Abstract
Biogenic amines are implicated in several mental disorders, many of which involve social interactions. Simple model systems, such as crustaceans, are often more amenable than vertebrates for studying mechanisms underlying behaviors. Although various cellular responses of biogenic amines have been characterized in crustaceans, the mechanisms linking these molecules to behavior remain largely unknown. Observed effects of serotonin receptor agonists and antagonists in abdomen posture, escape responses, and fighting have led to the suggestion that biogenic amine receptors may play a role in modulating interactive behaviors. As a first step in understanding this potential role of such receptors, we have cloned and fully sequenced two serotonin receptors, 5-HT1Mac and 5-HT2Mac, from the CNS of the freshwater prawn Macrobrachium rosenbergii, and have mapped their CNS immunohistochemical distribution. 5-HT1Mac was found primarily on the membranes of subsets of cells in all CNS ganglia, in fibers that traverse all CNS regions, and in the cytoplasm of a small number of cells in the brain, circum- and subesophageal ganglia (SEG), most of which also appear to contain dopamine. The pattern of 5-HT2Mac immunoreactivity was found to differ significantly, being found mostly in the central neuropil area of all ganglia, in glomeruli of the brain’s olfactory lobes, and in the cytoplasm of a small number of neurons in the SEG, thoracic and some abdominal ganglia. The observed differences in terms of localization, distribution within cells, and intensity of immunoreactive staining throughout the prawn’s CNS suggest that these receptors are likely to play different roles.
Keywords: aggressive behavior, dominance, serotonin, 5-HT type 1 and type 2 receptors, crustacean
Introduction
Serotonin, a biogenic amine that functions as a neurotransmitter and neurohormone, has been linked to several human disorders, such as alcoholism, anxiety, major depression, suicidal behavior, and aggression (Mann, 1999; Brummer et al., 2000). It has also been associated with various forms of social behaviors in a wide and diverse range of vertebrate and invertebrate species (Linnoila and Virkkunen, 1992; Edwards and Kravitz, 1997; Huber et al., 1997a, b; Stadler et al., 2007).
The neural mechanisms underlying social or interactive behaviors has been the focus of interest of studies conducted in several vertebrate model systems such as fishes (Adams et al., 1996; Lynn et al., 2007), chickens (Dennis et al., 2008), mice (Caramaschi, 2007; Faccidomo et al., 2008), rats (Nikulina, 1991; Albert et al., 2008), silver fox (Popova et al., 1991), prairie voles (Villalba et al., 1997; Insel et al., 1998; Young et al., 1998), dogs (van den Berg et al., 2008), and monkeys (Jarrell et al., 2008). One difficulty with vertebrate model systems, however, has been the complexity of their behaviors and their underlying neural circuitries. Thus, comparable simpler model systems, like invertebrates, have been used to better understand the circuitries involved in the regulation and modulation of social behaviors.
In crustaceans, serotonin has been shown to play important roles in feeding circuits (Ayali and Harris-Warrick, 1999), regulation of heart rate (Florey and Rathmayer, 1978), locomotion (Tierney and Mangiamele, 2001; Tierney et al., 2004), swimmeret beating (Barthe et al., 1993), abdominal posture (Livingstone et al., 1980; Harris-Warrick and Kravitz, 1984; Huber et al., 1997a,b; Tierney and Mangiamele, 2001; Tierney et al., 2004), claw opening (Qian and Delaney, 1997), escape circuits (Glanzman and Krasne, 1983; Yeh et al., 1996, 1997), and fighting behavior (Kravitz, 1993; Edwards and Kravitz, 1997; Sosa and Baro, 2002).
Although there is ample evidence linking serotonin to interactive behaviors in both vertebrates and invertebrates, the mechanisms by which this monoamine acts to modulate such behaviors remain largely unknown. One possible mode of action is through changes in receptor expression, distribution and/or function. A vertebrate serotonin type 1 receptor agonist has been shown to inhibit the LG neurons in dominant and subordinate crayfish, whereas a vertebrate serotonin type 2 receptor agonist enhances the LG neurons’ responses in both types of crayfish (Yeh et al., 1996, 1997). Based on these data, Yeh et al. (1996, 1997) have suggested that changes in social status may involve differences in the level of activity of specific aminergic receptor subtypes. It has also been shown that injection of vertebrate agonists for specific serotonin receptors can induce serotonin-like or dominant postures in the crayfish (Tierney and Mangiamele, 2001; Tierney et al., 2004). Preliminary experiments carried out in our laboratory have shown the increased aggression normally observed following injection of serotonin in submissive freshwater prawns (Sosa and Baro, 2002) can be blocked when serotonin is injected together with a 5-HT2, but not a 5-HT4, vertebrate antagonist.
There are multiple receptor subtypes that differ in affinity for serotonin, their location and action in the nervous system, and their pharmacology. Mammalian serotonin receptors are classified into seven classes: six are G-protein-coupled receptors (5-HT1, 5-HT2, 5-HT4, 5-HT5, 5-HT6, 5-HT7), and one is a ligand-gated ion channel (5-HT3) (Kroeze et al., 2002). Several serotonin receptors from various invertebrate species were cloned before those of crustaceans. Among these are the serotonin receptor of the fly (P28285, Saudou et al. 1992), pond snail (Q25414, Sugamori et al., 1993), silkworm and tobacco budworm (NP_001037502, Q25190, Von Nickisch-Rosennegk et al., 1996), nematode (AAB66360, Olde and McCombie, 1997), yellow fever mosquito (AF296125, Pietrantonio et al., 2001), sea hare (AAM46088, Barbas et al., 2002), swallowtail butterfly (AB182632, Ono & Yoshikawa, 2004), and tick (AAQ89933, Chen et al., 2004). The first partial sequences for two crustacean type 1 and type 2 serotonin receptors were cloned from the freshwater prawn by our laboratory (Sosa and Baro, 2002; AAS18606, Sosa et al., 2004), shortly thereafter followed by the cloning of one crayfish (AAS18608, partial sequence: Sosa et al., 2004) and two lobster (AAS18607, AAS57919, full sequence: Sosa et al., 2004; Clark et al., 2004) receptors. Since then, other serotonin receptors from various invertebrates have also been partially or fully cloned, including greasyback shrimp (AY462417, AY462418; Tiu et al., 2005), sea hare (AF372526; Barbas et al. 2005), tobacco hawkmoth (DQ840515, DQ840516; Dacks et al., 2006), black tiger shrimp (AY661549; Ongvarrasopone et al., 2007), pond snail (AY395746.1, AY395747.1; Mapara et al., 2008), and crayfish (ABX10972, ABX10973, Spitzer et al., 2008).
As an initial step in determining whether differences in distribution, expression, or function of serotonin receptors may play a role in modulating interactive behaviors in the prawn, we have cloned and fully sequenced two prawn serotonin receptors (5-HT1Mac and 5-HT2Mac) and have mapped their distribution throughout the animal’s CNS. This information is essential for laying down the groundwork to further develop the freshwater prawn as a model system in which to study the neural basis of aggression at the circuit / cellular / molecular level. A preliminary immunocytochemical localization of 5-HT1Mac in prawn thoracic ganglia has appeared previously (Sosa et al., 2004), comparing its pattern of expression with that of the crayfish. Here we greatly expand the immunohistochemical study to show a detailed map of 5-HT1Mac and 5-HT2Mac immunoreactivity throughout the entire ventral nerve cord of the prawn, and discuss how these compare with each other and with maps of similar receptors in other species.
Materials and Methods
Experimental animals and dissection
The freshwater prawn Macrobrachium rosenbergii is a crustacean model system, characterized by adults with three claw morphotypes that differ in color, relative size and spination, each of which correlates with a fixed dominance status and other defining features of their modes of interaction with others, including territoriality and courtship behavior (Ra’anan & Cohen, 1985; Ra’anan & Sagi, 1985; Kuris et al., 1987; Barki et al., 1991a; Barki et al., 1991b; Barki et al., 1992). The fixed nature of the dominance hierarchies established by the prawn increase the likelihood of detecting cellular or molecular changes that may underlie the observed differences in behavior among male morphotypes with differing dominance status.
Specimens of the three male morphotypes of M. rosenbergii were obtained from the Lajas Aquaculture Experimental Station of the University of Puerto Rico (UPR), Mayagüez Campus. They were housed in tanks with continuous filtration and aeration at the UPR Medical Sciences Campus Institute of Neurobiology Animal Care Facility. Temperature in the tanks was maintained at 29°C and the pH was adjusted to 7.4. Animals were fed a high protein (>40%) pelleted purina chow once every other day.
For dissection, prawns were immobilized by cooling on ice prior to dissection. After being weighed and measured, they were transected between the thorax and abdomen. Their claws, walking legs, and carapace were removed and one segment of the animal was placed in cold (4°C) prawn saline solution (PSS), while the other segment was dissected. The PSS had the following composition (in mM); NaCl 220; KCl 5.5, CaCl2 13.5; MgCl2 2.5; Tris 5; pH=7.4 (Miller et al., 1985). The dissection was carried out on ice. The thoracic and abdominal ventral nerve cord and the brain were isolated quickly with forceps by removing all surrounding organs and muscles and cutting all nerves.
All procedures involving the use of animals were approved by the University of Puerto Rico Medical Sciences Campus Institutional Animal Care and Use Committee (IACUC) prior to the start of the experiments.
RACE to obtain the 5-HT1Mac and 5-HT2Mac termini
On the basis of the partial sequences of the crustacean 5-HT type 1 and type 2 receptors that had previously been cloned in our laboratory (Sosa and Baro, 2002, Sosa et al., 2004), rapid amplification of cDNA ends (RACE) was performed to obtain the full sequence of each of these receptors in the prawn. For the 5-HT type 1 receptor, 5-HT1Mac, prawn’s mRNA was purified from CNS total RNA with Oligotex (Qiagen, Chatsworth, CA). 5’ and 3’ RACE reactions were performed with the SMART RACE amplification kit (BD Biosciences, San José, CA) according to the manufacturer’s instructions. RACE products were cloned with TOPO TA or TURBO cloning kits (Invitrogen, San Diego, CA) and sequenced at the Cornell University Life Sciences Core Laboratories Center. Data analysis and alignments were performed with VectorNTI Advance 10 software (Invitrogen). For the 5-HT2Mac receptor, prawn mRNA was purified from CNS total RNA using a RNAqueous Kit (Ambion, Austin, TX). 5’ and 3’ RACE reactions were performed with LAtaq enzyme (Takara, Madison, WI) in 50 µl volumes according to the manufacturer’s instructions. The primers used in the RACE reactions are listed in Table 1. Resulting RACE products were cloned with TOPO TA cloning kit (Invitrogen) and sequenced at the Cornell University Life Sciences Core Laboratories Center.
Table 1.
Primers used for amplification and cloning of 5-HT1Mac and 5-HT2Mac
| Oligo Name | Sequence |
|---|---|
| Lu4TRsa | 5’-CGACGTGGACTATCATGAACGCACGCAGTCGGTAC(T)13-3’ |
| NSLu4 | 5’-TCGAGCGGCCGCCCGGGCAGGTCGACGTGGACTATCCATGACGCA-3’ |
| For 5HT1Mac-6 | 5’-CCCAACGGCATCACGGAACACCAAACCA-3’ |
| NFor 5HT1Mac-8 | 5’-CCCACGTCAGCGAGGTCTCCAGAATGGA-3’ |
| For 5HT1Mac-10 | 5’-CTTCGTCACCGCCCTCCTCATGCCCATC-3’ |
| Rev 5HT1Mac-9) | 5’-GCCTTCTTCTCCCGCTTGGCTTCGAGGG-3’ |
| NRev 5HT1Mac-7) | 5’-CTGACGTGGGACGAGACGGAGACCGCCC-3’ |
| NRev 5HT1Mac-5 | 5’-ATGCCGTTGGGTTTGTCTCCGCTTCTGGG-3’ |
| For 5HT2Mac-1 | 5’-GCGGAGGCCGCCCTTAGTTAGGT-3’ |
| For 5HT2Mac-3 | 5’-GGTATTCCGTAAGAGAGCA-3’ |
For=forward; NFor=nested forward; Rev=reverse; NRev=nested reverse; Lu4TRsa=3’RACE adapter; NSLu4=nested 5’and 3’RACE adapter
BLAST analysis followed by paired alignments with invertebrate and vertebrate serotonin type 1 and type 2 receptors, as well as with other biogenic amine receptors, was conducted to confirm identity of the prawn’s two serotonin receptors. A phylogenetic tree was generated using default parameters and 10,000 iterations of the maximum likelihood algorithm implemented in the program TREE-PUZZLE (Schmidt et al., 2002; http://www.tree-puzzle.de). The initial multiple alignment was done using ClustalX (Thompson et al., 1997; Jeanmougin et al., 1998) with default parameters. All sequences were timed to include only the core part of the proteins along with additional gaps removed manually in GeneDoc (Nicholas et al., 1997) prior to tree construction. Numbers at branches represent bootstrap values for 10,000 iterations. Branch-length scale bar represents 0.1 amino acid substitutions per site. The graphical output was generated using Treeview (Page, 1996). Transmembrane domains for both receptor sequences were determined using SOSUI engine ver. 1.11 (http://www.expasy.org/cgi-bin&scanprosite (http://bp.nuap.nagoya-u.ac.jp/sosoui).
Antibody Characterization
For Serotonin (5-HT), a commercially produced polyclonal rabbit antibody raised against serotonin coupled to bovine serum albumin (BSA) with paraformaldehyde was used (Table 2). The resulting pattern of staining was consistent with that observed previously in other crustacean species such as crayfish and lobster (Beltz and Kravitz, 1983; Real and Czternasty, 1990; Beltz, 1999; Harzsch and Waloszek, 2000; Harzsch, 2003). 5-HT staining in the prawn’s ventral nerve cord was eliminated by preincubation of the diluted antibody (1:10,000) with 25 ug/ml of serotonin/BSA.
Table 2.
Primary antibodies used
| Antigen | Host | Source | Dilution |
|---|---|---|---|
| Serotonin (5-HT) | Rabbit | ImmunoStar, Hudson, WI (cat #20080, lot #051007) | 1:10,000 |
| 5-HT1crust receptor | Rabbit | Bethyl Laboratories, Montgomery, TX (lot #971314/1) | 5 ug/ml |
| 5-HT2crust receptor | Rabbit | Bethyl Laboratories, Montgomery, TX (lot #970304/1) | 5 ug/ml |
| Tyrosine Hydroxylase (TH) | Mouse | ImmunoStar, Hudson, WI (cat #22941, lot #21048) | 1:200 |
For the 5-HT receptors, two affinity-purified rabbit polyclonal antibodies were synthesized to order by a private company against portions of the 5-HT type 1 and type 2 receptor sequences that are conserved across the prawn and lobster (and also crayfish in the case of the type 1 receptor) (Sosa et al., 2004; Clark et al., 2004). Since these antibodies recognize these receptors in more than one crustacean species, they have been called anti-5-HT1crust and anti-5-HT2crust (Table 2). The immunogen for the 5-HT1crust receptor was the synthetic peptide KDPDFLVRVNEHKKCLVSQD (amino acids 366–385 from the N-terminus; see Fig. 1). This antiserum recognized the expected band at roughly 52–55 kD molecular weight on Western blots of prawn nervous tissue, with elimination of this band when the antiserum was preabsorbed with the immunogen peptide in a 1:1 proportion (w:w), as shown previously (Sosa et al., 2004). Immunostaining was also eliminated by preincubation of the diluted antibody (5ug/ml) with the immunogen peptide in a 1:20 mixture (w/w). The immunogen for the 5-HT2crust receptor was the synthetic peptide DRFLSLRYPMKFGRHKTRRR (amino acids 190–209 from the N-terminus; see Fig. 2). The published sizes of 5-HT2 orthologs vary with the species from 47–95 kD, most likely because of differences in the third intracellular or cytoplasmic loop (i3), as well as variations in lengths of the amino termini. The antiserum for the 5-HT2crust receptor recognized the expected band at roughly 79–83 kD molecular weight on Western blots of prawn nervous tissue, with elimination of this band when the antiserum was preabsorbed with the immunogen peptide in a 1:1 proportion (w:w), as shown in Figure 4A. Immunostaining was also eliminated by preincubation of the diluted antibody (5ug/ml) with the immunogen peptide in a 1:20 mixture (w/w). These data demonstrate that the antibodies for 5-HT1Crust and 5-HT2Crust are specific for the 5-HT1Mac and 5-HT2Mac receptor proteins, respectively.
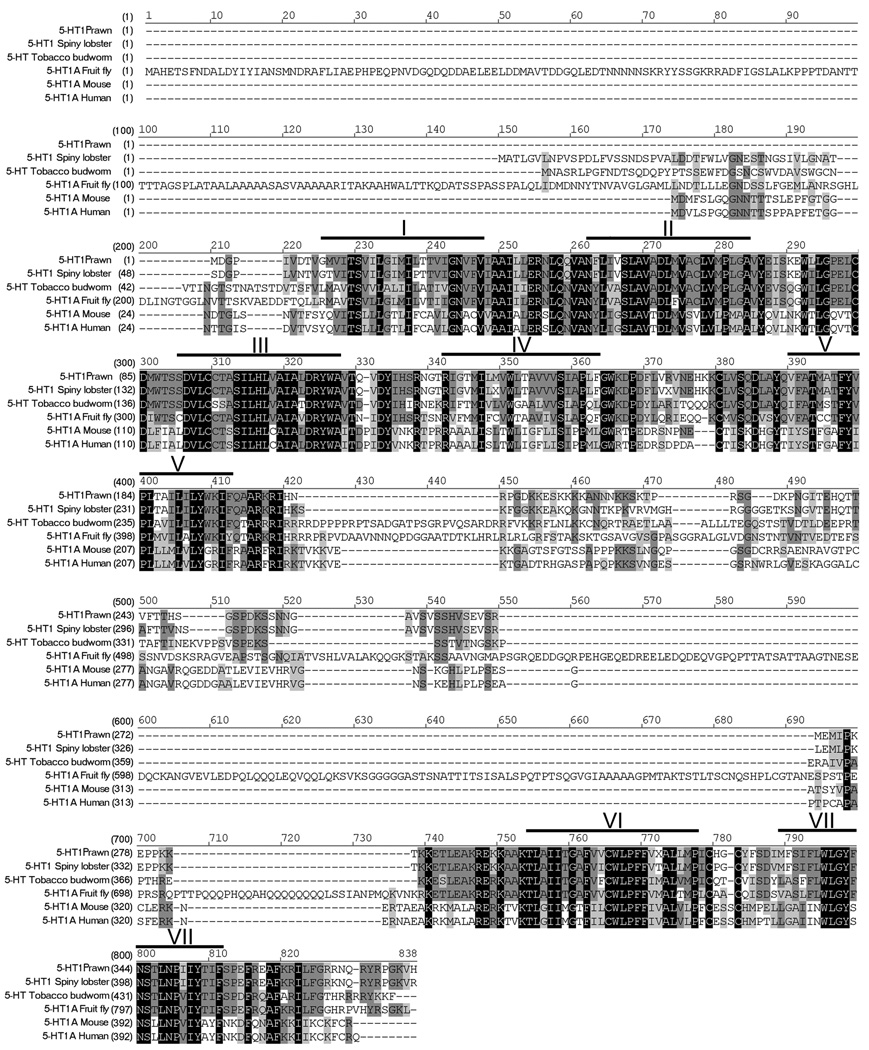
Figure 1. Alignment of 5-HT1Mac receptor.
The amino acid sequence of 5-HT1Mac receptor is aligned with the most highly homologous proteins found in each order. Heavy lines and roman numerals above the sequence indicate transmembrane regions 1 through 7. Black boxes with white letters represent areas of identical regions, dark gray boxes with black letters represent conserved regions, and light gray boxes with black letters represent blocks of similar regions. Accession numbers are: prawn, EU363466; lobster (Decapoda), AAS18607; tobacco budworm (Lepidoptera), CAA64863; fruit fly (Diptera), NP_476802; mouse (Rodentia), NP_032334; human (Primate), NP_000515.
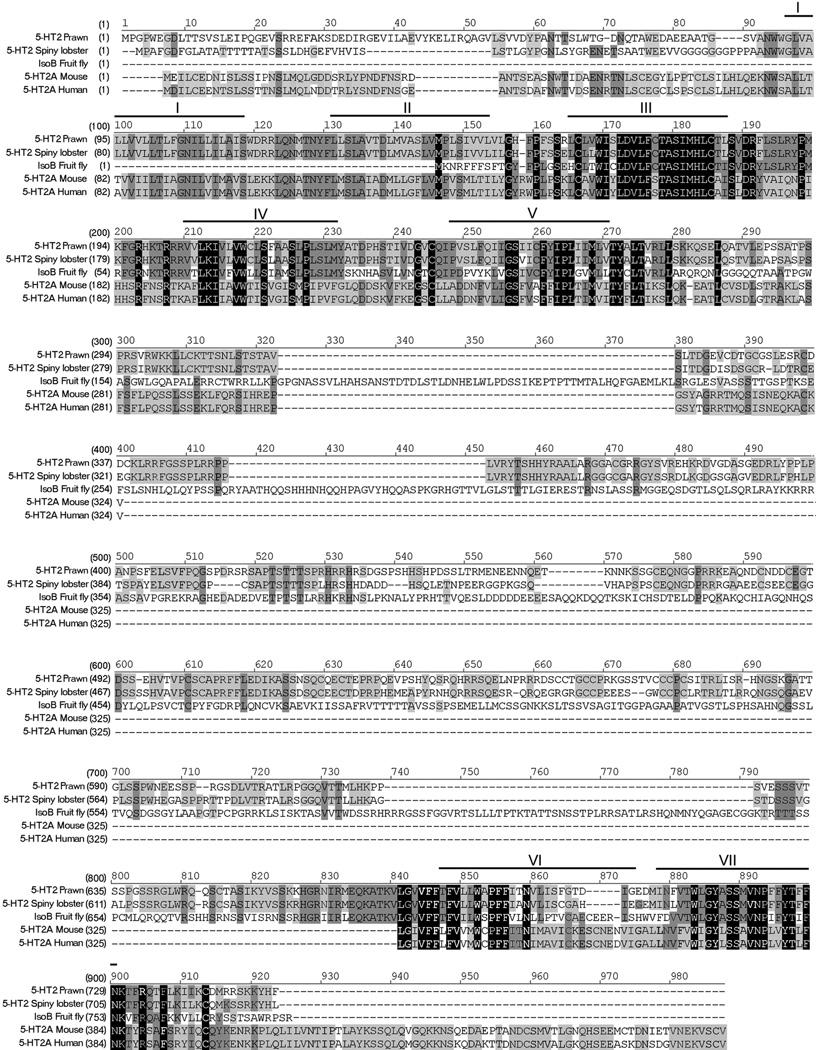
Figure 2. Alignment of 5-HT2Mac receptor.
The amino acid sequence of 5-HT2Mac receptor is aligned with the most highly homologous proteins found in each. Heavy lines and roman numerals above the sequence indicate transmembrane regions 1 through 7. Black boxes with white letters represent areas of identical regions, dark gray boxes with black letters represent conserved regions, and light gray boxes with black letters represent blocks of similar regions. Accession numbers are: prawn, EF033662; lobster, AAS57919; fruit fly, NP_649806; mouse, NP_766400; human, NP_000612.
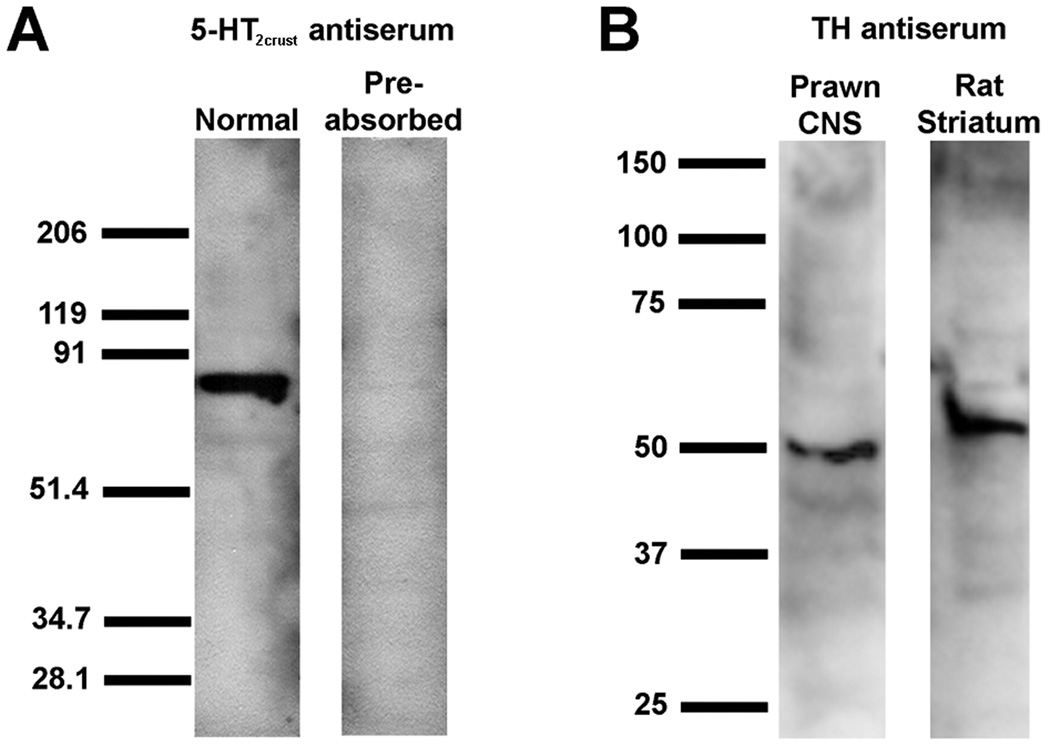
Figure 4. Anti-5-HT2crust and Anti-TH antibody specificity.
A: A representative Western blot experiment showing that the anti-5-HT2crust antibody recognizes the 5-HT2Mac receptor in the prawn nervous system. The blots containing a protein extract from the nervous system of the prawn were probed with anti-5-HT2crust or the same antibody preabsorbed with the peptide antigen used to generate the antibody. Molecular weight markers (numbers to the left) are in kD. The antibody produces a signal of the predicted size of the protein. This signal is lost when the antibody is first preabsorbed. B: A representative Western blot experiment showing that the anti-TH antibody recognizes the TH in the prawn nervous system. Blots containing a protein extract from the nervous system of the prawn and a protein extract from rat striatum (positive control) were probed with anti-TH. Molecular weight markers (numbers to the left) are in kD. The antibody produces a signal of the predicted size of the protein in both lanes.
For Tyrosine Hydroxylase (TH), a commercially produced mouse monoclonal antibody raised against full-length TH purified to homogeneity from rat PC12 cells was used (Table 2). The resulting pattern of staining in the prawn was consistent with that observed previously for tyrosine hydroxylase and/or dopamine in other crustacean species such as crayfish (Tierney et al., 2003; Alvarez-Alvarado et al., 2005), lobster (Cournil et al., 1994, 1995; Schmidt and Ache, 1997) and blue crab (Fort et al, 2004). Although no information is available on the specific protein sequence of crustacean TH, the published sizes of TH in both vertebrate and invertebrate (mostly insect) species vary from 45–75 kD. The TH antiserum used here recognized an expected band at roughly 50–55 kD molecular weight on Western blots of rat striatum, a rich source of TH (positive control; Wolf and Kapatos, 1989), and at roughly 48–50 kD molecular weight on Western blots of prawn nervous tissue, as shown in Figure 4B.
Immunocytochemical procedures
Ventral nerve cords were removed in PSS, immediately fixed by immersion in freshly prepared 4% paraformaldehyde in 0.1M PBS at room temperature for 1 hour with constant shaking, and rinsed overnight in phosphate buffered saline (PBS) containing 2% Triton X-100 and 0.1% sodium azide (PTA). Preparations were then incubated in normal goat serum (1:20) at 4°C for 3–5 hours, followed by the primary antibody for 5-HT, Tyrosine hydroxylase (TH), 5-HT1crust, and/or 5-HT2crust,, at a concentration or dilution of 1:10,000 for 5-HT, 1:200 for TH, and 5 µg/ml for 5-HT1crust, or 5-HT2crust, for 2–3 days. TH is the precursor synthesizing enzyme for dopamine and anti-TH antibodies have been shown to label primarily dopaminergic neurons in insects and crustaceans (Cournil et al., 1994; Neckameyer et al., 2000; Pulver and Marder, 2002; Pulver et al., 2003; Tierney et al., 2003; Alvarez-Alvarado et al., 2005). For double-labelling experiments, preparations were incubated simultaneously with two primary antibodies, TH and one of the antibodies for crustacean serotonin receptors. After washing 6 times, 30 minutes each, with PTA, tissues were incubated in the secondary antibody (goat anti-rabbit Alexa 488 and/or goat anti-mouse Alexa 594; Molecular Probes, Carlsbad, CA) in a 1:200 dilution at 4°C overnight. Preparations were washed in 0.1M PTA 6 times, 1 hour each, washed in PBS for 1 hour, and left overnight in 90% glycerol/PBS buffer. The next day they were mounted in Polyaquamount, coverslipped, and viewed with a Zeiss Axioskop using epifluorescent excitation and/or a Zeiss LSM Pascal confocal microscope. The brightness and contrast of the digital images obtained by the Zeiss LSM Pascal software were adjusted so that they’d be uniform in the figures presented here. Confocal stacks were reconstructed and analyzed in Adobe Photoshop 7.0 on a Dell PC computer. Colors were digitally adjusted to represent 5-HT1Mac ir or 5-HT2Mac ir as green in all dual-color images for consistency and clarity. Controls included preadsorption of the primary antibody with the peptide used to generate the antibody for 2 hr at room temperature before incubation with the preparation (peptide to antibody: 1:20, w/w).
Results
Sequence Analysis of 5-HT1Mac and 5-HT2Mac clones
The full length sequence for the 5-HT1Mac receptor encodes for a putative 381 amino acids protein and is shown in Figure 1, aligned with the most highly homologous proteins found in each class and order, namely 5-HT type 1 receptors of lobster, tobacco budworm, fruit fly, mouse and human. The predicted 5-HT2Mac receptor encodes for a 752 amino acids protein, almost twice as long as the 5-HT1Mac receptor, and is shown in Figure 2 aligned with other invertebrate (arthropod) and vertebrate (mammalian) 5-HT type 2 receptors.
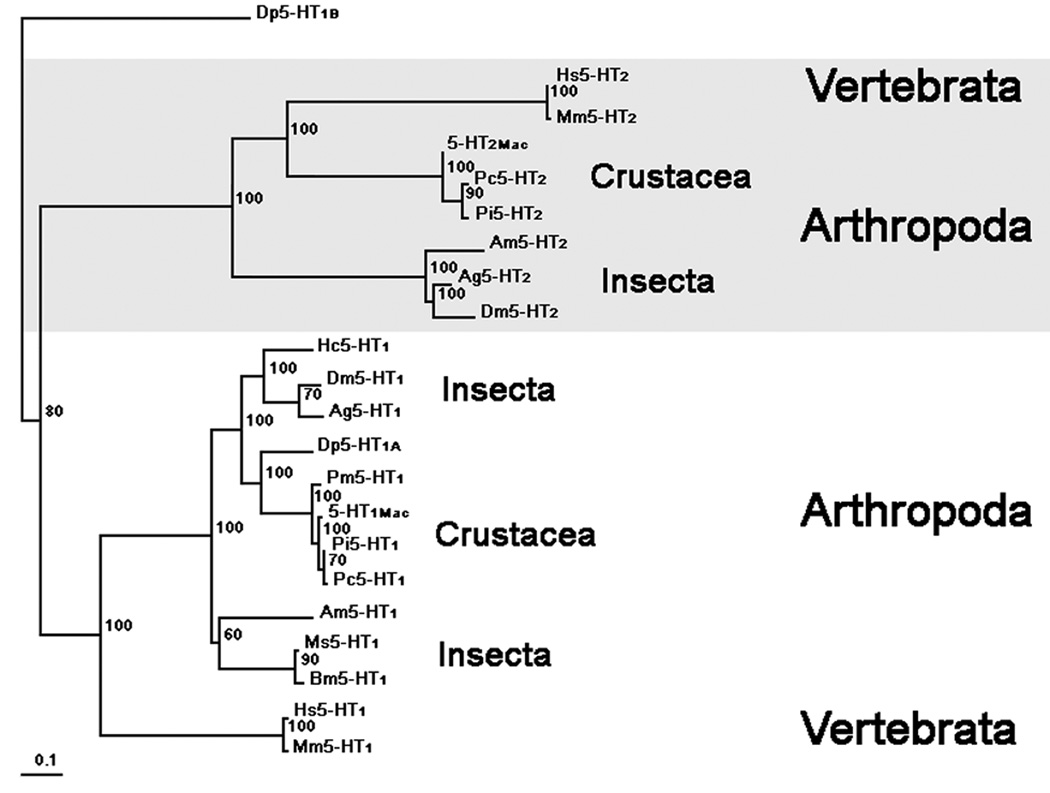
Our phylogenetic analysis (Fig. 3) shows that the putative prawn, 5-HT1Mac receptor shares highest identity with the 5-HT1 receptor gene family with high bootstrap support. Furthermore, all the crustacean 5-HT1 receptors cluster together, including our prawn 5-HT1Mac, receptor. Daphnia pulex, the common waterflea and the most basal crustacean in this analysis, contains two putative 5-HT receptors, Dp5-HT1A and Dp5-HT1B. This Dp5-HT1A receptor appears more basal compared to the more derived crustacean 5-HT1 receptors. The other putative Daphnia receptor Dp5-HT1B was used as an outgroup and shares highest identity to a 5-HT7-like receptor. Our analysis also supports two clades of 5-HT1 receptors for insects, as reported by Dacks et.al., 2006. Molecular studies by Dacks et.al. (2006) showed that the putative Manduca sexta Ms5-HT1A and Ms5-HT1B were split into two clades for insects 5-HT1 receptors. Interestingly, the prawn’s 5-HT1Mac receptor, as well as all crustacean 5-HT1 receptors, appears to be a 5-HT1A-like receptor and share highest identity with the 5-HT1A-like insect receptors. Our phylogenetic analysis shows that the putative prawn 5-HT2Mac receptor shares highest identity with the 5-HT2 receptor gene family, also with high bootstrap support. As shown above, all crustacean 5-HT2 receptors cluster together. It appears that the insect clade of 5-HT2 receptors may be more basal to the clade with the crustacean 5-HT2 receptors. It can thus be concluded from our phylogenetic analysis that the prawn’s two putative 5-HT receptors, 5-HT1Mac and 5-HT2Mac belong to different subtypes of 5-HT receptors.
Figure 3. Phylogenetic tree.
Evolutionary relationship of the prawn’s 5-HT1Mac and 5-HT2Mac receptors with 5-HT receptors of other species showing highest homology. The phylogenetic tree was generated using default parameters and 10,000 iterations of the maximum likelihood algorithm implemented in the program TREE-PUZZLE (Schmidt et al., 2002; http://www.tree-puzzle.de). The initial multiple alignment was done using ClustalX (Jeanmougin et al., 1998; Thompson et al., 1997) with default parameters. All sequences were timed to include only the core part of the proteins along with additional gaps removed manually in GeneDoc (Nicholas et al., 1997) prior to tree construction. Numbers at branches represent bootstrap values for 10,000 iterations. Branch-length scale bar represents 0.1 amino acid substitutions per site. The graphical output was generated using Treeview (Page, 1996). The name, abbreviation and accession number for all species used in this analysis are: Macrobrachium rosenbergii, 5-HT1Mac, EU363466; Daphnia pulex, Dp5HTR1B, jgi|Dappu1|23299|gw1.13.237.1; Daphnia pulex, Dp5HTR1A, jgi|Dappu1|42951|e_gw1.5.342.1; Penaeus monodon, Pm5HTR1, AAV48573.1; Heliothis virescens, Hv5HTR1, CAA64863.1; Panulirus interruptus, Pi5HTR1,AAS18607.1; Procambarus clarkii, Pc5HTR1, ABX10973.1; Apis mellifera, Am5HTR1, XP_393915.3; Anopheles gambiae, Ag5HTR1, XP_308623.4; Manduca sexta, Ms5HTR1A, ABI33826.1; Manduca sexta, Ms5HTR1B, ABI33827.1; Drosophila melanogaster, Dm5HTR1, NP_476802; Mus musculus, Mm5HTR1, NP_032334; Homo sapiens, Hs5HTR1A, NP_000515; Macrobrachium rosenbergii, 5-HT2Mac, EF033662; Panulirus interruptus, Pi5HTR2, AAS57919; Drosophila melanogaster, Dm5HTR2, NP_649806; Apis mellifera, Am5HTR2, XP_394798; Anopheles gambiae, Ag5HTR2, XP_307953; Procambarus clarkii, Pc5HTR2, ABX10972.1; Mus musculus, Mm5HTR2, NP_766400; Homo sapiens, Hs5HTR1A, NP_000612.
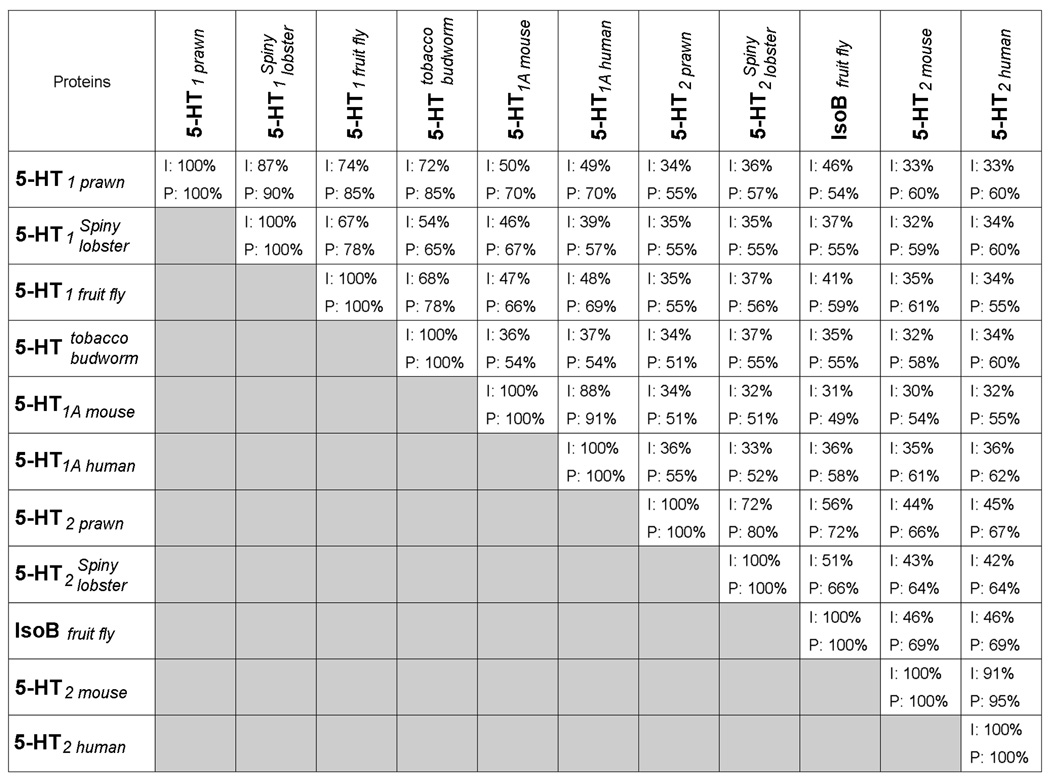
Table of Conservation analysis (Table 3) indicates that the putative 5-HT1Mac receptor is most closely related to the lobster 5-HT1Pan receptor, followed by the fruit fly 5-HT1A receptor and the tobacco budworm 5-HT receptor. It is also homologous to the mouse 5-HT1 receptor and the human 5-HT1A receptor. The putative 5-HT2Mac receptor is most closely related to the lobster 5-HT2Pan receptor and the fruit fly IsoB receptor (isoform B of the annotated Drosophila receptor CG8007), followed by the mouse 5-HT2 receptor, and the human 5-HT2A receptor (Table 3). It also shows a low identity between the prawn’s 5-HT1Mac and 5-HT2Mac receptors (Table 3).
Table 3.
Table of conservation. Amino acid identities (I:top) and positives (P:bottom) from Blast 2 Sequences for the prawn proteins (5-HT1Mac and 5-HT2Mac) and other crustacean, insect and mammalian related proteins. In BLAST, the term “identity” refers to the percent of amino acid residues in the alignment which are identical, a measure of the extent to which compared sequences are invariant. The term “positive” refers to the percent of amino acid residues for which the alignment scores (the sum of the scoring matrix values in each segment pair) have positive values, a measure of the similarity between sequences.
 |
Both the putative 5-HT1Mac and 5-HT2Mac receptors have the typical G-coupled protein receptor (GPCR) transmembrane (TM) signature motifs in the second (S(X)3D(X)6VMP), third (D(X)6SI(X)5DRY/F), fifth (FXXP), sixth (F(X)3WXPFF), and seventh (WXGY(X)2S(X)2NP(X)2Y) hydrophobic domains (reviewed in Kroeze et al., 2002). The transmembrane regions participate in both ligand binding and receptor activation, and many of these conserved residues contribute to those functions. The most highly conserved regions are found at the third, fourth, and fifth transmembrane domains. While the 5-HT1Mac receptor retains the typical DRY motif on its third TM region, Y has been substituted by F in the 5-HT2Mac receptor, as is also the case in the lobster (Clark et al., 2004) and nematode (Huang et al., 2002). As expected, the third intracellular loop in both receptors displays the greatest divergence. Although unconserved, the carboxy terminus (–COOH) is characteristically short, highly charged, and contains a PDZ domain-binding motif like most vertebrate and invertebrate 5-HT receptors (Songyang et al., 1997). The sequence and length of the amino termini (-NH2) of both receptors are also poorly conserved.
5-HT immunoreactivity in the prawn’s CNS
Serotonin immunoreactivity (5-HT ir) in the prawn’s CNS was found to be widely distributed, as has been reported to be the case in similar crustaceans, such as the lobster and crayfish (Beltz & Kravitz, 1983; Real & Czternasty, 1990). It was detected in more than 70 cell bodies, numerous fibers and neuropil regions within the ventral nerve cord (Fig. 5). No qualitative differences were observed in the staining pattern of the prawn’s male morphotypes.
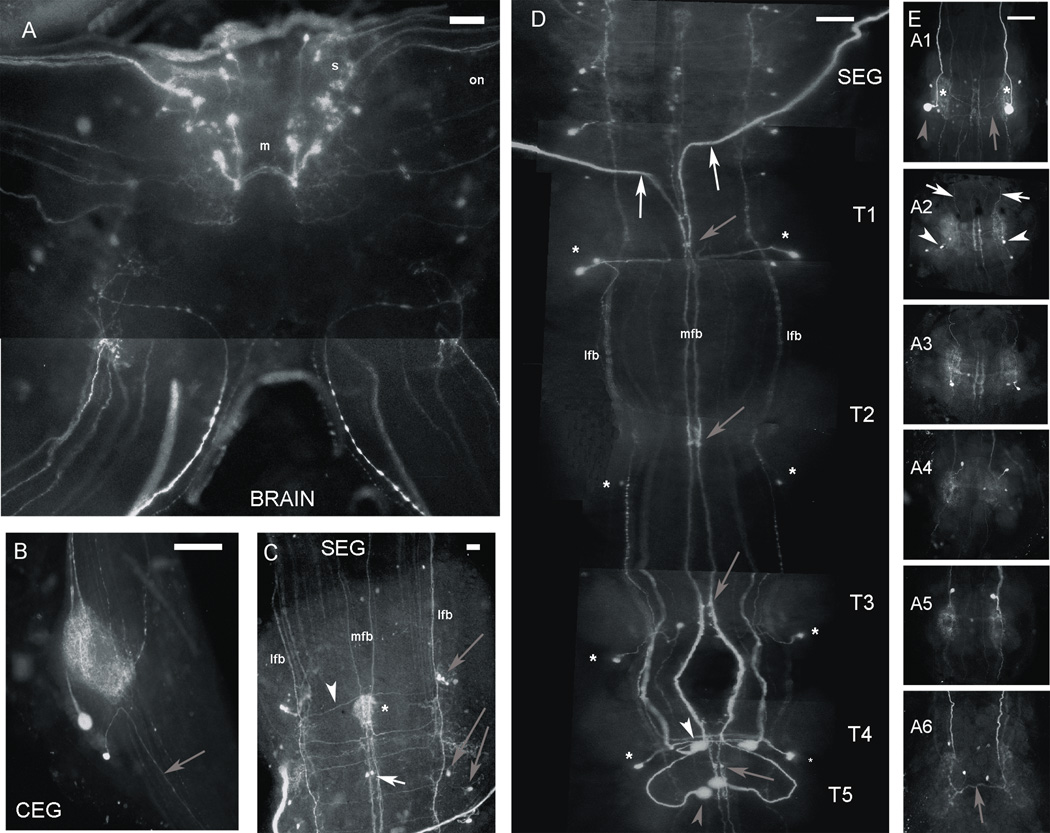
Figure 5. Serotonin immunoreactivity in the CNS of the prawn.
A: View of the brain, containing paired clusters of medium (m: 50–80 µm) and small (s: 20–50 µm) 5-HT immunoreactive (ir) cells located near the midline. The small-sized 5-HT ir cells tended to cluster near the base of the optic stalks, sending axons through the optic nerve (on). B: View of the circumesophageal ganglion (CEG), containing a pair of 5-HT ir cells, both of which sent their axons anteriorly towards the brain (gray arrow), and an extensive network of 5-HT ir terminal arborizations. C: View of the subesophageal ganglion (SEG), containing small bilaterally paired 5-HT ir cells found along the paths of immunoreactive midline and lateral fiber bundles (MFB and LFB, respectively). One pair of cells was consistently found along the MFB (white arrow), while at least three or four cells could be seen along each LFB (gray arrows). Thin 5-HT ir fibers could also be seen bridging the fibers of the LFB and MFB (white arrowhead). An area of dense 5-HT ir terminal arborizations (*) was also consistently labeled in the midline towards the center of the ganglion. D: View of the thoracic (T) ganglia, the first four containing at least one bilateral pair (in some cases two) of small 5-HT ir neurons with cell bodies placed laterally and axons projecting towards the midline (*). The fourth and fifth thoracic ganglia (T4–T5) also had pairs of large neurons, one in the midline (T5; gray arrowhead), the other halfway between the midline and the lateral edge of the ganglion (T4; white arrowhead). The axons of the latter pair of neurons projected horizontally for a short span and then anteriorly between the MFB and LFB. Cell bodies of axons comprising the MFB included two pairs of large 5-HT ir neurons: the T5 large midline neurons (gray arrowhead) and the two larger neurons of the first abdominal ganglion (gray arrowhead in Fig. 5E:A1). At least one pair of thick 5-HT ir fibers that form part of the MFB (white arrows) exited the ventral nerve cord through one of the SEG nerve roots. Two pairs of small 5-HT ir neurons were also consistently found along the MFB of the thoracic ganglia (gray arrows). E: Views of the six abdominal ganglia, each containing at least two pairs of small cells (white arrowheads), with fibers that turn anteriorly (white arrows) and branches that crossed the midline (gray arrows), with a neuropil region located laterally in each hemiganglion (*). The A1 ganglion appeared different only in that one pair of these 5-HT ir neurons was larger in size (gray arrowhead).All images shown were obtained from the ventral nerve cord of a male blue clawed prawn, except C, which was obtained from a male small clawed prawn. Images were obtained with a Zeiss Axioskop fluorescence microscope. (Scale bar = 250 µm for all images, except C, where it represents 100 µm).
The brain contained paired clusters of medium (m: 50–80 µm) and small (s: 20–50 µm) 5-HT ir cells located near the midline (Fig. 5A). The small-sized cells were mostly located near the base of the optic stalks, and some sent their axons towards the optic nerve (on).
Each circumesophageal ganglion (CEG) contained a pair of 5-HT ir cells (Fig. 5B), one medium-sized cell and another smaller cell, both of which sent their axons anteriorly towards the brain (gray arrow). There was also an extensive network of 5-HT ir terminal arborizations, presumably arising from fibers traveling through the connectives of each ganglion.
Small bilaterally paired 5-HT ir cells were found along the paths of immunoreactive midline and lateral fiber bundles (MFB and LFB, respectively) located within the subesophageal ganglion (SEG) (Fig. 5C). One pair of cells was consistently found along the MFB (white arrow), while at least three or four cells could be seen along each LFB (gray arrows). Thin 5-HT ir fibers could also be seen bridging the fibers of the LFB and MFB (white arrowhead). An area of dense 5-HT ir terminal arborizations (*) was also consistently labeled in the midline towards the center of the ganglion.
Each of the first four thoracic ganglia appeared to have at least one bilateral pair (in some cases two) of small 5-HT ir neurons with cell bodies placed laterally and axons projecting towards the midline (*; Fig. 5D). In addition to these small lateral neurons, the fourth and fifth thoracic ganglia (T4-T5) also had pairs of large (>80 µm) neurons, one in the midline (T5; gray arrowhea), the other halfway between the midline and the lateral edge of the ganglion (T4; white arrowhead). The axons of the latter pair of neurons projected horizontally for a short span and then anteriorly between the MFB and LFB. Cell bodies of axons comprising the MFB included two pairs of large 5-HT ir neurons: the T5 large midline neurons (gray arrowhead) and the two larger neurons of the first abdominal ganglion (gray arrowhead in Fig. 5E:A1). At least one pair of thick 5-HT ir fibers that form part of the MFB (white arrows) exited the ventral nerve cord through one (or more) of the SEG nerve roots. No 5-HT ir fibers could be seen exiting thoracic nerve roots. Also, two pairs of small 5-HT ir neurons were consistently found along the MFB of the thoracic ganglia (gray arrows).
The six abdominal ganglia all appeared to have a similar pattern of distribution of 5-HT ir neurons, with the exception of the first ganglion (A1) (Fig. 5E). The general pattern consisted of at least two pairs of small cells (white arrowheads), with fibers that turn anteriorly (white arrow) and branches that crossed the midline (gray arrows), with a neuropil region located laterally in each hemiganglion (*). The A1 appeared different only in that one pair of these 5-HT ir neurons was large in size (gray arrowhead). No 5-HT ir fibers could be seen exiting abdominal nerve roots.
5-HT1Mac immunoreactivity
5-HT1Mac immunoreactivity (ir) (Fig. 6) was observed around somata of specific subsets of neurons in all thoracic and abdominal ganglia and as punctate staining in the neuropil of the brain and all other ganglia. It was also found in a small number of neurons in the brain and the CEG, as well as in fibers that traversed all the ganglia, and terminal arborizations in the brain, CEG and A6. No qualitative differences were observed in the staining pattern of the different male morphotypes.
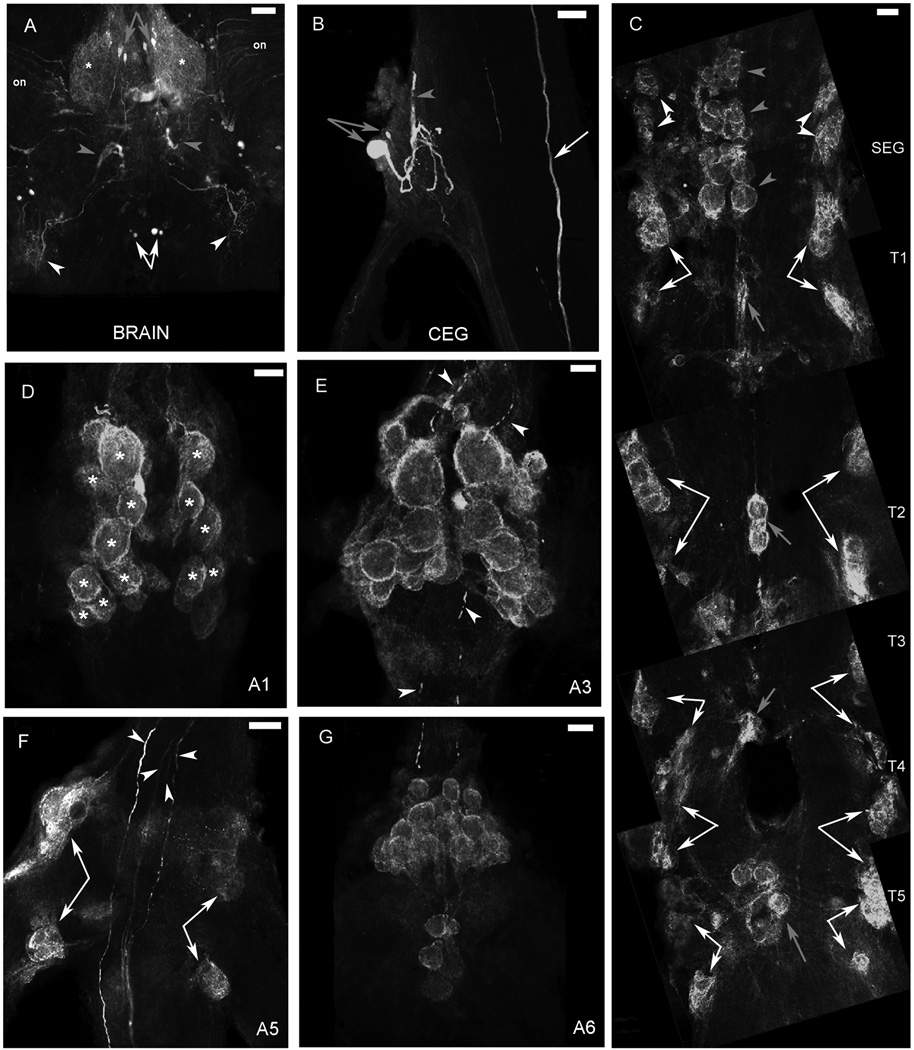
Figure 6. 5-HT1Mac immunoreactivity in the CNS of the prawn.
A: View of the brain, in which 5-HT1Mac immunoreactivity (ir) was observed as diffuse punctate staining, in the neuropil of the protocerebrum (*), within a group of 4–8 small-sized cells in the protocerebrum (gray arrows), a group of 4–8 medium-sized cells in the tritocerebrum (white arrows), in fibers in the optic nerves (on), within the deutocerebrum (gray arrowheads) and coming in through the connectives from the circumesophageal ganglia (CEG) (white arrowheads). B: View of the CEG, showing 5-HT1Mac ir within two cells (gray arrows), the largest of which sends its axon towards the brain (gray arrowhead), and in a fiber traversing the connective (white arrow). C: Ventral view of the subesophageal ganglion (SEG) and thoracic (T) ganglia, showing 5-HT1Mac ir on the membranes of SEG cells arranged in groups located on the lateralmost edges of the ganglion (white arrowheads) and along its midline (gray arrowheads), and on the membranes of cells located at the center of each thoracic ganglion (gray arrows), along with 4 clusters of medium- to large-sized cells, arranged as the wings of a butterfly, located towards the lateralmost edges of each ganglion (white arrows). The first (T1) and fifth (T5) thoracic ganglia had the larger of the centrally located clusters. D: Ventral view of the first abdominal ganglion (A1) showing 5-HT1Mac ir on the membranes of large-sized cells (*) arranged in clusters located at both sides of the midline. E: Ventral view of the third abdominal ganglion (A3) showing a pattern of 5-HT1Mac ir similar to that observed for A1. 5-HT1Mac ir could also be observed in centrally located fibers that traversed the ganglia along its connectives (white arrowheads). F: Dorsal view of the fifth abdominal ganglion (A5) showing 5-HT1Mac ir along 4 clusters of medium- to large-sized cells, located laterally and arranged as the wings of a butterfly (white arrows), and in 4 centrally located fibers that traversed the ganglia along its connectives (white arrowheads). G: Ventral view of the sixth abdominal ganglion (A6) showing 5-HT1Mac ir on the membranes of cells arranged in clusters (white arrows), following a pattern similar to that described for A1 and A3. All images shown were obtained from the ventral nerve cord of a male blue clawed prawn. Images A, B and F are each an individual frame or optical slice of sets of confocal stacks. Images C, D, E and G are each a composite of optical slices of sets of confocal stacks spanning the ventral aspect of the full dorsal-ventral axis of the ganglia. The fluorescence has been digitally brightened, and several pieces of surface debris have been digitally removed. (Scale bars = 250 µm).
In the brain, 5-HT1Mac ir was observed as diffuse punctate staining, in the neuropil of the protocerebrum (*). It was also found within a group of four to eight 20–30 µm cells in the protocerebrum (gray arrows; Fig. 6A and Fig. 8A), a group of four to eight 30–50 µm cells in the tritocerebrum (white arrows; Fig. 6A and Fig. 8A) and in fibers in the optic nerves (on), within the deutocerebrum (gray arrowheads) and coming in through the connectives from the circumesophageal ganglia (white arrowheads). In the CEG, 5-HT1Mac ir was found within two cells (gray arrows), the largest of which sends its axon towards the brain (gray arrowhead), and in a fiber that traverses the connective (white arrow).
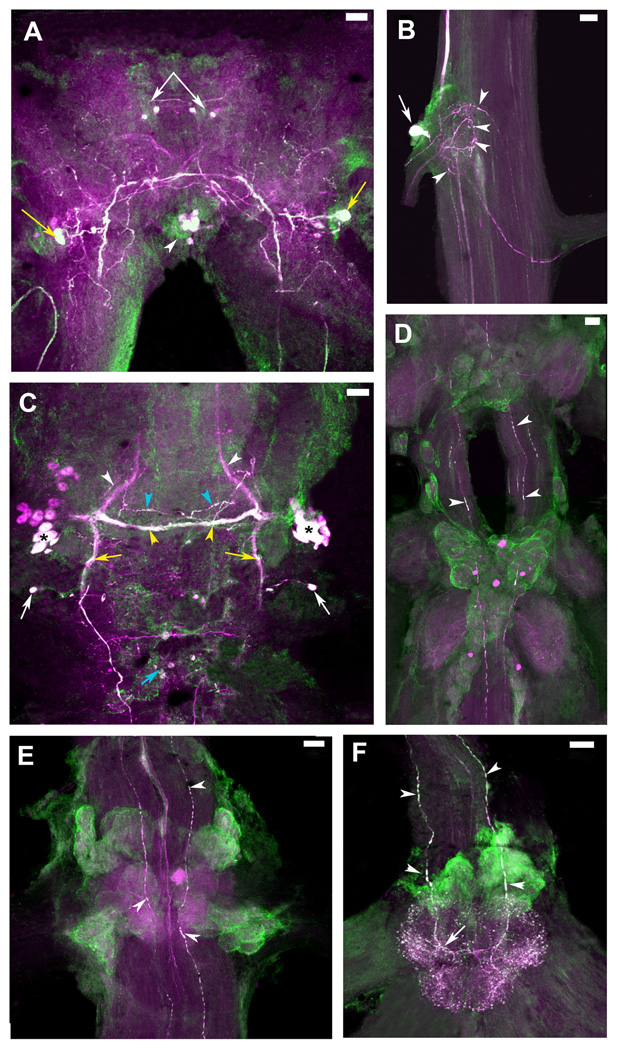
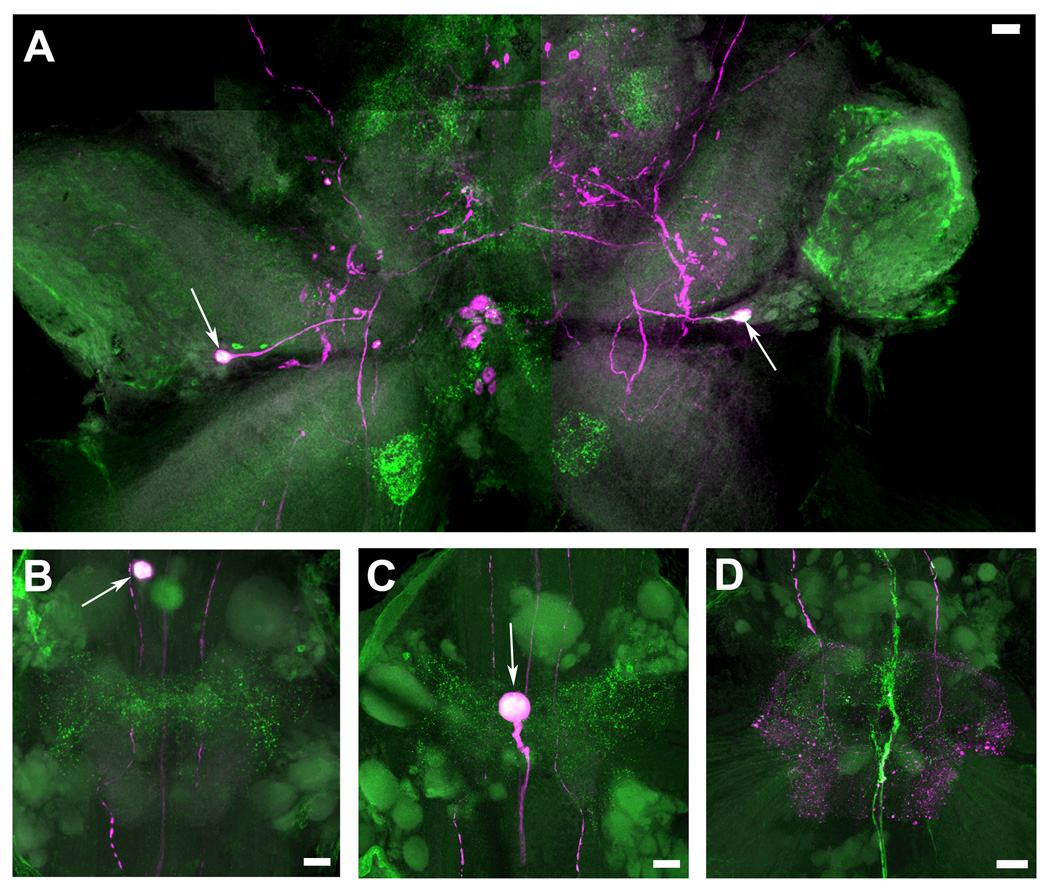
Figure 8. Colocalization of 5-HT1Mac and TH immunoreactivity in the CNS of the prawn.
5-HT1Mac immunoreactivity (ir) shown in green and Tyrosine Hydroxylase (TH) ir shown in magenta. TH ir is taken to represent dopamine (DA)-containing cells (see Materials & Methods). Cells and fibers showing immunoreactivity to both 5-HT1Mac and TH appear white. A: View of the brain, showing colocalization of both 5-HT1Mac and TH/DA in a group of four small-sized cells in the protocerebrum (white arrows), a pair of large- to medium-sized cells in the deutocerebrum (yellow arrows), and a cluster of 4–8 medium-sized cells in the tritocerebrum (white arrowheads). B: View of the circumesophageal ganglion (CEG), showing colocalization for both 5-HT1Mac and TH/DA in a large-sized cell (white arrow) that sends its axon towards the brain, as well as in terminal arborizations (white arrowheads) within the neuropil of the ganglion. C: View of the subesophageal ganglion (SEG), showing 5-HT1Mac and TH/DA colocalization in some (*), but not all, of the medium-sized cells within the bilateral clusters of the ganglion, in the fascicles of axons going to the contralateral (yellow arrowheads) side of the ganglion (but not in those going towards the CEG; white arrowheads), within longitudinal fibers communicating the SEG with the lower ganglia (yellow arrows), in some, but not all, of the horizontally-oriented fibers forming a ladder-like pattern (blue arrowheads), and in the small-sized cells on the edges of the ganglion (white arrows), inferior to the bilateral clustered cells, as well as in the centrally located cells (blue arrow) between the SEG and the first thoracic ganglion (T1). D: View of T3-T4, showing 5-HT1Mac and TH/DA colocalization only in the longitudinal fibers that traverse the ganglia (white arrowheads). E: View of the first abdominal ganglion (A1), showing 5-HT1Mac and TH/DA colocalization in the longitudinal fibers that traverse the ganglia (white arrowheads) and rather sparsely on the terminal arborizations within the neuropil. F: View of the sixth abdominal ganglion (A6), showing 5-HT1Mac and TH/DA colocalization in the expansive terminal arborization that forms at the end of the longitudinal fibers entering through the connective. All images shown were obtained from the ventral nerve cord of a male blue clawed prawn. All images are composites of optical slices of sets of confocal stacks spanning the full dorsal-ventral axis of the ganglia. The fluorescence has been digitally brightened, and several pieces of surface debris have been digitally removed. (Scale bars = 100 µm).
In the SEG, 5-HT1Mac ir was observed on what appears to be the membranes of cells on the ventral side of the ganglion (Fig. 6C). These cells are arranged in groups located on the lateralmost (white arrowheads) edges of the ganglion and along its midline (gray arrowheads). The lateralmost groups are formed by clusters of eight to twelve 30–50 µm cells, two on each side. The midline groups are formed by three consecutive clusters of four 80–100 µm cells, each surrounded by a few 30–50 µm cells. On the dorsal aspect of the ganglion (Fig. 8C), 5-HT1Mac ir was observed in bilateral clusters of six to twelve 30–50 µm cells located laterally at midlevel (*), in a pair of small cells, also located laterally and inferior to the clusters mentioned above (white arrows), and in fibers communicating the SEG with the thoracic ganglia (yellow arrows), as well as in a group of commissural fibers (yellow arrowheads) that appears to link the 5-HT1Mac ir bilateral clusters of cells, and in some (but not all) of a series of other commissural fibers that also cross the midline of the ganglion (blue arrowheads).
In the thoracic ganglia, 5-HT1Mac ir was observed on what appears to be the membranes of cells on the ventral side of the ganglion (Fig. 6C). These cells are arranged in clusters, following a pattern that repeats itself in each ganglion. The pattern consists of rows of 2 to 16 80–100 µm cells located at the center of each ganglion (gray arrows), along with 4 clusters of 20–80 µm cells, arranged as the wings of a butterfly, located towards the lateralmost edges of each ganglion (white arrows). T1 and T5 had the larger of the centrally located clusters. In a previous report (Sosa et al., 2004) it had been shown that at a more dorsal level, 5-HT1Mac ir could be observed in four centrally located fibers that traversed the thoracic ganglia along its connectives. These fibers showed 5-HT1Mac ir only in the prawn and not in the crayfish (Sosa et al., 2004).
In the first five abdominal ganglia (A1-A5), 5-HT1Mac ir was observed on what appears to be the membranes of cells on the ventral (Fig. 6D–E) and dorsal (Fig. 8F) sides of the ganglia. These cells are arranged in clusters, following a pattern that repeats itself in each ganglion (A2 and A4 are not shown but present the same staining pattern as the other ganglia). The pattern consists of clusters of 80–180 µm cells located ventrally at both sides of the midline (*; Fig. 6D–E), along with 4 clusters of 50–100 µm cells, located more dorsolaterally and arranged as the wings of a butterfly (white arrows; Fig. 6E–F and Fig. 8E). More dorsally, 5-HT1Mac ir was also found in four centrally located fibers that traversed the ganglia along its connectives (white arrowheads; Fig. 6F). (Two of these fibers are located right next to each other at the center of the ganglion). Punctate staining could also be observed in the area surrounding these filled fibers (Fig. 6F).
In the sixth abdominal ganglion (A6), 5-HT1Mac ir was observed on what also appears to be the membranes of cells on the ventral and dorsal sides of the ganglion (Fig. 6G). These cells are arranged in clusters, following a pattern similar to that described for A1–A5. At the dorsal level (Fig. 10F), 5-HT1Mac ir was also found in four centrally located fibers that enter the ganglion through its connective and terminate in an arborization of 5-HT1Mac ir boutons.
5-HT2Mac immunoreactivity
5-HT2Mac ir is also widely distributed in the ventral nerve cord of the prawn, but the pattern of staining differs from that of 5-HT1Mac. 5-HT2Mac ir was mostly observed as punctate staining in the neuropil of the brain and all other ganglia (Fig. 7). The distribution and intensity of this punctate staining throughout the neuropil of the various ganglia differed markedly for the two receptors. 5-HT2Mac ir was also found in a small number of brain (Fig. 7A and Fig. 9A), SEG (white arrows; Fig. 7C), thoracic (Fig. 7D) and some abdominal ganglia (Figs. 7E–G) cells with small somata, as well as in fibers in the brain (Fig. 7A), thoracic (Fig. 7D) and abdominal ganglia (Figs. 7E–G). No staining was observed around somata of specific subsets of neurons, as was the case with 5-HT1Mac. No qualitative differences were observed in the staining pattern of the different male morphotypes.
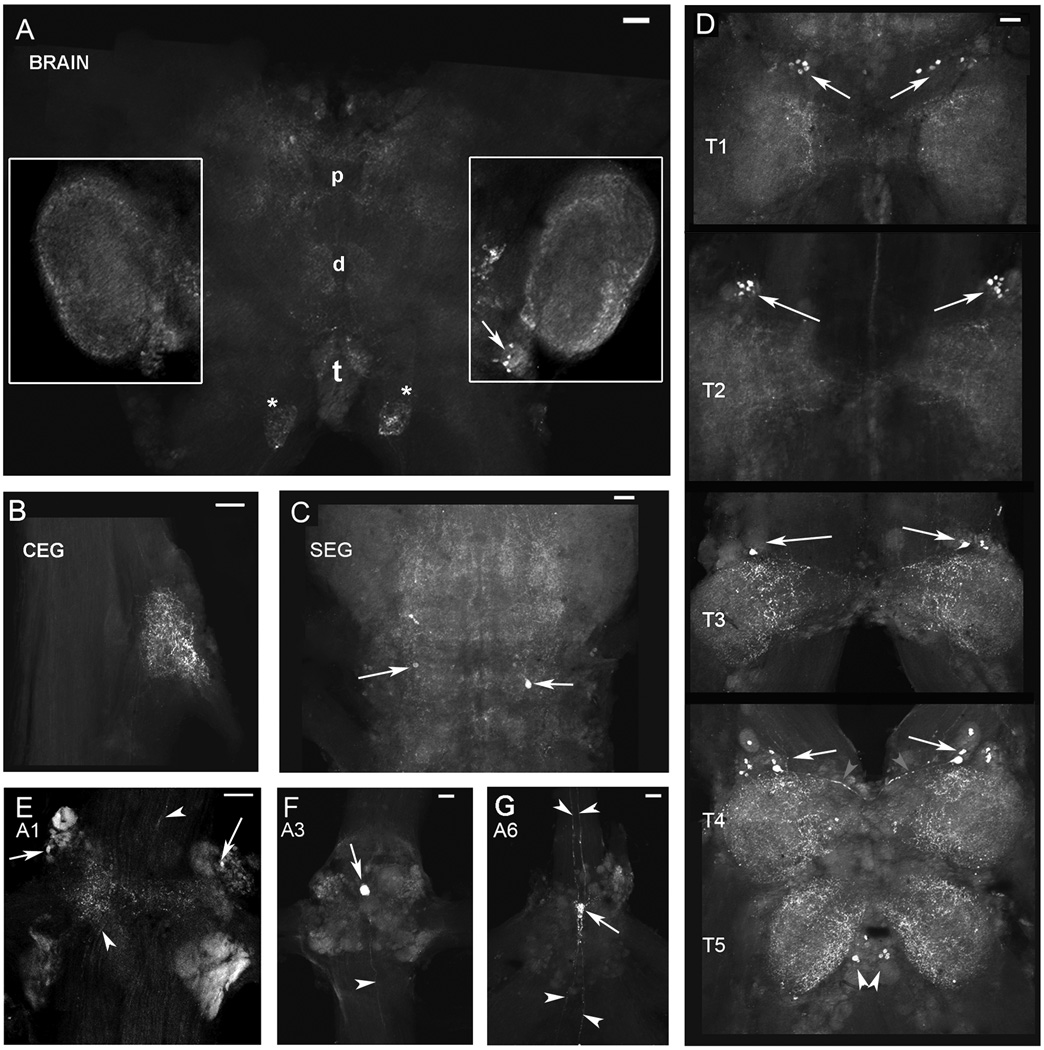
Figure 7. 5-HT2Mac immunoreactivity in the CNS of the prawn.
A: View of the brain, showing 5-HT2Mac immunoreactivity (ir) as very fine punctate staining throughout the neuropil of the centralmost regions of the proto- (p), deuto- (d) and tritocerebrum (t), surrounding the glomeruli of the olfactory lobes (white boxes), in a few small-sized cells medial and inferior to the olfactory lobes (white arrows), and in fibers and terminal arborizations (*) entering through the connectives near the tritocerebrum. B: View of the circumesophageal ganglion (CEG), showing 5-HT2Mac ir as punctate staining in the neuropil. C: Ventral view of the subesophageal ganglion (SEG), showing 5-HT2Mac ir as very fine punctate staining throughout the neuropil, and in a few small-sized cells and fibers placed laterally (white arrows). D: Ventral view of the thoracic (T) ganglia, showing 5-HT2Mac ir in groups of 2–8 small-sized cells located superolaterally on each side of the T1–T4 ganglia (white arrows) and inferomedially at T5 (white arrowheads), in the axons (gray arrowheads) of some neurons forming these clusters (e.g., see T4), and in the neuropil of all five thoracic ganglia, with a higher intensity of staining observed in T4 and T5. E: Dorsal view of the first abdominal ganglion (A1), showing 5-HT2Mac ir in a pair of small-sized cells located bilaterally in the superolateral aspect of the ganglion (white arrows), in the neuropil, and in fibers traversing the ganglion along its connectives (white arrowheads). F. Dorsal view of the third abdominal ganglion (A3), showing 5-HT2Mac ir in a single medium-sized cell (white arrow) in the midline between the two upper quadrants of the ganglion and in fibers traversing the ganglion along its connectives (white arrowhead). G: Dorsal view of the sixth abdominal ganglion (A6), showing 5-HT2Mac ir in a single medium-sized cell (white arrow) in the midline between the two upper quadrants of the ganglion, and in fibers traversing the ganglion along its connectives and nerve roots (white arrowheads). All images shown were obtained from the ventral nerve cord of a male blue clawed prawn. Images A, E, F and G are each an individual frame or optical slice of sets of confocal stacks. Images B, C and D are each a composite of optical slices of sets of confocal stacks spanning the full dorsal-ventral axis of the ganglia. The fluorescence has been digitally brightened, and several pieces of surface debris have been digitally removed. (Scale bars = 100 µm).
In the brain, 5-HT2Mac ir was observed as very fine punctate staining throughout the neuropil of the centralmost regions of the proto- (p), deuto- (d) and tritocerebrum (t) (Fig. 7A). It was also observed surrounding the glomeruli of the olfactory lobes (white boxes in Fig. 7A, and Fig. 9A). Fibers ending in an oval-shaped terminal arborization (*) as they enter the tritocerebrum through the connectives between the brain and CEG also show 5-HT2Mac ir (Fig. 7A and Fig. 9A). In the thoracic ganglia, 5-HT2Mac ir was observed in groups of two to eight 20–30 µm cells located superolaterally in each side of the T1-T4 ganglia (white arrows). Groups of three to four 20–30 µm cells also presented 5-HT2Mac ir at either side of the midline on the inferior aspect of the T5 ganglion (white arrowheads). 5-HT2Mac ir could also be observed in the axons of some neurons (see gray arrowheads in T4 in Fig. 7D). The neuropil of all five thoracic ganglia were 5-HT2Mac ir, with a higher intensity of staining observed in the T4 and T5 ganglia. In the abdominal ganglia, 5-HT2Mac ir was observed in a 10–20 µm cell (white arrow) located bilaterally in the superolateral aspect of A1 (Fig. 7E), in a single 30–50 µm cell (white arrow) in the midline between the two upper quadrants of the A3 (Fig. 7F) and A6 (Fig. 7G) ganglia. 5-HT2Mac ir was also observed in fibers (white arrowheads) traversing the abdominal ganglia and traveling along its connectives (Fig. 7E–G and Fig. 9D).
Figure 9. Colocalization of 5-HT2Mac and TH immunoreactivity in the CNS of the prawn.
5-HT2Mac immunoreactivity (ir) shown in green and Tyrosine Hydroxylase (TH) ir shown in magenta. TH ir is taken to represent dopamine (DA)-containing cells (see Materials & Methods). Cells and fibers showing immunoreactivity to both 5-HT2Mac and TH appear white. A: View of the brain, showing colocalization of both 5-HT2Mac and TH/DA only in the somas of a pair of medium-sized cells in the deutocerebrum (white arrows). B: View of the first abdominal ganglion (A1) showing colocalization of both 5-HT2Mac and TH/DA only at the soma of a single cell located at the midline (white arrow). C: View of the third abdominal ganglion (A3) showing colocalization of both 5-HT2Mac and TH/DA only at the soma of a single cell located at the midline (white arrows. D: View of the sixth abdominal ganglion (A6) showing there is no colocalization of 5-HT2Mac and DA (no white or overlapping staining). All images shown were obtained from the ventral nerve cord of a male blue clawed prawn. All images are composites of optical slices of sets of confocal stacks spanning the full dorsal-ventral axis of the ganglia. The fluorescence has been digitally brightened, and several pieces of surface debris have been digitally removed. (Scale bars = 100 µm).
Double labeling showing colocalization of 5-HT1Mac and Tyrosine Hydroxylase (Dopamine) immunoreactivity
We found that some (but not all) of the cells and fibers that showed 5-HT1Mac ir also appeared to contain tyrosine hydroxylase/dopamine (TH/DA) in several regions of the CNS of the prawn (Fig. 8). In the brain, a group of four 20–30 µm cells in the protocerebrum (white arrows), a pair of 60 µm cells in the deutocerebrum (yellow arrows), and a cluster of four to eight 40–50 µm cells in the tritocerebrum (white arrowheads) showed both 5-HT1Mac and TH/DA immunoreactivity (Fig. 8A). In the CEG, there was colocalization for both 5-HT1Mac and TH/DA in a large cell (70 µm) (white arrow) which sends its axon towards the brain, as well as in areas of the fibers that form terminal arborizations (white arrowheads) within the ganglion (Fig. 8B). In the SEG, both 5-HT1Mac and TH/DA were colocalized in some (*), but not all, of the 30–50 µm cells within the bilateral clusters of the ganglion, and in the fascicles of axons going to the contralateral side of the ganglion (yellow arrowheads), but not in those going towards the CEG (white arrowheads) (Fig. 8C). Colocalization was also observed within longitudinal fibers communicating the SEG with the lower ganglia (yellow arrows), in some, but not all, of the horizontally-oriented fibers forming a ladder-like pattern (blue arrowheads), and in the 20–30 µm cells on the edges of the ganglion, inferior to the bilateral clustered cells (white arrows), as well as in the centrally located cells between the SEG and T1 (blue arrow). In the thoracic and abdominal ganglia, there is colocalization of 5-HT1Mac and TH/DA only in the longitudinal fibers that traverse the ganglia (white arrowheads), and rather sparsely on the terminal arborizations within the neuropil areas of A1-A5 (Fig. 8D–E). Colocalization was also found in A6 on the expansive terminal arborization that stains much more intensely than those of the other ganglia (Fig. 8F). Fibers that cross to the contralateral side (white arrow) of A6 also show dual staining.
Double labeling showing colocalization of 5-HT2Mac and Tyrosine Hydroxylase (Dopamine) immunoreactivity
We found that a few of the cells that showed 5-HT2Mac ir also appeared to contain TH/DA in the brain and abdomen of the prawn (Fig. 9). Colocalization was observed in a pair of 60 µm cells in the deutocerebrum (white arrows) (Fig. 9A). These cells were also immunoreactive to 5-HT1Mac, although in that case staining was also observed throughout the fibers of these cells (Fig. 8A). No colocalization was observed in the CEG, SEG or any of the thoracic ganglia. In the abdominal ganglia, colocalization of 5-HT2Mac receptor and TH/DA was seen only at some of the centrally located cells of A1-A4 (white arrows) (Fig. 9B–C). The DA/TH immunoreactive fibers that form an expansive terminal arborization in A6 and show 5-HT1Mac ir (Fig. 9F) do not stain for 5-HT2Mac (Fig. 9D).
Discussion
Serotonin has been implicated in a wide variety of mental disorders (Mann, 1999). Various vertebrate model systems have been used to study the role of this amine in social behaviors. However, difficulties often arise when attempting to translate observations into specific mechanisms at the circuit or cellular levels, due to the inherent complexity of the vertebrate central nervous system. Simpler invertebrate model systems, such as crustaceans, are often better suited to understand the neural basis of interactive behaviors at the cellular and molecular levels because of the relative simplicity of their nervous systems.
The function of the serotonergic system depends on three major factors: (i) synthesis and degradation of the neurotransmitter, (ii) reuptake from the synaptic cleft by serotonin transporters (SERT); and (iii) the density and sensitivity of its receptors (Popova, 2006). It has been suggested that serotonin reuptake mechanisms in crustaceans may play an important role in behavioral changes since effects of serotonin infusion can be blocked by fluoxetine (Huber et al., 1997a; Huber, 2005), a serotonin transporter blocker. In addition, it is well known that injections of serotonin and some of its agonists activate specific serotonin receptors producing behaviors characteristics of dominant animals (Yeh et al., 1997; Tierney et al., 2004), suggesting that the effects of serotonin in modulation of interactive behavior may be mediated by specific responses of its receptors. In addition, it has been proposed that these serotonin effects may be mediated by several receptor subtypes (Tierney and Mangiamele, 2001; Tierney et al., 2004), and that there may be species-specific differences in responses to exogenous serotonin in different crustacean systems (Tierney et al., 2004). As a first step to study the role of serotonin receptors in the mechanisms underlying social behaviors of the freshwater prawn, we have cloned two of the prawn’s 5-HT receptors and mapped out their distribution in the CNS.
In Sosa et al. (2004) we described the cloning of a fragment of a type 1 serotonin receptor in the prawn. Here we present results on the cloning and sequencing of the full cDNA encoding this type 1 and a type 2 serotonin receptor in the prawn. We have named the prawn forms of these receptors as 5-HT1Mac and 5-HT2Mac.
The 5-HT1Mac receptor cDNA encodes a polypeptide of 380 amino acids in length, which is more closely related to lobster, budworm, fruit fly, mouse, and human 5-HT1-like receptors. The 5-HT2Mac receptor gene encodes a polypeptide of 752 amino acids, which is more closely related to lobster, fruit fly, mouse, and human 5-HT2-like receptors. It is interesting to note that the type 1 serotonin receptor of the prawn appears to be more closely related to the 5-HT1A receptor of the human than the equivalent receptors from the lobster and tobacco budworm. It will be of interest to determine whether the function, pharmacological profile and cascade of second messenger systems activated by the 5-HT1Mac receptor are also closer to the human receptor than the receptors from these other model systems. The degree of relationship between the prawn and the human 5-HT2 receptor was similar to that of the type 1 receptor, and it was comparable with that of the lobster.
In terms of the distribution of 5-HT neurons in the CNS of the prawn, the overall map is similar, although not identical, to that previously described for other crustaceans such as the lobster (Beltz and Kravitz, 1983) and crayfish (Real and Czternasty, 1990). There are at least three major differences: 1) the CEG has an additional smaller serotonergic neuron; 2) there is an additional pair of large-sized serotonergic neurons, with somas located near the midline between T4 and T5 ganglia, whose axons project anteriorly along the LLF; and 3) no serotonergic axons appear to exit the CNS through abdominal or thoracic nerves (this type of axon is observed only in nerves that arise from the SEG, CEG and brain). At first it might be thought that the latter observation may be due to experimental artifact if such axons were to be very thin or were very labile and thus damaged upon cutting of the nerve roots during dissection. This is unlikely though because very thin 5-HT axons are indeed observed in other regions throughout the whole nerve cord of the prawn, at least of the diameter observed in thoracic and abdominal nerves of the lobster and crayfish, serotonergic axons survive the cutting of nerve roots in other regions, and axons containing other transmitters (e.g., GABA, unpublished observations) or receptor molecules (e.g. 5HT1Mac; Sosa et al., 2004) show immunoreactivity in the cut roots of thoracic and/or abdominal nerves. It would seem reasonable to speculate that the serotonergic system of the prawn is circumscribed to internal (within the CNS) regulatory or modulatory functions caudal to the SEG and that its external influences are to be carried out through peripheral nerves at more rostral levels of the CNS. Alternatively, external functions at the thoracic and abdominal levels might also be carried out through a neurohormonal route.
The staining of 5-HT1Mac receptor showed a consistent pattern in the prawn’s CNS, from the SEG to the last abdominal ganglion. In these regions of the ventral nerve cord, associated with regulation and coordination of motor activities, such as mouth parts, leg, claw and swimmerette movements, and the initiation of the tail-flip escape response (Rossi-Durand, 1993; Edwards et al., 1999), the 5-HT1Mac receptor appears to be located on the membranes of clusters of medium- to large-sized cells and inside fibers that traverse all ganglia. In the brain and CEG, the 5-HT1Mac receptor was found primarily in the cytoplasm of small- to medium-sized cells and their axons. This type of cytoplasmic staining may be indicative of receptor units or subunits being synthesized for later insertion in the membrane of the cell body or dendritic extensions, and/or packed for transport to locations away from the soma, or vesicles containing recycled receptors being brought towards the soma from other regions. A similar type of type 1 5-HT receptor somatic staining has also been observed in some regions of the crayfish ventral nerve cord (Spitzer et al., 2005). If the receptor is being transported from the cell body towards neuronal terminals along axons, it is possible that it is being inserted in terminals to act as autoreceptors. It could also be speculated that the synthesized receptor might be transferred across the synaptic cleft or via glial or other type of supporting cells to other neurons, through some yet undescribed process, although no evidence exists in the literature to this date of such a novel transfer mechanism. It is interesting to note that the somas and fibers that showed 5-HT1Mac receptor staining were shown to be most likely dopaminergic in nature, suggesting a functional link between the dopaminergic and serotonergic neural systems in the CNS of the prawn.
The staining of 5-HT2Mac receptor showed a markedly different pattern to that observed for the 5-HT1Mac receptor. The 5-HT2Mac receptor was found mostly in the central neuropil regions of each ganglion throughout the CNS and not concentrated on the membranes of the somas of cells that form the outer ventrolateral shell of each ganglion, as was the case with the 5-HT1Mac receptor. It was also observed in the cytoplasm and fibers of a small number of small-sized cells in the brain, SEG and thoracic ganglia, and some abdominal ganglia. The somas of the few cells in the brain and abdominal ganglia that contain the 5-HT2Mac receptor also contain dopamine, and have been shown to also contain 5-HT (preliminary data not shown here). It is interesting to note that the 5-HT2Mac receptor is not observed in the axons of these particular cells. The 5-HT2Mac receptor was also found in glomeruli of the olfactory lobes of the brain, whereas in the case of the crayfish, these glomeruli show staining for a type 1 5-HT receptor (Spitzer et al., 2005). The 5-HT1Mac receptor was not found in these glomeruli in the prawn.
Our data demonstrate that both type 1 and type 2 serotonin receptors in the prawn differ in terms of localization, distribution within cells, and intensity of immunoreactive staining throughout the CNS. It is thus likely that both receptors play different roles within the CNS. Although no qualitative differences were found in the patterns of staining of these receptors amongst the three male morphotypes of the prawn, it is possible that differences in levels of expression, function or in the interplay or balance between the activities of both types of receptors may play a role in modulating the differing interactive behaviors that characterize these morphotypes. The data we present here on the sequence and immunohistochemical localization of the prawn’s 5-HT1Mac and 5-HT2Mac receptors will serve as the basis for developing experimental tools to next focus on these types of questions.
Acknowledgments
Other Acknowledgement
We are thankful to Ms. Clarissa Martínez-Rubio and Mr. Néstor Reyes-Colón for technical help obtaining and/or analyzing confocal microscope images.
This work was supported by NIH/MBRS S06GM008224 (Sosa), NIH/MRISP MH48190 (Sosa), NIH/RCMI G12RR03051 (Institute of Neurobiology), NSF DBI 0115825 (Institute of Neurobiology), NIH RO1 NS39103 (Moroz).
Literature Cited
- Adams CF, Liley NR, Gorzalka BB. PCPA increases aggression in male firemouth cichlids. Pharmacology. 1996;53(5):328–330. doi: 10.1159/000139446. [DOI] [PubMed] [Google Scholar]
- Albert FW, Shchepina O, Winter C, Römpler H, Teupser D, Palme R, Ceglarek U, Kratzsch J, Sohr R, Trut LN, Thiery J, Morgenstern R, Plyusnina IZ, Schöneberg T, Pääbo S. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm Behav. 2008;53(3):413–421. doi: 10.1016/j.yhbeh.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Alvarez-Alvarado R, Porras Villalobos MG, Calderón Rosete G, Rodríguez Sosa L, Aréchiga H. Dopaminergic modulation of neurosecretory cells in the crayfish. Cell Mol Neurobio. 2005;25(2):345–370. doi: 10.1007/s10571-005-3064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayali A, Harris-Warrick RM. Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci. 1999;19(15):6712–6722. doi: 10.1523/JNEUROSCI.19-15-06712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M. Functional characterization of a novel serotonin receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. doi: 10.1046/j.0022-3042.2001.00703.x. [DOI] [PubMed] [Google Scholar]
- Barbas D, Campbell A, Castellucci VF, DesGroseillers L. Comparative localization of two serotonin receptors and sensorin in the central nervous system of Aplysia californica. J Comp Neurol. 2005;490:295–304. doi: 10.1002/cne.20666. [DOI] [PubMed] [Google Scholar]
- Barki A, Karplus I, Goren M. The agonistic behavior of the three male morphotypes of the freshwater prawn Macrobrachium rosenbergii (Crustacea, Palaemonidae) Behavior. 1991a;116(3–4):252–277. [Google Scholar]
- Barki A, Karplus I, Goren M. Morphotype related dominance hierarchies in males of Macrobrachium rosenbergii (Crustacea, Palaemoidae) Behavior. 1991b;117(3–4):145–160. [Google Scholar]
- Barki A, Karplus I, Goren M. Effects of size and morphotype on domiance hierarachies and resource competition in the freshwater prawn Macrobrachium rosenbergii. Animal Behavior. 1992;44:547–555. [Google Scholar]
- Barthe JY, Bevengut M, Clarac F. In vitro, proctolin and serotonin induced modulations of the abdominal motor system activities in crayfish. Brain Res. 1993;623(1):101–109. doi: 10.1016/0006-8993(93)90016-g. [DOI] [PubMed] [Google Scholar]
- Beltz BS. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc Res Tech. 1999;44:105–120. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<105::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Kravitz EA. Mapping of serotonin-like immunoreactivity in the lobster nervous system. J Neurosci. 1983;3:585–602. doi: 10.1523/JNEUROSCI.03-03-00585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer M, Lowe E, Raore B. Serotonin increases flexion and excitatory junction potential amplitude while octopamine decreases average excitatory junction potential amplitude in crayfish. Pioneering Neuroscience. 2000;1:25–33. [Google Scholar]
- Chen A, Holmes SP, Pietrantonio PV. Molecular cloning and functional expression of a serotonin receptor from the southern cattle tick, Boophilus microplus (Acari: Ixodidae) Insect Mol Biol. 2004;13:45–54. doi: 10.1111/j.1365-2583.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- Clark MC, Dever TE, Dever JJ, Xu P, Rehder V, Sosa MA, Baro DJ. Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J Neurosci. 2004;24(13):3421–3435. doi: 10.1523/JNEUROSCI.0062-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette M, Parent A, Levesque D. Tyrosine hydroxylase-positive neurons intrinsic to the human striatum express the transcription factor Nurr1. Eur J Neurosci. 2004;20:2089–2095. doi: 10.1111/j.1460-9568.2004.03661.x. [DOI] [PubMed] [Google Scholar]
- Cournil I, Helluy SM, Beltz BS. Dopamine in the lobster Homarus gammarus. I. Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the nervous system of the juvenile. J Comp Neurol. 1994;344(3):455–469. doi: 10.1002/cne.903440308. [DOI] [PubMed] [Google Scholar]
- Cournil I, Casasnovas B, Helluy SM, Beltz BS. Dopamine in the lobster Homarus gammarus: II. Dopamine-immunoreactive neurons and development of the nervous sytem. J Comp Neurol. 1995;362:1–16. doi: 10.1002/cne.903620102. [DOI] [PubMed] [Google Scholar]
- Croll RP, Finney JL, Smith FM. Innervation of the swim bladder in the zebrafish, Danio rerio. Integr Comp Biol. 2003;43:1035. doi: 10.1002/cne.20948. [DOI] [PubMed] [Google Scholar]
- Dacks AM, Dacks JB, Christensen TA, Nighorn AJ. The cloning of one putative octopamine receptor and two putative serotonin receptors from the tobacco hawkmoth, Manduca sexta. Insect Biochem Mol Biol. 2006;36(9):741–747. doi: 10.1016/j.ibmb.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis RL, Chen ZQ, Cheng HW. Serotonergic mediation of aggression in high and low aggressive chicken strains. Poult Sci. 2008;87(4):612–620. doi: 10.3382/ps.2007-00389. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr Opin Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–161. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek KA. Escalated Aggression after Alcohol Drinking in Male Mice: Dorsal Raphé and Prefrontal Cortex Serotonin and 5-HT(1B) Receptors. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.7. [Epub ahead of print] PMID: 18305458. [DOI] [PubMed] [Google Scholar]
- Finney JL, Croll RP, Smith FM. Autonomic innervation of the swim bladder in the zebrafish Danio rerio. Bull Can Soc Zool. 2004;35:77. doi: 10.1002/cne.20948. [DOI] [PubMed] [Google Scholar]
- Florey E, Rathmayer M. The effects of octopamine and other amines on the heart and on neuromuscular transmission in decapod crustaceans: further evidence for a role as neurohormone. Comp Biochem Physiol. 1978;61C(1):229–237. doi: 10.1016/0306-4492(78)90136-3. [DOI] [PubMed] [Google Scholar]
- Fort TJ, Brezina V, Miller MW. Modulation of an integrated central pattern generator-effector system: dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus. J Neurophysiol. 2004;92(6):3455–3470. doi: 10.1152/jn.00550.2004. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Krasne FB. Serotonin and octopamine have opposite modulatory effects on the crayfish’s lateral giant escape reaction. J Neurosci. 1983;3(11):2263–2269. doi: 10.1523/JNEUROSCI.03-11-02263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Kravitz EA. Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci. 1984;4(8):1976–1993. doi: 10.1523/JNEUROSCI.04-08-01976.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzsch S. Evolution of identified arthropod neurons: the serotonergic system in relation to engrailed-expressing cells in the embryonic ventral nerve cord of the American lobster Homarus americanus Milne Edwards, 1873 (malacostraca, pleocyemata, homarida) Dev Biol. 2003;258:44–56. doi: 10.1016/s0012-1606(03)00113-1. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Waloszek D. Serotonin-immunoreactive neurons in the ventral nerve cord of Crustacea: a character to study aspects of arthropod phylogeny. Arthropod Struct Dev. 2000;29:307–322. doi: 10.1016/s1467-8039(01)00015-9. [DOI] [PubMed] [Google Scholar]
- Huang X, Xiao H, Rex EB, Hobson RJ, Messer WS, Komuniecki PR, Komuniecki RW. Functional characterization of alternatively spliced 5-HT2 receptor isoforms from the pharynx and muscle of the parasitic nematode, Ascaris suum. J Neurochem. 2002;83:249–258. doi: 10.1046/j.1471-4159.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- Huber R. Amines and motivated behaviors: a simpler systems approach to complex behavioral phenomena. J Comp Physiol A. 2005;191:231–239. doi: 10.1007/s00359-004-0585-5. [DOI] [PubMed] [Google Scholar]
- Huber R, Orzeszyna M, Pokory N, Kravitz EA. Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav & Evol. 1997a;50(1):60–68. doi: 10.1159/000113355. [DOI] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci USA. 1997b;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv Exp Med Biol. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23(10):403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. On violence and the value of animal models of aggression. The Harvard Mahoney Neuroscience Institute Letter: Commentary. 1993;2(1):2–3. [Google Scholar]
- Kroeze WK, Kristiansen KK, Roth BL. Molecular biology of serotonin receptors structure and funnction at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- Kuris AM, Ra’anan Z, Sagi A, Cohen D. Morphotypic differentiation of male Malaysian giant prawns Macrobrachium rosenbergii. J Crust Biol. 1987;7:219–237. [Google Scholar]
- Linnoila VM, Virkkunen M. Aggression, suicidality, and serotonin. J Clin Psychiatry. 1992;53:46–51. [PubMed] [Google Scholar]
- Livingstone MS, Harris-Warrick RM, Kravitz EA. Serotonin and octopamine produce opposite postures in lobsters. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- Lynn SE, Egar JM, Walker BG, Sperry TS, Ramenofsky M. Fish on Prozac: a simple, noninvasive physiology laboratory investigating the mechanisms of aggressive behavior in Betta splendens. Adv Physiol Educ. 2007;31(4):358–363. doi: 10.1152/advan.00024.2007. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharm. 1999;21(2S) doi: 10.1016/S0893-133X(99)00040-8. 99S–105S. [DOI] [PubMed] [Google Scholar]
- Mapara S, Parries S, Quarrington C, Ahn KC, Gallin WJ, Goldberg JI. Identification, molecular structure and expression of two cloned serotonin receptors from the pond snail, Helisoma trivolvis. J Exp Biol. 2008;211(Pt 6):900–910. doi: 10.1242/jeb.013953. [DOI] [PubMed] [Google Scholar]
- Miller MW, Parnas H, Parnas I. Dopaminergic modulation of neuromuscular transmission in the prawn. J Physiol. 1985;363:363–375. doi: 10.1113/jphysiol.1985.sp015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiol Aging. 2000;21(1):145–152. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB, Jr, Deerfield DW., II GeneDoc: Analysis and Visualization of Genetic Variation, EMBNEW.NEWS. 1997;4:14. [Google Scholar]
- Nikulina EM. Neural control of predatory aggression in wild and domesticated animals. Neurosci Biobehav Rev. 1991;15(4):545–547. doi: 10.1016/s0149-7634(05)80146-0. [DOI] [PubMed] [Google Scholar]
- Olde B, McCombie WR. Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J Mol Neurosci. 1997;8(1):53–62. doi: 10.1007/BF02736863. [DOI] [PubMed] [Google Scholar]
- Ongvarrasopone C, Roshorm Y, Somyong S, Pothiratana C, Petchdee S, Tangkhabuanbutra J, Sophasan S, Panyim S. Molecular cloning and functional expression of the Penaeus monodon 5-HT receptor. Biochim biophys Acta. 2006;1759:328–339. doi: 10.1016/j.bbaexp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ono H, Yoshikawa H. Identification of amine receptors from a swallowtail butterfly, Papilio xuthus L.: cloning and mRNA localization in foreleg chemosensory organ for recognition of host plants. Insect Biochem Mol Biol. 2004;34:1247–1256. doi: 10.1016/j.ibmb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12(4):357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Peeke HV, Blank GS, Figler MH, Chang ES. Effects of exogenous serotonin on a motor behavior and shelter competition in juvenile lobsters (Homarus americanus) J Comp Physiol. 2000;186(6):575–582. doi: 10.1007/s003590000113. [DOI] [PubMed] [Google Scholar]
- Pietrantonio PV, Jagge C, McDowell C. Cloning and expression analysis of a 5HT7-like serotonin receptor cDNA from mosquito Aedes aegypti female excretory and respiratory systems. Insect Mol Biol. 2001;10(4):357–369. doi: 10.1046/j.0962-1075.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- Popova NK, Voitenko NN, Kulikov AV, Avgustinovich DF. Evidence for the involvement of central serotonin in mechanism of domestication of silver foxes. Pharmacol Biochem Behav. 1991;40(4):751–756. doi: 10.1016/0091-3057(91)90080-l. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Marder E. Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus. J Comp Neurol. 2002;451:79–90. doi: 10.1002/cne.10331. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Thirumalai V, Richards KS, Marder E. Dopamine and histamine in the developing stomatogastric system of the lobster Homarus americanus. J Comp Neurol. 2003;462(4):400–414. doi: 10.1002/cne.10767. [DOI] [PubMed] [Google Scholar]
- Qian SM, Delaney KR. Neuromodulation of activity-dependent synaptic enhancement at crayfish neuromuscular junction. Brain Res. 1997;771(2):259–270. doi: 10.1016/s0006-8993(97)00812-3. [DOI] [PubMed] [Google Scholar]
- Ra’anan Z, Cohen D. The ontogeny of social structure and population dynamics in the freshwater prawn Macrobrachium rosenbergii (de Man) In: Schram FM, Wenner A, editors. Crustacean Issues II. Crustacean Growth. Rotterdam: A.A. Balkema; 1985. pp. 277–311. [Google Scholar]
- Ra’anan Z, Sagi A. Alternative mating strategies in male morphotypes of the freshwater prawn Macrobrachium rosenbergii (de Man) Biol Bull. 1985;169:592–601. [Google Scholar]
- Real D, Czternasty G. Mapping of serotonin-like immunoreactivity in the ventral nerve cord of crayfish. Brain Res. 1990;521:203–212. doi: 10.1016/0006-8993(90)91544-q. [DOI] [PubMed] [Google Scholar]
- Rossi-Durand C. Peripheral proprioceptive modulation in crayfish walking leg by serotonin. Brain Res. 1993;632:1–15. doi: 10.1016/0006-8993(93)91131-b. [DOI] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat J-L, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Ache BW. Immunocytochemical analysis of glomerular regionalization and neuronal diversity in the olfactory deutocerebrum of the spiny lobster. Cell Tissue Res. 1997;287:541–563. doi: 10.1007/s004410050778. [DOI] [PubMed] [Google Scholar]
- Schmidt HA, Strimmer K, Vingron M, von Haeseler A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18(3):502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Baro DJ. Amine Effects on aggression in the giant tropical freshwater prawn Macrobrachium rosenbergii. In: Konrad Wiese, editor. The Crustacean Nervous System. New York: Springer; 2002. pp. 143–155. [Google Scholar]
- Sosa MA, Spitzer N, Edwards DH, Baro DJ. A crustacean serotonin receptor: cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J Comp Neurol. 2004;473:526–537. doi: 10.1002/cne.20092. [DOI] [PubMed] [Google Scholar]
- Spitzer N, Antonsen BL, Edwards DH. Immunocytochemical mapping and quantification of expression of a putative type 1 serotonin receptor in the crayfish nervous system. J Comp Neurol. 2005;484:261–282. doi: 10.1002/cne.20456. [DOI] [PubMed] [Google Scholar]
- Spitzer N, Cymbalyuk G, Zhang H, Edwards DH, Baro DJ. Serotonin transduction cascades mediating variable changes in pyloric network cycle frequency in response to the same modulatory challenge. J Neurophys. 2008 doi: 10.1152/jn.00986.2007. [Epub ahead of print] PMID:18400960. [DOI] [PubMed] [Google Scholar]
- Stadler C, Zepf FD, Demisch L, Schmitt M, Landgraf M, Poustka F. Influence of rapid tryptophan depletion on laboratory-provoked aggression in children with ADHD. Neuropsychobiology. 2007;56(2–3):104–110. doi: 10.1159/000112951. [DOI] [PubMed] [Google Scholar]
- Sugamori KS, Sunahara RK, Guan HC, Bulloch AG, Tensen CP, Seeman P, Niznik HB, Van Tol HH. Serotonin receptor cDNA cloned from Lymnaea stagnalis. Proc Natl Acad Sci USA. 1993;90(1):11–15. doi: 10.1073/pnas.90.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ, Mangiamele LA. Effects of serotonin and serotonin analogs on posture and agonistic behavior in crayfish. J Comp Physiol A. 2001;187:757–767. doi: 10.1007/s00359-001-0246-x. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Kim T, Abrams R. Dopamine in crayfish and other crustaceans: Distribution in the central nervous system and physiological functions. Microsc Res Tech. 2003;60:325–335. doi: 10.1002/jemt.10271. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Greenlaw MA, Dams-O’Connor K, Aig SD, Perna AM. Behavioral effects of serotonin and serotonin agonists in two crayfish species, Procambarus clarkii and Orcoectes rusticus. Comp Biochem Physiol Part A. 2004;139:495–502. doi: 10.1016/j.cbpb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Tiu SH, He JG, Chan SM. Organization and expression study of the shrimp (Metapenaeus ensis) putative 5-HT receptor: up-regulation in the brain by 5-HT. Gene. 2005;353:41–52. doi: 10.1016/j.gene.2005.03.037. [DOI] [PubMed] [Google Scholar]
- van den Berg L, Vos-Loohuis M, Schilder MB, van Oost BA, Hazewinkel HA, Wade CM, Karlsson EK, Lindblad-Toh K, Liinamo AE, Leegwater PA. Evaluation of the serotonergic genes htr1A, htr1B, htr2A, and slc6A4 in aggressive behavior of golden retriever dogs. Behav Genet. 2008;38(1):55–66. doi: 10.1007/s10519-007-9179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba C, Boyle PA, Caliguri EJ, De Vries GJ. Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster) Horm Behav. 1997;32(3):184–191. doi: 10.1006/hbeh.1997.1420. [DOI] [PubMed] [Google Scholar]
- Von Nickisch-Rosenegk E, Kubick KS, Laage R, Strobel J, Strotmann J, Breer H. Cloning of biogenic amine receptors from moths (Bombyx mori and Heliothis virescens) Insect Biochem Molec Biol. 1996;26:817–827. doi: 10.1016/s0965-1748(96)00031-8. [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311(2):175–185. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- Winberg S, Winberg Y, Fernald RD. Effect of social rank on brain monoaminergic activity in a cichlid fish. Brain Behav Evol. 1997;49(4):230–236. doi: 10.1159/000112994. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci. 1997;17(2):697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21(2):71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]