Abstract
Allogeneic blood and marrow transplantation (alloBMT) remains the only curative treatment for patients with myelodysplastic syndromes (MDS), but its application has been limited by the older age range of patients with this disease. T cell depletion decreases transplant-related toxicity related to graft-versus-host disease (GVHD), but does not improve overall survival because of increased risk for relapse and graft failure. Myeloid growth factors have been used to speed engraftment following alloBMT, but data suggest that they may also have anti-tumor properties. We treated 43 patients (median age 56) with MDS/AML with high risk features using a myeloablative T cell depleted alloBMT followed by prolonged systemic GM-CSF. The current event-free survival at 1 and 3 years was 47% and 34% respectively with a median follow-up of 22.8 months in surviving patients. The toxicities compared favorably with those seen using reduced intensity conditioning regimens and included grade III/IV GVHD (10%), graft failure (9%), and cumulative treatment related mortality (28%). The cumulative incidence of relapse remained high at 38%; however, 3/10 patients receiving donor lymphocyte infusions achieved durable complete remissions. These results suggest that it is possible to maintain treatment intensity while minimizing toxicity in older, high-risk MDS patients.
Keywords: allogeneic bone marrow transplantation, t cell depletion, myelodysplastic syndrome, acute myeloid leukemia, GM-CSF
Introduction
Myelodysplastic syndromes (MDS) are a complex and heterogeneous group of clonal hematopoietic disorders characterized by ineffective hematopoiesis. The overall incidence of MDS is approximately 2-10 cases per 100,000 people and increases to nearly 50 cases per 100,000 among people over age 70.[1] Currently, the only potential cure for MDS is allogeneic blood or marrow transplantation (alloBMT). A recent review of IBMTR data evaluating 452 patients with MDS undergoing myeloablative transplant from 1989 through 1997 reported transplant-related mortality of 37%, relapse rates of 23%, and an overall survival of 42% at 3 years.[2] However, the median age of patients in this series was only 38 years, whereas the median age of patients with this disease is 70 years. Because increasing age is a risk factor for both conditioning regimen toxicities and graft-versus-host disease (GVHD), concerns about transplant-related toxicities often prevent older patients from being offered alloBMT. [3],[4],[5] Consequently, minimizing toxicity while maintaining efficacy remains the major challenge in treating older MDS patients with alloBMT.
Reduced intensity conditioning (RIC) regimens decrease early treatment related mortality (TRM) secondary to conditioning regimen toxicity, but GVHD remains a major problem. [6],[7],[8] Relapses and graft failure also appear to be increased in MDS patients receiving reduced intensity conditioning.[9],[10],[7],[6] T cell depletion, another approach that can significantly decrease the treatment related morbidity and mortality associated with GVHD, also increases the risk of relapse. [2] While several trials indicate that conditioning regimen intensity is important for disease control in MDS,[11],[12] numerous analyses also show similar overall survivals with myeloablative and RIC regimens, suggesting that the relative advantages and disadvantages of the two approaches counterbalance one another.[6],[7],[9],[10],[8]
Both preclinical and clinical data suggest that GM-CSF may have activity against MDS by inducing differentiation[13],[14] and/or augmenting graft-versus-leukemia reactivity. [15] Thus, we initiated a clinical trial combining a myeloablative preparative regimen, T cell depleted allograft, and prolonged post-transplant GM-CSF to improve alloBMT outcomes in patients with high-risk MDS.
Materials and Methods
Patients
Between August 1998 and December 2004, 43 patients were accrued to this trial (J9852) at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital. Patients age 18 and older diagnosed with MDS with high risk features or AML arising out of MDS were eligible. High risk MDS features were protocol defined to include patients with RAEB, RAEB-T or CMML by FAB classification, those with poor-risk cytogenetic abnormalities, patients with ≥ 2 cytopenias, a diagnostic International Prognostic Scoring System (IPSS) ≥1, history of progression to AML, extended duration between diagnosis and treatment, or progression from initial IPSS score. Diagnostic IPSS is included to portray baseline disease. Morphologic classification at time of trial enrollment is included to portray disease stage at time of transplant. Due to the evolution of classification schema post trial initiation, all pathology specimens were re-reviewed by Johns Hopkins Hematopathology and classified according to WHO criteria for standardization. Cytogenetic data was available on all but two patients. Poor-risk cytogenetic abnormalities were defined as monosomy 7, complex cytogenetics, or chromosome 5 abnormalities outside of the 5q- syndrome.
Methods
Patients within this single institution clinical trial (J9852) at the Sidney Kimmel Cancer Center at Johns Hopkins underwent an HLA-matched sibling donor transplant using standard conditioning regimen including busulfan (Myleran, Glaxosmithkline, USA) (starting at 1 mg/kg orally every 6 hours) on Days -9 through -6 and cyclophosphamide (Cytoxan, Bristol-Myers Squibb, USA) (50mg/kg/day) days -5 through -2.[16] First dose busulfan pharmacokinetics were determined and dosing was adjusted to achieve an area under the curve between 800 and 1400 micromole-minutes/L.[17] Following a day of rest, patients received the T cell depleted bone marrow allograft on Day 0. T cell depletion was performed by counterflow centrifugal elutriation and CD34+ cell add-back as previously described.[18],[19] All patients signed protocol consent forms as per the Johns Hopkins Institutional Review Board.
In addition to T cell depletion for GVHD prophylaxis, patients were treated with cyclosporine (CsA) (Novartis, USA) for 180 days post-BMT. The initial dose (5mg/kg IV beginning Day -1) was decreased to 2.5mg/kg IV for Day +3-14 post BMT. Patients were converted to CsA 10mg/kg orally on Day +15. The dose was decreased to 7.5mg/kg by Day +51, and prior to discontinuation on Day +180 the dose was tapered over the preceding 7 days. In cases of cyclosporine intolerance or concern for hepatic injury, tacrolimus (Prograf/FK506, Fujisawa, USA) was substituted. Supportive care was not protocolized but followed routine monitoring per the Johns Hopkins BMT plan of care and included 1) peri-transplant antibacterial, antifungal, and antiviral prophylaxis; 2) pneumocystis prophylaxis following count recovery; and 3) weekly CMV PCR monitoring with gancyclovir treatment when indicated. Patients received GM-CSF (Leukine, Berlex, USA) 250mcg/m2/day as a subcutaneous injection beginning Day +5, continued at that dose until an absolute neutrophil count (ANC) >2000/mm3 × 3 days, and then decreased to 125mcg/m2/day through Day +60 post-BMT. The dose of GM-CSF was dose modified for grade 3 and 4 toxicity or held for ANC >20,000/mm3 and restarted when ANC decreased to ≤ 10,000/mm3. Patients were taught to self administer the injections as outpatients.
Peripheral blood counts were monitored on a routine basis. Bone marrow assessments were conducted at protocol defined time points post transplant (Day +60, 6 months and 1 year) for cytogenetic analysis and chimerism studies using restriction fragment length polymorphism (RFLP). Peripheral blood chimerism studies were completed at the same time points. Patients who had not reached an ANC ≥ 100 by Day +21 underwent bone marrow aspirate and biopsy evaluation earlier. Additionally, patients with falling counts post-engraftment underwent additional marrow evaluations as clinically indicated. Donor lymphocyte infusion (DLI) was planned at first evidence of MDS recurrence, without additional chemotherapy.
Definitions
Hematopoietic engraftment was defined as time to an ANC of 500/mm3 for 3 consecutive days and a sustained platelet count of 20,000/mm3 untransfused for 7 days. Patients who failed to recover neutrophils or platelets or who continued to require transfusion support with no evidence of chimerism were determined to have engraftment failure. Grades I through IV of acute GVHD were evaluated in patients who survived at least 21 days with evidence of engraftment. Chronic GVHD was evaluated in patients who engrafted and survived at least 90 days after alloBMT. Grading of both acute and chronic GVHD followed standard consensus guidelines.[20],[21] Event free survival was defined as time to event described as relapse or death. Current event free survival included remissions induced by DLI and defined relapse as time of relapse post BMT and DLI, if DLI was received.[22] For analysis of overall survival (OS), the event was death from any cause; surviving patients were censored at the date of last contact. Criteria for relapse were based on bone marrow morphology and peripheral blood counts. Relapse was defined by recurrence of multilineage dysplasia, recurrence of cytogenetic abnormality, or emergence of a phenotypic blast population. Complete remission (CR) was defined as resolution of any prior cytogenetic abnormalities, resolution of dysplasia, and normalization of blood counts.
Statistical Analysis
All patients entered on the trial are included in this analysis. The study's primary endpoint was event free survival (EFS) at one year post-transplant. In addition, time to hematopoietic recovery, overall survival (OS), current EFS, cumulative incidence of relapse, acute and chronic GVHD, and overall and transplant-related mortality (TRM) were assessed. Kaplan-Meier curves were used to describe current EFS and OS, as well as current EFS in terms of diagnostic IPSS score, % marrow blasts at transplant, and disease status at the time of transplant. Competing risk analysis was used to calculate the cumulative incidence of aGVHD with death as a competing risk and to assess TRM with relapse as a competing risk. Patients who did not achieve engraftment were excluded from analysis of GVHD. 95% confidence intervals and p-values were used for making inferences.[23]
Results
Patient Characteristics
A total of 43 patients were enrolled on the proposed trial. Retrospective morphologic analysis performed by hematopathologists blinded to clinical outcomes confirmed initial diagnoses in 40 patients and upgraded 3 patients to de novo AML. The 40 MDS patients met at least one protocol defined poor risk characteristic (FAB class RAEB, RAEBt, CMML or AML from MDS, IPSS ≥ 1, cytopenias in ≥2 lineages, poor-risk cytogenetics, history of progression to AML, extended duration between diagnosis and treatment, or progression from initial IPSS score). As expected, the blinded pathologic review altered the final analysis for patients with MDS. We did include all patients enrolled on the trial in the summary OS and EFS curves to limit selection bias. The three subjects with AML did fall out of the subsequent analyses based on IPSS and FAB subtype.
The median age of the patients was 56 (range 30-65) years with 32% (n=14) of patients age 60 or above and the majority of patients (84%, n=36) age 50 years or above. Forty-two percent (n=18) of the total patients enrolled were women. Calculations of IPSS scores[24] at diagnosis were possible in 38 of the 40 MDS patients. The interval between diagnosis and transplant was less than or equal to 12 months in 84% of patients (n=36) and 81% (n=35) of patients had active disease at time of their transplant. The majority of patients who underwent alloBMT in CR1 were treated with Ara-C based induction and included patients with AML/MDS (n=5), CMML (n=1), RAEB-2 (n=1), and de novo AML (n=1). Patient characteristics including cytogenetics, IPSS at diagnosis, WHO classification and percentage bone marrow blasts at time of transplant, are included in Table 1.
Table 1. Patient Characteristics (n=43).
| Age | Range 30-65 (median = 56 yrs) | |||
|---|---|---|---|---|
| Sex | Male 25 (54%) Female 18 (42%) | |||
| IPSS at Diagnosis (n = 38) | Low: | 0 | Int-1: | 18 (42%) |
| Int-2: | 16 (37%) | High: | 4 (9%) | |
| Unknown: | 2 (5%) | de novo AML: | 3 (7%) | |
| Cytogenetics (n = 41) | Normal: | 22 (51%) | Trisomy 8: | 2 (5%) |
| Complex: | 10 (23%) | Chromosome 7: | 4 (9%) | |
| Misc: | 3 (7%) | Unknown: | 2 (5%) | |
| WHO Classification at Time of BMT | RAEB-1: | 8 (18.5%) | RAEB-2: | 7 (16%) |
| RCMD: | 8 (18.5%) | CMML: | 2 (5%) | |
| MDS-U: | 2 (5%) | de novo AML: | 3 (7%) | |
| AML w/MLD: | 13 (30%) | |||
| % Marrow Blasts at Time of BMT | Less than 5% | 17 (40%) | ||
| 5-10% | 15 (35%) | |||
| 11-20% | 4 (9%) | |||
| 21-30% | 3 (7%) | |||
| >30% | 4 (9%) | |||
| Time from diagnosis to BMT | ≤ 12 months: | 36 (84%) | ||
| > 12 months: | 5 (12%) | |||
| Unknown | 2 (4%) | |||
| Disease Status at Time of BMT | Active Disease: | 35 (81%) | ||
| Remission: | 8 (19%) | |||
WHO = World Health Organization, RAEB-1 = Refractory Anemia with Excess Blasts -1, RAEB-2 = Refractory Anemia with Excess Blasts -2, RCMD = Refractory Cytopenias with Multilineage Dysplasia, CMML = Chronic Myelomonocytic Leukemia, MDS-U = Myelodysplastic Syndrome Unspecified, AML w/ MLD = Acute Myelogenous Leukemia with Multilineage Dysplasia, BMT = Blood and Marrow Transplantation
A median of 2.82 × 106/kg (range 1.43 – 5.88 × 106/kg) CD34+ cells and a median of 1.08 × 105/kg (range 2.29 × 104/kg – 7.62 × 105/kg) CD3+ cells were transplanted. All patients completed the full course of post-transplant GM-CSF with one patient receiving half dose GM-CSF (62.5mcg/m2/day) from Day 26 on. Toxicity attributed to the GM-CSF was low with mild myalgias the most common symptom, comparable to other trials. Busulfan dose adjustments were required in 8 patients, with 2 requiring dose increases and 6 requiring dose reductions.
Hematopoietic Recovery
The median time to neutrophil recovery was 15 (range 9-26) days and to platelet recovery was 19 (range 8-279) days. A total of 4 (9%) patients experienced engraftment failure: one with graft rejection and the remaining 3 patients never achieving engraftment. At the time of alloBMT, 3 of the 4 engraftment failures had active disease and 2 had a marrow blast percentage >15%. All 4 of these patients subsequently died between days 30 and 53.
GVHD
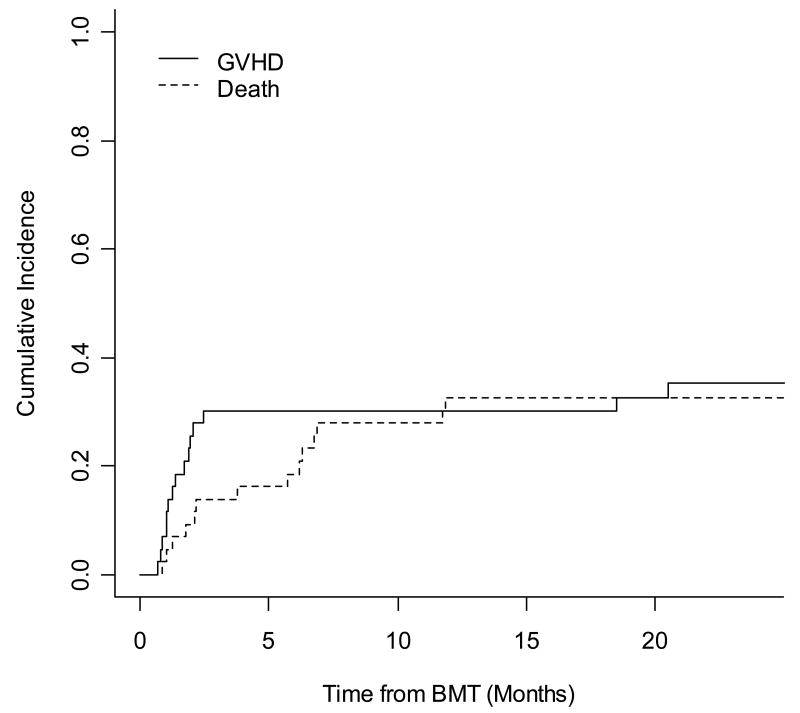
The cumulative incidence of acute GVHD (aGVHD) was 30% (95% CI, 16%, 44%) at 1 year. Of the 39 patients evaluable for aGVHD (engrafted and survived 21 days), only 10% (4/39) experienced grade ≥ 3 aGVHD. The majority of aGVHD was skin only (n=12) and the remaining cases included 1 case of liver only GVHD and 2 cases with multisystem aGVHD. Within the cohort of patients younger than age 50 (n=7) all engrafted and the majority (71%) developed some form of aGVHD; however, only 14% developed advanced aGVHD. Thirty-two out of the 36 patients over the age of 50 showed evidence for engrafted and were therefore evaluable for aGVHD. Overall, this older cohort had a lower overall incidence of aGVHD (31%) as well as an extremely low incidence of severe aGVHD (9%). The incidence of chronic GVHD was also rare with only 1 of the 35 patients who engrafted and survived 90 days post transplant developing chronic GVHD. Competing risk analysis for aGVHD and death is shown in Figure 1. There was no correlation noted between the CD3+/kg dose and the incidence of either acute or chronic GVHD in our patient population when analyzed using CD3+/kg as a continuous variable (p=0.68) as well as in dose groups (p=0.33).
Figure 1.
Competing risk analysis for acute GVHD with death as a competing risk. The cumulative incidence of aGVHD at one year was 30% (95% CI, 16%, 44%).
As expected, new aGVHD was seen following DLI with half of the 10 patients developing either skin only aGVHD (n=3) or liver aGVHD (n=2), including 1 patient with grade IV. There were no cases of chronic GVHD post DLI.
Relapse
The cumulative incidence of relapse at 1 and 3 years was 26% (95% CI, 12%, 39%) and 38% (95% CI, 23%, 53%) respectively. Median time to relapse was 172 days with 18% (n=3) of relapses occurring within 3 months. Specifically, 2 of the 3 early relapses were patients with the longest duration of MDS prior to the BMT (17 and 22 months) and the third entered transplant with the largest volume of disease (blast percentage approximating 70%). Of all the patients who eventually relapsed, 38% did so beyond a year. Relapse initially appeared to track with diagnostic IPSS with a notable exception that only 14% (1/7) of patients in the high risk MDS or de novo AML groups relapsed compared to 39% (7/17) of the INT-1 and 50% (8/16) of the INT-2 patients.
Ten patients who relapsed were treated with donor lymphocyte infusion with 3 of those patients achieving a remission: 1 with a two-year remission and subsequent relapse after receiving 3 escalating DLI infusions and 2 responders currently in complete remission 9 and 12 months after DLI. Every attempt was made to utilize DLI at low disease burden, and 50% (3/6) of patients with ≤5% bone marrow blasts at relapse went into CR following DLI compared to none of the 4 patients with > 5% blasts. Detailed data on all patients receiving DLI are included in Table 2.
Table 2. Characteristics and Treatment Outcomes of Patients Receiving DLI.
| Pt # | Age | WHO Class at Transplant | Disease Status at Transplant | Time to Relapse (days) | Chimerism at Relapse | % BM Blasts at Relapse | DLI CD3+/kg Dose | GVHD Post DLI | Outcomes Post DLI |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 57 | RCMD | Active | 227 | 7% D | 2% |
1st : 1×107 2nd : 5×107 3rd : 1×108 |
Grade II | CR×2 yr then relapse |
| 17 | 65 | RCMD | Active | 52 | 20-40% D | NL | 1st: 1×107 2nd: 4.7× 107 |
None | NR @ 14 mos |
| 21 | 63 | RAEB-1 | Active | 1455 | 56-60% D | 5% | 1×107 | Grade II | CR at 9 mos |
| 23 | 64 | AML w/MLD | Active | 130 | 21-27% D | 27% | 1×108 | None | NR Died 1 mos post |
| 24 | 45 | AML from CMML | Active | 105 | 5-9% D | 20% PB blasts | 5×107 | Grade III | NR: Hospice |
| 29 | 56 | RCMD | Active | 888 | 91-94% D | 6% | 1.13×108 | None | NR Died 6 mos post |
| 31 | 56 | RAEB-2 | Active | 923 | 6-7% D | 3% |
1st : 1×107 2nd : 5×107 |
Grade II | CR at 12 mos from 2nd DLI |
| 33 | 47 | AML from CMML | Active | 123 | 22-27% D | 50%+ blasts | 1×108 | None | NR – Died 1 mos post |
| 36 | 48 | RAEB-1 | Active | 848 | 2-7% D | 2% | 1×107 | None | NR – Died 2 mos post |
| 42 | 50 | RAEB-2 | Active | 492 | 79-85% D | 4% | Data N/A | Grade IV | NR – Died 6 mos post |
D = Donor Chimerism, CR = Complete Response, NR = No response, Yr = Years, Mos = months
Mortality
The cumulative TRM at 1 year was 28% (95% CI, 14%, 42%) and remained unchanged at 3 years. The causes of TRM included 2 deaths from VOD, 4 due to engraftment failure, 2 deaths due to GVHD, 3 secondary to infectious complications (disseminated Aspergillous, fungal pneumonia, typhlitis), and 1 death from multisystem organ failure. Although the majority (11) of 12 treatment-related deaths occurred in the patient cohort age 50 or above, the oldest patients (age 60 or above, n=14) tolerated the transplant fairly well and accounted for only 4 treatment-related deaths. Death related to relapse was the cause of the majority (14) of the 26 deaths, producing a cumulative overall mortality of 54% (95% CI, 33% - 73%) at 3 years with a median time to death of 28.8 months.
Survival
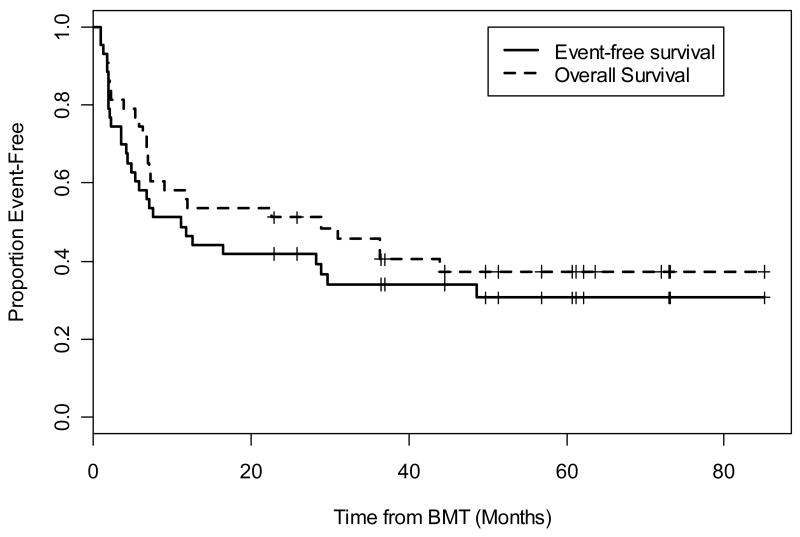
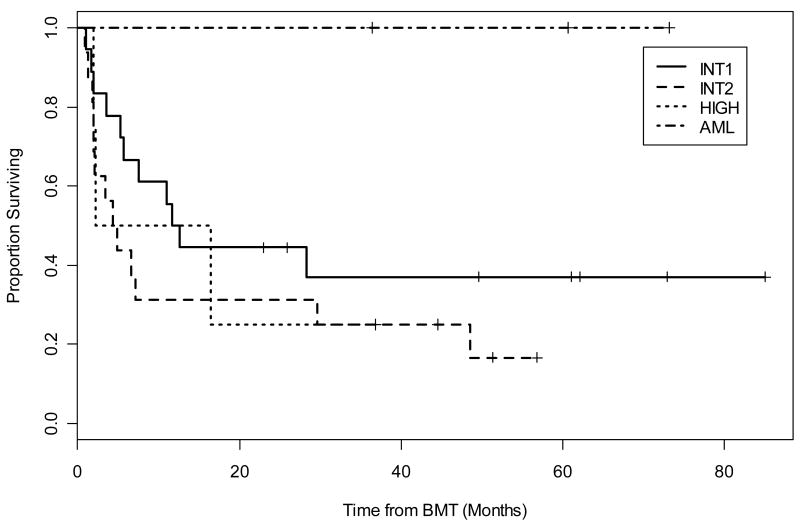
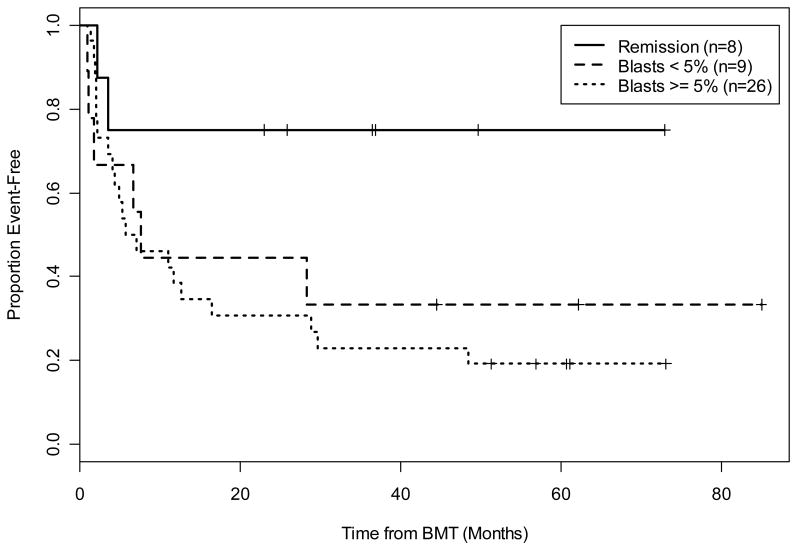
The actuarial OS at 1 and 3 years was 54% (95% CI, 41%, 71%) and 46% (95% CI, 33%, 64%) respectively. Current EFS, including those patients who obtained remission from DLI, at 1 and 3 years were 47% (95% CI, 34%, 64%) and 34% (95% CI, 22%, 52%) respectively (Figure 2). Calculations of EFS without DLI responses were similar with 1 year percentages the same at 47% and 3 year outcomes slightly less at 31%. Clinical characteristics, including IPSS at diagnosis as well as WHO classification, blast percentage, and disease status at time of transplant, were evaluated for any significant relationship to current EFS. Stratification by diagnostic IPSS score suggested improved current EFS in the lower risk group of patients (INT-1) compared to high risk (INT-2, High); however, this did not reach statistical significance (p=0.16) (Figure 3). The patients reclassified as de novo AML showed 100% EFS and remain in CR at 6, 5, and 3 years including one patient who underwent alloBMT with active AML (Figure 3). There did not appear to be a difference in the current EFS based on WHO class morphology possibly due to small patient numbers in each group (data not shown). Stratification by tumor burden as defined by marrow status (CR, <5% marrow blasts and >5% marrow blasts) showed improved EFS between patients transplanted in CR compared with those having ≥ 5% blasts (p=0.03) (Figure 4). The importance of tumor burden was also noted with a trend towards statistical significance in EFS in the patients with active disease and <5% or >5% marrow blasts (p=0.12) (Figure 4).
Figure 2.
The actuarial OS at 1 and 3 years was 54% (95% CI, 41%, 71%) and 46% (95% CI, 33%, 64%) respectively. Current EFS of at 1 and 3 years were 47% (95% CI, 34%, 64%) and 34% (95% CI, 22%, 52%) respectively and included those patients who obtained a remission with DLI. Note: EFS calculations excluding the remissions obtained with DLI were the same at 1 year = 47% and similar at 3 years = 31%; thus, an additional curve was not included.
Figure 3.
Current EFS further categorized by IPSS category at diagnosis or de novo AML. Stratification by diagnostic IPSS score suggested improved current EFS in the INT-1 patients compared to INT-2 and High patients but did not reach statistical significance. (p=0.16)
Figure 4.
Current EFS by tumor burden at the time of transplant showed a clinically significant EFS in those patients entering transplant in remission versus those with the heaviest tumor burden defined by marrow blasts >5% (p = 0.03). There was a trend towards statistical significance with the group in CR versus low tumor burden defined by marrow blasts <5% (p = 0.12).
Discussion
We found that myeloablative conditioning, a T-cell depleted allograft, post-transplant systemic GM-CSF, and early use of DLI was well-tolerated in an older (median age of 56 years) cohort of patients with MDS with high risk features. The “overall toxicity” as measured by TRM and 3 year EFS in our cohort were similar to a group of younger patients (median age 38 years) reported by the IBMTR receiving myeloablative alloBMT[2] and multiple reported series using reduced intensity regimens. [6],[7],[11],[12],[9],[8] Comparing the results of alloBMT trials for MDS is difficult because of significant patient and disease heterogeneity, the use of different staging systems for MDS subgroups, and the use of different preparative regimens and GVHD prophylaxis among trials. Nevertheless, it is apparent that younger patients tolerate both preparative regimen and GVHD toxicities better than the older patients, and efforts to lessen these alloBMT toxicities have decreased upfront mortality at the expense of increased post-BMT relapse. Several components of our alloBMT approach likely contribute to its relative tolerability, including low rates of graft failure, limited preparative regimen toxicity, and minimal GVHD. The roles of TCD in minimizing aGVHD and close monitoring of busulfan levels in decreasing VOD (only 2 patients with clinically significant VOD) are well recognized. Moreover, it is possible that the CD34+ add back in combination with the prolonged GM-CSF also improved expected engraftment success with our resultant graft failure <10%.
Our low overall cumulative incidence of aGVHD (30%) and low severe aGVHD (10%) likely played a significant role in tolerability of the transplant. Interestingly, most of the younger patients (age < 50) in our study developed some form of aGVHD (71%) but only one experienced advanced stage aGVHD and subsequently died of disseminated aspergillous infection. These outcomes are in contrast to only 31% of patients age 50 and older developing some form of GVHD. However, the rate of advanced aGVHD was not statistically significantly different between the younger and older cohort (14% versus 9%). The reason for the overall difference in aGVHD incidence is unclear as all clinical features such as diagnosis, disease status, and blast percentage at the time of transplant appeared well matched between the two groups. The use of prolonged, systemic GM-CSF might have been expected to substantially increase the risk of aGVHD through its well recognized effects on dendritic cell maturation and antigen-presenting cell recruitment[25] with the expectation that the older patients would be the most vulnerable to these effects. However, studies evaluating the role of high dose GM-CSF in the context of vaccine trials now suggest a threshold dose of GM-CSF, that once breached, creates an inhibitory immune environment by inducing a population of myeloid suppressor cells (Gr1+/CD11b+) that functionally impair antigen specific CD4+ T cell response.[26] Thus, it is plausible that the systemic GM-CSF levels achieved in our study surpassed the “theoretical threshold” and may have had a protective effect resulting in the low severe GVHD rates.
As has been reported in other series using T cell depletion [27],[2], relapse remained relatively high in our trial. The prolonged administration of GM-CSF following alloBMT was utilized to provide anti-tumor effects and prevent re-emergence of MDS; we have shown previously that growth factors alone and in combination with pharmacologic differentiating agents can induce terminal differentiation in both leukemia cell lines and primary leukemia cells.[13],[28] However, as described above, GM-CSF may have dose dependent activity with low levels stimulating dendritic cells and increasing antigen presentation and higher levels stimulating production of an inhibitory CD11b+ myeloid population which can dampen T cell and other immune effects. To that end, most relapses occurred outside the planned GM-CSF treatment window with a significant proportion (38%) doing so beyond a year in our study. These results are improved compared to other series showing fewer delayed relapses (25%)[2] and support the potential biologic activity of the growth factor. The 3 early relapses seen in our study may be partially explained by potentially resistant disease with 2 of them representing patients with the longest disease duration (17 and 22 months) and 1 having significant disease burden (70% blasts) at the time of transplant. In balancing the opposing forces of GM-CSF: 1) the potential increased anti-tumor activity and stimulation of aGVHD through increased antigen presentation or through differentiation of the residual malignant clone versus 2) the potential loss of anti-tumor effects and limited aGVHD through the development of myeloid suppressor populations suppressing the immune response, it appeared that the GM-CSF in our trial did not increase aGVHD and may have delayed time to relapse. The excellent toxicity profile of our transplant platform combined with the finding that most relapses occurred outside of the window of GM-CSF administration suggest that extending the duration of planned growth factor may further delay or eliminate relapse in some of our patients. A second intervention that may lead to lower rates of relapse and overall better outcomes made possible in the setting of this well-tolerated transplant platform (and its low incidence of severe aGVHD) is the earlier discontinuation of immunosuppression that might lead to an increased GVL effect.
On the other hand, DLI clearly showed activity as primary salvage therapy for patients who relapsed following transplant. There are limited data regarding the impact of DLI in patients with MDS, but most reports suggest a limited effect on durable disease control.[29],[30] Of the 18 initial relapses on this trial, ten received DLI (without chemotherapy). The eight patients who did not receive DLI had rapidly progressive disease or refused (2 patients). Of the ten patients getting DLI for relapsed MDS, three (30%) achieved complete remission. All three patients developed grade II GVHD that resolved with therapy. The impact of DLI was most evident in early and low disease burden relapse with 50% of patients treated with ≤5% bone marrow blasts achieving a CR. Close monitoring and early intervention with DLI may improve the historically poor results with DLI in MDS patients.
Traditionally, the role of induction chemotherapy prior to alloBMT for MDS patients has been controversial.[31],[32],[33] Our data supports the finding that patients entering alloBMT in remission have a lower risk of relapse.[34],[31],[8] Unfortunately, there are limited randomized data in this setting and a number of trials are heavily weighted towards AML patients making the direct comparison difficult.[8] Thus, determining a true denominator of subjects eligible for either approach, rather than having chemotherapy serve as a selection tool for the “best risk” patients, has been challenging. Although the denominator in our series doesn't allow for definitive conclusions, our data would support the position that when successful, induction therapy seems to impact the success of alloBMT. Our data further substantiate the importance of low tumor burden noting an improved EFS in patients transplanted in CR compared to those with marrow blasts > 5%. Other groups have also showed improved outcomes in patients transplanted with lower with lower tumor burden[2] and suggests that this is an important element in determining the optimal timing for allogeneic transplant.
The curative potential of alloBMT in MDS has primarily been attributed to immunologic anti-tumor effects. However, while reduced intensity conditioning regimens minimize the chemotherapy-related side effects of alloBMT for older patients, emerging literature supports the contributions of the conditioning regimen intensity to disease control in MDS, especially in patients with high risk, advanced disease.[6],[7],[12],[11], The use of T cell depletion and busulfan monitoring not only appeared to reduce the toxicity of alloBMT while maintaining dose intensity, but also allows for consideration of additional post-BMT interventions to further improve disease control. For example, the low incidence of GVHD with T cell depletion allows consideration of preemptive DLI for patients at high risk of relapse. Alternatively, one of the newly FDA-approved agents for MDS (azacitidine, decitabine, or lenalidomide) may also be incorporated into the post-BMT setting as another means to decrease possible relapses.
Acknowledgments
This work was supported in part by a grant from the NIH entitled “Bone Marrow Transplantation in Human Disease” (PO1CA15396). There are no financial conflicts of interest to disclose in regards to all authors included.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Aul C, Giagounidis A, Germing U. Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital-based statistics. Int J Hematol. 2001;73(4):405–410. doi: 10.1007/BF02994001. [DOI] [PubMed] [Google Scholar]
- 2.Sierra J, Perez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100(6):1997–2004. [PubMed] [Google Scholar]
- 3.Sutton L, Chastang C, Ribaud P, Jouet JP, Kuentz M, Attal M, et al. Factors influencing outcome in de novo myelodysplastic syndromes treated by allogeneic bone marrow transplantation: a long-term study of 71 patients Societe Francaise de Greffe de Moelle. Blood. 1996;88(1):358–365. [PubMed] [Google Scholar]
- 4.Cahn JY, Labopin M, Schattenberg A, Reiffers J, Willemze R, Zittoun R, et al. Allogeneic bone marrow transplantation for acute leukemia in patients over the age of 40 years. Acute Leukemia Working Party of the European Group for Bone Marrow Transplantation (EBMT) Leukemia. 1997;11(3):416–419. doi: 10.1038/sj.leu.2400573. [DOI] [PubMed] [Google Scholar]
- 5.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23(15):3439–3446. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 6.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104(3):865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 7.Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20(1):128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 8.Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13(4):454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108(3):836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 10.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23(36):9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 11.Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20(2):322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 12.Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12(10):1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Matsui WH, Gladstone DE, Vala MS, Barber JP, Brodsky RA, Smith BD, et al. The role of growth factors in the activity of pharmacological differentiation agents. Cell Growth Differ. 2002;13(6):275–283. [PubMed] [Google Scholar]
- 14.Smith BD, Matsui WH, Gladstone DE, Karp JE, Gore SD, Huff CA, et al. Differentiation Therapy for Poor Risk Myeloid Malignancies: Results of a Dose Finding Study of Bryostatin-1 (BRYO) + GM-CSF. Blood. 2005;106(11):783a. [Google Scholar]
- 15.Platzbecker U, Thiede C, Freiberg-Richter J, Helwig A, Mohr B, Prange G, et al. Treatment of relapsing leukemia after allogeneic blood stem cell transplantation by using dose-reduced conditioning followed by donor blood stem cells and GM-CSF. Ann Hematol. 2001;80(3):144–149. doi: 10.1007/s002770000258. [DOI] [PubMed] [Google Scholar]
- 16.Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309(22):1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 17.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20(4 Suppl 4):18–25. [PubMed] [Google Scholar]
- 18.Huff CA, Fuchs EJ, Noga SJ, O'Donnell PV, Ambinder RF, Diehl L, et al. Long-term follow-up of T cell-depleted allogeneic bone marrow transplantation in refractory multiple myeloma: importance of allogeneic T cells. Biol Blood Marrow Transplant. 2003;9(5):312–319. doi: 10.1016/s1083-8791(03)00075-2. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell PV, Jones RJ, Vogelsang GB, Seber A, Ambinder RF, Flinn I, et al. CD34+ stem cell augmentation of elutriated allogeneic bone marrow grafts: results of a phase II clinical trial of engraftment and graft-versus-host disease prophylaxis in high-risk hematologic malignancies. Bone Marrow Transplant. 1998;22(10):947–955. doi: 10.1038/sj.bmt.1701476. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 21.Akpek G, Zahurak ML, Piantadosi S, Margolis J, Doherty J, Davidson R, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood. 2001;97(5):1219–1226. doi: 10.1182/blood.v97.5.1219. [DOI] [PubMed] [Google Scholar]
- 22.Craddock C, Szydlo RM, Klein JP, Dazzi F, Olavarria E, van Rhee F, et al. Estimating leukemia-free survival after allografting for chronic myeloid leukemia: a new method that takes into account patients who relapse and are restored to complete remission. Blood. 2000;96(1):86–90. [PubMed] [Google Scholar]
- 23.Kalbfleisch P. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 24.Greenberg P, Cox C, Lebeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 25.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110(1):9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 27.Mattijssen V, Schattenberg A, Schaap N, Preijers F, de Witte T. Outcome of allogeneic bone marrow transplantation with lymphocyte-depleted marrow grafts in adult patients with myelodysplastic syndromes. Bone Marrow Transplant. 1997;19(8):791–794. doi: 10.1038/sj.bmt.1700739. [DOI] [PubMed] [Google Scholar]
- 28.Matsui W, Smith BD, Vala M, Beal N, Huff CA, Diehl LF, et al. Requirement for myeloid growth factors in the differentiation of acute promyelocytic leukaemia. Br J Haematol. 2005;128(6):853–862. doi: 10.1111/j.1365-2141.2005.05395.x. [DOI] [PubMed] [Google Scholar]
- 29.Depil S, Deconinck E, Milpied N, Sutton L, Witz F, Jouet JP, et al. Donor lymphocyte infusion to treat relapse after allogeneic bone marrow transplantation for myelodysplastic syndrome. Bone Marrow Transplant. 2004;33(5):531–534. doi: 10.1038/sj.bmt.1704381. [DOI] [PubMed] [Google Scholar]
- 30.Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transplant. 2000;26(7):769–774. doi: 10.1038/sj.bmt.1702596. [DOI] [PubMed] [Google Scholar]
- 31.de Witte T, Zwaan F, Hermans J, Vernant J, Kolb H, Vossen J, et al. Allogeneic bone marrow transplantation for secondary leukaemia and myelodysplastic syndrome: a survey by the Leukaemia Working Party of the European Bone Marrow Transplantation Group (EBMTG) Br J Haematol. 1990;74(2):151–155. doi: 10.1111/j.1365-2141.1990.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 32.de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, et al. Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110(3):620–630. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 33.Alessandrino EP, Amadori S, Barosi G, Cazzola M, Grossi A, Liberato LN, et al. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002;87(12):1286–1306. [PubMed] [Google Scholar]
- 34.de Witte T, Suciu S, Verhoef G, Labar B, Archimbaud E, Aul C, et al. Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood. 2001;98(8):2326–2331. doi: 10.1182/blood.v98.8.2326. [DOI] [PubMed] [Google Scholar]