Abstract
Aims/Hypothesis
Islet amyloid in type 2 diabetes contributes to loss of beta cell mass and function. Since islets are susceptible to oxidative stress-induced toxicity, we sought to determine whether islet amyloid formation is associated with induction of oxidative stress.
Methods
Human islet amyloid polypeptide transgenic and non-transgenic mouse islets were cultured for 48 or 144 hours with or without the antioxidant N-acetyl-L-cysteine (NAC) or the amyloid inhibitor Congo red. Amyloid deposition, reactive oxygen species (ROS) production, beta cell apoptosis, and insulin secretion, content and mRNA were measured.
Results
After 48 hours, amyloid deposition was associated with increased ROS levels and increased beta cell apoptosis, but no change in insulin secretion, content or mRNA expression. Antioxidant treatment prevented the rise in ROS but did not prevent amyloid formation or beta cell apoptosis. In contrast, inhibition of amyloid formation prevented both the induction of oxidative stress and beta cell apoptosis. After 144 hours, amyloid deposition was further increased and was associated with increased ROS levels, increased beta cell apoptosis and decreased insulin content. At this time point, both antioxidant treatment and inhibition of amyloid formation were effective in reducing ROS levels, amyloid formation and beta cell apoptosis. Inhibition of amyloid formation also increased insulin content.
Conclusions/Interpretation
Islet amyloid formation induces oxidative stress, which in the short term does not mediate beta cell apoptosis but in the longer term may feed back to further exacerbate amyloid formation and contribute to beta cell apoptosis.
Keywords: islet amyloid, oxidative stress, beta cell apoptosis, IAPP, insulin secretion
INTRODUCTION
Islet amyloid deposits are a characteristic morphological feature of the pancreas in type 2 diabetes, comprising fibrils formed from islet amyloid polypeptide (IAPP) [1, 2]. Factors affecting the amyloidogenicity of IAPP include species-specific differences in the amino acid sequence, in addition to a permissive environment. Specifically, human but not rodent IAPP is capable of forming amyloid fibrils [3–5], and amyloid deposition from human IAPP (hIAPP) has been shown to be more frequent in the typical type 2 diabetes milieu of chronically elevated glucose and free fatty acids [6–10].
A large number of studies have demonstrated that islet amyloid formation is cytotoxic [11–14], with some evidence that IAPP oligomers may be the toxic species [13, 15–18]. Examination of autopsy pancreas specimens from subjects with type 2 diabetes has suggested a positive relationship between the amount of islet amyloid and beta cell loss [19–21]. In addition, there is growing support for involvement of oxidative stress in islet dysfunction and death. Typically, the generation of damaging reactive oxygen species (ROS) during oxidative stress has been strongly linked to defective insulin gene expression, reduced insulin content and impaired insulin secretion (reviewed in [22]). Further, in autopsy tissue from Japanese subjects with type 2 diabetes, islets that contained amyloid also stained positive for oxidative stress markers [23]. In other studies, treatment of immortalized beta cells with exogenous hIAPP resulted in intracellular ROS accumulation [24] and lipid peroxidation [25]. Also, antioxidant treatment inhibited the progression of hIAPP-induced apoptosis [24].
While the data linking hIAPP-induced toxicity to oxidative stress are intriguing, all the work has been performed using autopsy samples enabling only a retrospective evaluation [23], or by applying exogenous hIAPP to immortalized cells [24–26] which in some studies [27, 28] were not pancreatic beta cells. These approaches limit the ability to reach a definitive conclusion about whether oxidative stress is a cause or effect of islet amyloid formation, which in humans is derived from endogenous IAPP. Thus, in the present study, we employed an in vitro model using isolated islets from our hIAPP transgenic mice [10] to investigate the link between islet amyloid formation and oxidative stress, and the consequences for beta cell function and survival.
METHODS
Transgenic mice
Hemizygous transgenic mice with beta cell expression of hIAPP [29] on an F1 C57BL/6 × DBA/2J background were used in this study. Non-transgenic littermates were used as controls. Transgenic status was determined by polymerase chain reaction, as previously described [30]. Mice were fed a diet containing 18% kcal from fat (9% fat by weight; Purina #5021, IN, USA) or 45% kcal from fat (Research Diets D12290, NJ, USA). The study was approved by the Institutional Animal Care and Use Committee at the VA Puget Sound Health Care System.
Immunohistochemistry for nitrotyrosine
Five-µm paraffin pancreas sections were cut from three non-transgenic and three hIAPP transgenic mice after 12 months of high fat (45%) feeding [9]. Sections were treated with 0.05% (v/v) trypsin for antigen retrieval and non-specific immunoreactivity was blocked with 10% (v/v) normal goat serum. Sections were reacted overnight with mouse monoclonal anti-nitrotyrosine antibody (1:100; Chemicon International, CA, USA), followed by goat anti-mouse Cy3 (1:250) and thioflavin S staining to visualise amyloid deposits. To visualise cell nuclei, sections were counterstained with Hoechst 33258 (2 µg/ml). An average of 18 islets per mouse were examined. For negative controls, the primary antibody was omitted.
Isolation and culture of pancreatic islets
Islets were isolated from the pancreata of 10-week old female and male mice by collagenase digestion using methods previously described [31]. Islets were cultured overnight in RPMI-1640 medium (containing 11.1 mmol/L glucose), in a 37°C humidified atmosphere of 95% air:5% CO2 to allow them to recover from the isolation procedure. Islets were then either harvested for measurements described below or transferred to media containing either 5.5 mmol/L or 16.7 mmol/L glucose, and cultured for an additional 48 or 144 hours in the absence or presence of the antioxidant N-acetyl-L-cysteine (5 mmol/L), or one of two amyloid inhibitors 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-α-D-xylohexopyranose (WAS-406; 100 µmol/L) or Congo Red (200 µmol/L). WAS-406 [32, 33] and Congo red [34, 35] have previously been shown to inhibit IAPP oligomer and/or amyloid formation. The choice of dose for each amyloid inhibitor was made based on dose-response experiments we have performed in this model ([32] and unpublished observations). The culture periods were chosen based on our previous studies demonstrating light microscopy-visible amyloid at 48 hours and enhanced levels after 144 hours in 16.7 mmol/L glucose culture [10].
Measurement of ROS production
ROS production was measured in islets following overnight recovery or after 48-hour or 144-hour culture as previously described [36] with minor modifications. Briefly, 70 islets were dispersed by treatment with 0.0075% trypsin and then cells were loaded with 100 µmol/L oxidant-sensitive fluorescent carboxy-H2DCFDA dye (Invitrogen, CA, USA) for 30 minutes. After washing with phosphate-buffered saline, cells were resuspended in islet culture medium without phenol red, transferred to a 96-well plate and incubated at 37°C for 2 hours. For a positive control, a parallel sample of dispersed cells from 70 islets was treated with 100 µmol/L hydrogen peroxide for 2 hours. ROS levels were measured by quantifying the fluorescence intensity in each well at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Histological assessment of islet amyloid and beta cell apoptosis
Islets were fixed in 4% (w/v) phosphate-buffered paraformaldehyde (PFA) and embedded in paraffin [10]. Five-µm sections were cut and stained with thioflavin S to visualise amyloid deposits. For quantification of beta cell apoptosis, sections were stained with propidium iodide and anti-insulin antibody as previously described [37]. Histological assessments for amyloid and apoptosis were made in a blinded manner on an average of 19 islets per culture condition per experiment. Amyloid prevalence (% of islets containing amyloid) and severity (% of islet area occupied by amyloid) were determined using a computer-based quantitative method as reported previously [38], with islet area being determined morphometrically by manually outlining each islet when viewed under fluorescence at excitation 480 nm and emission 505 nm (channel used for thioflavin S staining). The proportion of apoptotic beta cells was determined by manual counting of condensed and fragmented nuclei in insulin-positive cells.
Assessment of 51Cr release
51Cr release was used to assess cell viability after 48-hour culture of islets in 5.5 or 16.7 mmol/L glucose [31]. This method is based on the principle that over 48 hours, viable cells retain preloaded 51Cr intracellularly because cell membranes are intact. In contrast, non-viable cells develop leaky membranes and thus release preloaded 51Cr into the media. Data were expressed as fractional 51Cr release in terms of total incorporation (cpm in medium/[cpm in medium + cpm in islets] × 100).
Evaluation of insulin secretion and content
Following 48 hour cultures, insulin secretion was measured by islet perifusion as previously described [10]. Effluent fractions were collected at 2–5 minute intervals during perifusion with 1.67 mmol/L glucose for 8 minutes (basal), then with 16.7 mmol/L glucose for 30 minutes (glucose-stimulated). Following 144 hour cultures, insulin secretion was measured in static incubations as previously described [31]. Supernatant fractions were collected after 60-minute incubation of islets in either 2.8 mmol/L (basal) or 20 mmol/L (glucose-stimulated) glucose. Samples were stored at −20°C before determination of insulin by radioimmunoassay [30]. Islet insulin content was measured after acid-ethanol extraction.
Real-time quantitative reverse-transcription PCR
Gene expression of insulin in isolated islets was determined with real-time quantitative RT-PCR performed using the TaqMan system (ABI Prism 7000, Applied Biosystems, CA, USA) as previously described [39]. TaqMan Assays on Demand insulin II gene expression mix was from Applied Biosystems (# Mm00731595_gH). TaqMan eukaryotic 18S rRNA (Applied Biosystems # Hs99999901_s1) was used as an endogenous control. Each sample was run in triplicate.
Statistical Analyses
Data are presented as mean ± standard error of the mean (SEM) for the number of experiments indicated. Statistical significance was determined using analysis of variance or Mann-Whitney U tests where appropriate, with non-parametric tests being used where data were not normally distributed. A P value <0.05 was considered statistically significant.
RESULTS
Detection of oxidative stress markers in vivo
To determine whether oxidative stress occurs in association with amyloid formation in vivo, pancreas sections from year-old, high fat fed non-transgenic and hIAPP transgenic mice [9] were stained for nitrotyrosine, a widely used marker of oxidative stress [36, 40, 41]. Amyloid formation and nitrotyrosine immunoreactivity were only observed in hIAPP transgenic islets (Figure 1a).
Figure 1.
(a) Representative islets from pancreata of non-transgenic and hIAPP transgenic mice after 12 months on a high fat diet. Nitrotyrosine (red) and amyloid (green) staining is seen in hIAPP transgenic islets only.
(b) Representative islets from non-transgenic (NT) and hIAPP transgenic (T) mice following 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose. Only hIAPP transgenic islets cultured in 16.7 mmol/L glucose developed amyloid deposits (lower right-hand panel arrows). Red=insulin, blue=nuclei, green=amyloid.
(c) ROS levels in non-transgenic (open bars) and hIAPP transgenic (closed bars) islets following 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose. Data are expressed relative to the fluorescence from 5.5 mmol/L glucose-cultured islets. Only hIAPP transgenic islets cultured in 16.7 mmol/L glucose had significantly elevated ROS levels. n=11; *p<0.0005 vs NT 16.7 mmol/L glucose.
Analysis of islets after isolation and overnight recovery, prior to treatment
Having demonstrated the presence of oxidative stress markers in vivo, an in vitro culture model was then adopted to directly examine the relationship between amyloid formation and oxidative stress. To ensure hIAPP transgenic islets did not differ from non-transgenic islets prior to culture, ROS levels, amyloid deposition, beta cell apoptosis and insulin content were determined following overnight recovery of isolated islets. hIAPP transgenic and non-trangsenic islets had comparable ROS levels, beta cell apoptosis rates and insulin content. As expected, amyloid was not detected in any of the non-transgenic or hIAPP transgenic islets examined (Table 1).
Table 1.
ROS levels (n=3), beta cell apoptosis (n=5), insulin content (n=5) and amyloid prevalence (n=5) in islets following isolation and overnight recovery.
| Non-transgenic | hIAPP transgenic | |
|---|---|---|
| ROS Levels (fold over non-transgenic) |
1.00 | 1.01 ± 0.01 |
| % Apoptotic Beta Cells | 0.13 ± 0.01 | 0.10 ± 0.05 |
| Insulin Content (nmol/100 islets) |
1171 ± 165 | 1347 ± 266 |
| Amyloid Prevalence (% islets with amyloid) |
0 | 0 |
Islet amyloid and ROS levels post 48-hour culture in 5.5 and 16.7 mmol/L glucose
Figure 1b shows representative thioflavin S staining of islet amyloid in non-transgenic and hIAPP transgenic islets cultured for 48 hours in 5.5 mmol/L or 16.7 mmol/L glucose. Islet amyloid was present only in hIAPP transgenic islets cultured in 16.7 mmol/L glucose. After 48 hours, ROS levels were comparable in non-transgenic and hIAPP transgenic islets cultured in 5.5 mmol/L glucose and in non-transgenic islets cultured in 16.7 mmol/L glucose (Figure 1c). In contrast, hIAPP transgenic islets cultured in 16.7 mmol/L glucose for 48 hours had significantly elevated ROS levels, compared to both 5.5 mmol/L glucose and to non-transgenic islets at 16.7 mmol/L glucose.
Islet amyloid and ROS levels after 48-hour antioxidant treatment
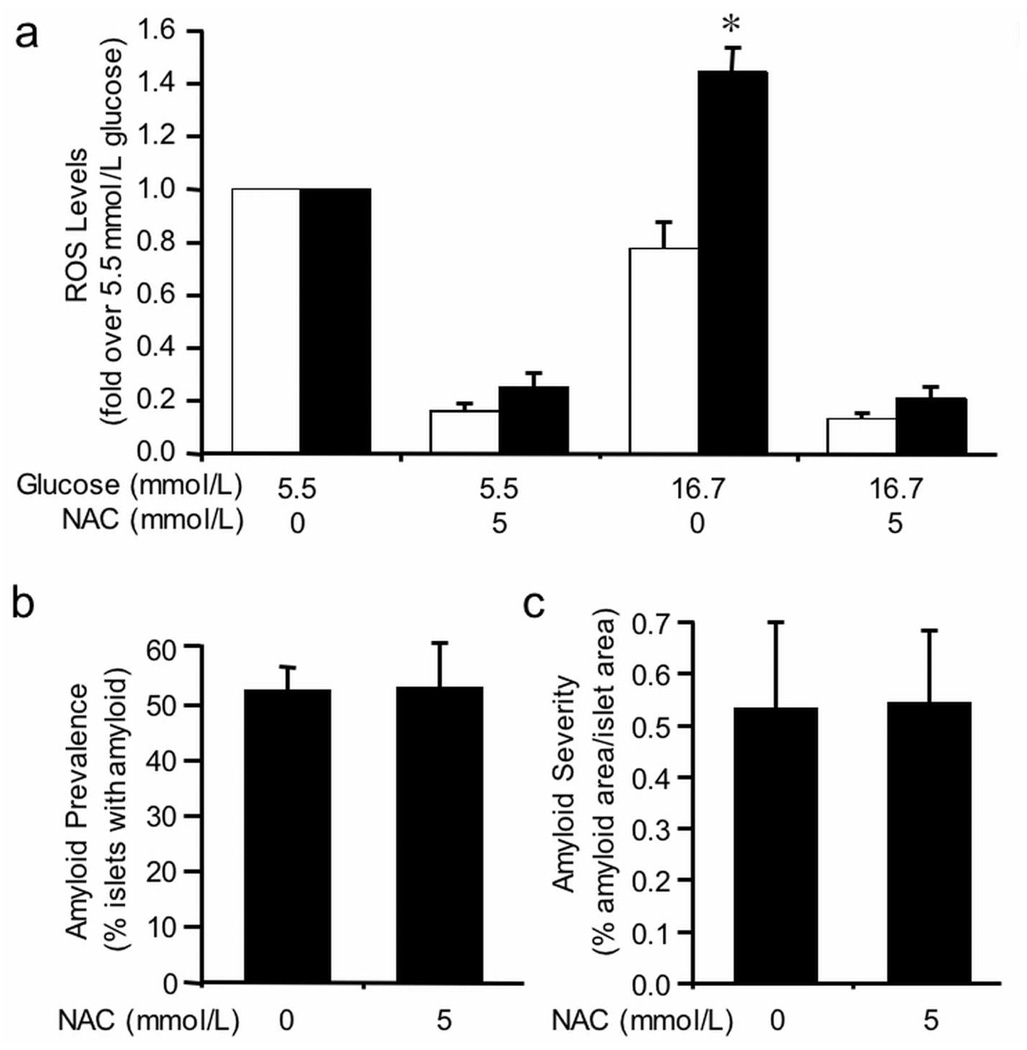
To investigate whether reducing ROS levels over 48 hours could prevent islet amyloid formation, islets were co-cultured with the antioxidant N-acetyl-L-cysteine (NAC). NAC significantly decreased ROS levels in both non-transgenic and hIAPP transgenic islets cultured in 5.5 mmol/L or 16.7 mmol/L glucose when compared to either glucose concentration alone (Figure 2a).
Figure 2.
(a) ROS levels in non-transgenic (open bars) and hIAPP transgenic (closed bars) islets following 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose +/− the antioxidant, N-acetyl-L-cysteine (NAC; 5 mmol/L). Data are expressed relative to the fluorescence from 5.5 mmol/L glucose-cultured islets. NAC significantly decreased ROS levels in all islets, regardless of genotype or media glucose concentration. n=3; *p<0.01 vs non-transgenic.
Amyloid prevalence (b) and severity (c) in hIAPP transgenic islets following 48-hour culture in 16.7 mmol/L glucose +/− 5 mmol/L NAC. NAC treatment had no effect on amyloid prevalence or severity. n=5.
hIAPP transgenic islets cultured in 16.7 mmol/L glucose developed islet amyloid with a prevalence of 52 ± 4% (Figure 2b) and severity of 0.53 ± 0.17% (Figure 2c). Treatment with NAC did not alter either amyloid prevalence (Figure 2b) or severity (Figure 2c), suggesting amyloid formation occurs upstream or independent of increased ROS production. As anticipated, islet amyloid was not present in non-transgenic islets or in hIAPP transgenic islets cultured in 5.5 mmol/L glucose (data not shown).
Islet amyloid and ROS levels after 48-hour treatment with amyloid inhibitors
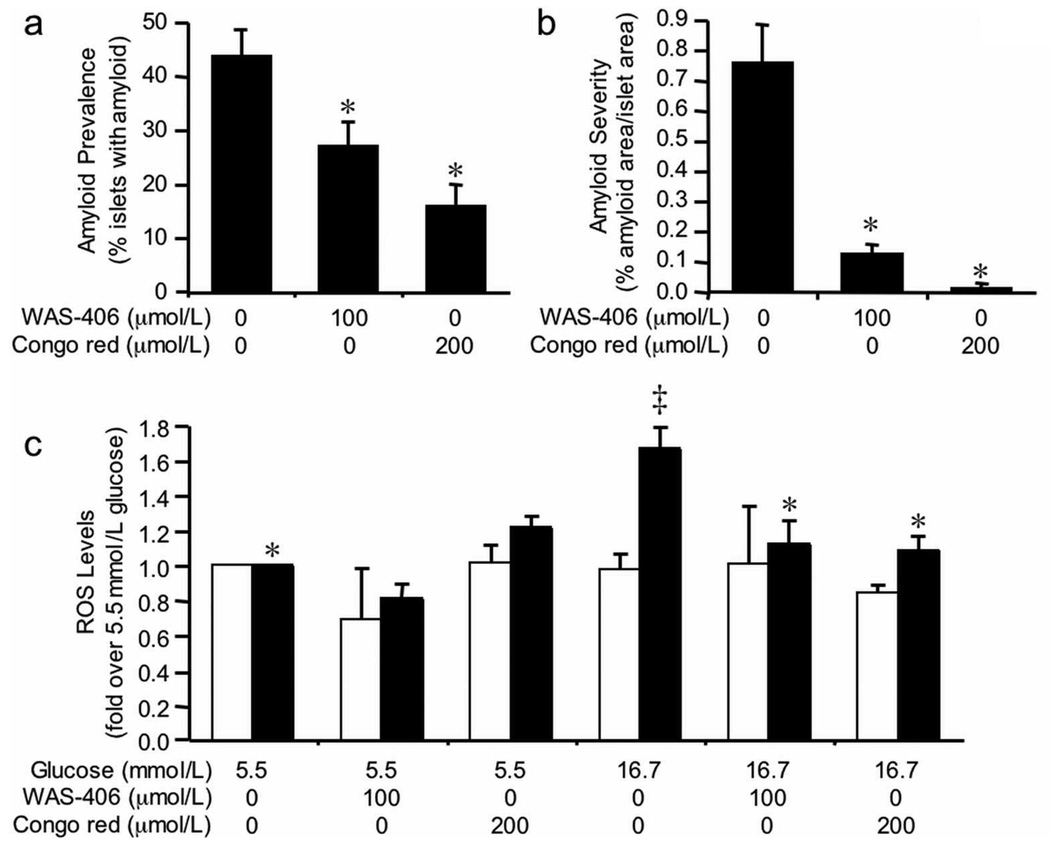
To test the hypothesis that islet amyloid formation results in increased ROS levels, we co-cultured islets with one of two amyloid inhibitors (WAS-406 or Congo red), then measured ROS levels. WAS-406 treatment of hIAPP transgenic islets cultured in 16.7 mmol/L glucose for 48 hours significantly decreased amyloid prevalence (Figure 3a) from 43.9 ± 4.8% to 27.2 ± 4.4% (p=0.015). Similarly, Congo red treatment decreased amyloid prevalence to 16.1 ± 3.9% (p=0.016). Amyloid severity in hIAPP transgenic islets cultured in 16.7 mmol/L glucose for 48 hours was decreased from 0.76 ± 0.13% to 0.13 ± 0.03% (p=0.0004) and 0.02 ± 0.01% (p=0.01) following WAS-406 and Congo red treatment, respectively (Figure 3b). Both WAS-406 and Congo red prevented the increase of ROS levels in hIAPP transgenic islets cultured in 16.7 mmol/L glucose, while having no effect in non-transgenic islets or hIAPP transgenic islets cultured in 5.5 mmol/L glucose (Figure 3c).
Figure 3.
Amyloid prevalence (a) and severity (b) in hIAPP transgenic islets following 48-hour culture in 16.7 mmol/L glucose +/− the amyloid inhibitors, 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-α-D-xylohexopyranose (WAS-406; 100 µmol/L) or Congo red (200 µmol/L). Both inhibitors significantly decreased amyloid prevalence and severity, compared to culture in 16.7 mmol/L glucose alone. n=4–5; *p<0.05 vs 16.7 mmol/L glucose.
(c) ROS levels in non-transgenic (open bars) and hIAPP transgenic (closed bars) islets following 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose +/− WAS-406 or Congo red. Data are expressed relative to the fluorescence from 5.5 mmol/L glucose-cultured islets. Inhibition of amyloid with WAS-406 and Congo red significantly decreased ROS levels in hIAPP transgenic islets cultured in 16.7 mmol/L glucose. n=4; *p<0.05 vs transgenic 16.7 mmol/L, ‡p<0.05 vs non-transgenic.
51Cr release after 48-hour treatment with Congo red or NAC
We next investigated whether amyloid formation and subsequent induction of oxidative stress is associated with reduced cell viability in hIAPP transgenic islets. Figure 4a and 4b demonstrate that 51Cr release did not differ between non-transgenic and hIAPP transgenic islets cultured in 5.5 mmol/L glucose for 48 hours. When cultured in 16.7 mmol/L glucose, both non-transgenic and hIAPP transgenic islets released significantly more 51Cr than at 5.5 mmol/L glucose, indicating a decrease in cell viability. However, hIAPP transgenic islets released significantly more 51Cr than non-transgenic islets at 16.7 mmol/L glucose, demonstrating a further reduction in hIAPP transgenic islet cell viability.
Figure 4.
51Cr release from non-transgenic (open bars) and hIAPP transgenic (closed bars) islets after 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose +/− (a) 200 µmol/L Congo red or (b) 5 mmol/L NAC. hIAPP transgenic islets cultured in 16.7 mmol/L glucose showed lowest viability, i.e. greatest 51Cr release. The amyloid inhibitor, Congo red, prevented this increased 51Cr release while having no effect on non-transgenic islets at either glucose concentration or hIAPP transgenic islets cultured in 5.5 mmol/L glucose. The antioxidant, NAC, also reduced 51Cr release in both non-transgenic and hIAPP transgenic islets cultured in 16.7 mmol/L glucose, while having no effect in 5.5 mmol/L glucose. n=4; *p<0.02 vs non-transgenic, ‡p<0.0005 vs transgenic 16.7 mmol/L.
To inhibit amyloid formation in this series of experiments, Congo red was utilized. 51Cr release from hIAPP transgenic islets cultured in 16.7 mmol/L glucose plus Congo red decreased significantly compared to hIAPP transgenic and non-transgenic islets in 16.7 mmol/L glucose. 51Cr release from non-transgenic islets and islets cultured in 5.5 mmol/L glucose was unchanged in the presence versus the absence of Congo red (Figure 4a).
To determine whether ROS could mediate the amyloid-induced reduction in cell viability, islets were treated with NAC. 51Cr release from both hIAPP transgenic and non-transgenic islets cultured in 16.7 mmol/L glucose plus NAC decreased significantly compared to islets in 16.7 mmol/L glucose alone. NAC did not change 51Cr release from islets cultured in 5.5 mmol/L glucose (Figure 4b).
Beta cell apoptosis after 48-hour treatment with Congo red or NAC
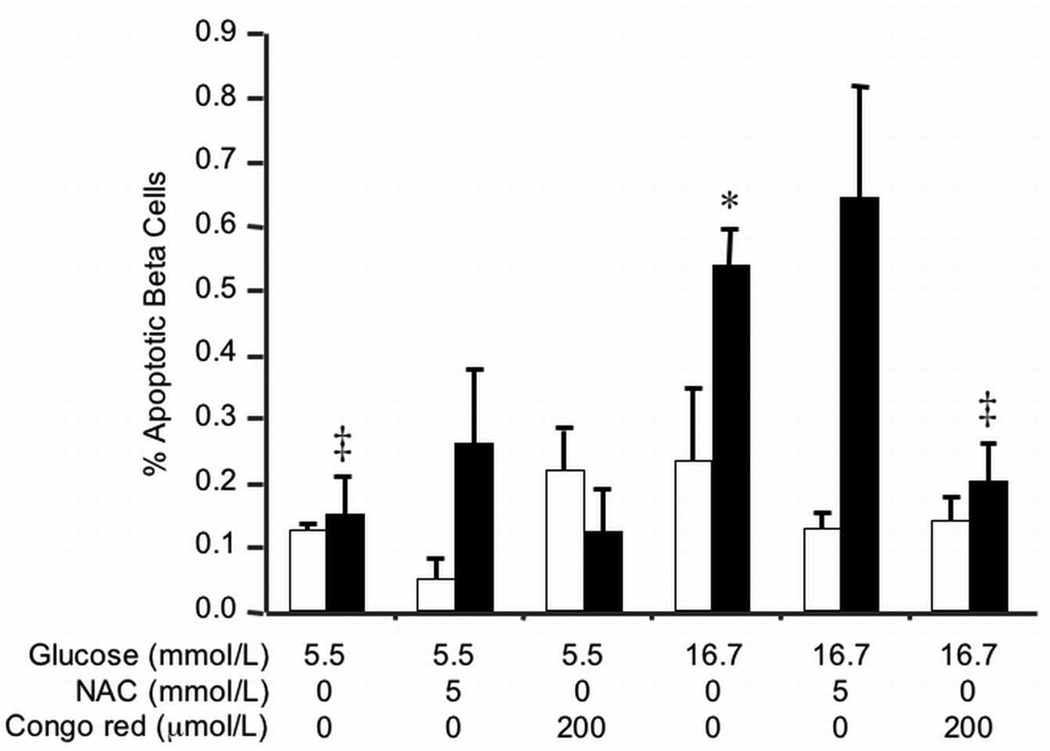
To determine whether amyloid-containing islets demonstrated increased beta cell apoptosis, we determined the proportion of condensed or fragmented nuclei in insulin-positive cells following 48-hour cultures. Beta cell apoptosis did not differ between non-transgenic and hIAPP transgenic islets cultured in 5.5 mmol/L glucose (Figure 5). hIAPP transgenic islets cultured in 16.7 mmol/L glucose exhibited increased beta cell apoptosis compared to non-transgenic islets and transgenic islets cultured in 5.5 mmol/L glucose. When amyloid formation was inhibited with Congo red, beta cell apoptosis in hIAPP transgenic islets decreased significantly compared to hIAPP transgenic islets in 16.7 mmol/L glucose alone, while remaining unchanged in non-transgenic islets and islets cultured in 5.5 mmol/L glucose. Interestingly, when islets were treated with the antioxidant NAC, beta cell apoptosis did not change in hIAPP transgenic islets.
Figure 5.
Beta cell apoptosis in non-transgenic (open bars) and hIAPP transgenic (closed bars) islets after 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose +/− 200 µmol/L Congo red or 5 mmol/L NAC. Only hIAPP transgenic islets cultured in 16.7 mmol/L glucose showed increased beta cell apoptosis that could be reduced by co-culture with the amyloid inhibitor, Congo red. The antioxidant, NAC, failed to reduce beta cell apoptosis in hIAPP transgenic islets cultured in 16.7 mmol/L glucose. n=3–5; *p<0.05 vs non-transgenic, ‡p<0.01 vs transgenic 16.7 mmol/L.
Insulin secretion, content and mRNA expression after 48-hour culture
To determine whether amyloid-induced oxidative stress alters beta cell function, insulin secretion was measured after 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose. Neither basal (response to 1.67 mmol/L glucose) nor glucose-stimulated (response to 16.7 mmol/L glucose) insulin secretion differed between genotypes (Figure 6a). Islets cultured in 16.7 mmol/L glucose exhibited an enhanced insulin response to stimulatory glucose compared to islets in 5.5 mmol/L glucose, regardless of genotype (Figure 6a). Total insulin content (Figure 6b) and insulin mRNA expression (Figure 6c) were not different amongst culture conditions or between genotypes.
Figure 6.
(a) Insulin secretion in response to 1.67 mmol/L and 16.7 mmol/L glucose, (b) islet insulin content and (c) insulin mRNA expression from non-transgenic (open symbols/bars) and hIAPP transgenic (closed symbols/bars) islets after 48-hour culture in 5.5 mmol/L or 16.7 mmol/L glucose. There were no differences in insulin secretion, content or mRNA expression between non-transgenic and hIAPP transgenic islets after either 5.5 mmol/L or 16.7 mmol/L glucose culture. n=4–5.
Effect of NAC or Congo red treatment during prolonged 16.7 mmol/L glucose culture
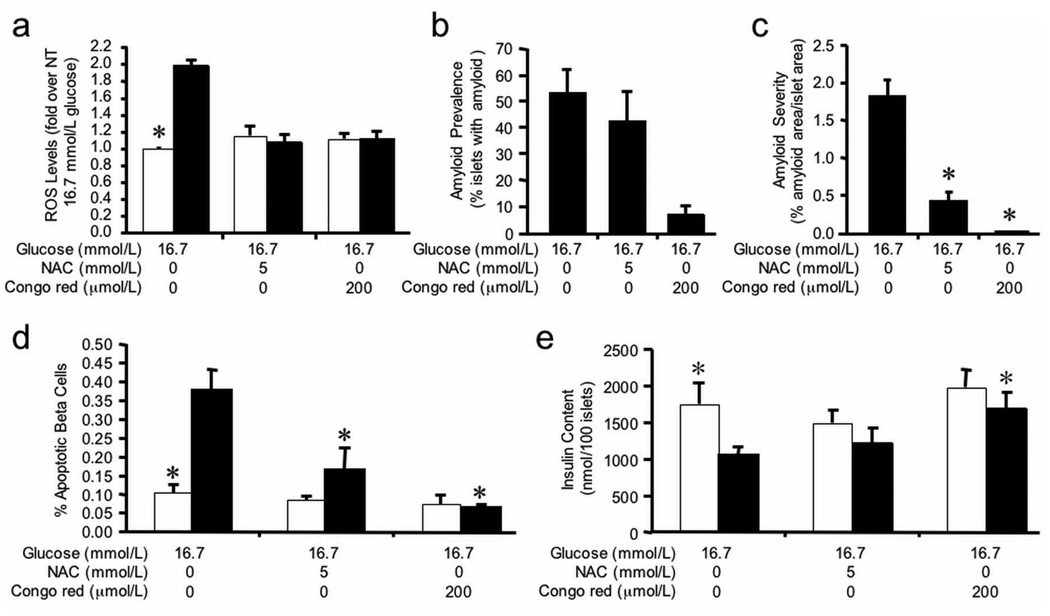
As culture of hIAPP transgenic islets in 16.7 mmol/L glucose results in a time-dependent increase in amyloid deposition [10], we investigated the relationship between amyloid and oxidative stress during prolonged culture. Islet amyloid and ROS levels were determined in non-transgenic and hIAPP transgenic islets cultured for 144 hours in 16.7 mmol/L glucose with or without NAC or Congo red. hIAPP transgenic islets had a 1.9-fold increase in ROS levels compared to non-transgenic islets (Figure 7a), and this was associated with an amyloid prevalence of 52.7 ± 9.2 (Figure 7b) and severity of 1.81 ± 0.23 (Figure 7c). Non-transgenic islets did not contain amyloid.
Figure 7.
(a) ROS levels, (b) amyloid prevalence, (c) amyloid severity, (d) beta cell apoptosis and (e) insulin content in non-transgenic (open bars) and hIAPP transgenic (closed bars) islets following 144-hour culture in 16.7 mmol/L glucose +/− NAC or Congo red. ROS data are expressed relative to the fluorescence from 16.7 mmol/L glucose-cultured non-transgenic islets. Compared to non-transgenic islets, hIAPP transgenic islets cultured in 16.7 mmol/L glucose developed amyloid and had significantly elevated ROS levels, accompanied by increased beta cell apoptosis and reduced insulin content. NAC or Congo red treatment of hIAPP transgenic islets reduced ROS levels, amyloid deposition and beta cell apoptosis. Also, Congo red significantly increased insulin content in hIAPP transgenic islets compared to non-transgenic islets. n=4–5; *p<0.05 vs transgenic 16.7 mmol/L.
NAC significantly decreased ROS levels (Figure 7a) and interestingly also prevented the increase of amyloid severity (Figure 7c) in hIAPP transgenic islets. As anticipated, Congo red treatment significantly decreased amyloid prevalence (Figure 7b) and severity (Figure 7c), and also prevented the increase of ROS levels in hIAPP transgenic islets, while having no effect in non-transgenic islets (Figure 7a).
hIAPP transgenic islets cultured in 16.7 mmol/L glucose for 144 hours had elevated rates of beta cell apoptosis compared to non-transgenic islets (Figure 7d). When co-cultured with either NAC or Congo red, beta cell apoptosis was reduced by 56% and 83% respectively.
To investigate whether increased amyloid formation, ROS levels and beta cell apoptosis were associated with impaired beta cell function after 144 hours of culture, insulin secretion and content were measured. Neither basal (non-transgenic 14.8 ± 4.8 vs transgenic 11.7 ± 2.3 pmol/min/100 islets, p=0.58, n=4) nor glucose-stimulated insulin secretion (non-transgenic 105.4 ± 16.3 vs transgenic 107.2 ± 11.7 pmol/min/100 islets, p=0.93, n=4) differed between genotypes. Compared to non-transgenic islets, total insulin content was significantly reduced in hIAPP transgenic islets cultured in 16.7 mmol/L glucose (Figure 7e). Co-culture of hIAPP transgenic islets with NAC tended to increase insulin content, whereas Congo red completely restored insulin content to levels seen in non-transgenic islets (Figure 7e).
DISCUSSION
The current study provides evidence for a causative role of amyloidogenesis in the induction of oxidative stress in hIAPP transgenic mouse islets. Further, it demonstrates that in the short term, amyloid-induced beta cell apoptosis is independent of oxidative stress, while in the long term, oxidative stress may feed back to exacerbate amyloid formation and thus contribute to beta cell apoptosis. We also demonstrate that oxidative stress, islet viability, beta cell apoptosis and insulin content can be markedly improved by inhibition of amyloid formation. While this is not the first report to reveal a link between islet amyloid formation and oxidative stress, it is the first to show causality utilizing a model that is particularly relevant to islet amyloid formation in humans since the amyloid deposits form from endogenous hIAPP and are histologically comparable to the classical light microscopy-visible deposits observed in human type 2 diabetes [8].
The association between amyloid fibril formation and oxidative stress has been extensively investigated in Alzheimer’s disease [42], but has not been pursued with the same intensity for islet amyloid. A limited number of studies using immortalised beta cell lines demonstrated an elevation of oxidative stress markers with exogenous application of hIAPP [24–26], while examination of amyloid-positive islets obtained at autopsy from Japanese subjects with type 2 diabetes has also revealed increased oxidative stress [23]. In our study, we demonstrate increased markers of oxidative stress in the islets of hIAPP transgenic mice both in vivo after a year of high fat feeding and in vitro when islets from hIAPP transgenic islets are exposed to 16.7 mmol/L glucose. As amyloid forms in vitro under conditions of elevated glucose, i.e. 16.7 mmol/L but not 5.5 mmol/L glucose [10], the lack of elevated ROS levels in hIAPP transgenic islets immediately following isolation and recovery or after culture in 5.5 mmol/L glucose indicates that expression of amyloidogenic hIAPP per se is not sufficient to induce oxidative stress. Additionally, non-transgenic islets (incapable of forming amyloid) did not exhibit elevated ROS levels when cultured in 16.7 mmol/L glucose. Thus, collectively these observations suggest that independent of any effects of elevated glucose, amyloid formation in islets induces oxidative stress.
To investigate this possibility and in particular, the causality, we used the antioxidant NAC, that has been shown to protect islets from oxidative stress both in vitro and in vivo [43–45]. While we did see a marked reduction of ROS levels with 48 hours of NAC treatment in all islets regardless of genotype or media glucose concentration, NAC did not prevent or reduce the formation of amyloid in hIAPP transgenic islets cultured in 16.7 mmol/L glucose. This is consistent with the report of hIAPP-treated RINm5F cells [24] and strongly supports the notion that in the short term, amyloid formation occurs prior to the increase of ROS levels. Further evidence for this hypothesis was provided by measurement of ROS levels following inhibition of islet amyloid formation using the amyloid inhibitors Congo red and WAS-406. Both compounds markedly inhibited islet amyloid formation, consistent with previous reports in the literature [32–34]. Treatment of hIAPP transgenic islets with either of the amyloid inhibitors abolished the increase in ROS levels, consistent with a causative role for amyloid deposition in the induction of oxidative stress.
While detrimental effects of elevated ROS levels on the beta cell are well documented [22], involvement of oxidative stress in the toxicity of islet amyloid remains controversial. In a cell line derived from rat brain tumours, exogenous hIAPP treatment resulted in increased hydrogen peroxide levels and decreased cell viability that could be prevented with the antioxidant vitamin E [27]. In a similar study utilizing primary rat islets, hIAPP-induced toxicity could not be prevented with vitamin E [11]; however ROS levels were not measured and so it is unclear whether the experimental conditions were associated with oxidative stress. In our study, hIAPP transgenic islets that developed amyloid and elevated ROS levels after culture in 16.7 mmol/L glucose had decreased cell viability and increased beta cell apoptosis. Inhibition of amyloid formation with Congo red, improved cell viability and reduced beta cell apoptosis. However, 48 hours of NAC treatment did not reduce beta cell apoptosis. This implies that initially, amyloid-induced beta cell apoptosis occurs independent of oxidative stress. In contrast, 144 hours of NAC treatment resulted in reductions in both amyloid severity and beta cell apoptosis. Thus it appears that over time, amyloid-induced oxidative stress potentiates further amyloid formation and thereby contributes to beta cell apoptosis. A model describing this time-dependent paradigm is shown in Figure 8.
Figure 8.
(a) Short- and (b) long-term consequences of islet amyloid formation. Culture of hIAPP transgenic islets in 16.7 mmol/L glucose induces amyloid formation. In the short term (48 hours; panel a), amyloid formation induces oxidative stress and beta cell apoptosis via independent mechanisms. In the long term (144 hours; panel b), amyloid-induced oxidative stress feeds back to potentiate amyloid formation and thereby mediates amyloid-induced beta cell apoptosis.
Non-transgenic islets cultured in 16.7 mmol/L glucose were less viable (measured as increased 51Cr release) than islets cultured in 5.5 mmol/L glucose. This observation was most likely due to a glucotoxic effect mediated by 48-hour exposure to 16.7 mmol/L glucose – an effect that could not be ascribed to oxidative stress since non-transgenic islets cultured in 16.7 mmol/L glucose did not exhibit the same elevation in ROS levels as the hIAPP transgenic islets. This non-amyloid-related glucotoxicity was also seen in hIAPP transgenic islets cultured in 16.7 mmol/L glucose, as even when the amyloid-associated increase in ROS levels was inhibited, the islets were still significantly less viable than transgenic or non-transgenic islets cultured at 5.5 mmol/L glucose. These findings indicate additive cytotoxic effects of amyloid formation and glucose.
While oxidative stress due to amyloid formation was seen in hIAPP transgenic islets after 48-hour culture, no changes in insulin secretion, content or mRNA levels were observed. This is somewhat surprising given the extensive literature describing decreased insulin secretion and suppressed insulin mRNA levels following prolonged elevations of ROS. The reason for the discrepancy between our data and other published data is unclear. However, the most likely explanation would be the relatively short period of high glucose exposure. Consistent with this idea, the longer 144-hour culture of hIAPP transgenic islets in 16.7 mmol/L glucose resulted in reduced insulin content compared to non-transgenic islets in both the present study, and our previous study [10]. However, insulin secretion in response to stimulatory glucose was not decreased after either culture period. Thus, the 48-hour and perhaps even the 144-hour period utilized in the current study may be insufficient to produce measurable beta cell dysfunction, even though ROS levels and beta cell apoptosis are elevated. Another possible explanation for the difference between our data and other published data is the genetic background of the mice in our study, i.e. C57BL/6 × DBA/2J F1. The DBA/2 strain is genetically susceptible to high glucose-induced oxidative stress and impaired insulin secretion, whereas the C57BL/6 strain is resistant [45, 46]. Therefore, some genetic component(s) from the C57BL/6 parental strain may have protected the F1 hIAPP transgenic islets from impaired insulin secretion despite amyloid-induced ROS production. The fact that non-transgenic islets cultured in 16.7 mmol/L glucose do not exhibit elevated ROS levels is consistent with the concept of protective mechanisms against glucose-induced elevations in ROS levels. Moreover, this lack of an ROS response to 16.7 mmol/L glucose culture is an advantage of our in vitro model, as it enables the clear separation of amyloid-induced versus glucose-induced effects.
In summary, we have shown that amyloid formation induces oxidative stress in hIAPP transgenic islets, as well as decreased cell viability, increased beta cell apoptosis and reduced insulin content but no change in insulin secretory function. In addition, prolonged oxidative stress may potentiate islet amyloid formation and its toxic effects. Given that type 2 diabetes is a progressive disorder, it can be perceived that continuous amyloid formation and prolonged amyloid-associated oxidative stress would present a detrimental state for the beta cell. Interventions aimed at reducing and/or preventing the formation of amyloid could prove valuable for beta cell preservation in type 2 diabetes.
ACKNOWLEDGEMENTS
We thank Breanne Barrow, Rahat Bhatti, Tina Braddock, Maria Cone, Mike Peters, Jeanette Teague, Melissah Watts and Joshua Willard for excellent technical support. This work was supported by research funding from the Department of Veterans Affairs and NIH grants DK-75998 and DK-17047. S.Z. was supported by a Juvenile Diabetes Research Foundation Postdoctoral Fellowship and an American Diabetes Association Mentor-Based Fellowship. R.L.H. was supported by NIH grant DK-74404. Preparation and development of WAS-406 was funded by the Canadian Institutes for Health Research grant MOP-3153, the Natural Sciences and Engineering Research Council of Canada and the Institute for the Study of Aging.
Abbreviations
- hIAPP
human islet amyloid polypeptide
- IAPP
islet amyloid polypeptide
- NAC
N-acetyl-L-cysteine
- PFA
paraformaldehyde
- ROS
reactive oxygen species
Footnotes
DUALITY OF INTEREST
The authors declare that there is no duality of interest associated with this manuscript.
REFERENCES
- 1.Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betsholtz C, Christmansson L, Engstrom U, et al. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251:261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- 4.Betsholtz C, Svensson V, Rorsman F, et al. Islet amyloid polypeptide (IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res. 1989;183:484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opie E. The relation of diabetes mellitus to lesions of the pancreas: hyaline degeneration of the islets of Langerhans. J Exp Med. 1901;5:527–540. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westermark P. Amyloid and polypeptide hormones: what is their relationship? Amyloid Int J Exp Clin Invest. 1994;1:47–60. [Google Scholar]
- 8.Verchere CB, D'Alessio DA, Palmiter RD, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull RL, Andrikopoulos S, Verchere CB, et al. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Zraika S, Hull RL, Udayasankar J, et al. Glucose-and time-dependence of islet amyloid formation in vitro. Biochem Biophys Res Commun. 2007;354:234–239. doi: 10.1016/j.bbrc.2006.12.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 12.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 13.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 14.Bai JZ, Saafi EL, Zhang S, Cooper GJ. Role of Ca2+ in apoptosis evoked by human amylin in pancreatic islet beta-cells. Biochem J. 1999;343(Pt 1):53–61. [PMC free article] [PubMed] [Google Scholar]
- 15.Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 16.Janson J, Soeller WC, Roche PC, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. Febs J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 18.Meier JJ, Kayed R, Lin CY, et al. Inhibition of human IAPP fibril formation does not prevent beta-cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. Am J Physiol Endocrinol Metab. 2006;291:E1317–E1324. doi: 10.1152/ajpendo.00082.2006. [DOI] [PubMed] [Google Scholar]
- 19.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- 20.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 21.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 22.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 24.Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. Thiol reducing compounds prevent human amylin-evoked cytotoxicity. Febs J. 2005;272:4949–4959. doi: 10.1111/j.1742-4658.2005.04903.x. [DOI] [PubMed] [Google Scholar]
- 25.Janciauskiene S, Ahren B. Fibrillar islet amyloid polypeptide differentially affects oxidative mechanisms and lipoprotein uptake in correlation with cytotoxicity in two insulin-producing cell lines. Biochem Biophys Res Commun. 2000;267:619–625. doi: 10.1006/bbrc.1999.1989. [DOI] [PubMed] [Google Scholar]
- 26.Janciauskiene S, Ahren B. Different sensitivity to the cytotoxic action of IAPP fibrils in two insulin-producing cell lines, HIT-T15 and RINm5F cells. Biochem Biophys Res Commun. 1998;251:888–893. doi: 10.1006/bbrc.1998.9574. [DOI] [PubMed] [Google Scholar]
- 27.Schubert D, Behl C, Lesley R, et al. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci U S A. 1995;92:1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker HM, Rydel RE, Wright S, Estus S. Human amylin induces “apoptotic” pattern of gene expression concomitant with cortical neuronal apoptosis. J Neurochem. 1998;71:506–516. doi: 10.1046/j.1471-4159.1998.71020506.x. [DOI] [PubMed] [Google Scholar]
- 29.D'Alessio DA, Verchere CB, Kahn SE, et al. Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes. 1994;43:1457–1461. doi: 10.2337/diab.43.12.1457. [DOI] [PubMed] [Google Scholar]
- 30.Andrikopoulos S, Verchere CB, Terauchi Y, Kadowaki T, Kahn SE. Beta-cell glucokinase deficiency and hyperglycemia are associated with reduced islet amyloid deposition in a mouse model of type 2 diabetes. Diabetes. 2000;49:2056–2062. doi: 10.2337/diabetes.49.12.2056. [DOI] [PubMed] [Google Scholar]
- 31.Zraika S, Dunlop M, Proietto J, Andrikopoulos S. The hexosamine biosynthesis pathway regulates insulin secretion via protein glycosylation in mouse islets. Arch Biochem Biophys. 2002;405:275–279. doi: 10.1016/s0003-9861(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 32.Hull RL, Zraika S, Udayasankar J, et al. Inhibition of glycosaminoglycan synthesis and protein glycosylation with WAS-406 and azaserine result in reduced islet amyloid formation in vitro. Am J Physiol Cell Physiol. 2007;293:C1586–C1593. doi: 10.1152/ajpcell.00208.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisilevsky R, Szarek WA, Ancsin JB, et al. Inhibition of amyloid A amyloidogenesis in vivo and in tissue culture by 4-deoxy analogues of peracetylated 2-acetamido-2-deoxy-alpha- and beta-d-glucose: implications for the treatment of various amyloidoses. Am J Pathol. 2004;164:2127–2137. doi: 10.1016/s0002-9440(10)63771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aitken JF, Loomes KM, Konarkowska B, Cooper GJ. Suppression by polycyclic compounds of the conversion of human amylin into insoluble amyloid. Biochem J. 2003;374:779–784. doi: 10.1042/BJ20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green JD, Goldsbury C, Kistler J, Cooper GJ, Aebi U. Human amylin oligomer growth and fibril elongation define two distinct phases in amyloid formation. J Biol Chem. 2004;279:12206–12212. doi: 10.1074/jbc.M312452200. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004;279:765–771. doi: 10.1074/jbc.M307907200. [DOI] [PubMed] [Google Scholar]
- 37.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Hull RL, Vidal J, Cnop M, Kahn SE. Islet amyloid develops diffusely throughout the pancreas before becoming severe and replacing endocrine cells. Diabetes. 2001;50:2514–2520. doi: 10.2337/diabetes.50.11.2514. [DOI] [PubMed] [Google Scholar]
- 39.Zraika S, Hull RL, Udayasankar J, et al. Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes. 2007;56:304–310. doi: 10.2337/db06-0430. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Li X, Epstein PN. MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes. 2005;54:1437–1446. doi: 10.2337/diabetes.54.5.1437. [DOI] [PubMed] [Google Scholar]
- 41.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes. 2004;53:989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 42.Leuner K, Hauptmann S, Abdel-Kader R, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 43.Kaneto H, Kajimoto Y, Miyagawa J, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zraika S, Aston-Mourney K, Laybutt DR, et al. The influence of genetic background on the induction of oxidative stress and impaired insulin secretion in mouse islets. Diabetologia. 2006;49:1254–1263. doi: 10.1007/s00125-006-0212-9. [DOI] [PubMed] [Google Scholar]
- 46.Kooptiwut S, Kebede M, Zraika S, et al. High glucose-induced impairment in insulin secretion is associated with reduction in islet glucokinase in a mouse model of susceptibility to islet dysfunction. J Mol Endocrinol. 2005;35:39–48. doi: 10.1677/jme.1.01720. [DOI] [PubMed] [Google Scholar]