Abstract
The combination of less positive and more negative expectations for the future (i.e., lower optimism and higher pessimism) increases risk for disease and early mortality. We tested the possibility that expectancies might influence health outcomes by altering the rate of biological aging, specifically of the immune system (immunosenescence). However, no studies to date have examined associations between optimism or pessimism and indicators of immunosenescence such as leukocyte telomere length (TL) and interleukin-6 (IL-6) levels. We investigated whether dispositional tendencies towards optimism and pessimism were associated with TL and IL-6 in a sample of 36 healthy post-menopausal women. Multiple regression analyses where optimism and pessimism were entered simultaneously, and chronological age and caregiver status was controlled, indicated that pessimism was independently associated with shorter TL (β = −.68, p =.001) and higher IL-6 concentrations (β =.50, p =.02). In contrast, optimism was not independently associated with either measure of immunosenescence. These findings suggest that dispositional pessimism may increase IL-6 and accelerate rate of telomere shortening. Mechanistic causal relationships between these parameters need to be investigated.
Keywords: optimism, pessimism, telomere length, interleukin-6, aging
Expecting less positive and/or more negative outcomes in the future (i.e., lower optimism and/or higher pessimism) is associated with faster progression of diseases of aging (Allison et al., 2003; Matthews et al., 2004) and earlier mortality (Giltay et al., 2006), but the mechanisms underlying this association have been elusive. Immunosenescence (aging of the immune system) has been implicated in health defects associated with aging, including susceptibility to infectious disease, diseases of aging, and earlier mortality (Effros, 2004). Telomeres, consisting of repetitive DNA sequences complexed with proteins, cap chromosome ends and protect against chromosomal damage (Blackburn, 2001). Human population studies show a general decline of leukocyte telomeric DNA length (TL) with age, suggesting that leukocyte telomere shortness may serve as an indicator of immunosenescence (Effros, 2004).
Chronic psychological stress has been associated with accelerated leukocyte telomere shortening (Damjanovic et al., 2007; Epel et al., 2004). In one study, caregivers had shorter TL than controls (Damjanovic et al., 2007). In another study, caregivers and non-caregivers had similar mean TL, but perceived stress was a significant predictor of TL in both caregiving and non-caregiving women and across the combined sample (Epel et al., 2004). No studies have examined how individual differences in perceived stress vulnerability might promote telomere shortening.
An individual’s scores on optimism and pessimism measures generally remain stable across time (Giltay et al., 2006; Scheier et al., 1994), and the combination of low optimism and/or high pessimism is associated with more negative mood, less positive mood and poorer coping with stress (Carver et al., 1993; Scheier & Carver, 1985). Generalized negative expectations for the future could increase vulnerability to high perceived stress and consequently, accelerated telomere shortening.
Inflammatory cytokines including interleukin-6 (IL-6) can promote inflammatory processes, and both acute and chronic psychological stress have been associated with increased IL-6 (Kiecolt-Glaser et al., 2003; Steptoe et al., 2001). Repeated episodes of inflammation are associated with telomere shortening in leukocytes (Carrero et al., 2008; Wu et al., 2000). Thus, inflammatory processes may provide an important link between psychological stress and TL.
Positive and negative future thinking have different neural correlates (Sharot et al., 2007), and it has been suggested that optimism and pessimism have divergent genetic and environmental determinants (Plomin et al., 1992). Thus, it is important to separately examine associations between optimism, pessimism and health-related variables. The primary goal of the current study was to assess optimism vs. pessimism, in relation to TL, and in secondary analyses, whether this relationship might be mediated by neuroticism, perceived stress, and health behaviors.
Methods
Participants and Procedure
We recruited 36 healthy postmenopausal women aged between 50 and 80 years through flyers and posters in the community, and from elderly service providers in the Bay Area, California. This was a different sample from the premenopausal sample in which we previously reported a link between stress and telomere length (Epel et al., 2004). The postmenopausal sample described here included 30 White (81.1%), one Black, one Hispanic/Latina and four Asian women. Exclusion criteria included the presence of major medical conditions such as heart disease, cancer, or diabetes, and use of medications containing agents known to affect stress hormone levels, and regular smoking. Participants underwent a fasting blood draw between 8.00AM and 10.00AM, and completed self-report questionnaires within one week of the blood draw. Blood was collected from the non-dominant arm using an indwelling catheter, and processed within one hour. The study protocol was approved by the Institutional Review Board of the University of California, San Francisco. Written, informed consent was obtained from all participants.
Materials and Measures
Optimism/Pessimism
The Revised Life Orientation Test (LOT-R) was used to measure optimism and pessimism (Scheier et al., 1994). The instrument comprises three positively worded items, which comprise the optimism scale (e.g., “In uncertain times, I usually expect the best”); three negatively worded items, which comprise the pessimism scale (e.g., “If something can go wrong for me, it will”); and four filler items. Participants are asked to rate their agreement or disagreement with each of the statements on a five-point scale from 0 (“strongly disagree”) to 4 (“strongly agree”). Internal consistency for the optimism (α =.74) and pessimism (α =.78) subscales was high.
Perceived Stress
The 10-item Perceived Stress Scale was used to assess appraisals of psychological stress experienced during the last month, including the extent to which situations are experienced as unpredictable, uncontrollable and overwhelming (Cohen et al., 1983). Participants are asked to rate the extent to which they felt or thought a particular way in the previous month on a 5-point Likert scale ranging from 0 (“never”) to 4 (“very often”). Internal consistency was high (α =.93).
Neuroticism
The Big Five Inventory was used to assess neuroticism (Donahue & Kentle, 1993). This scale comprises eight items that assess emotional stability or neuroticism (e.g., “I see myself as someone who worries a lot”). Participants rate their agreement with the items on a 5-point Likert scale ranging from 1 (“disagree strongly”) to 5 (“agree strongly”). Internal consistency for the scale was high (α =.84).
Health behaviors and demographics
Questions from the Yale Physical Activity Scale were used to assess frequency and duration of vigorous activities during the previous month (Dipietro et al., 1993). Scores were computed by multiplying frequency of vigorous activities by duration. The Insomnia Severity Index (ISI), a valid and reliable measure in older people, was used to assess sleep difficulties (Bastien et al., 2001). Body mass index (BMI) was calculated as weight in kg (measured on a balance beam scale in hospital gown) divided by height in meters squared.
Telomere Length (TL)
Samples were collected in 10ml heparin tubes (Becton Dickinson, Franklin Lakes, NJ). Leukocytes were isolated using density-gradient centrifugation (with Ficoll-Paque PLUS) and frozen at −80°C. DNA was extracted from leukocytes by the University of California, San Francisco DNA bank. Genomic DNA isolation was performed using a standardized and quality-controlled PureGene DNA isolation system (Gentra Systems, Minneapolis). The quantity and quality of the genomic DNA isolate was determined by 260/280 UV spectrophotometery. At regular intervals, the integrity of isolated DNA was evaluated by agarose gel electrophoresis performed on randomly selected isolates.
DNA was analyzed for TL using quantitative polymerase chain reaction (qPCR) as previously described (Cawthon, 2002) with the following modifications. The primers for the telomere qPCR were tel1b [5′-CGGTTT(GTTTGG)5GTT-3′] and tel2b [5′-GGCTTG(CCTTAC)5CCT-3′], each used at a final concentration of 900nM. Single-copy gene (human beta-globin) qPCR primers were: hbg1 [5′-GCTTCTGACACAACTGTGTTCACTAGC-3′], used at a final concentration of 300nM, and hbg2 [5′-CACCAACTTCATCCACGTTCACC-3′], used at a final concentration of 700nM. The final reaction mix was: 20mM Tris-HCl, pH 8.4; 50mM KCl; 200nM each dNTP; 1% DMSO; 0.4x Sybr Green I; 44ng E. coli DNA; 0.8 units Platinum Taq DNA polymerase (Invitrogen) per 22μl reaction; 1.5 – 20ng genomic DNA. Tubes containing 40, 13.3, 4.4, and 1.5ng of reference DNA from Hela cells were included in each qPCR run so that the quantity of targeted templates in each research sample could be determined relative to a single reference DNA sample by the standard curve method. All qPCRs were carried out on a MX3000P (Stratagene, La Jolla, CA) real-time PCR instrument.
To adjust for batch-to-batch variation, the same four control DNA samples covering the normal range of T/S ratios were included on each of six independent runs. A conversion factor was calculated based on the average T/S ratio of the four control DNA samples in each run compared to the established T/S ratio.
To develop the conversion factor for the calculation of approximate base pair telomere length from the T/S ratio, the T/S ratios of a set of genomic DNA samples from primary human cell line IMR90 at different population doubling were determined. The terminal restriction fragment length of these DNA samples was measured by Southern blot analysis to create the TRF and T/S ratio plot. The slope of the plot was used as the conversion factor. The CV of the TL method was 6.7%.
IL-6
Samples were collected in 10ml SST tubes (Becton Dickinson, Franklin Lakes, NJ). A high sensitivity enzyme-linked immunosorbent assay was used to quantify IL-6 (R&D Systems, Minneapolis, MN) in the laboratory of Dr. Dhabhar. Assay sensitivity is < 0.1 pg/ml, and average intra- and inter-assay coefficients of variation were 7% and 8% respectively. IL-6 levels were available for 21 participants.
Data analysis
Zero-order Pearson’s correlations were used to assess associations among optimism, pessimism, TL and IL-6. In a series of separate hierarchical linear regression models for TL and IL-6, optimism and pessimism were entered simultaneously in the second step of the models, controlling for chronological age and caregiver status (caregiver/control) in the first step.
In secondary analyses, to demonstrate relationships between high and low pessimism and TL, adjusted only for age, we examined differences between groups in the top and bottom tertiles for pessimism. Studies that examine telomere shortening across the lifespan indicate that approximately 31 to 63 base pairs are lost per year (Hastie et al., 1990; Iwama et al., 1998). We used these estimates of base pair loss per year to examine approximate differences in years of telomere shortening between participants scoring in the top and bottom tertiles for pessimism.
Secondary analyses were conducted to assess the contribution of potential mediating variables including perceived stress, neuroticism, BMI, sleep and exercise. In order to assess the contribution of these variables, we entered them in the first step of hierarchical linear regression equations with optimism and pessimism entered in the second step, and separate equations computed for TL and IL-6. We also controlled for these variables in ANCOVA to assess the difference in TL between participants in the top and bottom tertiles for pessimism. Because we did not have sufficient power to conduct these multivariate analyses, and health behaviors are current rather than reflecting lifetime (see Discussion below), these secondary results should be interpreted with caution. SPSS software was used. Two tailed p tests were used throughout.
Results
The sample comprised 36 postmenopausal women ranging from 51 to 79 years (M age = 60.73, SD = 6.65; M BMI = 26.05, SD = 5.04). Of the women, 23 were caregivers for a relative with dementia and 13 were not caregiving and considered controls. Caregivers and controls were not significantly different on demographics, TL or IL-6, and the pattern of findings did not differ between groups. Accordingly, for the analyses reported here, we pooled data from caregivers and controls.
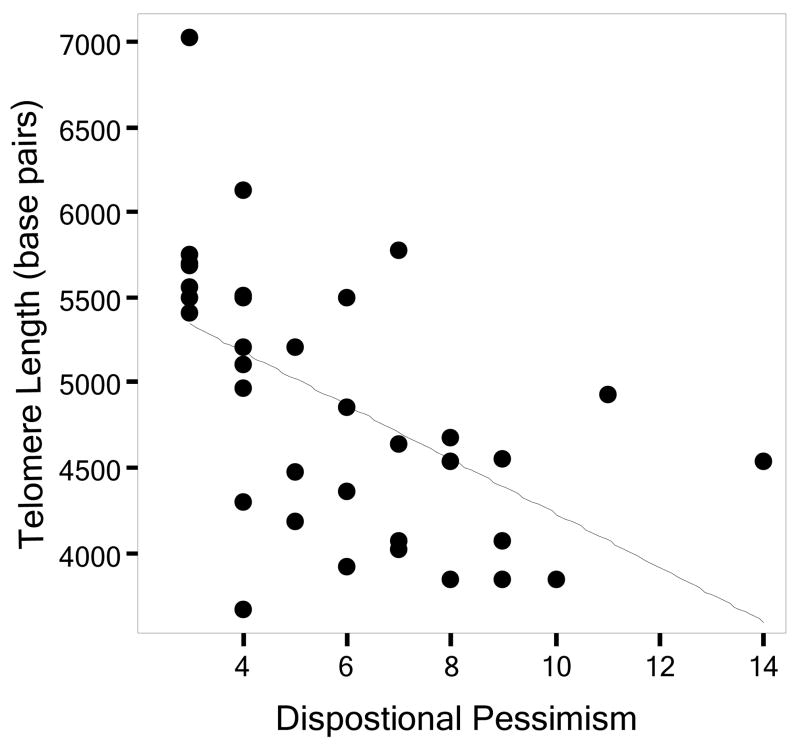
TL and IL-6 were significantly and negatively associated (r = −.51, p =.01). Optimism was marginally associated with longer TL (r =.31, p =.07), and there was no association between optimism and IL-6 (r = −.34, p =.14). In contrast, pessimism was strongly associated with shorter TL (r = −.55, p =.001; Fig. 1) and higher IL-6 (r =.43, p =.05). Controlling for age and caregiver status, and entering optimism and pessimism simultaneously, optimism was not uniquely associated with either TL or IL-6. Pessimism, on the other hand, remained significantly associated with both TL and IL-6 (Table 1).
Figure 1.
Scatterplot of dispositional pessimism and telomere length (r = −.55, p =.001).
Table 1.
Multiple Regression Analyses Assessing Relationships of Optimism and Pessimism with Telomere Length and Interleukin-6
| R | ΔR2 | β | t | p | |
|---|---|---|---|---|---|
| Telomere length | |||||
| Step 1: Age | −.05 | −.34 | .73 | ||
| Caregiver status | .26 | .07 | −.20 | 1.23 | .23 |
| Step 2: Optimism | −.17 | −.93 | .36 | ||
| Pessimism | .64 | .33 | −.68 | −3.72 | .001** |
| Interleukin-6 | |||||
| Step 1: Age | .28 | 1.33 | .20 | ||
| Caregiver status | .47 | .22 | −.19 | −.88 | .39 |
| Step 2: Optimism | .22 | −1.09 | .29 | ||
| Pessimism | .77 | .37 | .50 | 2.58 | .02* |
Note. p =.001,
p <.05
Comparing those participants with scores in the top and bottom tertiles for pessimism, and controlling for age, we found that participants with the highest pessimism scores had mean TL (n = 12; M TL = 4697bp, SD = 849bp) 705 base pairs shorter than those participants with the lowest pessimism scores (n = 16; M TL = 5402bp, SD = 734bp). This between group difference was significant, F(1,24) = 6.36, η2 =.21, p =.02. Using the same methods as in previous work (Epel et al., 2004), we estimated that highly pessimistic participants demonstrated roughly 11 to 23 years’ equivalent of additional telomere shortening compared with non-pessimistic participants. Participants in the top tertile for pessimism also had significantly higher levels of IL-6 than participants in the bottom tertile, F(1,12) = 9.95, η2 =.52, p =.01. Higher levels of perceived stress were associated with shorter TL (p =.01), which replicated our previous finding (Epel et al., 2004). Perceived stress was not associated with IL-6 (p =.31).
In the secondary analyses, the association between pessimism and TL remained significant when we controlled for age, caregiver status, optimism, perceived stress, neuroticism, BMI, exercise and sleep in multiple regression (β = −.66, t = −2.60, p =.02). However, the association between pessimism and IL-6 was not significant when these variables were included (β =.64, t = 1.93, p =.11). The between group (high vs. low pessimism) difference in TL also remained significant when we entered potential mediators as covariates, F(1,10) = 5.04, η2 =.35, p =.049. The TL/pessimism effect sizes for both the correlation (Cohen’s d = 1.32) and the adjusted coefficient from the multiple regression (f = 1.32) were both large effects. However, given the small sample size, and multiple covariates these secondary analyses should be viewed as preliminary and true tests of mediation will require further studies.
Discussion
This study is the first demonstration that a personality trait, specifically higher pessimism, is associated with shorter TL in leukocytes. It is also the first demonstration that pessimism is associated with higher basal levels of IL-6, an indicator of systemic inflammation and possibly immune system aging (Effros, 2004; Franceschi et al., 2000). Diminished TL and higher IL-6 level each alone predict mortality (Cawthon et al., 2003; Kimura et al., 2008; Volpato et al., 2001). Hence, our data support the possibility that immunosenescence, as measured by shorter TL and higher IL-6, is one of the mediators of the relationships between expectancies, disease risk (Allison et al., 2003; Matthews et al., 2004) and mortality (Giltay et al., 2006).
The combination of high optimism and/or low pessimism has previously been associated with markers of immune functioning (Cruess et al., 2000; Segerstrom, 2005). Interestingly, in our study the absence of pessimism was more important than the presence of optimism in predicting a “younger”-appearing immune system; we found no unique association between optimism and either TL or IL-6. These results are consistent with previous studies that have distinguished between optimism and pessimism, in which linear associations were reported between pessimism, but not optimism, and objective indicators of health (Milam et al., 2004; Schulz et al., 1996).
Although pessimism could exert some of its effects on immunosenescence through perceived stress or health behaviours, the observed associations between pessimism and markers of immunosenescence were independent of current perceived stress and health behaviours as measured. While current perceived stress and health behaviours are likely to share variance with lifetime history, they will not fully estimate exposure over decades. Consequently, the current findings do not rule out the possibility that psychological stress and health behaviours mediate at least some of the relationships we have shown to exist between pessimism, shorter TL and higher IL-6.
Given observed associations between both acute and chronic psychological stress and raised IL-6 levels (Kiecolt-Glaser et al., 2003; Steptoe et al., 2001), we propose that exposure to psychological stressors in pessimists could contribute to chronic low-level increases in circulating pro-inflammatory cytokines, which may in turn contribute to telomere shortening across the lifespan. However, leukocytes with short telomeres tend to release greater quantities of pro-inflammatory cytokines, including IL-6 (Effros, 2004), and thus we cannot rule out the possibility that the direction of causality is from short telomeres to greater IL-6 and not vice versa. Longitudinal research will be needed to assess causality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison PJ, Guichard C, Fung K, Gilain L. Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. J Clin Oncol. 2003;21:543–548. doi: 10.1200/JCO.2003.10.092. [DOI] [PubMed] [Google Scholar]
- 2.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–73. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 4.Carrero JJ, Stenvinkel P, Fellstrom B, Qureshi AR, Lamb K, Heimburger O, Barany P, Radhakrishnan K, Lindholm B, Soveri I, Nordfors L, Shiels PG. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med. 2008;263:302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 5.Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, Ketcham AS, Moffat J, Frederick L, Clark KC. How coping mediates the effect of optimism on distress: A study of women with early stage breast cancer. J Pers Soc Psych. 1993;65:375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- 6.Cawthon RM. Telomere measurement by quantitative PCR. Nucl Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 9.Cruess S, Antoni M, Kilbourn K, Ironson G, Klimas N, Fletcher MA, Baum A, Schneiderman N. Optimism, distress, and immunologic status in HIV-infected gay men following Hurricane Andrew. Int J Behav Med. 2000;7:160–182. [Google Scholar]
- 10.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng N. Accelerated telomere erosion is associated with a declining immune function of caregivers of alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exer. 1993;25:628–642. [PubMed] [Google Scholar]
- 12.John OP, Donahue EM, Kentle RL. The Big Five Inventory: versions 4a and 54. Berkeley: University of California, Berkeley, Institute of Personality and Social Research; 1991. [Google Scholar]
- 13.Effros RB. From Hayflick to Walford: The role of T cell replicative senescence in human aging. Exper Gerontol. 2004;39:885–890. doi: 10.1016/j.exger.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Nat Acad Sci USA. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschi C, Bonafe M, Valensin S, Oliveri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 16.Giltay EJ, Kamphuis MH, Kalmijn S, Zitman FG, Kromhout D. Dispositional optimism and the risk of cardiovascular death: The Zutphen Elderly Study. Arch Intern Med. 2006;166:431–6. doi: 10.1001/archinte.166.4.431. [DOI] [PubMed] [Google Scholar]
- 17.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 18.Iwama H, Ohyashiki K, Ohyashiki JH, Shigifumi H, Yahata N, Ando K, Taoyama K, Hoshika A, Takasaki M, Mori M, Shay JW. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Human Genet. 1998;102:397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: A study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews KA, Raikkonen K, Sutton-Tyrrell K, Kuller LH. Optimistic attitudes protect against progression of carotid atherosclerosis in healthy middle-aged women. Psychosom Med. 2004;66:640–644. doi: 10.1097/01.psy.0000139999.99756.a5. [DOI] [PubMed] [Google Scholar]
- 22.Milam JE, Richardson JL, Marks G, Kemper CA, Mccutchan AJ. The roles of dispositional optimism and pessimism in HIV disease progression. Psychol Health. 2004;19:167–181. [Google Scholar]
- 23.Plomin R, Scheier MF, Bergeman CS, Pedersen NL, Nesselroade JR, McClearn GE. Optimism, pessimism and mental health: A twin/adoption analysis. Pers Indiv Diffs. 1992;13:921–930. [Google Scholar]
- 24.Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 25.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 26.Schulz R, Bookwala J, Knapp JE, Scheier M, Williamson GM. Pessimism, age, and cancer mortality. Psychol Aging. 1996;11:304–309. doi: 10.1037//0882-7974.11.2.304. [DOI] [PubMed] [Google Scholar]
- 27.Segerstrom SC. Optimism and immunity: Do positive thoughts always lead to positive effects? Brain Behav Immun. 2005;19:195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature Med. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- 29.Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci. 2001;101:185–192. [PubMed] [Google Scholar]
- 30.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular Disease, Interleukin-6, and Risk of Mortality in Older Women: The Women’s Health and Aging Study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 31.Wu K, Higashi N, Hansen ER, Lund M, Bang K, Thestrup-Pedersen K. Telomerase Activity Is Increased and Telomere Length Shortened in T Cells from Blood of Patients with Atopic Dermatitis and Psoriasis. J Immunol. 2000;165:4742–4747. doi: 10.4049/jimmunol.165.8.4742. [DOI] [PubMed] [Google Scholar]