Abstract
Background
Previous research has shown that a manufacturer’s promotional strategy for a brand-name drug is typically affected by generic entry. However, little is known about how newer strategies to extend patent life, including product reformulation introduction or obtaining approval to market for additional clinical indications, influence promotion.
Objective
To examine the relationship between promotional expenditures, generic entry, reformulation entry, and new indication approval.
Study Design/Setting
We used quarterly data on national product-level promotional spending (including expenditures for physician detailing and direct-to-consumer advertising (DTCA), and the retail value of free samples distributed in physician offices) for selective serotonin reuptake inhibitors (SSRIs) over the period 1997 through 2004. We estimated econometric models of detailing, DTCA, and total quarterly promotional expenditures as a function of the timing of generic entry, entry of new product formulations, and Food and Drug Administration (FDA) approval for new clinical indications for existing medications in the SSRI class.
Main Outcome Measure
Expenditures by pharmaceutical manufacturers for promotion of antidepressant medications.
Results
Over the period 1997–2004, there was considerable variation in the composition of promotional expenditures across the SSRIs. Promotional expenditures for the original brand molecule decreased dramatically when a reformulation of the molecule was introduced. Promotional spending (both total and detailing alone) for a specific molecule was generally lower after generic entry than before, although the effect of generic entry on promotional spending appears to be closely linked with the choice of product reformulation strategy pursued by the manufacturer. Detailing expenditures for Paxil were increased after the manufacturer received FDA approval to market the drug for generalized anxiety disorder (GAD), while the likelihood of DTCA outlays for the drug was not changed. In contrast, FDA approval to market Paxil and Zoloft for social anxiety disorder (SAD) did not affect the manufacturers’ detailing expenditures but did result in a greater likelihood of DTCA outlays.
Conclusion
The introduction of new product formulations appears to be a common strategy for attempting to extend market exclusivity for medications facing impending generic entry. Manufacturers that introduced a reformulation before generic entry shifted most promotion dollars from the original brand to the reformulation long before generic entry, and in some cases manufacturers appeared to target a particular promotion type for a given indication. Given the significant impact pharmaceutical promotion has on demand for prescription drugs, these findings have important implications for prescription drug spending and public health.
I. Introduction
Pharmaceutical manufacturers employ a variety of strategies to promote their medications, including direct-to-consumer advertising (DTCA), physician detailing, journal advertising, and the provision of free samples for physicians to distribute in their offices. The aims of these activities are to enhance profitability by shifting demand and perhaps altering the shape of demand curves for their products. Pharmaceutical promotional expenditures have been increasing steadily in recent years, and represent a significant portion of the industry’s post-launch non-research and development (R&D) costs (Donohue et al., 2007).1 In 2005, manufacturers spent over $11 billion on DTCA, detailing, and journal advertising; the retail value of free samples provided in physicians offices was estimated to be $18.4 billion (Donohue et al., 2007).1
The level of pharmaceutical promotional expenditures for a given drug is sensitive to competitive market conditions, among other factors. Because generic entry involves the marketing of “chemical carbon copies” at substantially lower prices than those of branded originators, a substantial drop in total brand plus generic sales revenue results following generic entry (e.g., Frank and Salkever, 1997; CBO 1998; Grabowski and Vernon, 1992; Hudson, 2000).2–5 Previous theoretical models have shown that manufacturers’ promotional strategies are likely to be affected by entry of generic equivalents (Frank and Salkever, 1992).6 In fact, empirical research has generally found that a branded drug’s promotional spending decreases in anticipation of and immediately following entry by a generic equivalent (e.g., Hurwitz and Caves, 1988; Caves, Whinston, and Hurwitz, 1991; Iizuka, 2004; Berndt, Kyle, and Ling, 2002).7–10 Less is known about the effects on a branded drug’s promotional strategy when a competing brand molecule in the class faces generic entry. Branded products can extend the life of their marketing exclusivity by introducing product reformulations and obtaining Food and Drug Administration (FDA) approval to market an existing drug for additional clinical indications in advance of generic entry. These “product life cycle” strategies may be undertaken to extend market exclusivity and preserve profitability. Over the past decade, several manufacturers have introduced reformulated products or obtained FDA approval to market their drug for additional indications (e.g., Glaxo SmithKline received FDA approval to market Paxil for generalized anxiety disorder in April 2001). Product reformulation and expansion of clinical indications can be expected to affect promotional expenditures. There is little empirical evidence, however, on the effect of these strategies on manufacturer promotion for the originator product.
In this research, we examine the relationship between promotional expenditures for a class of antidepressants called selective serotonin reuptake inhibitors (SSRIs), and generic competition, entry of reformulated products, and new indication approval. SSRIs were among the most heavily promoted drugs, with total promotional spending for SSRIs and selective norepinephrine reuptake inhibitors (SNRIs) together equaling over $1 billion in 2005 (Donohue et al., 2007).1 Four commonly-used SSRIs (Prozac, Paxil, Zoloft, and Celexa) lost patent protection between 2001 and 2006, resulting in generic competition in the class. In addition to generic competition, there has also been increased therapeutic competition due to the introduction of several new product formulations of existing brand molecules, including Lexapro (an isomer of the brand drug Celexa), Paxil CR, and Prozac Weekly. Several SSRI manufacturers have also obtained FDA approval to market their SSRI for clinical indications other than depression. We examine how therapeutic and generic competition within the SSRI class affects the type and level of promotional spending by SSRI manufacturers. We also explore the timing of new product entry and subsequent promotional strategies.
II. Unique Characteristics of the Pharmaceutical Industry
The pharmaceutical industry has a number of distinguishing characteristics that have important implications for explaining spending on promotional activities. First, consumers must obtain a physician’s prescription in order to buy most pharmaceuticals. The importance of physician decision-making in pharmaceutical markets is underscored by the fact that well over 60% of the combined promotional expenditures are aimed at physicians (IMS Health, 2005).11
A second distinguishing feature of the market for pharmaceuticals is that, like most health care services, the majority of prescription drug expenditures are paid for by third party insurers. Currently, nearly all (98%) covered workers in employer-sponsored health insurance plans have prescription drug benefits (Kaiser Family Foundation, 2005).12 Prescription drug benefits are also covered under Medicaid and Medicare (as of 2006). In 2002, third party payers accounted for 70% of prescription drug expenditures (Smith, 2004).13 In recent years, insurers/pharmacy benefit managers (PBMs) have sought to encourage use of lower cost generics as well as stimulate price competition among brand name drugs through the use of tiered or incentive-based formularies (Gibson, Ozminkowski, and Goetzel, 2005).14 Incentive-based formularies have been shown to reduce demand for drugs placed on the highest copay tier by 22% to 65% depending on the therapeutic class of drugs (Huskamp et al., 2003).15
These two demand side characteristics interact with a third important characteristic of prescription drug markets -- the presence of monopoly power on the supply-side -- to support prices that well exceed marginal production costs (Scherer, 2000).16 Monopoly power is conferred by patent protection, which makes it illegal for any other company to sell the same drug between the time the FDA approves a drug and its patent expires (or is deemed invalid). The Hatch Waxman Act extended the duration of monopoly protection, granting exclusive marketing rights (known as exclusivity) to pharmaceutical manufacturers for a period of at least five and not more than 14 years from FDA approval date for new drugs (referred to as new chemical entities (NCEs) or new molecular entities (NMEs)). Pharmaceutical firms may obtain three years of additional exclusivity for new indications or formulations. The introduction of new product formulations and applications for approval for new indications have become increasingly common in recent years, yet the implications for promotional spending for the originator product and the new formulation are unknown.
Finally, marginal production costs for many prescription drugs are very low, literally “pennies a pill.” An implication of this is that any promotional activities that generate more sales than their promotion and relatively small marginal production costs will be profitable for a manufacturer whose product is patent protected.
III. Theoretical Considerations
In their classic paper on optimal advertising, Dorfman and Steiner (1954) showed that the optimal advertising to sales ratio for a profit-maximizing monopolist facing a downward-sloping linear demand curve is equal to the ratio of the elasticity of demand with respect to advertising and the (absolute value of the) elasticity of demand with respect to price.17 Although the Dorfman-Steiner theorem assumes that advertising lasts only one time period, it can be generalized to a dynamic model where the effects of advertising are long-lived and persist into the future (Schmalensee, 1972).18
The Dorfman-Steiner result implies that the marginal profitability from advertising increases as the price elasticity of demand for the good falls (in absolute value). An implication of this theorem for pharmaceutical markets is that the optimal level of advertising intensity will decline with generic entry. When generic equivalents enter the market, the numerator of the ratio (the elasticity of demand with respect to advertising) is expected to decrease, because of the potential spillover of any branded promotional efforts onto the generic alternative. At the same time, the denominator of the ratio (the price elasticity of demand) is expected to increase, as generic entry allows a set of nearly perfect substitutes into the market. As a result, the Dorfman-Steiner theorem implies that the optimal advertising to sales ratio for a brand-name drug facing generic competition will decrease.
The Dorfman-Steiner theorem describes the optimal level of total promotional expenditures relative to sales. It does not, however, shed light on the optimal ratios of different types of promotional spending. Palda (1969) showed that when there are several types of promotional instruments and constant unit promotional costs, the optimal ratio of spending for any two types of promotion equals the ratio of their promotional elasticities.19 Thus, generic entry should result in proportional declines in all forms of promotion intensity.
IV. Prior Empirical Literature on Pharmaceutical Promotion
In this section, we review the empirical studies of determinants of pharmaceutical promotional spending. Direct-to-consumer advertising of prescription drugs has only constituted a significant portion of pharmaceutical promotional spending since the mid-1990s (Rosenthal et al., 2002);20 therefore, earlier studies focused exclusively on promotion to physicians.
Hurwitz and Caves (1988) studied the determinants of pharmaceutical promotional spending in a study of 150 products from 29 multisource drug markets (in which both brand name and generic equivalents were sold) between 1978 and 1983.7 Promotional spending included detailing, journal advertising, and direct-mail advertising. They examined the impact of a number of structural and competitive factors on product-specific advertising to sales ratios. Their cross-sectional regressions showed that the number of years since the first generic competitors entered the market had the largest impact on a brand name drug’s advertising to sales ratio. Caves, Whinston and Hurwitz (1991) examined branded and generic drug prices, market shares, quantities sold, and brand advertising (defined as detailing and journal advertising) for a panel of 30 drugs that lost patent protection between 1976 and 1987.8 They found that generic entry had a negative impact on advertising expenditures, with advertising outlays falling during the two years prior to generic entry. Branded advertising fell 20% with entry of the first generic, an additional 40% when the number of generic entrants reached five, and an additional 20% when the number of generic competitors reached ten. Similarly, in an analysis of 98 drugs that lost patent protection between 1986 and 1992, Scott-Morton (2000) found that the amount of time on patent was strongly negatively correlated with expenditures on detailing and journal advertising, meaning that the longer a drug had been on the market the less was spent on promotion.21
In a study of the product- and market-level determinants of spending on DTCA, Iizuka (2004) examined a total of 606 drug-year observations for 169 unique brand name central nervous agents, respiratory agents, and renal and genitourinary agents over the period 1996 through 1999.9 He found that the number of branded drugs in the therapeutic class had a negative and significant effect on DTC advertising outlays. In addition, the coefficient for generic entry was large, negative and statistically significant. These findings are consistent with the Dorfman-Steiner theorem, which predicts lower advertising to sales ratios in more competitive markets where demand is more price elastic. Iizuka (2004) examined the impact of product-level generic entry but not generic entry elsewhere in the class as a whole or among close competitors, on an individual firm’s DTCA outlays.
In one of the only analyses of the effect of generic entry on a competitor’s promotional spending, Berndt, Kyle and Ling (2003) examined changes in detailing minutes and pages of journal advertising associated with Zantac (an H2 blocker used to treat acid reflux) losing patent protection in the late 1990s.10 In the months leading up to Zantac’s patent expiration date and in the months afterward, the manufacturers of Axid and Pepcid (two other H2 blockers that had not yet lost patent protection) decreased detailing for their products. Journal advertising increased for Pepcid in the months before Zantac generic entry and dropped afterward, while journal advertising for Axid dropped before Zantac generic entry and continued to drop afterward. The case of the H2 blockers is somewhat unique because the H2 market was already in decline during the period that the H2 blockers began losing patent protection due to the introduction of proton pump inhibitors (PPIs), which are generally regarded as more potent therapeutic substitutes, and the launch of over-the-counter versions of the H2 blockers. Hence, the results are difficult to interpret in light of the theory set out above.
While most studies have focused on the potential for advertising to affect demand for a given drug, Ellison and Ellison (2000) hypothesized that advertising and new product formulation introduction might also be used as tools to deter generic entry, particularly in medium-sized markets (in very large and very small markets, deterring generic entry was unlikely or unnecessary, respectively).22 Using a panel of drugs that lost patent protection between 1986 and 1992, they report some empirical evidence suggesting that incumbents in medium-sized markets facing generic entry were more likely than incumbents in similarly-sized markets not facing entry to decrease detailing expenditures. Those facing generic entry were also more likely to introduce reformulations as patent expiration approached.
Thus, the few studies that have examined the determinants of pharmaceutical promotional spending directed at physicians and consumers have consistently found time left on patent and generic entry to have strong negative effects on own product promotion, a finding that is consistent with the Dorfman-Steiner theorem. It is reasonable to expect that the price elasticity is greater in absolute value for drugs that have generic substitutes because mandatory substitution policies, tiered formularies, and other financial incentives that favor generics induce demand for the generic versions of these molecules. The studies of pharmaceutical promotion reviewed above, however, do not examine the impact of generic entry by a competitor or new product formulation entry on promotional outlays. Studies of price competition in pharmaceutical markets point to strong price competition among generics and weaker competition among brand name drugs (Ellison et al, 1997; Frank and Salkever, 1997; Reiffen and Ward, 2005; Lu and Comanor, 1998).2, 23–25 This suggests that generic competition has large effects on demand elasticities, whereas therapeutic competition has more modest demand response effects. We therefore expect own generic entry to have much stronger impacts on promotional outlays than entry by other brands.
V. The SSRI Market
SSRIs are medications used commonly to treat depression. The first SSRI, Prozac, received FDA approval in 1987. Zoloft, the second SSRI approved by the FDA, entered the market in 1991. Paxil entered the market in 1992 and Celexa in 1998. A fifth SSRI, Luvox, received FDA approval in December 1994 to treat obsessive compulsive disorder only. Although an SSRI, Luvox was never approved for the treatment of depression and was thus excluded from our analysis.
Prozac was the first SSRI to lose patent protection (Table 1). Prozac’s patent expired in August 2001 and generic fluoxetine products entered at that time. Generic entry followed for Paxil in June 2003, Celexa in October 2004, and Zoloft in June 2006.
Table 1.
New Indications and Product Entry for SSRIs
| SSRI | Initial FDA Approval | Generic Entry | New Indication | New Formulation Entry |
|---|---|---|---|---|
| Prozac* | 12/87 | 8/01 | PMDD 7/00 | Prozac Weekly 3/01 |
| Panic Disorder 7/02 | ||||
| Zoloft | 12/91 | 6/06 | Panic Disorder 7/97 | -- |
| PTSD 12/99 | ||||
| PMDD 5/02 | ||||
| SAD 2/03 | ||||
| Paxil | 12/92 | 6/03 | SAD 5/99 | Paxil CR 4/02 |
| GAD 4/01 | ||||
| Celexa | 7/98 | 10/04 | -- | Lexapro 8/02 |
PMDD=premenstrual dysphoric disorder; PTSD=post-traumatic stress disorder; SAD=social anxiety disorder; GAD=generalized anxiety disorder
Prozac also has an indication for use in pediatric populations.
Data sources: FDA Orange Book of Approved Therapeutic Equivalents; FDA New and Generic Drug Approvals, http://www.fda.gov/cder/approval/index.htm; FDA Safety Related Drug Labeling Changes,http://www.fda.gov/medwatch/
New product formulations of three of the four SSRIs indicated for the treatment of depression have been introduced over the past several years (Table 1). In March 2001, Eli Lilly introduced Prozac Weekly, a once-weekly formulation of Prozac. In April 2003, GlaxoSmithKline (then SmithKline Beecham) introduced Paxil CR, a controlled-release version of Paxil. In August 2002, Forest Labs introduced Lexapro (an isomer of the Celexa molecule) and claimed that Lexapro may have a slightly shorter onset of action than Celexa (Burke, Gergel, and Bose, 2002).26
Moreover, manufacturers of three of the four SSRIs initially approved for the treatment of depression have sought and obtained FDA approval to market the drugs for additional indications, including panic disorder, generalized anxiety disorder (GAD), premenstrual dysphoric disorder (PMDD), and post traumatic stress disorder (PTSD). Two of the four drugs (Paxil in May 1999 and Zoloft in February 2003) received FDA approval for social anxiety disorder (SAD), an illness whose diagnosis and treatment are somewhat controversial (Scott, 2006).27
VI. Data and Methods
We examined factors affecting promotional spending for the four molecules in the SSRI class that are indicated for the treatment of depression: Prozac, Paxil, Zoloft, and Celexa. We employed quarterly data on product-level promotional spending over the period 1997 through 2004. DTCA expenditure data were obtained from TNS Media Intelligence (formerly Competitive Media Reporting), physician detailing expenditure data were obtained from Verispan, and data on the retail value of free samples were obtained from IMS Health.
We specified and estimated two types of empirical models of promotion. First, we estimated drug-specific models of total quarterly promotional expenditures (DTCA, detailing, and sampling combined) and detailing expenditures alone. Second, we estimated a pooled (across drug products over time) logit model of the likelihood of any DTCA expenditures in a given quarter. Unlike detailing expenditures, which are consistently non-zero from quarter to quarter, DTCA expenditures can be highly variable over time. As a result, we estimated the probability of any DTCA expenditures in a quarter rather than the level of spending. We do not estimate separate models for sampling expenditures because of the high correlation between detailing and sampling spending.
Quarterly total and detailing spending models
As noted above, we estimated multivariate regression models of the logarithm of expenditures for each SSRI and promotional type combination (e.g., Celexa total promotional expenditures, Celexa detailing expenditures). We estimated two specifications for these models, which differ based on the treatment of generic entry. In the first specification, we included a dummy variable that equaled one if the drug had lost patent protection and zero otherwise. In the second specification, we used two variables to measure the effects of generic entry. First, each model included a variable controlling for the number of quarters until patent expiration for the molecule. This variable equals one divided by the number of quarters until expiration (1/Nq) and one for all quarters after patent expiration. [Therefore its maximum value was constrained to one.] We hypothesized that as patent expiration approached, promotional spending would decrease, which would result in a negative coefficient for this variable. Recognizing the pre/post patent expiration asymmetry, we controlled for the number of quarters since the molecule’s patent expired in the Paxil and Prozac models. This variable equals zero for all quarters before patent expiration, one in the quarter of patent expiry, and is a counter variable thereafter. Because Celexa’s patent expired in the last quarter of our study period, we did not add this variable to the Celexa models. Zoloft remained on patent throughout our study period, so the Zoloft models do not contain this variable.
To explore the effects of generic entry by a competitor, in both specifications we included dummy variables indicating whether generic entry by a particular competitor molecule had occurred. For example, the Paxil models included a dummy variable indicating whether generic entry by fluoxetine had occurred; the Celexa models included the same variable, as well as a dummy variable indicating whether generic entry by paroxetine had occurred. The Prozac models do not include the paroxetine entry variable because paroxetine entered the market almost two years after Prozac lost its patent, and at that time there was very little promotional spending for Prozac.
Models also included dummy variables indicating whether a reformulated product using the same molecule had been launched. Thus, the Prozac models included a dummy variable for entry of Prozac Weekly, the Celexa models included a dummy variable for Lexapro entry, and the Paxil models included a dummy variable for Paxil CR entry. Finally, the models also included dummy variables representing whether the drug had received FDA approval for indications other than depression. We focused on two indications -- generalized anxiety disorder (GAD) and social anxiety disorder (SAD) -- that were likely to represent sizeable markets for SSRIs due to the prevalence of these conditions (Kessler et al, 2005).28 The Zoloft models contained a variable indicating approval of an indication for SAD, while the Paxil models contained variables for approval of an indication for SAD and for GAD. We hypothesized that the GAD indication would be associated with increased detailing expenditures because of the prevalence of GAD, particularly among patients seen in primary care settings. We hypothesized that the SAD indication would be associated with increased DTCA expenditures since manufacturers wished to convey the availability of a treatment for this previously unapproved indication.
Because we were concerned about the possibility of partial adjustment in promotional spending over time since media promotion contracts frequently are longer in length than one quarter, we estimated partial adjustment models of the logarithm of expenditures for each drug and promotion combination. This involved including an explanatory variable for the lagged value of the dependent variable to the models described above. The coefficient on the lagged expenditure variable was statistically significant only in the partial adjustment model of Paxil detailing expenditures, so we selected a model with contemporaneous spending as the dependent variable (i.e., the logarithm of quarterly spending at time t) for the expenditure analyses. The partial adjustment model results for Paxil promotional expenditures were implausible (i.e., a negative adjustment of promotional spending) so we do not present these results. We also tested for the presence of autocorrelation using Durbin’s m statistic. In all models but one (the model of Paxil detailing expenditures), we could not reject the null hypothesis of no autocorrelation.
Probability of DTCA spending model
As noted above, because manufacturers’ DTCA expenditures are highly variable compared to those for detailing (i.e., a drug may have high DTCA spending in one month and none in the next), we estimated a pooled logit model of the probability of any DTCA spending in a given quarter. The unit of observation was a drug-quarter. The model included as explanatory variables the time to patent expiration variable used in the models of promotional expenditures as well as a dummy variable indicating whether a new formulation of the molecule had entered and a variable indicating the order of entry for the molecule (e.g., for Prozac, equals “1” since Prozac was the first to be approved by the FDA; for Zoloft, equals “2” since Zoloft was the second, and so on). The model also included a dummy variable indicating whether the molecule had received FDA approval for an indication to treat social anxiety disorder, and a time counter variable named “Quarter.”
In all these models, we assumed that generic entry, which we postulate is largely beyond the control of the branded drug manufacturer, is exogenous to manufacturer decisions about promotional spending. As a result, we interpret the coefficients on the generic entry variables as representing causal effects. By contrast, the decision to introduce a reformulated product is jointly determined along with promotional spending decisions. In examining coefficients on the reformulation entry variables, we look for a partial correlation that is consistent with the Dorfman-Steiner theorem described above rather than a causal relationship. We hypothesize that manufacturer promotional spending on the original product will drop substantially after the introduction of a product reformulation, as the manufacturer attempts to shift demand from the originator brand product (which has limited time left on patent) onto the reformulation.
VII. Results
Over the period 1997–2004, there was considerable variation in the composition of promotional expenditures across these four products. Prozac and Zoloft, the first two entrants, followed similar strategies. The manufacturers of each spent approximately four times more on physician detailing than they spent on DTCA over this time period. DTCA expenditures were approximately 8% of total promotional spending for both drugs, while detailing expenditures were 30% for Prozac and 27% for Zoloft. Note that the estimated share spent on free samples (62% for Prozac and 65% for Zoloft) is likely an overestimate of promotion costs to the manufacturer since IMS Health estimates this promotion cost as equaling the retail value of free samples, computed as the average wholesale (list) price (for more discussion of this issue see Berndt 200729). Paxil, the third entrant, devoted a larger share of its promotional expenditures to DTCA. Paxil’s manufacturer spent less than twice as much on detailing as on DTCA. Celexa, the last entrant, employed no DTCA, focusing instead on detailing and free samples.
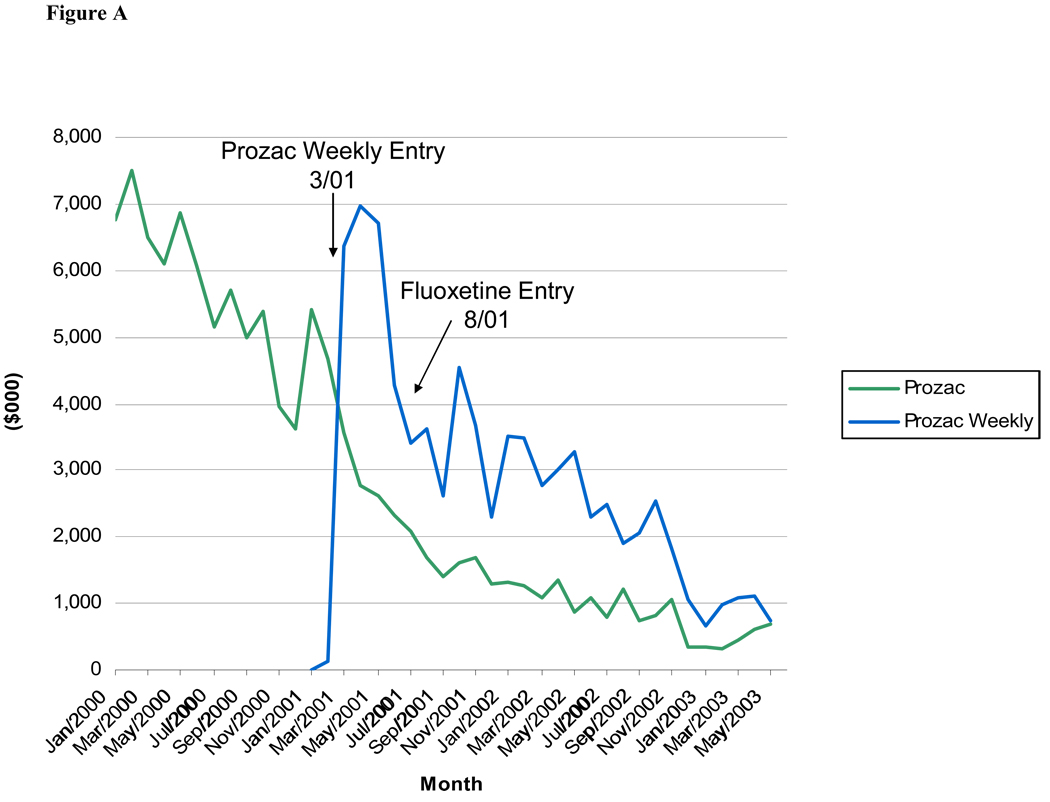
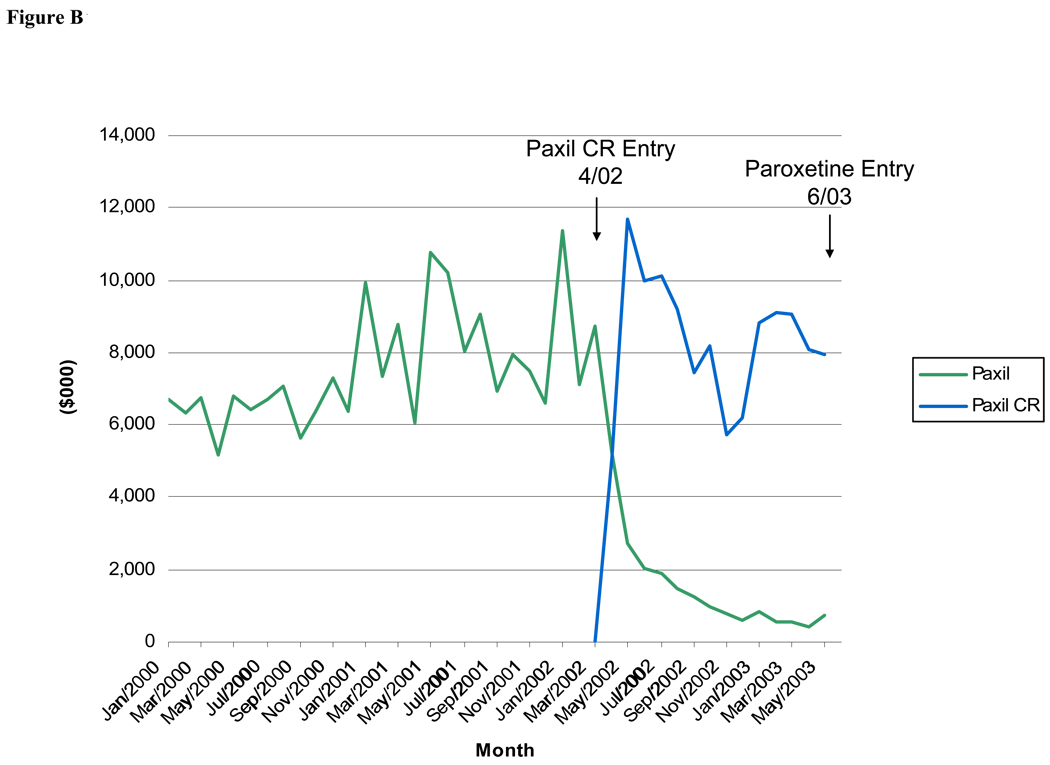
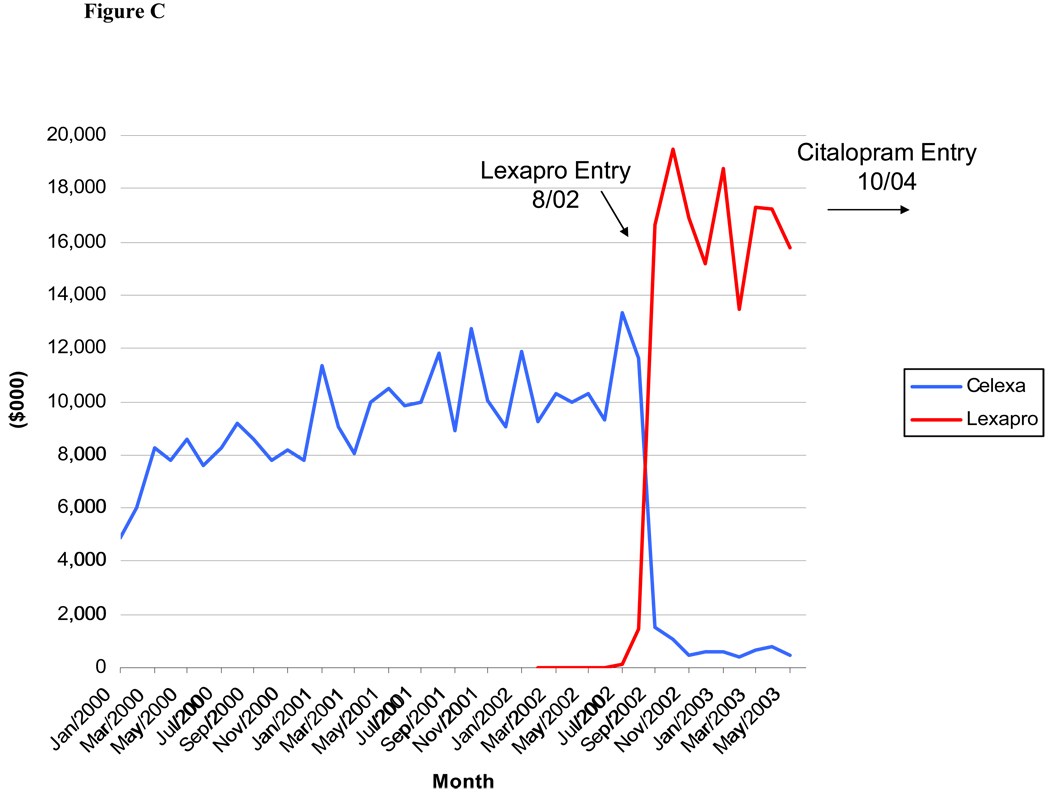
As seen in Figures A – C, detailing expenditures for the originator products Prozac, Paxil and Celexa dropped dramatically when a reformulation of the molecule was introduced (there were no reformulations of Zoloft). For Paxil and Celexa, promotional expenditures had virtually stopped by the time of generic entry for these molecules (15 and 27 months after new formulation entry, respectively).
Figure.
A: Monthly Detailing Expenditures for Prozac and Prozac Weekly, January 2000-June 2003
B: Monthly Detailing Expenditures for Paxil and Paxil CR, January 2000-June 2003
C: Monthly Detailing Expenditures for Celexa and Lexapro, January 2000-June 2003
Generic entry and total promotion and detailing expenditures
In the initial specification of the models of promotional spending (Table 2), we found that own generic entry had a large negative effect on detailing and total promotional spending for Prozac and on total promotional spending for Paxil. The coefficient on the own generic entry variable for Paxil detailing was negative but small relative to other coefficients in the model and not statistically significant. In the second specification (Table 3), we found that the time to patent expiration variable was negative and statistically significant for detailing and total promotional spending for Paxil and for detailing spending for Prozac.
Table 2.
Multivariate Regression Model Results for Logarithm of Quarterly Detailing and Total Promotion Expenditures
| Detailing | Total Promotion | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient | Standard Error | P-values | Coefficient | Standard Error | P-values |
| Prozac | ||||||
| Own generic entry | −1.96 | 0.25 | 0.004 | −2.93 | 0.14 | <0.001 |
| Prozac Weekly entry | −0.63 | 0.24 | 0.08 | −0.11 | 0.16 | 0.55 |
| Constant | 9.78 | 0.05 | <0.001 | 11.00 | 0.02 | <0.001 |
| Paxil | ||||||
| Own generic entry | −0.48 | 0.30 | 0.21 | −1.63 | 0.46 | 0.04 |
| Fluoxetine generic entry | −0.11 | 0.06 | 0.17 | 0.24 | 0.15 | 0.21 |
| Paxil CR entry | −1.96 | 0.38 | 0.01 | −2.31 | 0.58 | 0.03 |
| SAD indication | 0.12 | 0.07 | 0.16 | 0.40 | 0.07 | 0.01 |
| GAD indication | 0.31 | 0.06 | 0.01 | 0.10 | 0.07 | 0.26 |
| Constant | 9.68 | 0.02 | <0.001 | 10.77 | 0.02 | <0.001 |
| Celexa | ||||||
| Fluoxetine generic entry | 0.77 | 0.41 | 0.16 | 0.98 | 0.48 | 0.14 |
| Paroxetine generic entry | −1.14 | 0.72 | 0.21 | −1.86 | 0.78 | 0.10 |
| Lexapro entry | −2.20 | 0.73 | 0.06 | −2.26 | 0.80 | 0.07 |
| Constant | 9.47 | 0.43 | <0.001 | 10.03 | 0.46 | <0.001 |
| Zoloft | ||||||
| Fluoxetine generic entry | 0.07 | 0.05 | 0.22 | 0.47 | 0.06 | 0.01 |
| Paroxetine generic entry | 0.10 | 0.04 | 0.11 | −0.81 | 0.06 | 0.001 |
| SAD indication | 0.19 | 0.09 | 0.12 | 0.23 | 0.09 | 0.08 |
| Constant | 9.78 | 0.02 | <0.001 | 10.93 | 0.03 | <0.001 |
SAD=social anxiety disorder
GAD=generalized anxiety disorder
Table 3.
Multivariate Regression Model Results for Logarithm of Quarterly Detailing and Total Promotion Expenditures
| Detailing | Total Promotion | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient | Standard Error | P-values | Coefficient | Standard Error | P-values |
| Prozac | ||||||
| Time to patent expiration | −1.16 | 0.11 | 0.002 | −0.41 | 0.44 | 0.42 |
| Time after expiration | −0.23 | 0.01 | <0.001 | −0.37 | 0.02 | <0.001 |
| Prozac Weekly entry | 0.13 | 0.11 | 0.34 | 0.08 | 0.30 | 0.81 |
| Constant | 9.93 | 0.03 | <0.001 | 11.01 | 0.06 | <0.001 |
| Paxil | ||||||
| Time to patent expiration | −1.29 | 0.29 | 0.02 | −3.56 | 0.37 | 0.002 |
| Time after expiration | 0.07 | 0.06 | 0.31 | 0.09 | 0.07 | 0.32 |
| Fluoxetine generic entry | −0.07 | 0.08 | 0.46 | 0.36 | 0.19 | 0.15 |
| Paxil CR entry | −1.57 | 0.40 | 0.03 | −1.20 | 0.69 | 0.18 |
| SAD indication | 0.16 | 0.07 | 0.11 | 0.51 | 0.08 | 0.01 |
| GAD indication | 0.35 | 0.06 | 0.01 | 0.22 | 0.10 | 0.11 |
| Constant | 9.74 | 0.02 | <0.001 | 10.94 | 0.02 | <0.001 |
| Celexa | ||||||
| Time to patent expiration | 1.50 | 1.21 | 0.30 | 1.76 | 1.31 | 0.27 |
| Time after expiration | -- | -- | -- | -- | -- | -- |
| Fluoxetine generic entry | −0.05 | 0.26 | 0.86 | 0.02 | 0.24 | 0.93 |
| Paroxetine generic entry | −1.14 | 0.74 | 0.22 | −1.86 | 0.80 | 0.10 |
| Lexapro entry | −2.20 | 0.74 | 0.06 | −2.25 | 0.82 | 0.07 |
| Constant | 8.79 | 0.98 | <0.001 | 9.23 | 1.06 | 0.003 |
| Zoloft | ||||||
| Time to patent expiration | 1.17 | 0.52 | 0.11 | 0.01 | 2.39 | 0.99 |
| Time after expiration | -- | -- | -- | -- | -- | -- |
| Fluoxetine generic entry | 0.04 | 0.04 | 0.43 | 0.47 | 0.06 | 0.004 |
| Paroxetine generic entry | 0.04 | 0.05 | 0.50 | −0.81 | 0.17 | 0.02 |
| SAD indication | 0.16 | 0.10 | 0.19 | 0.23 | 0.13 | 0.18 |
| Constant | 9.73 | 0.02 | <0.001 | 10.93 | 0.06 | <0.001 |
SAD=social anxiety disorder
GAD=generalized anxiety disorder
The coefficient on the variable indicating the number of quarters since patent expiration, included in the Prozac and Paxil models only, was negative and statistically significant only in the detailing and total expenditure models for Prozac. A reformulation of Prozac (Prozac Weekly) was introduced just five months before generic entry for Prozac, and promotional spending for the originator drug remained fairly high at the time of generic entry, after which it dropped off. By contrast, promotional spending for Paxil dropped off to a very low level when Paxil CR was introduced over a year before generic entry of paroxetine.
For both specifications, in most cases generic entry by a competitor did not have a significant effect on promotional spending. The coefficients on these variables were statistically significant only for total promotional spending for Zoloft (the only product that did not experience generic entry during our study period) – generic entry for Prozac (fluoxetine) had a positive effect, while generic entry for Paxil (paroxetine) had a negative effect.
New product formulations, new indication approval, and total promotion and detailing expenditures
The entry of a new product formulation for Celexa and Paxil was associated with a lower level of detailing and total promotional spending for the original product, although the coefficient for the Lexapro entry variable only approached significance in the Celexa models (p=0.06 or 0.07, Table 2 and Table 3). The coefficient on the variable indicating approval for a SAD indication was not statistically significant in the detailing models for either Paxil or Zoloft but was significant for total promotion of Paxil (Table 2 and Table 3). For Paxil, the only SSRI with a GAD indication, the coefficient on the GAD indication variable was positive and statistically significant for detailing expenditures (Table 2 and Table 3).
Generic entry, new formulation and indication approval, and DTCA spending
The results of the pooled logit model of DTCA spending in a quarter (Table 4) suggest that the probability of any DTCA expenditures decreases as patent expiration nears, although this coefficient estimate was not statistically significant. The presence of a product reformulation is associated with a significantly lower likelihood of DTCA for the original brand. In fact, Figures A, B, and C show that detailing expenditures drop precipitously after entry of a reformulated product, suggesting a shift in the promotional strategy.
Table 4.
Logit Model Results for Probability of Any DTCA Spending in a Given Quarter
| Variable | Estimate | Standard Error | P-value |
|---|---|---|---|
| Time to patent expiration | −1.21 | 1.30 | 0.35 |
| New formulation | −3.33 | 1.33 | 0.01 |
| Social anxiety (SAD) indication | 4.06 | 1.04 | <0.001 |
| Molecule order of entry | −1.03 | 0.33 | 0.002 |
| Quarter | 0.10 | 0.05 | 0.04 |
| Intercept | 0.71 | 0.82 | 0.38 |
FDA approval for a SAD indication is associated with a substantially greater likelihood of DTCA expenditures. Order of entry has a negative, statistically significant effect on probability of DTCA spending, which is consistent with previous findings that earlier entrants are more likely to advertise directly to consumers (Iizuka, 2004).9 Finally, the time trend variable is positive and significant, suggesting a small increase in the likelihood of any DTCA spending over the time period.
VIII. Conclusions
SSRI manufacturers spend substantial amounts promoting their products through the use of DTCA, detailing, free samples, and other strategies. The amount and composition of promotional spending differs both across SSRIs and over time for a given drug, with changes occurring with own generic entry, reformulation entry, and new indications.
Promotional spending (both total and detailing alone) is generally lower after own generic entry than before, a finding consistent with the Dorfman Steiner theorem and other empirical literature, although the impact of generic entry has become more nuanced in the wake of regulatory changes permitting extension of marketing exclusivity. Promotional expenditures generally dropped as patent expiration approached for the drugs that lost patent protection during our study period.
The introduction of a new product formulation appears to be a common strategy for attempting to extend market exclusivity for medications facing impending generic entry. Because the Hatch-Waxman Act allows for an additional three years of market exclusivity for a new formulation, manufacturers have an incentive to shift demand for the original formulation of a brand drug that will soon lose patent protection onto a reformulation of the drug. The manufacturers of Paxil and Celexa shifted almost all promotion dollars from the original brand to the new formulation (Paxil CR and Lexapro, respectively) when the new formulation was introduced. In both cases, the reformulation was introduced long before generic entry (15 months before paroxetine’s entry in the case of Paxil CR and 27 months before citalopram’s entry in the case of Lexapro), which is consistent with the hypothesis that manufacturers attempted to shift demand for the original brand onto the new formulation in advance of generic entry. Eli Lilly released Prozac Weekly, the first SSRI reformulation to enter the market, just five months before generic entry of fluoxetine. Promotional spending for Prozac decreased somewhat gradually in the months after patent expiration rather than stopping more abruptly once the reformulated product entered or right before patent expiration. One reason could be that Eli Lilly may have expected to win a patent litigation case that would have delayed patent expiration beyond the actual expiration date of August 2001 (Angell, 2004).30 Eli Lilly’s detailing and DTCA contracts may have been fixed in the short term and non-cancellable immediately after generic entry. Also, Prozac was the first SSRI to lose patent protection, and the past six years have likely generated considerable experimentation and learning in the marketing of antidepressants.
It is clear that the manufacturers of these products faced a trade-off between lost profits today from brand cannibalization versus increased profits tomorrow stemming from brand protection. Our empirical work can offer some insights on the optimal timing of the launch of a reformulated product, viewed from the manufacturer’s perspective (not necessarily the consumer’s). Future work should examine manufacturer strategies regarding the timing of new product formulation entry relative to the originator brand’s patent expiration, recognizing that the observed effect of generic entry on promotional spending is conditional on a particular reformulation introduction strategy pursued by manufacturers.
Receiving FDA approval to market a drug for a new clinical indication may represent another way for a brand manufacturer to extend market exclusivity for its product. The results for the SAD and GAD indications suggest that manufacturers may target a particular type of promotion for a given indication. Paxil’s manufacturer increased detailing expenditures for Paxil after receiving FDA approval to market the drug for GAD, but did not appear to change its use of DTCA for Paxil. This suggests that the primary promotional target for GAD, a condition seen often in both primary care and specialty mental health settings, may have been physicians instead of consumers. In contrast, FDA approval to market Paxil and Zoloft for SAD did not affect the manufacturers’ detailing expenditures but did result in a greater likelihood of using DTCA in a given quarter. This result suggests that the primary target for promotion of the drugs as treatments for SAD may have been consumers rather than physicians, with manufacturers perhaps hoping to convince consumers that social anxiety is a treatable clinical condition. A complementary explanation may be that Paxil was the first drug to receive an FDA indication approval for SAD. Therefore, its manufacturer needed to convince patients to ask their doctors for the medication but did not have a need to persuade doctors that its drug was the best to treat that condition.
It is important to place our findings into the context of what is known about the welfare effects of pharmaceutical promotion for antidepressant medications. Most studies that shed light on this issue focus only on DTCA. Donohue and colleagues (2004) found that DTCA for antidepressants results in increased antidepressant prescribing.31 Donohue and Berndt (2004) found that DTCA has little effect on the choice of a specific antidepressant among individuals who initiated antidepressant treatment, although detailing does have an important effect on medication choice.32
The evidence on the effect of promotion and patient requests stemming from those promotions on the appropriateness of antidepressant use is mixed. Donohue and colleagues (2004) found that DTCA expenditures were associated with a small increase in appropriate duration of antidepressant use among individuals diagnosed with depression who initiated antidepressant therapy. Kravitz and colleagues (2005) found that standardized patients (actors following strict protocol for presenting their condition) who presented with symptoms of major depression were more likely to receive appropriate medications if they made a request either for any antidepressant or one that they had seen advertised (e.g. Paxil) than if they made no medication request.33 To the extent that DTCA results in patients requesting medication from their doctors, this suggests that DTCA may result in more appropriate treatment for major depression for some patients. However, the study also found that patients who presented with symptoms of adjustment disorder with depressed mood (a condition for which there is no consensus supporting antidepressant use) and who made general or DTCA related requests for an antidepressant were also more likely to receive an antidepressant than those who made no requests and merely presented with symptoms. Thus, DTCA may alleviate problems with underuse of antidepressants but could result in increased utilization of these medications for conditions for which there is no clinical consensus on the appropriateness of treatment. Thus, the net welfare effects of increased promotional spending for antidepressants are unclear.
There are several limitations of our work. First, findings from the antidepressant market may not generalize to other therapeutic categories. Second, when time series analysis is employed, it can be difficult to distinguish effects of multiple events occurring approximately simultaneously. Third, there may be partial adjustment of Paxil detailing expenditures that we do not account for with our specification. Fourth, we ignore price in these models because we lack data on transactions prices. Manufacturers seeking to maximize profits have several instruments available to affect profits, including promotion, new product formulations, new clinical indications, and prices. Fifth, our analyses of total promotional spending do not include data on journal advertising expenditures. However, journal advertising expenditures for SSRIs and SNRIs represented a very small share (0.4%) of total promotional spending for these medication classes in 2005.1
A number of changes in the competitive environment for SSRIs and other drugs are likely to affect manufacturer promotional strategies in the future. For example, in response to increased generic competition, brand drug manufacturers are increasingly releasing authorized generics (generic versions introduced by the original brand manufacturer) in an attempt to maintain revenues after generic entry, albeit at a lower level. The release of an authorized generic is likely to affect the promotional strategy of a brand drug manufacturer.34 Because promotions for the original brand could have positive spillovers onto a brand manufacturer’s authorized generic, promotional spending for the original brands might actually increase right before and after generic entry. Also, three-tiered formularies have no doubt led to stronger price competition in some classes. As pharmacy management tightens further and there are more generic SSRIs available, pressure by payers for consumers to use generics may cause manufacturers to invest even more in development and promotion of reformulations. If manufacturers can convince consumers and payers that the reformulations are sufficiently different from the original brand product, payers may be more likely to include the reformulations in preferred tiers of their formularies or be more willing to grant prior authorization or formulary exceptions for the reformulations.
Pharmaceutical manufacturers have developed a number of strategies for extending their effective patent life that have important effects on promotional spending. Given the significant impact pharmaceutical promotion has on prescription drug spending and public health it is important to gain an understanding and appropriate interpretation of these relationships. This study provides an important first look at how the promotional strategies of branded SSRI manufacturers are affected by generic competition by a competitor in the same class, by new product formulations, and by new clinical indications.
Acknowledgements
Financial support was provided by the Agency for Healthcare Research and Quality (P01 HS10803). Dr. Huskamp acknowledges financial support from the National Institute of Mental Health (K01 MH 66109). Dr. Donohue acknowledges financial support from the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12 RR023267). A previous version of this paper was presented at the 13th NIMH Biennial Research Conference on the Economics of Mental Health in September 2006. We thank our discussant, Daniel Eisenberg, and other participants for their helpful comments.
Contributor Information
Haiden A. Huskamp, Associate Professor of Health Economics, Harvard Medical School, Department of Health Care Policy, 180 Longwood Avenue, Boston, MA 02115, (617) 432-0838 (phone), (617) 432-0173 (fax), huskamp@hcp.med.harvard.edu
Julie M. Donohue, University of Pittsburgh Graduate School of Public Health, Department of Health Policy and Management.
Catherine Koss, Northwestern University, Feinberg School of Medicine.
Ernst R. Berndt, Louis E. Seley Professor in Applied Economics, Alfred P. Sloan School of Management, Massachusetts Institute of Technology
Richard G. Frank, Margaret T. Morris Professor of Health Economics, Harvard Medical School, Department of Health Care Policy
References
- 1.Donohue J, Cevasco M, Rosenthal M. A decade of direct-to-consumer advertising of prescription drugs. New England Journal of Medicine. 2007;357(7):673–681. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- 2.Frank RG, Salkever DS. Generic entry and the pricing of pharmaceuticals. Journal of Economics and Management Strategy. 1997;6(1):75–90. Spring. [Google Scholar]
- 3.Washington, D.C; Jul, 1998. U.S. Congressional Budget Office, How increased competition from generic drugs has affected prices and returns in the pharmaceutical industry. USGPO ISBN 0-16-049681-0. [Google Scholar]
- 4.Grabowski H, Vernon J. Brand loyalty and price competition in pharmaceuticals after the 1984 Drug Act. Journal of Law and Economics. 1992;35(2):331–350. [Google Scholar]
- 5.Hudson J. Generic take-up in the pharmaceutical market following patent expiry: a multi-country study. International Review of Law and Economics. 2000;20:205–221. [Google Scholar]
- 6.Frank RG, Salkever DS. Pricing, patent loss and the market for pharmaceuticals. Southern Economic Journal. 1992;59(2):165–179. [Google Scholar]
- 7.Hurwitz MA, Caves RE. Persuasion or information? promotion and the shares of brand name and generic pharmaceuticals. Journal of Law and Economics. 1988;31(2):299–320. [Google Scholar]
- 8.Caves RE, Whinston MD, Hurwitz MA. Patent expiration, entry and competition in the U.S. pharmaceutical industry. Brookings Papers on Microeconomics. 1991:1–66. [Google Scholar]
- 9.Iizuka T. Whatexplains the use of DTCA of prescription drugs? Journal of Industrial Economics. 2004;52(3):349–379. [Google Scholar]
- 10.Berndt ER, Kyle MK, Ling D. The long shadow of patent expiration: generic entry and rx to OTC switches,” ch. 8. In: Robert C, Freenstra, Matthew DShapiro, editors. Scanner Data and Price Indexes. Chicago: University of Chicago Press for the National Bureau of Economic Research; 2003. pp. 229–267. [Google Scholar]
- 11.IMS Health. Top-line Industry Data: Total U.S. Promotional Spend by Type. 2005 Available at http://www.imshealth.com/ims/portal/front/articleC/0,2777,6599_78084568_78152318,00.html. [Google Scholar]
- 12.Kaiser Family Foundation and Health Research Education Trust. Employer Health Benefits 2005 Annual Survey. 2005 September; Section 9 http://www.kff.org/insurance/7315/index.cfm. [Google Scholar]
- 13.Smith C. Retail prescription drug spending in the national health accounts. Health Affairs. 2004;23(1):160–167. doi: 10.1377/hlthaff.23.1.160. [DOI] [PubMed] [Google Scholar]
- 14.Gibson TB, Ozminkowski JR, Geotzel RZ. The effects of prescription drug cost sharing: a review of the evidence. American Journal of Managed Care. 2005;11(11):730–740. [PubMed] [Google Scholar]
- 15.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, et al. The effect of incentive-based formularies on prescription-drug utilization and spending,”. New England Journal of Medicine. 2003;349(23):2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 16.Scherer FM. The pharmaceutical industry. In: AJ Culyer, JP Newhouse., editors. Handbook of Health Economics. Elsevier: Amsterdam; 2000. lume 1B. [Google Scholar]
- 17.Dorfman R, Steiner PO. Optimal advertising and optimal quality. American Economic Review. 1954;44(5):826–836. [Google Scholar]
- 18.Schmalensee R. The economics of advertising. North-Holland: Amsterdam; 1972. [Google Scholar]
- 19.Palda K. Economic analysis for marketing decisions. New York: Prentice-Hall; 1969. [Google Scholar]
- 20.Rosenthal MB, Berndt ER, Donohue JM, Epstein AM, et al. Promotion of prescription drugs to consumers. New England Journal of Medicine. 2002;346(70):498–505. doi: 10.1056/NEJMsa012075. [DOI] [PubMed] [Google Scholar]
- 21.Scott-Morton F. Barriers to entry, brand advertising, and generic entry in the U.S. pharmaceutical industry. Int J Indus Org. 2000;18:1085–1104. [Google Scholar]
- 22.Ellison SF, Ellison G. Strategic entry deterrence and the behavior of pharmaceutical incumbents prior to patent expiration. Working paper. 2000 Available at http://econ-www.mit.edu/faculty/download_pdf.php?id=217. [Google Scholar]
- 23.Ellison SF, Cockburn I, Griliches Z, Hausman J. Characteristics of demand for pharmaceutical products: an examination of four cephalosporins. RAND Journal of Economics. 1997;28(3):426–446. [PubMed] [Google Scholar]
- 24.Reiffen D, Ward MR. Generic drug industry dynamics. Review of Economics and Statistics. 2005;87(1):37–49. [Google Scholar]
- 25.Lu JL, Comanor WS. Strategic pricing of new pharmaceuticals. Review of Economics and Statistics. 1998;80:108–118. [Google Scholar]
- 26.Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. Journal of Clinical Psychiatry. 2002;63(4):331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- 27.Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133–153. doi: 10.1111/j.1467-9566.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 28.Kessler RC, Berglund P, Demler O, Jin R, et al. Lifetime prevalence and age of onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(5):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 29.Berndt ER. The United States’ experience with direct-to-consumer advertising of prescription drugs: what we have we learned? In: Sloan FA, Hsieh C, editors. Pharmaceutical innovation: incentives, competition, and cost-benefit analysis in international perspective. Cambridge, UK: Cambridge University Press; 2007. pp. 174–195. [Google Scholar]
- 30.Angell M. The truth about drug companies. New York: Random House; 2007. pp. 187–188. [Google Scholar]
- 31.Donohue JM, Berndt ER, Rosenthal M, Epstein AM, et al. Effects of pharmaceutical promotion on adherence to the treatment guidelines for depression. Medical Care. 2004;42(12):1176–1185. doi: 10.1097/00005650-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Donohue JM, Berndt ER. Effects of direct-to-consumer advertising on medication choice: the case of antidepressants. Journal of Public Policy and Marketing. 2004;23(2):115–127. [Google Scholar]
- 33.Kravitz RL, Epstein RM, Feldman MD, Franz CE, et al. Influence of patients’ requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. Journal of the American Medical Association. 2005;293(16):1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berndt ER, Mortimer R, Bhattacharjya A, Parece A, et al. Authorized generic drugs, price competition, and consumers’ welfare. Health Affairs. 2007;26(3):790–799. doi: 10.1377/hlthaff.26.3.790. [DOI] [PubMed] [Google Scholar]