Abstract
The Hedgehog signaling pathway plays a key role in directing growth and patterning during embryonic development and is required in vertebrates for the normal development of many structures, including the neural tube, axial skeleton, skin, and hair. Aberrant activation of the Hedgehog (Hh) pathway in adult tissue is associated with the development of basal cell carcinoma (BCC), medulloblastoma, and a subset of pancreatic, gastro-intestinal, and other cancers. This review will provide an overview of what is known about the mechanisms by which activation of Hedgehog signaling leads to the development of BCCs and will review two recent papers suggesting that agents that modulate sterol levels might influence the Hh pathway. Thus, sterols may be a new therapeutic target for the treatment of BCCs, and readily available agents such as statins (HMG-CoA reductase inhibitors) or vitamin D might be helpful in reducing BCC incidence.
Epidemiology of Basal cell carcinoma (BCC)
Basal cell carcinoma (BCC), the most common of all human cancers, affects close to 1 million Americans a year. Over the past 40 years there has been a dramatic increase in the incidence of BCC, and it is estimated that nearly 30% of Caucasians living in areas of high ambient sun exposure will develop a BCC (Miller and Weinstock, 1994). Furthermore, the incidence of BCC is rising in younger populations, especially among women (Christenson et al., 2005). Although the case fatality rate of BCCs is low, the high incidence and frequent occurrence of multiple primary tumors in affected individuals leads to significant morbidity. BCCs characteristically arise in sun-exposed body areas, most commonly on the head and neck, but also occur on the trunk and extremities. There are three accepted environmental insults for BCC development: ultraviolet radiation (UV), ionizing radiation (IR), and arsenic. Inter-individual differences in the susceptibility to BCC development have been recognized for many years. Epidemiologic studies have identified phenotypic features such as fair skin and freckling tendency that are associated with an increased susceptibility to BCCs (Rubin et al., 2005).
It is generally accepted that BCC can be caused by UV exposure from the sun. While the risk of squamous cell carcinoma is strongly related to cumulative sun exposure, sunlight’s exact role in BCC development remains less clear (Armstrong and Kricker, 2001). Thus, epidemiologic studies have shown that BCC risk correlates better with intermittent sun exposure (i.e. childhood sunburns, weekend sun exposure) than with cumulative lifetime sun exposure (Corona et al., 2001). Hence, the timing, dose, and duration of UV exposure are critical to carcinogenesis, but UV seems to have a different role in SCC versus BCC carcinogenesis development. Consistent with this, a randomized clinical trial of daily sunscreen use showed that sunscreen reduced the incidence of SCC but not BCC. The daily application of an SPF 15 sunscreen to the head, neck, arms, and hands over a 4–5 year period had no effect on the risk of basal cell carcinoma development on these sites (Green et al., 1999).
No effective agent exists yet for prevention of BCC. Oral retinoids are only moderately effective as a chemopreventive agent for BCC, and their multiple side effects limit their widespread use (Wright et al., 2006). Treatment of sporadic BCCs with topical retinoids such as tazarotene can be successful; however complete tumor regression occurs in less than 50% of BCCs so treated (Bianchi et al., 2004). A retinoid precursor, β–carotene, and other less toxic agents such as non-steroidal anti-inflammatory agents (NSAIDs), do not have a significant effect in lowering BCC risk (Frieling et al., 2000) (Grau et al., 2006). One provocative study has suggested that BCC may also be prevented with a low-fat diet. In a randomized clinical trial, one hundred patients who had already developed one non-melanoma skin cancer were placed on either a normal or a low-fat diet, and followed for 2 years for the development of skin cancer (Black et al., 1995). A low-fat diet was found to reduce significantly the occurrence of BCCs in this randomized, cohort study (Black et al., 1994; Black et al., 1995). However, subsequent observational studies using questionnaires to assess diet failed to find a protective effect of a low fat diet against BCC carcinogenesis (McNaughton et al., 2005). Thus, it is unclear whether diet has a significant role in BCC development.
Hedgehog signaling pathway
The pivotal molecular abnormality in BCC carcinogenesis is inappropriate activation of the Hedgehog (Hh) signaling pathway. The Hh signaling pathway was first described in genetic studies of embryonic segmentation and imaginal disk specification in Drosophila. It is highly conserved from insects to vertebrates, and vertebrates have multiple homologs of a number of components of the pathway (Hooper and Scott, 2005). In mammals, three hedgehog homologs: Sonic (Shh), Indian (Ihh), and Desert hedgehog (Dsh) are known. Shh is the most commonly expressed and the best characterized homolog; it is crucial for the development and maintenance of the nervous system, axial skeleton, lungs, skin, hair, and stem cell populations. Shh is synthesized as a 45 kDa precursor protein that is auto-catalytically cleaved and covalently modified by palmitate and cholesterol. Shh is secreted and binds to its receptor Patched 1 (Ptch1).
The Ptch1 protein is a 12-transmembrane receptor for Shh, and is the key inhibitor of Shh signaling. Most BCC tumors have an overactive Shh signaling pathway, most commonly due to mutational inactivation of Ptch1; hence Ptch1 can act as a tumor suppressor gene (Quinn and Epstein, 2003). Ptch1 inhibits Shh signaling by inhibiting activity of the 7-transmembrane receptor, Smoothened (Smo), a positive regulator of signaling (Figure 1A). The repression of Smo by Ptch1 inactivates downstream Shh effectors, the glioma-associated (Gli) family of transcription factors. The Gli transcription factors (Gli1, Gli2, Gli3) control the expression of Shh target genes including Ptch1 and Gli1 themselves, which provide negative and positive feedback regulation of Hh signaling, respectively.
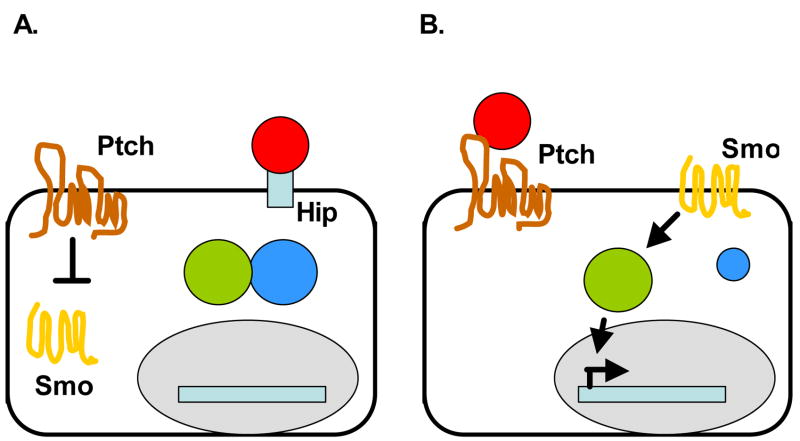
Figure 1.
Model of Hedgehog signaling in vertebrates.
A. Ptch inhibits Smo in the absence of Hh. SMO is unable to activate Gli transcription factors as Sufu binds Gli and prevents the transcription of Hh target genes. Hh ligand is bound by Hip on the membrane surface.
B. Binding of the Hh ligand inhibits Ptch, and relieves the inhibition of Smo by Ptch. Smo localizes to the membrane and is now able to activate Gli transcription factors. Hh target genes are transcribed.
Signaling events downstream of Smo are not fully defined, but the suppressor of fused, (Sufu), acts as a negative regulator of the pathway by binding to Gli to prevent the activation of Hh target genes (Kogerman et al., 1999). Other negative regulators of Gli include protein kinase A which phosphorylates Gli, leading to proteosomal-mediated cleavage of Gli to a truncated form (Chen et al., 1998). The amount of Shh available to bind Ptch1 is tightly regulated by Hh-binding proteins, such as Hh-interacting protein (Hip) which sequester Hh (Chuang and McMahon, 1999).
Binding of the Shh ligand to Ptch1 causes both Shh and Ptch1 to be sequestered into endocytic vesicles; this relieves the inhibition of Smo and Smo becomes localized to the membrane (Figure 1B)(Incardona and Roelink, 2000). Smo activates the Gli transcription factors through an unknown mechanism that includes the release of Gli from Sufu (Kogerman et al., 1999). In mammalian cells, Gli1 and Gli2 serve mainly as positive transcriptional regulators, whereas Gli3 acts as a repressor (Ingham and McMahon, 2001). Overexpression of Gli1 or Gli2 leads to BCC tumors in murine skin (Grachtchouk et al., 2000; Grachtchouk et al., 2003; Nilsson et al., 2000). As transcription factors, Gli molecules can regulate target gene expression by direct association with a consensus binding site (5′-tgggtggtc-3′) located in the promoter region of the target genes. Direct downstream targets of Hh signaling include Bcl2, Cyclin D1, Cyclin D2, FoxM1, FoxE1, Hip, PDGFR a, MIM (Athar et al., 2006; Eichberger et al., 2004; Regl et al., 2004; Teh et al., 2002). As many of these genes are directly involved in cell cycle regulation (cyclins) and cell survival (Bcl2), activation of these genes are one mechanism that lead to BCC tumor development and proliferation.
Basal Cell Nevus Syndrome and Ptch1 heterozygous (+/−) mice
Most BCC tumors have PTCH1 gene inactivating mutations that cause Shh signaling to be activated constitutively (Aszterbaum et al., 1998; Johnson et al., 1996; Ling et al., 2001; Xie et al., 1998). In general, more than 70% of sporadic BCCs have detectable genetic mutations in components of the Shh signaling pathway that lead to elevated levels of the Gli1 transcription factor (Dahmane et al., 1997; Ling et al., 2001). Patients with Basal Cell Nevus Syndrome (BCNS or Gorlin syndrome, MIM # 109400), a rare multi-system disease whose hallmark is the development of dozens to thousands of BCCs, inherit one defective copy of PTCH1 and lose the other copy somatically in tumors. BCNS is a highly penetrant autosomal dominant cancer syndrome in which affected individuals develop multiple BCCs at an early age. BCNS patients also develop other tumors: e.g. odontogenic cysts, medulloblastomas, meningiomas, fibrosarcomas, ovarian fibromas, bone hamartomas, cardiac fibromas and rhabdomyosarcomas.
Ptch1+/− mice have been generated by replacing exons from one allele of Ptch1 with the lacZ gene (Goodrich et al., 1997; Hahn et al., 1996), and these mice recapitulate many of the clinical abnormalities found in BCNS patients (Aszterbaum et al., 1999; Mancuso et al., 2004). Thus, Ptch1+/− mice develop multiple BCCs after exposure to UV or IR, and approximately 10–15% of the mice develop medulloblastomas and rhabdomyosarcomas. Thus, the skin of the Ptch1 +/− mice appears normal at birth, and the only cutaneous abnormality detectable is the presence of tiny, microscopically-detectable clusters of basaloid cells in the superficial dermis at around 16 months of age. However, if exposed to repeated doses of UV radiation or to a single dose of IR, the Ptch1 +/− mice develop large numbers of BCCs and other closely-related tumors whose histology resembles more that of cells of various regions of the hair follicle (Aszterbaum et al., 1999; Mancuso et al., 2004). When Ptch1+/− mice are exposed to UV 3 times/week from age 6 weeks, the microscopic lesions are easily seen by 6 months of age and arise in 100% of the mice. From 9–12 months of UV treatment, Ptch1 +/− mice develop grossly-visible skin tumors in the irradiated areas. Of these, approximately 25% are BCCs, another 25% are squamous cell carcinomas (SCCs), and 50% are fibrosarcomas. Since one allele of Ptch1 was mutated by the insertion of the lacZ gene into exons 1 and 2, cells with up-regulated Ptch1 expression will have β-galactosidase enzyme activity which can be detected by cells staining blue when a yellow substrate (X-gal) is added (Goodrich et al., 1997; Hahn et al., 1996). The BCCs and fibrosarcomas, like the medulloblastomas and rhabdomyosarcomas, stain blue (positive for β-galactosidase activity) whereas the SCCs do not. Consistent with this staining pattern, loss of the wild type Ptch1 allele occurs frequently in both BCCs and fibrosarcomas, but such loss does not occur in the SCCs (Aszterbaum et al., 1999). The grossly visible fibrosarcomas and SCCs are inconsistent with the human BCNS phenotype and suggest that the Ptch1 +/− mice may have a currently unexplained tendency to skin tumorigenesis beyond BCC formation. However, consistent with the hypersensitivity to IR-induced BCCs of BCNS patients, mice exposed to Cs137 IR also develop microscopic BCCs in a dose-dependent manner, and some of these eventually develop into visible BCCs. In the skin, IR induces only BCCs and not the fibrosarcomas and SCCs seen after UV exposure. The cause of this difference is not clear (Aszterbaum et al., 1999). Similar to the Ptch1 +/− mice, transgenic mice overexpressing Shh, Smo, Gli1, or Gli2 in keratinocytes all develop BCC-like lesions (Fan et al., 1997; Grachtchouk et al., 2000; Grachtchouk et al., 2003; Hutchin et al., 2005; Nilsson et al., 2000). The deletion of Sufu, a repressor of Gli, also led to BCC-like basaloid proliferations in mice (Svard et al., 2006). Taken together, the studies of these genetically engineered mice confirm that activation of the Shh pathway is an early event in BCC tumor formation.
Smo: the target of small molecule inhibitors of the Shh pathway
Despite the central role of Smo as the upstream mediator of all Hh signaling, the mechanisms by which Smo activation is regulated and coupled to downstream components remain unclear. The mechanism by which Ptch1 inhibits Smo does not appear to involve binding of Ptch1 to Smo. Binding of Shh to Ptch1 regulates the distribution of Smo from intracellular vesicles to the cell membrane and perhaps to specialized structures such as the cilia (Corbit et al., 2005). Therefore, there is evidence that Ptch1 regulates Smo catalytically (Taipale et al., 2002). Ptch1 is related to the resistance-nodulation-cell division (RND) family of bacterial pumps and to the Niemann-Pick C1 cholesterol transporter that is capable of transporting hydrophobic small molecules, including cholesterol (Corcoran and Scott, 2006; Taipale et al., 2002). It is possible that Smo activity is regulated by an endogenous small molecule rather than by direct protein-protein interactions (Chen et al., 2002a). Cyclopamine, a plant-derived steroidal alkaloid, binds directly to the transmembrane helices of Smo and inhibits Hh signaling. Cyclopamine likely changes the conformation and/or subcellular localization of Smo such that Smo becomes inactive.
The discovery of small molecule antagonists of Smo such as cyclopamine has opened up exciting new prospects for molecularly targeted BCC therapy. Oral cyclopamine can block the growth of UV-induced BCCs in Ptch1 +/− mice by 50%, perhaps by increasing Fas-induced apoptosis (Athar et al., 2004). Cyclopamine administration reduced BCCs but not SCCs in these mice, highlighting the specificity of cyclopamine for the Shh pathway. Cyclopamine also can inhibit the proliferation of murine BCC cell lines (ASZ, BSZ, CSZ). These cell lines have been valuable for testing for drug inhibition of BCCs (So et al., 2006). Other synthetic Smo antagonists, such as CUR61414 from Curis/Genentech, have also been found to be effective in reducing BCCs in model systems. Thus, using an ex vivo model of BCC, CUR61414 causes the regression of UV-induced basaloid lesions in punch biopsies taken from Ptch1 +/− mice (Williams et al., 2003). A topical formulation of this compound was tested against sporadic BCCs in a Phase I clinical trial. Unfortunately no clinical effect was seen but the lack of down regulation of Hh target gene expression indicates that the compound failed to inhibit Hh signaling. The reason for this failure is not known. Other Smo antagonists have been identified using a small molecule screen for Shh pathway inhibitors using Shh-LIGHT2 cells, a clonal NIH3T3 cell line stably transfected with a Gli-dependent reporter (Chen et al., 2002b). These compounds, SANT-1, SANT-2, SANT-3, SANT-4, bind directly to Smo but are structurally distinct from cyclopamine.
Of note, cyclopamine, CUR61414, and the SANT compounds all act at the level of Smo. For example, cyclopamine could not inhibit the reporter activity in a murine BCC cell line (ASZ001) overexpressing the more downstream component Gli1 (Athar et al., 2004). Therefore, tumors with genetic mutations downstream of Smo would not be expected to be sensitive to cyclopamine and its analogs. Beyond the treatment of BCCs, inhibitors of the Shh pathway may target other malignancies of aberrant Shh activation, including subsets of medulloblastoma, pancreatic, lung and gastrointestinal cancers (Rubin and de Sauvage, 2006).
Sterols activate Shh signal transduction
The role for sterol synthesis in Shh signaling was first suggested by the finding that the Shh ligand is covalently modified by cholesterol. Further evidence linking cholesterol to Shh transduction came from human syndromes with genetic defects in the sterol synthesis pathway (Smith-Lemli-Opitz syndrome and lathosterolosis) which are characterized by holoprosencephaly, a developmental phenotype also associated with Shh deficiency (Chiang et al., 1996). Cyclopamine was first discovered when a group of lambs were born with cyclopia, the most severe form of holoprosencephaly. The pregnant ewes had ingested cyclopamine-containing plants (California corn lily) (Cooper et al., 1998). The lack of cholesterol in genetic syndromes and the exposure to cyclopamine both caused fetal holoprosencephaly. Furthermore, holoprosencephaly has been found in fetuses from pregnant women taking lovastatin (an HMG-CoA reductase inhibitor) (Edison and Muenke, 2004). This suggested that cholesterol may be important for Hh signal transduction. In fact, several laboratories have suggested that a hydrophobic molecule like cholesterol could be the ligand that binds Smo and modulates its switch between active versus inactive states.
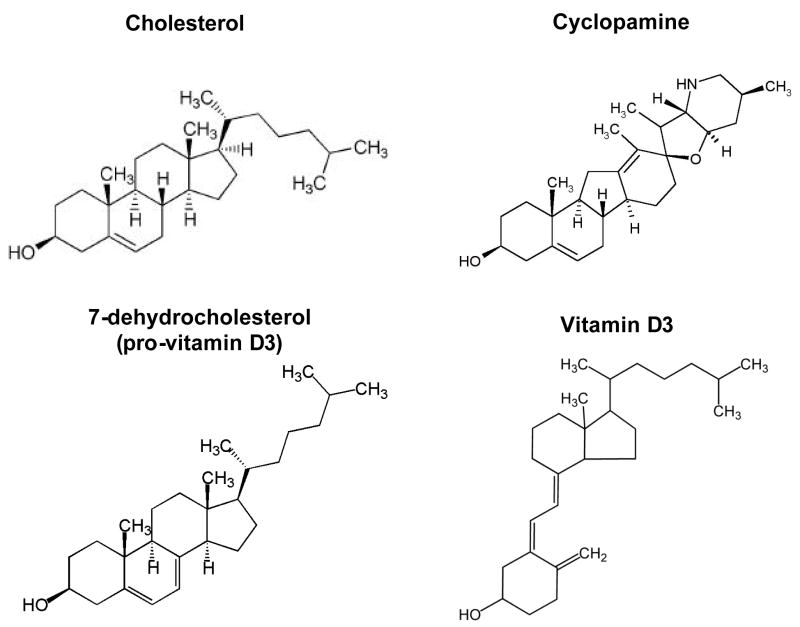
Beachy et al. first showed that depletion of cholesterol by cyclodextrin or a statin (HMG-CoA reductase inhibitor) caused a decrease in Shh signal transduction in Shh-expressing cells (Cooper et al., 2003). Sterol depletion did not cause a decrease in autoprocessing of Shh signal, contrary to the expectation that depletion of cholesterol would most affect the generation of the cholesterol-modified Shh ligand. Cyclodextrin and statin could inhibit Shh target gene transcription in Ptch1−/− fibroblasts but not in cells expressing a constitutively active mutant Smo. Thus, cholesterol depletion acts at the level of Smo, or theoretically between Ptch1 and Smo. Subsequently, Corcoran et al. showed that various pharmacologic inhibitors of the sterol synthesis pathway, including statins, could decrease cellular proliferation and Gli1 expression in a medulloblastoma cell line derived from Ptch1+/− p53−/− mice (PZp53MED) (Corcoran and Scott, 2006). This indicated that cholesterol or a cholesterol derivative was required for Shh signal transduction and proliferation. Sterol synthesis inhibitors could also inhibit Ptch1−/− fibroblasts’ proliferation, showing that the effect was not limited only to the Ptch1+/− medulloblastoma cell line. Addition of cholesterol or derivatives of cholesterol (25-hydroxycholesterol, which is downstream of the statin induced block) could potently activate Shh signal pathway as measured by lacZ expression in the PZp53MED cell line. Other sterols such as 7β-hydroxycholesterol could not activate Shh target genes. As some and not all sterols activated the Shh pathway, pathway activation is dependent on specific sterols rather than due to total sterol concentration. The effect of addition or depletion of sterols occurs at the level of Smo since addition of activating sterols could overcome, to some extent, the inhibitory effects of cyclopamine. Of note, sterols are similar in structure to cyclopamine, and may bind directly to Smo (Figure 2). Sterols and cyclopamine both contain a well-conserved four-ring core structure, and their ability to activate or inhibit Smo may depend on their differing side chain structures. 7-dehydrocholesterol (pro-vitamin D3) (Figure 2) resembles the structure of cyclopamine and inhibits Smo (Bijlsma et al., 2006). In fact, other cholesterol derivatives, 20-hydroxycholesterol and 25-hydroxycholesterol, are as potent in activating Smo as the known Smo-agonist SAG (Chen et al., 2002b).
Figure 2.
Structural similarities between cholesterol, cyclopamine, 7-dehydrocholesterol (pro-vitamin D3), and vitamin D3
There are several possible mechanisms by which sterols might activate Smo. Sterols may act as a direct agonist, binding to Smo to induce a conformational change, or they could induce an activating conformational change in Smo by influencing nearby membrane properties. Sterols also may regulate the localization of Smo to the cell membrane, perhaps to the primary cilium. Smo recently has been shown to localize to the primary cilia in some cells (Corbit et al., 2005). Addition of Shh promotes ciliary localization, whereas cyclopamine inhibits that localization. Ptch1 could then regulate the cellular levels of sterols available for activation of Smo (Taipale et al., 2002).
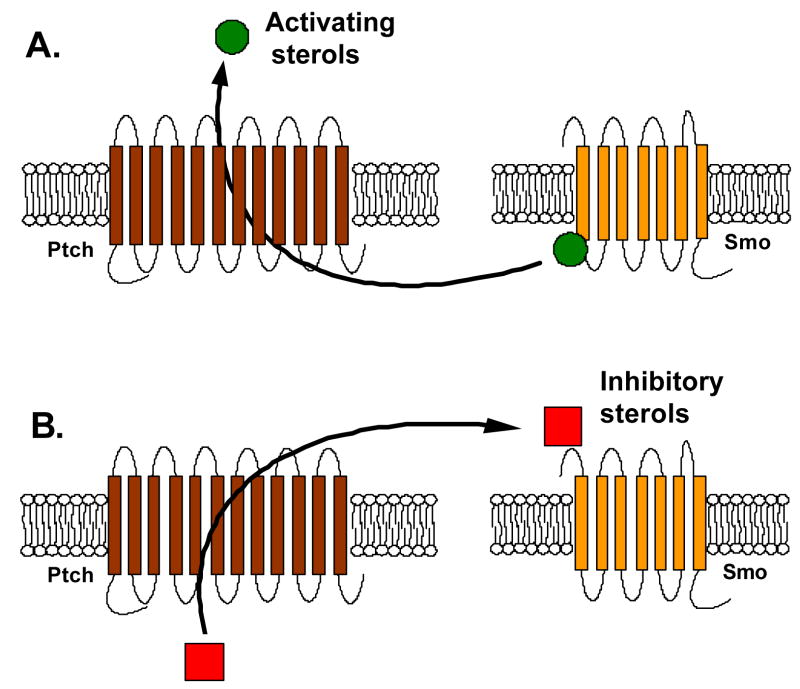
There are at least two models for how Ptch1 might inhibit Smo activation by sterols in the absence of Shh (Figure 3). The first is that Ptch1 pumps activating sterols away from Smo. Addition of Shh would inhibit the transport function of Ptch1 and thereby allow activating sterols to accumulate and bind to Smo. The second is that Ptch1 pumps an unknown inhibitor of Smo towards Smo. This inhibitor could be a direct Smo antagonist or could be a ligand which prevents binding of activating sterols.
Figure 3.
Two models for Ptch regulation of Smo activation by sterols based on Ptch homology to pump and transport proteins. (Adapted from Corcoran and Scott, 2006)
A. Ptch could pump activating sterols (cholesterol, other oxysterols) away from Smo, preventing Smo activation. (Corcoran and Scott, 2006)
B. Alternatively, Ptch could pump inhibitory sterols (vitamin D3) towards Smo. (Bijlsma and Peppelenbosch, 2006)
Possible role of vitamin D in Shh signaling
Recently, experiments testing the non-cell autonomous effects of Ptch1 have produced evidence that vitamin D is a potential Smo inhibitor. These studies suggest that Ptch1 may translocate 7-dehydrocholesterol (pro-vitamin D3) to the extracellular medium (Bijlsma et al., 2006). Ptch1 in one cell may pump vitamin D3 or a related sterol into the extracellular medium that then acts as a Smo antagonist either in the same cell (cell-autonomous) or in a neighboring cell (non-cell autonomous). Addition of synthetic pro-vitamin D3 or vitamin D3 inhibited Gli activity in reporter cells. Vitamin D3 also competed with cyclopamine for binding to Smo. Thus, vitamin D3 is capable of binding to Smo and inhibiting the Shh pathway, at least in vitro. One caveat to these studies is that it remains to be determined whether intercellular inhibition of Smo by Ptch1 action actually occurs in vivo.
A new interpretation of the role of sunlight and diet in BCC development
If vitamin D does indeed have an effect on the Shh pathway, this may explain some of the relationship of UV exposure to BCC development. As noted above, constant UV exposure results in SCC and intermittent exposure results in BCC; constant UV exposure might maintain high vitamin D levels in the skin and thereby specifically prevent BCC carcinogenesis through inhibition of proliferation induced by constitutive activation of HH signaling.
In general, vitamin D has been shown to decrease tumor cell proliferation and to promote differentiation in vitro (Mokady et al., 2000). Cutaneous synthesis of vitamin D depends on UV light to convert 7-dehydrocholesterol (pro-vitamin D3) to its active form, vitamin D3. Epidemiologic evidence substantiate the importance of adequate vitamin D levels and UV exposure in reducing the risk of breast, prostate, and colon cancer (Garland et al., 2006) (Martinez et al., 1996) (Garland et al., 1985). These observational studies have not shown that vitamin D can prevent skin cancer. One potential complication in the interpretation of these studies is that UV exposure has the dual effect of promoting vitamin D3 synthesis and of causing tumorigenic mutations. If vitamin D does indeed inhibit Smo and Shh target genes, then supplemental vitamin D might inhibit Shh pathway associated tumors like BCCs and perhaps even extra-cutaneous tumors with significant abnormalities of HH signaling. This could explain why BCC risk does not increase with cumulative UV exposure. Greater UV exposure would increase levels of vitamin D that may be protective for BCCs. In fact, in vitamin D receptor knock-out mice, multiple skin tumors arise after treatment with the carcinogen DMBA, and many of these tumors were BCCs (Zinser et al., 2002).
As mentioned earlier, BCC risk may be decreased with a low-fat diet (Black et al., 1995). It is unclear what component of a low-fat diet may be responsible for the anti-BCC effect. A low-fat diet significantly reduces serum cholesterol, and serum cholesterol also can be reduced by statins. Observational studies have shown that statin therapy is associated with reduced cancer risk for breast cancer and melanoma (Dellavalle et al., 2005). Statins have potent effects on cancer cells in vitro and in animal models, but their anti-cancer effect remains debatable in randomized clinical trials (Demierre et al., 2005). Two groups have shown that statins can inhibit the Shh signaling pathway in vitro (Cooper et al., 2003; Corcoran and Scott, 2006). Simvastatin decreased the proliferation of a medulloblastoma cell line that had increased Shh target gene expression. Addition of cholesterol and other oxysterols could potently activate the Shh signal pathway. These findings suggest that lowering cholesterol via statins may be a potential therapy for Shh pathway driven tumors. Similarly, a low-fat diet may have had anti-BCC effects due to lowered cholesterol levels which thereby decreased the Shh signal pathway.
Future directions for chemoprevention of BCC
Statins and vitamin D may prevent BCC tumors by inhibiting Smo signaling. Both statins and vitamin D have relatively few side effects and could be the first practical chemopreventive drugs for BCCs. Systemic high-dose isotretinoin has been reported to have a prophylactic effect on non-melanoma skin cancer, although it is associated with significant toxicity. In contrast, vitamin D supplementation and statins are relatively well tolerated and inexpensive and could be a new therapy for prevention of BCC. Clearly, more work needs to be done to explore the possibility of statins and vitamin D as anti-BCC agents. Statins, by inhibiting HMG-CoA reductase, lower both cholesterol and 7-dehydrocholesterol (pro-vitamin D3). It is unclear if the actual effects of statins would be to enhance the Hh pathway by reducing the production of an inhibitor (vitamin D3) or inhibit the Hh pathway by reducing activating oxysterols and cholesterol. The pre-clinical effect remains to be tested in Ptch1+/− mice and other animal models of BCC tumorigenesis. If these results are promising, clinical trials for statins and vitamin D would be indicated in patients at high risk for BCC development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH., Jr Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- Athar M, Li C, Tang X, Chi S, Zhang X, Kim AL, Tyring SK, Kopelovich L, Hebert J, Epstein EH, Jr, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Experimental Dermatology. 2006;15:667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Orlandi A, Campione E, Angeloni C, Costanzo A, Spagnoli LG, Chimenti S. Topical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of cases. British journal of dermatology. 2004;151:148. doi: 10.1111/j.1365-2133.2004.06044.x. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of Smoothened by Patched-Dependent (Pro-)Vitamin D3 Secretion. PLoS Biol. 2006:4. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black HS, Herd JA, Goldberg LH, Wolf JE, Jr, Thornby JI, Rosen T, Bruce S, Tschen JA, Foreyt JP, Scott LW, et al. Effect of a low-fat diet on the incidence of actinic keratosis. N Engl J Med. 1994;330:1272–1275. doi: 10.1056/NEJM199405053301804. [DOI] [PubMed] [Google Scholar]
- Black HS, Thornby JI, Wolf JE, Jr, Goldberg LH, Herd JA, Rosen T, Bruce S, Tschen JA, Scott LW, Jaax S, et al. Evidence that a low-fat diet reduces the occurrence of non-melanoma skin cancer. Int J Cancer. 1995;62:165–169. doi: 10.1002/ijc.2910620210. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002a;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002b;99:14071. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gallaher N, Goodman RH, Smolik SM. Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. PNAS. 1998;95:2349–2354. doi: 10.1073/pnas.95.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, Roenigk RK. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. Jama. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona R, Dogliotti E, D’Errico M, Sera F, Iavarone I, Baliva G, Chinni LM, Gobello T, Mazzanti C, Puddu P, Pasquini P. Risk factors for basal cell carcinoma in a Mediterranean population: role of recreational sun exposure early in life. Arch Dermatol. 2001;137:1162–1168. doi: 10.1001/archderm.137.9.1162. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- Dellavalle R, Drake A, Graber M, Heilig L, Hester E, Johnson K, McNealy K, Schilling L, Dellavalle R. Statins and fibrates for preventing melanoma. Cochrane Database Syst Rev. 2005:CD003697. doi: 10.1002/14651858.CD003697.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med. 2004;350:1579–1582. doi: 10.1056/NEJM200404083501524. [DOI] [PubMed] [Google Scholar]
- Eichberger T, Regl G, Ikram MS, Neill GW, Philpott MP, Aberger F, Frischauf AM. FOXE1, a new transcriptional target of GLI2 is expressed in human epidermis and basal cell carcinoma. J Invest Dermatol. 2004;122:1180–1187. doi: 10.1111/j.0022-202X.2004.22505.x. [DOI] [PubMed] [Google Scholar]
- Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- Frieling UM, Schaumberg DA, Kupper TS, Muntwyler J, Hennekens CH. A Randomized, 12-Year Primary-Prevention Trial of Beta Carotene Supplementation for Nonmelanoma Skin Cancer in the Physicians’ Health Study. Arch Dermatol. 2000;136:179–184. doi: 10.1001/archderm.136.2.179. [DOI] [PubMed] [Google Scholar]
- Garland C, Barrett-Connor E, Rossof A, Shekelle R, Criqui M, Paul O. Dietary Vitamin D and Calcium and Risk of Colorectal Cancer: A 19-Year Prospective Study In Men. The Lancet. 1985;325:307. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The Role of Vitamin D in Cancer Prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- Grachtchouk V, Grachtchouk M, Lowe L, Johnson T, Wei L, Wang A, de Sauvage F, Dlugosz AA. The magnitude of hedgehog signaling activity defines skin tumor phenotype. Embo J. 2003;22:2741–2751. doi: 10.1093/emboj/cdg271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Langholz B, Karagas M, Greenberg ER, Stukel TA, Mandel JS. Effect of NSAIDs on the recurrence of nonmelanoma skin cancer. Int J Cancer. 2006;119:682–686. doi: 10.1002/ijc.21878. [DOI] [PubMed] [Google Scholar]
- Green A, Williams G, Nale R, Hart V, Leslie D, Parsons P, Marks GC, Gaffney P, Battistutta D, Frost C. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. The Lancet. 1999;354:723. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Hutchin ME, Kariapper MS, Grachtchouk M, Wang A, Wei L, Cummings D, Liu J, Michael LE, Glick A, Dlugosz AA. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005;19:214–223. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Roelink H. The role of cholesterol in Shh signaling and teratogen-induced holoprosencephaly. Cell Mol Life Sci. 2000;57:1709–1719. doi: 10.1007/PL00000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG. Mammalian Suppressor-of-Fused modulates nuclear-cytoplasmic shuttling of GLI-1. Nat Cell Biol. 1999;1:312. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- Ling G, Ahmadian A, Persson A, Unden AB, Afink G, Williams C, Uhlen M, Toftgard R, Lundeberg J, Ponten F. PATCHED and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene. 2001;20:7770–7778. doi: 10.1038/sj.onc.1204946. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pazzaglia S, Tanori M, Hahn H, Merola P, Rebessi S, Atkinson MJ, Di Majo V, Covelli V, Saran A. Basal Cell Carcinoma and Its Development: Insights from Radiation-Induced Tumors in Ptch1-Deficient Mice. Cancer Res. 2004;64:934–941. doi: 10.1158/0008-5472.can-03-2460. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Giovannucci EL, Colditz GA, Stampfer MJ, Hunter DJ, Speizer FE, Wing A, Willett WC. Calcium, vitamin D, and the occurrence of colorectal cancer among women. Journal Of The National Cancer Institute. 1996;88:1375. doi: 10.1093/jnci/88.19.1375. [DOI] [PubMed] [Google Scholar]
- McNaughton SA, Marks GC, Green AC. Role of dietary factors in the development of basal cell cancer and squamous cell cancer of the skin. Cancer Epidemiol Biomarkers Prev. 2005;14:1596–1607. doi: 10.1158/1055-9965.EPI-05-0026. [DOI] [PubMed] [Google Scholar]
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- Mokady E, Schwartz B, Shany S, Lamprecht SA. A protective role of dietary vitamin D3 in rat colon carcinogenesis. Nutr Cancer. 2000;38:65–73. doi: 10.1207/S15327914NC381_10. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, Toftgard R. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. PNAS. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn AG, Epstein E., Jr Patched, hedgehog, and skin cancer. Methods Mol Biol. 2003;222:85–95. doi: 10.1385/1-59259-328-3:085. [DOI] [PubMed] [Google Scholar]
- Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger C, Frischauf AM, Aberger F. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- So PL, Langston AW, Daniallinia N, Hebert JL, Fujimoto MA, Khaimskiy Y, Aszterbaum M, Epstein EH., Jr Long-term establishment, characterization and manipulation of cell lines from mouse basal cell carcinoma tumors. Exp Dermatol. 2006;15:742–750. doi: 10.1111/j.1600-0625.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Svard J, Henricson KH, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic Elimination of Suppressor of Fused Reveals an Essential Repressor Function in the Mammalian Hedgehog Signaling Pathway. Developmental Cell. 2006;10:187. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–4780. [PubMed] [Google Scholar]
- Williams JA, Guicherit OM, Zaharian BI, Xu Y, Chai L, Wichterle H, Kon C, Gatchalian C, Porter JA, Rubin LL, Wang FY. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci U S A. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. Journal of the American Academy of Dermatology. 2006;54:933. doi: 10.1016/j.jaad.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]