memories appear to become labile upon retrieval, and as a result, become destabilized and ultimately weakened in the presence of certain pharmacological agents (see Introduction, and reviews by Dudai, 2006; Nader, 2007; Sara, 2000).
The broad-spectrum MMP inhibitor FN-439 has been shown to disrupt the consolidation of spatial tasks (Meighan et al., 2006; Wright et al., 2007), the consolidation and reconsolidation of cocaine-CPP (Brown et al., 2007), and the stability of LTP (Meighan et al., 2007). Also, MMP-9 or broad MMP inhibitors prevent the induction and/or stability of long-term potentiation (LTP) (Nagy, Bozdagi, Matynia, Balcerzyk, Okolski, Dzwonek, Costa, Silva, Kaczmarek, & Huntley, 2006; Meighan et al., 2007). Knockout of MMP-9 impairs contextual fear conditioning (Nagy et al., 2006), and Nagy et al. (2007) and Olson (Olson, Meighan, Brown, Asay, Benoist, Harding, & Wright, 2008) have demonstrated a critical role for MMP-9 and MMP-3 in inhibitory avoidance learning. These studies have focused on hippocampal-dependent behavior that relies on a spatial orientation strategy or contextual cues (Myers, Zavala & Neisewander, 2003; Wright, Murphy, Elijah, Holtfreter, Davis, Olson, Muhunthan, & Harding, 2004). In contrast, the conditioned fear paradigm generates tone-shock-associations, and these associations have been shown to be hippocampus-independent (Kim and Fanselow, 1992; Phillips and LeDoux, 1992). The only study we are aware of that examined a role for MMPs in freezing behavior reported that MMP-9 knockout mice have a deficit in the acquisition of contextual fear conditioning but not in the acquisition of cued fear conditioning to a CS (Nagy et al. 2006), consistent with our present findings from the consolidation experiment. Here we demonstrate for the first time that MMP inhibition in the brain is capable of disrupting the reconsolidation of a tone-shock association memory that does not depend on contextual cues. Thus, the role of brain MMPs in learning and memory can now be expanded to include the reconsolidation of memory for a fear-associated CS.
FN-439 did not disrupt the consolidation of the tone-shock memory. This was an unexpected finding since our previous experiments using the same dose of FN-439 demonstrated the ability of FN-439 to disrupt the consolidation of cocaine-CPP (Brown et al., 2007). Our present findings were further surprising, given that we used only a single tone-shock pairing for the consolidation experiment and 4 tone-shock pairings for the reconsolidation experiment. It may be that consolidation of cocaine-CPP in our previous study, but not the tone-shock memory in the present study, was disrupted by FN-439 because the cocaine-memory was weaker than the tone-shock memory. A single pairing of tone with shock is effective in forming long-lasting associations that can last for up to 1 yr (Hoffman, Fleshler, & Jensen, 1962; Fanselow and Gale, 2003). However, in our hands, a single pairing of cocaine (12 mg/kg) with a neutral chamber does not produce CPP (data not shown), suggesting that consolidation of the cocaine-associated chamber may be more easily disrupted by FN-439 given during the training (acquisition) period. As a result, associations made in fear conditioning may become consolidated more intensely than those in cocaine-CPP, and these associations may therefore be harder to disrupt.
There are several possible explanations for the dissociation between the effects of FN-439 on consolidation and reconsolidation of fear conditioning. One possibility is that consolidation of the tone-shock association requires different MMPs from those necessary for reconsolidation of this association. Alternatively, different brain regions may be involved in the consolidation vs. the reconsolidation process of fear memory, and i.c.v. infusion of FN-439 may not have reached high enough concentrations to inhibit MMPs in the brain region(s) involved in consolidation of the tone-shock pairing. Based on previous studies that examined the consolidation and reconsolidation of fear memory, we would expect the amygdala to contribute to these processes (Bucherelli, Baldi, Mariottini, Passani & Blandina, 2006; Lee, Milton, & Everitt, 2006; Nader et al., 2000), and future studies would need to explore the effects of FN-439 directly applied to this brain region. A third possibility is that, as discussed by Nader (2007), the protocol used to study consolidation of fear conditioning is different from the protocol used to study reconsolidation. This is because when testing for consolidation, the drug (FN-439 in our case) is administered, and then both the CS and the US are applied during the training phase, whereas when testing for reconsolidation, only the CS is given during the reactivation session. This was the case for the fear conditioning study described here, but not for our previous study with cocaine-CPP (Brown et al., 2007). In that study, we administered both the CS (the CPP compartment) and the US (cocaine) during both the consolidation (training) period and the reactivation session to measure subsequent reconsolidation. While it is not apparent why we observed different actions of FN-439 on consolidation of fear conditioning vs. consolidation of cocaine-CPP, different neural circuitry mediates these two different behaviors, and the discrepancy in protocols discussed above may explain why consolidation and reconsolidation of memories are differently affected by FN-439 when examining fear conditioning but are similarly affected by FN-439 when examining cocaine-CPP.
Regardless of which MMPs or brain regions are involved with memory consolidation, i.c.v. infusion of FN-439 was capable of disrupting reconsolidation of the tone-shock association in a reactivation-dependent manner (Figure 2). There is some possibility that the reduction in freezing behavior 7 days after reactivation was due to enhanced extinction of the fear response. However, this seems an unlikely explanation because animals demonstrated no significant extinction between the reactivation session and the 3 hr or 24 hr sessions. The reduction in freezing 7 days later was not due to a reduction in general anxiety-like behavior. There were also no changes in anxiety-like behavior during the reactivation session (given 30 min after i.c.v. infusion of FN-439) or 3 hr after the infusion, since there was no difference in performance on the elevated plus-maze at these times. These results are consistent with our observation in the non-reactivated group in which FN-439 treated rats showed no differences with controls in freezing behavior at any of the three time points tested.
Other studies demonstrating disruption of reconsolidation typically have reported a decrease in freezing response 24 hr after the reactivation session (Lee et al., 2004; Lee et al., 2006; Nader et al., 2000), and some studies have demonstrated the persistence of this disruption by testing animals for conditioned fear several days later (Debiec & LeDoux, 2004; Lee et al., 2006). We did not observe disruption 24 hr after conditioning, suggesting that this period of time was too brief to produce the changes that are necessary to suppress the expression of the tone-shock-associated memory. In our previous study examining the reconsolidation of cocaine-CPP, animals were always administered two infusions of FN-439 and then tested 24 hr later (Brown et al., 2007). It is thus possible that we would also have observed a reduction in cued fear conditioning if we had tested for freezing behavior 2 days after the reactivation session.
The possibility that FN-439 has very long-acting effects cannot be ruled out, since in vitro, FN-439 has prolonged inhibitory effects on gelatinases even after overnight incubation with several proteases (Odake, Morita, Morikawa, Yoshida, Hori, & Nagai, 1994). The in vivo stability of FN-439 in the brain is not known, but it seems unlikely that FN-439 itself persists for 7 days. FN-439 has broad inhibitory effects on MMPs, including MMP-1, MMP-3, MMP-8, and MMP-9 (Odake et al., 1994; Hagemann, Robinson, Schulz, Trümper, Balkwill, & Binder, 2004; Wright et al., 2007). We are not aware that FN-439 inhibits proteases other than MMPs (Odake et al., 1994), although FN-439 may inhibit additional MMPs that have not yet been tested. MMPs impact the functioning of a wide range of molecules involved in neural plasticity, including brain-derived neurotrophic factor, cadherins, integrins, ephrins, laminins, tenascin-R, beta-dystroglycan, N-methyl-D-aspartate receptors, and other MMPs (Dityatev & Schachner, 2003; Dzwonek et al., 2004; Agrawal, Lau, & Yong, 2008; Szklarczyk, Ewaleifoh, Beique, Wang, Knorr, Haughey, Malpica, Mattson, Huganir, & Conant, 2008). Therefore, the most likely possibility is that FN-439 impairs one or more of these downstream molecules necessary for the long-term stability of fear memory associated with the CS.
In conclusion, FN-439 disrupted reconsolidation for a tone-shock association but had no effect on consolidation. This disruption was reactivation-specific, because FN-439 had no effect on subsequent freezing when it was given in the absence of the tone CS presentation during the reactivation session. Therefore, inhibition of MMP activity during memory reactivation may provide a new therapeutic approach for disrupting the pervasive memories associated with PTSD.
Acknowledgments
The authors thank Brian Lee for assistance with some of the experiments, Dr. Bryan K. Slinker for help with the statistical analysis, and Ms. Jenny Baylon for assistance with the manuscript. This work was supported by National Institutes of Health grants ES 09135 and DA 023729.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal SM, Sau L, Yong VW. MMPs in the central nervous system: Where the good guys go bad. Seminars in Cell & Developmental Biology. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends in Neuroscience. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bahar A, Dorfman N, Dudai Y. Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. European Journal of Neuroscience. 2004;19:1115–1118. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learning and Memory. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucherelli C, Baldi E, Mariottini C, Passani MB, Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learning and Memory. 2006;13:426–430. doi: 10.1101/lm.326906. [DOI] [PubMed] [Google Scholar]
- Cloutier S, Forquer MR, Sorg BA. Low level lindane exposure alters extinction of conditioned fear in rats. Toxicology. 2006;217:147–154. doi: 10.1016/j.tox.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption and reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nature Reviews. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Current Opinion in Neurobiology. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Dzwonek J, Rylski M, Kaczmarek L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Letters. 2004;567:129–135. doi: 10.1016/j.febslet.2004.03.070. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Gawlak M, Górkiewicz T, Gorlewicz A, Konopacki FA, Kaczmarek L, Wilczynski GM. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience. 2008 June 7; doi: 10.1016/j.neuroscience.2008.05.045. [EPub ahead of print] [DOI] [PubMed] [Google Scholar]
- Guess KF. Posttraumatic stress disorder: early detection is key. The Nurse Practioner. 2006;31:26–27. doi: 10.1097/00006205-200603000-00008. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteinases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Fleshler M, Jensen JK. Aversive training: long-term effects. Science. 1962;138:1269–1270. doi: 10.1126/science.138.3546.1269. [DOI] [PubMed] [Google Scholar]
- Hoffman KB, Martinez J, Lynch G. Proteolysis of cell adhesion molecules by serine proteases: a role in long term potentiation? Brain Research. 1998a;811:29–33. doi: 10.1016/s0006-8993(98)00906-8. [DOI] [PubMed] [Google Scholar]
- Hoffman KB, Pinkstaff JK, Gall CM, Lynch G. Seizure induced synthesis of fibronectin is rapid and age dependent: implications for long-term potentiation and sprouting. Brain Research. 1998b;812:209–215. doi: 10.1016/s0006-8993(98)00727-6. [DOI] [PubMed] [Google Scholar]
- Iribarren J, Prolo P, Neagos N, Chiappelli F. Post-traumatic stress disorder: evidence-based research for the third millennium. Evidence-Based Complementary and Alternatative Medicine. 2005;2:503–512. doi: 10.1093/ecam/neh127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Kaukinen S, Kinnunen T, Ylinen A, Imai S, Kaila K, Taira T, Rauvala H. Regulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. Journal of Neuroscience. 1999;19:1226–1235. doi: 10.1523/JNEUROSCI.19-04-01226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. Journal of Neuroscience. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiology of Learning and Memory. 1998;70:82–100. doi: 10.1006/nlme.1998.3840. [DOI] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. Journal of Neurochemistry. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. Journal of Neurochemistry. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–2131. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Nader K. A single standard for memory; the case for reconsolidation. Debates in Neuroscience. 2007;1:2–16. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000a;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nature Reviews Neuroscience. 2000b;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. Journal of Neuroscience. 2006;26:1923–34. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odake S, Morita Y, Morikawa T, Yoshida N, Hori H, Nagai Y. Inhibition of matrix metalloproteinases by peptidyl hydroxamic acids. Biochemical and Biophysicl Research Communications. 1994;199:1442–1446. doi: 10.1006/bbrc.1994.1392. [DOI] [PubMed] [Google Scholar]
- Okulski P, Jay TM, Jaworski J, Duniec K, Dzwonek J, Konopacki FA, Wilczynski GM, Sánchez-Capelo A, Mallet J, Kaczmarek L. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biological Psychiatry. 2007;62:359–362. doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Olson ML, Meighan PC, Brown TE, Asay AL, Benoist CC, Harding JW, Wright JW. Hippocampal MMP-3 elevation is associated with passive avoidance conditioning. Regulatory Peptides. 2008;146:19–25. doi: 10.1016/j.regpep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioural Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Research. 1997;84:241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. Journal of Neuroscience. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H, Peng HB. HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Progress in Neurobiolgy. 1997;52:127–144. doi: 10.1016/s0301-0082(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Riccio DC, Millin PM, Bogart AR. Reconsolidation: a brief history, a retrieval view, and some recent issues. Learning and Memory. 2006;13:536–544. doi: 10.1101/lm.290706. [DOI] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. Journal of Neuroscience. 2003;23:8034–8040. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learning and Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Ewaleifoh O, Beique JC, Wang Y, Knorr D, Haughey NMalpica T, Mattson MP, Huganir R, Conant K. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. FASEB Journal. 2008 Aug. 6; doi: 10.1096/fj.07-101402. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nature Neuroscience. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. Journal of Cell Biology. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller J, Kyrios M, Bennett P. Post traumatic stress disorder. Australian Family Physician. 1996;25:1569–1573. [PubMed] [Google Scholar]
- Wright JW, Harding JW. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Progress in Neurobiology. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wright JW, Brown TE, Harding JW. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plasticity. 2007;2007:73813. doi: 10.1155/2007/73813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Reichert JR, Davis CJ, Harding JW. Neural plasticity and the brain renin-angiotensin system. Neuroscience and Biobehavioral Reviews. 2002;26:529–552. doi: 10.1016/s0149-7634(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Wright JW, Murphy ES, Elijah IE, Holtfreter KL, Davis CJ, Olson ML, Muhunthan K, Harding JW. Influence of hippocampectomy on habituation, exploratory behavior, and spatial memory in rats. Brain Research. 2004;1023:1–14. doi: 10.1016/j.brainres.2004.06.083. [DOI] [PubMed] [Google Scholar]