Abstract
Background
Loss of expression of fragile gene products, Fhit and Wwox, occurs in many cancer types, with loss exhibited early in the neoplastic process in some. Wwox has been understudied in pancreatobiliary cancers, especially in relation to other involved tumor suppressors. We have assessed the status of the Fhit and Wwox proteins encoded by DNA damage susceptible chromosome fragile sites encompassed by FHIT and WWOX tumor suppressor genes.
Methods
Pancreatic, gallbladder and ampullary cancers, normal pancreas, chronic pancreatitis, and benign gall-bladder specimens were stained for expression of Fhit, Fhit effector protein Fdxr, Wwox, and other tumor suppressors by immunohistochemistry, and comparisons were made between benign and malignant tissue. Correlations of expression among proteins and clinicopathologic features were sought using Spearman’s rank order. Survival curves were created using the Kaplan-Meier method and compared by log-rank analysis. Predictors of survival were determined using multivariate Cox proportional hazards analysis.
Results
Fhit and Wwox were ubiquitously expressed in benign samples and significantly and coordinately reduced in pancreatic, gallbladder, and ampullary cancers. In pancreatic cancers, Fdxr expression was positively correlated with Fhit and Wwox expression. Neither Fhit nor Wwox expression correlated with expression of other tumor suppressors or with clinicopathologic characteristics measured.
Conclusion
Loss of Fhit and Wwox expression does not predict tumor progression or patient survival, suggesting that loss of expression of genes at the exquisitely replication stress sensitive chromosome fragile regions is an early event in the pathogenesis of cancers of the gallbladder, pancreas, and ampulla.
Pancreatobiliary cancers are among the deadliest of gastrointestinal malignancies with nearly all patients diagnosed with pancreatic and gallbladder cancer dying of disease within a year.1 Ampullary cancer, though the least aggressive of these tumors, is still highly lethal once disease has spread to regional lymph nodes or distant organs. The inherent resistance of these tumors to chemotherapy and radiation in addition to their propensity to present in an advanced stage underline the need for novel genetic and molecular therapies. Understanding the molecular alterations, especially the early events, involved in development and progression of these cancers may provide the basis for therapies targeting specific altered signal pathways.
The fragile histidine triad (FHIT) gene, at chromosome 3p14.2, and the WW-domain oxidoreductase gene, WWOX at 16q23.3, are the two most active of the human common chromosome fragile sites; these loci, sites that show chromosome gaps or breaks under conditions of replicative stress, are highly susceptible to DNA alterations, such as deletions, translocations, and amplifications.2 WWOX and FHIT loci are involved in such chromosome alterations in more than 50% of cancers of many types.2,3 Shortly after discovery of the FHIT gene and protein, Shridhar et al. reported loss of alleles of 3pl4.2 markers in 16 of 25 pancreatic tumors and loss of heterozygosity of 3p markers outside of 3pl4.2 in only 2 of 25 tumors.4 There was a “dramatic clustering of chromosomal breakpoints at 3pl4.2 in and immediately distal to FRA3B in pancreatic cancer.” In a study of pancreatic cancer-derived cell lines, loss of heterozygosity (LOH) within FHIT was found in 41% of 93 pancreatic carcinomas and homozygous deletions within FHIT were found in some.5 There were significantly more homozygous deletions within FHIT in pancreatic cancers showing microsatellite instability, suggesting FHIT-related selective pressures that act during tumorigenesis of pancreatic cancers with mismatch repair deficiency.
Sorio et al examined a few normal pancreata, 21 primary pancreatic ductal cancers, and 19 pancreatic cancer cell lines for Fhit expression and FHIT gene status and reported that normal pancreas expressed Fhit protein in the cytoplasm of ductal cells, whereas interlobular and larger ducts, acini, and insulae of Langerhans were negative.6 Fhit protein was detected by immunoblot assay in 11 of 19 pancreatic cancer cell lines. DNA from 5 of these 8 cell lines showed homozygous loss of FHIT exon 5.
Similarly, the WWOX gene has been shown to be altered in several cancer types, including pancreatic cancer, and Wwox protein has been reported to be reduced or lost in a large fraction of these cancers; in vitro studies of pancreatic cancer-derived cell lines showed that expression of exogenous Wwox in Wwox-deficient pancreatic cancer cells increased the number of cells with subG1 DNA content, upregulated caspase-3 activity and reduced procaspase-3 levels. Restoration of Wwox expression suppressed cell growth in vitro and tumorigenicity in vivo.7,8
In a recent review of advancing knowledge of molecular alterations in ductal cancers, it is noted that common ductal cancers have a distinct molecular fingerprint showing alterations of Kras, p53, p16, DPC4/SMAD4, and Fhit, compared with other pancreatic tumor types with different aberrations.9 The SMAD4 gene is reportedly inactivated in approximately half of pancreatic cancers, either by homozygous deletion or by intragenic mutations plus loss of the second allele (reviewed in Ref.10) The SMAD4 protein plays a role in signaling through the transforming growth factor-β (TGF-β) pathway, that triggers a signal cascade with growth-inhibitory effects; so loss of SMAD4 in pancreatic cancer cells provides a selective growth advantage.
Wwox and Fhit are coordinately lost in breast cancers and in early breast lesions, suggesting coordinate inactivation of these fragile gene products early in breast cancer development.11,12
We have compared changes in expression of Fhit, Wwox, and other tumor suppressors in the related pancreatobiliary cancers. We have also assessed expression of the ferredoxin reductase (Fdxr) protein in these cancers. Fhit binds and stabilizes Fdxr in a complex in the mitochondria and thus contributes to the production of reactive oxygen species (ROS) in the presence of exogenous stress.13 Fhit-deficient cells are predicted to express reduced Fdxr levels and to have alterations in control of ROS production; p53, on the other hand, is a transcriptional activator of Fdxr, and thus p53 and Fdxr expression might be correlated in these cancers.14,15
We were interested in the early molecular changes in development of cancers in the anatomically and morphologically related pancreas, gallbladder, and ampulla tissues and have examined the loss of Fhit and Wwox fragile gene products in comparison with other known tumor suppressors for these tissues.
MATERIALS AND METHODS
Tissues
After receiving approval by the institutional review board at the Ohio State University, paraffin blocks were identified from the Department of Pathology archive for pancreatic, ampullary, and gallbladder cancers resected between 1991 and 2005. Only malignant tumor samples with adequate volume of tissue for tissue microarray creation were used. Blocks were considered inadequate if the volume of tissue to be used for the tissue microarray left no residual tumor for future histologic or immunohistochemical studies. Demographic, pathologic, and clinical data on the 170 cases meeting these criteria were collected.
Tissue Microarrays
The method for tissue microarray (TMA) production has been described.16 Briefly, two tissue cores (2 mm diameter each) were punched out of each paraffin block and transferred to each of the recipient TMA blocks using a precision instrument (Beecher Instruments, Silver Spring, MD). Paraffin-embedded tissue was cut at 4 µm and placed on positively charged slides, then heated to 40°C for 30 min. After leveling paraffin and cores, the array was cooled to 4°C for 15 min. The presence of tumor was confirmed, and immunohistochemistry was undertaken.
Immunohistochemistry
The methods for immunohistochemistry (IHC) have been described.17,18 Fhit, Wwox, and Fdxr rabbit polyclonal antisera were obtained from the Huebner lab and were used at dilutions of 1:2000, 1:4000, and 1:200, respectively.11 Primary antibodies for p53 (catalog No. M7001, clone DO-7, Dako, Carpinteria, CA), p16INK4a (catalog No. CMC802, clone JC2, Cell Marque Corp., Rocklin, CA), and SMAD4/DPC4 (catalog No. sc-7966, clone B-8, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were obtained commercially and used at dilutions of 1:50, 1:20, and 1:100, respectively. Slides were counter-stained in Richard Allen hematoxylin, dehydrated through graded ethanol solutions, and cover-slipped. The positive and negative controls stained appropriately.
Staining for p53 was considered positive if nuclear staining in at least 5% of cells was seen; cytoplasmic staining in at least 5% of cells was considered positive for Fhit, Wwox, Fdxr, and p16INK4a and less than 10% of nuclear and cytoplasmic staining of cells for SMAD4/DPC4 was considered to be loss of expression. All stains were read by a single pathologist (WLF) blinded to tumor stage and clinical characteristics.
Statistical Analysis
Continuous variables were compared by Student t-test while categorical variables were compared by chi-square analysis or Fisher exact test, where appropriate. Kaplan-Meier overall survival curves were generated using SPSS v14.0 software (SPSS Inc., Chicago, IL) and compared by log-rank analysis. Predictors of survival were determined using Cox proportional hazards. Only the variables with P ≤ .2 by univariate analysis were entered into the multivariate model. Significance was accepted at the P ≤ .05 level. Data are presented as mean ± standard deviation unless stated otherwise.
RESULTS
Tissue Samples
Between 1991 and 2005 inclusive, 397 patients underwent resection for tumors of the pancreas, ampulla of Vater, or gallbladder. Of these, 276 tumors were malignant, 170 of which had paraffin block suitable for utilization in our TMAs (Table 1). For each tumor type, patients were of similar gender and ages, though those with gallbladder cancer were more likely to be women. Preoperative carbohydrate antigen (CA) 19-9 levels were available in 74 patients. While fewer patients with ampullary cancer had abnormally elevated levels (i.e.>37 U/ml), this was not statistically different. Patients with gallbladder cancer were significantly less likely to present with jaundice. Although tumor grade and nodal status was similar between tumor types, margin negative resections were more common in ampullary cancers (Table 1). More gallbladder cancers were found to have advanced disease resulting in incomplete (i.e., R2) resection. Pancreatic cancers tended to be larger than ampullary and gallbladder cancers. Complication rates were not significantly different based on tumor type, but patients undergoing resection for gallbladder cancer had the shortest hospitalizations while those with ampullary cancer had the longest. Postoperative deaths were similar between tumor types.
TABLE 1.
Characteristics of 170 patients with pancreatobiliary carcinoma
| All | Pancreatic cancer | Ampullary cancer | Gallbladder cancer | |
|---|---|---|---|---|
| N | 170 | 98 | 49 | 23 |
| Sex (% men) | 54% | 54% | 65% | 26%a,b |
| Age (y) | 64.4 ± 10.5 | 64.5 ± 9.3 | 63.9 ± 11.2 | 65.3 ± 13.8 |
| Comorbidities | 83 (49%) | 50 (51%) | 19 (39%) | 14 (61%) |
| Elevated CA19-9c | 48 (65%) | 33 (72%) | 10 (45%) | 5 (83%) |
| Jaundice | 124 (73%) | 75 (77%) | 38 (78%) | 11 (48%)a,b |
| Pain | 61 (36%) | 38 (39%) | 16 (33%) | 7 (30%) |
| Tumor graded | ||||
| Well | 24 (14%) | 10 (11%) | 10 (20%) | 4 (17%) |
| Moderate | 89 (53%) | 50 (53%) | 26 (53%) | 13 (57%) |
| Poor | 54 (32%) | 35 (37%) | 13 (27%) | 6 (26%) |
| Node status | ||||
| N0 | 72 (42%) | 40 (41%) | 28 (57%) | 4 (17%) |
| N1 | 88 (52%) | 58 (59%) | 21 (43%) | 9 (39%) |
| Nx | 10 (6%) | 0 | 0 | 10 (43%) |
| Margins | ||||
| Neg | 127 (75%) | 68 (69%) | 47 (96%)a,c | 13 (57%) |
| Pos | 42 (25%) | 30 (31%) | 2 (4%) | 10 (43%) |
| Resection extent | ||||
| R0 | 123 (72%) | 68 (69%) | 47 (96%)ae | 8 (35%)a |
| R1 | 30 (18%) | 25 (26%) | 2 (4%)a,e | 3 (13%)a |
| R2 | 17 (10%) | 5 (5%) | 0ae | 12 (52%)a |
| Tumor size (cm) | 3.2 ± 2.1 | 3.8 ± 2.2 | 2.4 ± 1.5a | 2.7 ± 1.6a |
| Complications | 79 (46%) | 46 (47%) | 25 (51%) | 8 (35%) |
| Length of stay (d) | 12.6 ± 5.8 | 12.5 ± 5.7 | 13.6 ± 6.1e | 9.9 ± 4.3a |
| Mortality | 10 (6%) | 7 (7%) | 1 (2%) | 2 (9%) |
Note: Some categories do not add up to 100% due to rounding
P < .05 vs. pancreatic cancer
P < .05 vs. ampullary cancer
Data available for 74 patients
Data missing for 3 pancreatic cancers
P < .05 vs. gallbladder cancer
Immunohistochemical Analyses
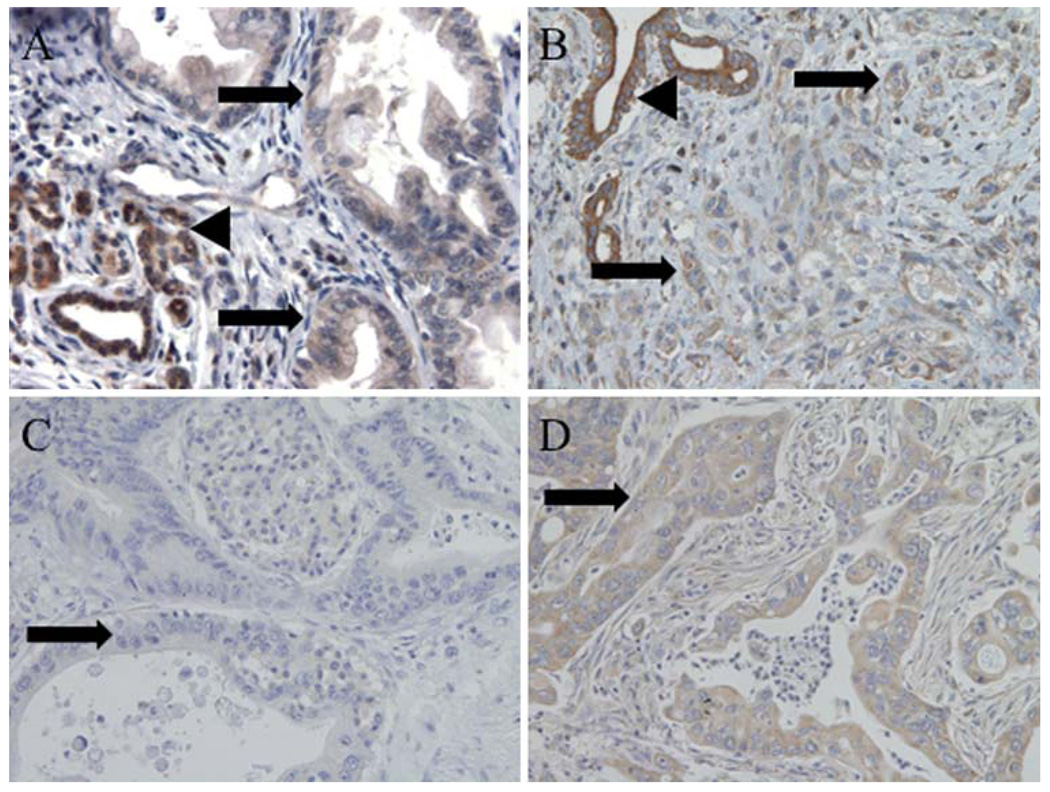
Fhit and Wwox were expressed at moderate to high levels in all benign samples including normal pancreas, normal gallbladder, and chronic pancreatitis and were markedly reduced in malignant tissues (Table 2). Ampullary cancers showed the highest frequency of reduced or absent Fhit expression, with only 2% of tumors showing unaltered expression relative to normal ducts, while gallbladder and pancreatic cancers showed very similar frequencies (78%) of tumors with reduced expression (Table 2, Fig. 1a). Wwox expression was reduced in >70% of tumors of each type (Fig. 1b). Fdxr, a Fhit mitochondrial effector, was reduced in expression in 40% of pancreatic tumors (Fig. 1c,d).
TABLE 2.
Immunohistochemical assessment of protein expression in pancreatobiliary tissues
| Fhit | Wwox | Fdxr | P53 | P16INK4a | SMAD4 | |
|---|---|---|---|---|---|---|
| Tumor type | ||||||
| All | 28/170 (16%)a | 40/141 (28%)a | 90/159 (57%)a | 42/165 (25%) | 57/162 (35%)a | |
| Pancreatic cancer | 22/98 (22%)b,c,d | 15/75 (20%)b,c | 52/86 (60%) | 54/89 (61%)b | 11/94 (12%)d,e | 26/93 (28%)b,e |
| Ampullary cancer | 1/49 (2%)b,e | 9/44 (20%)b | 27/47 (57%)b | 21/48 (44%) | 19/47 (40%) | |
| GB cancer | 5/23 (22%)f | 6/22 (27%) | 9/23 (39%)f | 10/23 (43%) | 12/22 (55%) | |
| Benign samples | ||||||
| All | 44/61 (72%) | 35/38 (92%) | 0/26 (0%) | 5/23 (22%) | 17/24 (71%) | |
| Pancreas | 8/11 (73%) | 8/10 (80%) | 16/24 (67%) | 0/11 (0%) | 1/9 (11%) | 9/11 (82%) |
| GB | 12/15 (80%) | - | 0/15 (0%) | 4/14 (29%) | 14/14 (100%) | |
| CP | 24/35 (69%) | 27/28 (96%) | N/A | N/A | N/A |
Data for Fhit and Wwox represent proportion of samples with unaltered (i.e., 3+) expression level
The numbers and percentages are the number/total number (%) of tissue cores that showed strong positive expression of the specific proteins, i.e., expression equal to the expression level in the benign tissues
GB gallbladder, CP chronic pancreatitis
P < .001 vs. all benign samples
P < .001 vs. normal pancreas
P < .0001 vs. chronic pancreatitis
P < .01 vs. ampullary cancer
P < .05 vs. gallbladder cancer
P < .01 vs. normal gallbladder
FIG. 1.
Immunohistochemical staining of proteins in pancreatic cancers. Micrographs (100×) of pancreatic cancers, illustrating. a Reduced expression of Fhit in tumor (arrows) and strong expression in benign ducts (arrowhead). b Reduced expression of Wwox in tumor (arrows) and strong expression in benign ducts (arrowhead). c Absence of expression of Fdxr in pancreatic cancer (arrow). d Expression of Fdxr in tumor (arrow)
Table 3 shows the correlation coefficients (Spearman rho) between Fhit, Wwox, Fdxr, p53, and SMAD4. Interestingly, Fhit and Wwox expression was significantly positively correlated in pancreatic cancer (P = .008) and gallbladder cancer (P = .024), as well as when considering all cancers together (P = .001). Similarly, Fhit and Fdxr expression was correlated in pancreatic cancer (P = .03), as was Wwox and Fdxr expression (P = .019), suggesting that the Fhit and Wwox-deficient cancers would be less affected by ROS-induced apoptosis.
TABLE 3.
Protein expression correlation matrix
| Fhit | Wwox | Fdxr | p53 | Smad4 | |
|---|---|---|---|---|---|
| Fhit | 1 | 0.275* | 0.234* | 0.062 | 0.099 |
| Wwox | 1 | 0.279* | 0.002 | 0.111 | |
| Fdxr | 1 | 0.183 | 0.141 | ||
| p53 | 1 | 0.099 | |||
| Smad4 | 1 |
Data represent Spearman’s rho. A positive value signifies a positive correlation. A value of 1 is equivalent to prefect correlation whereas a value of 0 represents no correlation at all
P < .05
No benign tissues demonstrated abnormal p53 expression. Significantly increased p53 expression was seen in each tumor type, however (Table 2). Similar p53 expression was seen in ampullary and pancreatic cancers. Although p53 was expressed in few gallbladder cancers, there was no significant difference in expression among the three tumor types. As expected, significant reduction is SMAD4 expression in pancreatic cancers compared with normal pancreas. There was no significant reduction in the degree of expression of p16INK4a relative to benign controls. No correlation, except those described among Fhit, Wwox, and Fdxr, was observed in pairwise comparisons of expression of Fhit, Wwox, Fdxr, p53, p16INK4a, and SMAD4 in any of the tumor types. While there was a trend toward an inverse correlation between Fhit and p53 expression in gallbladder cancer, this did not reach statistical significance (Spearman’s rho = −0.375, P = .078). Of the tumor suppressors assessed, Fhit, Wwox, p53, and SMAD4 were significantly reduced in tumors relative to benign tissues; and of these, Fhit and Wwox loss occurred much more frequently in tumors relative to benign tissue than the other tumor suppressors, suggesting that Fhit and Wwox loss is a very early event, possibly associated with development of preneoplastic changes in pancreatobiliary tissues.
Correlations Among Clinical Features, Markers, and Survival
Fhit and Wwox expression levels did not correlate with tumor size, T stage, node status, or differentiation. Fhit and Wwox did not predict survival. T stage, node status, and tumor size all correlated with survival for all tumor types together. Individually, nodes predicted survival for ampullary only while tumor size was predictive of survival for gallbladder and pancreatic cancer (summarized in Table 4).
TABLE 4.
Predictors of survival in 170 patients with pancreatobiliary cancer
| Univariate | Multivariate | |
|---|---|---|
| Age (continuous) | 0.024 | 0.07 |
| Comorbidity (yes vs. no) | 0.254 | - |
| Jaundice (yes vs. no) | 0.383 | - |
| Abdominal pain (yes vs. no) | 0.053 | 0.463 |
| CA19-9 (≤37 U/ml vs. >37 U/ml) | 0.008 | 0.717 |
| Tumor type (PCA vs. Amp vs. GB) | <0.001 | 0.189 |
| Tumor grade (well vs. moderate vs. poor) | 0.359 | - |
| Tumor size (continuous) | <0.001 | 0.482 |
| T stage (1 vs. 2 vs. 3 vs. 4) | 0.748 | - |
| Nodal status (positive vs. negative) | 0.001 | 0.004 |
| Margin status (positive vs. negative) | 0.002 | 0.177 |
| Extent of resection (R0 vs. R1 vs. R2) | 0.004 | 0.372 |
| Complication (yes vs. no) | 0.097 | 0.097 |
| Fhit (positive vs. negative) | 0.885 | - |
| Wwox (positive vs. negative) | 0.571 | - |
| Fdxr (positive vs. negative)* | 0.782 | - |
| P53 (positive vs. negative) | 0.998 | - |
| P16 (positive vs. negative) | 0.002 | 0.063 |
| SMAD4 (positive vs. negative) | 0.584 | - |
PCA pancreatic cancer, Amp ampullary cancer, GB gallbladder cancer
Fdxr data only available for pancreatic cancer specimens
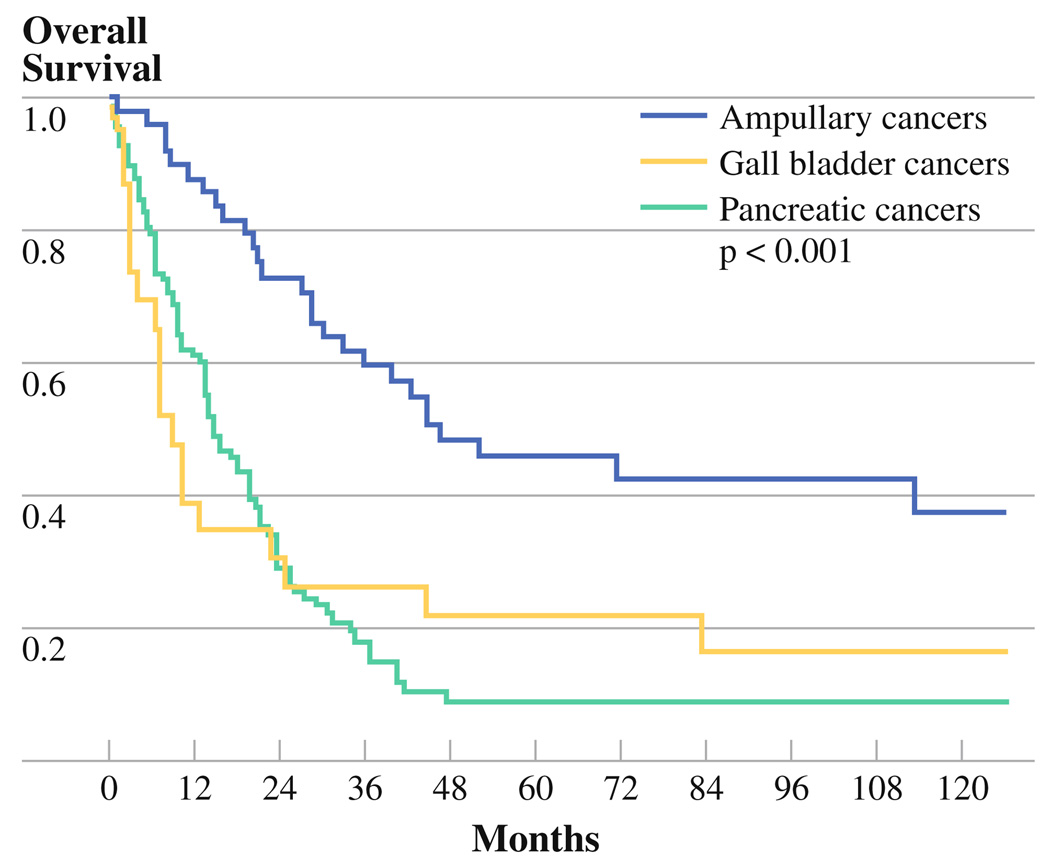
Overall median survival for all patients was 20.6 months (95% confidence interval [CI] 15.6–25.6 months) with 2-and 5-year survival of 44% and 22%, respectively. Patients with ampullary cancers demonstrated the longest overall survival (median 46.8 months [95% CI 13.5–80.0]) compared with pancreatic cancer (median 14.6 months [95% CI 10.4–18.9]) and gallbladder cancer (median 8.9 months [95% CI 4.4–13.4]) (Fig. 2). Other predictors of decreased survival by univariate analysis included increasing age, the presence of abdominal pain at presentation, elevated CA19-9, increasing tumor size, positive nodes, positive surgical margin, and p16INK4a loss (Table 4). By multivariate analysis, however, only the presence of lymph node metastases predicted decreased survival (median, 14.9 vs. 30.3 months). When each tumor type was considered separately by multivariate analysis, age and nodal status were significant predictors of survival in ampullary cancers. In pancreatic cancer, postoperative complications, positive margins, incomplete resection, and increasing tumor size were predictive of decreased survival. Finally, abdominal pain, the absence of jaundice, and increasing tumor size were predictors of decreased survival in gallbladder cancer.
FIG. 2.
Kaplan-Meier overall survival curve comparing pancreatic cancers (PCA), ampullary cancers (Amp), and gallbladder cancers (GB)
As a final analysis, we evaluated the expression of Fhit and Wwox in patients with rapid recurrence and death (i.e., survival less than 12 months) compared with those living longer than 24 months. There were 49 patients who died within 12 months of resection compared with 70 who lived more than 24 months. Fhit (14% vs. 19%, respectively) and Wwox (20% vs. 16%, respectively) were not significantly different between and long-term and short-term survivors. Of note, in patients who survived greater than 60 months (N = 22), Fhit was lost in all but 2 and Wwox was lost in all but 3. These too were not significantly different from those who died within 12 months.
DISCUSSION
Loss of Fhit and Wwox has been shown to be an important step in the initiation of tumorigenesis in a variety of solid tumors including lung, breast, colorectal, and gastric cancers to name a few.19–22 In lung cancer sections, Fhit loss has been reported to occur in dysplasia and prior to alterations in p53.23 Fhit and Wwox are lost coordinately in early breast lesions.12 In pancreatic cancer tumors and cell lines, FHIT mRNA and protein expression have been shown to be frequently lost or reduced, often as the result of loss of heterozygosity.4–6,24 FHIT cDNA alterations have been identified in 73% of nitrosamine-induced Syrian golden hamster pancreatic ductal adenocarcinomas, providing further evidence that abnormal Fhit expression may contribute to pancreatic tumorigenesis.25 Correlation of Fhit expression with clinicopathologic parameters has not been reported. Replacement of the FHIT gene, using recombinant adenoviral and adenoassociated viral FHIT vectors resulted in apoptosis via the caspase pathway in pancreatic cancer cell lines, in accord with a role for Fhit in pancreatic cancer initiation.26 The role of Fhit or Wwox in other biliary tumors has not been explored extensively, though there are two reports of Fhit locus silencing by methylation in gallbladder cancers.27,28 Herein, we have shown that Fhit and Wwox proteins are lost in cancers arising from the pancreas, ampulla of Vater, and gallbladder, independent of other well known genetic alterations.
Tumor samples suitable for use in our TMAs were selected based on availability of the paraffin blocks, adequate volume of malignant tissue not heavily contaminated by surrounding inflammatory changes, and tissue that must be able to be obtained without interfering with potential future diagnostic studies. Thus, we could not use all of the cancerous tissue from each case for our study. This left us with 170 patients over the 15-year time period. The demographics and presentation of these patients were typical for the respective tumor types. Not surprisingly, ampullary cancers had fewer elevations of CA19-9, while patients with gallbladder cancer were less likely to present with jaundice. The histologic grade of the resected tumors was similar for each tumor type, but ampullary cancers tended to present with earlier T stage than pancreatic and gallbladder cancers. While this could reflect differences in the respective staging systems, their smaller size at resection, lower incidence of nodal metastases, and increased likelihood of margin negative resection suggest a more indolent biologic behavior for ampullary cancers. This point is also evident in their significantly prolonged survival relative to the other tumor types.
Across all tumor types loss of Fhit and Wwox expression was proportionally more common than abnormalities in p53, p16, or SMAD4 expression. Only aberrant p53 expression, a known early event in carcinogenesis, occurs with similar frequency (57%) as Fhit and Wwox loss in these cancers. This is even more pronounced with Fhit loss in ampullary cancers, where only 2% of cancers demonstrated unaltered Fhit expression. Wwox loss was the most common molecular alteration with a reduction in expression from 92% in benign samples to only 28% in the pancreatobiliary cancers. This suggests that Fhit and Wwox loss may be early events in pancreatobiliary cancers. Given that all of the resected lesions in this study represented established cancers, often with advanced stage, and not precursor lesions, definitive conclusions about the timing of Fhit and Wwox loss relative to other molecular events is not possible.
Although Fhit was commonly lost in our pancreatobiliary tumors, its loss did not predict survival in any tumor type. In fact, none of the genetic alterations were predictive of survival. This is not an unexpected finding given that few reliable molecular predictors of survival have been described in these aggressive tumors, particularly pancreatic cancer. In ampullary cancers, where the prognosis is much better than in pancreatic and gallbladder cancers, several genetic and/or protein alterations have been described that impact survival including allelic loss of chromosome 17p, expression of epithelial cell adhesion molecule (Ep-CAM), vascular endothelial growth factor (VEGF), and stromal osteonectin.18,29–31 Still, the frequency of Fhit loss in these tumors along with previous reports of its impact on outcome in other tumor types and in preclinical models emphasizes its potential importance in the pathogenesis of pancreatobiliary cancers and warrants continued investigation; particularly considering that Lucito et al have very recently shown copy-number variants in the FHIT locus in some patients with a strong family history of pancreatic cancer.32 Copy-number variants such as germ-line deletions and amplifications are associated with inherited genetic disorders including familial cancer. Lucito et al. used representational oligonucleotide microarray analysis to characterize germ-line copy number variants in 60 cancer patients from 57 familial pancreatic cancer kindreds. Among the unique genomic regions with copy-number variants were 25 deletions. Two deleted regions were observed in two different patients, and 1 in 3 patients. Examples of genes included in the germ-line deletions included the FHIT gene.
The role of Wwox in pancreatobiliary tumors is less defined. Kuroki et al. demonstrated decreased Wwox expression by deletion and/or aberrant gene expression and promoter hypermethylation in 6 of 15 (40%) resected pancreatic cancers.7 Furthermore, transfection of WWOX into a pancreatic cancer cell line resulted in apoptosis. Decreased Wwox expression is suggested to be an early event with its loss being seen in precursor pancreatic intraepithelial neoplasia (PanIN) in accord with grade.8 Wwox downregulation also seemed to be associated with downregulation of Smad4. We too saw a positive correlation between Smad4 and Wwox expression, but this was not statistically significant. The complexity of interaction between Wwox and other intracellular proteins is well known. Interrelationships between Wwox and other proteins such as protein kinase A inhibitory subunit (PrkaRIα), transcription factors Ap2α and Ap2γ, ErbB4, and Her2 have been shown to play a role in tamoxifen resistance in resected breast cancer specimens.33 Furthermore, interaction with growth regulatory proteins such as p53 has been suggested.34,35 However, such an interaction was not suggested in our pancreatobiliary tumors.
In summary, we have shown that loss of expression of Fhit and Wwox tumor suppressor proteins is highly correlated in pancreatobiliary cancers. Their loss often occurs prior to other genetic alterations, suggesting that the exquisite susceptibility to DNA damage of their fragile site chromosomal loci contributes to their coordinated inactivation. Although expression of these proteins is not predictive of clinical tumor characteristics or survival, reduction of expression of these proteins is likely to be a very early event in tumor development and play important roles in pathogenesis of these malignancies. Thus, Fhit and Wwox signal pathway proteins may provide targets for therapy and FHIT or WWOX gene replacement may become an option in treatment of pancreatobiliary cancers.
ACKNOWLEDGMENT
Grant support: National Cancer Institute CA133250 (MB) and CA132453 and CA115965 (CMC, FP, KH). We thank Teresa Druck for assistance with figure preparation.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Iliopoulos D, Guler G, Han SY, Druck T, Ottey M, McCorkell KA, et al. Roles of FHIT and WWOX fragile genes in cancer. Cancer Lett. 2006;232:27–36. doi: 10.1016/j.canlet.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Kuroki T, Tajima Y, Furui J, Kanematsu T. Common fragile genes and digestive tract cancers. Surg Today. 2006;36:1–5. doi: 10.1007/s00595-005-3094-4. [DOI] [PubMed] [Google Scholar]
- 4.Shridhar R, Shridhar V, Wang X, Paradee W, Dugan M, Sarkar F, et al. Frequent breakpoints in the 3p14.2 fragile site, FRA3B, in pancreatic tumors. Cancer Res. 1996;56:4347–4350. [PubMed] [Google Scholar]
- 5.Hilgers W, Koerkamp BG, Geradts J, Tang DJ, Yeo CJ, Hruban RH, et al. Genomic FHIT analysis in RER + and RER- adenocarcinomas of the pancreas. Genes Chromosomes Cancer. 2000;27:239–243. [PubMed] [Google Scholar]
- 6.Sorio C, Baron A, Orlandini S, Zamboni G, Pederzoli P, Huebner K, et al. The FHIT gene is expressed in pancreatic ductular cells and is altered in pancreatic cancers. Cancer Res. 1999;59:1308–1314. [PubMed] [Google Scholar]
- 7.Kuroki T, Yendamuri S, Trapasso F, Matsuyama A, Aqeilan RI, Alder H, et al. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin Cancer Res. 2004;10:2459–2465. doi: 10.1158/1078-0432.ccr-03-0096. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama S, Semba S, Maeda N, Aqeilan RI, Huebner K, Yokozaki H. Role of the WWOX gene, encompassing fragile region FRA16D, in suppression of pancreatic carcinoma cells. Cancer Sci. 2008;99:1370–1376. doi: 10.1111/j.1349-7006.2008.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore PS, Beghelli S, Zamboni G, Scarpa A. Genetic abnormalities in pancreatic cancer. Mol Cancer. 2003;2:7. doi: 10.1186/1476-4598-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, et al. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer. 2004;100:1605–1614. doi: 10.1002/cncr.20137. [DOI] [PubMed] [Google Scholar]
- 12.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, McCue P, et al. Concordant loss of fragile gene expression early in breast cancer development. Pathol Int. 2005;55:471–478. doi: 10.1111/j.1440-1827.2005.01855.x. [DOI] [PubMed] [Google Scholar]
- 13.Trapasso F, Pichiorri F, Gaspari M, Palumbo T, Ageilan RI, Gaudio E, et al. Fhit interaction with ferredoxin reductase triggers generation of reactive oxygen species and apoptosis of cancer cells. J Biol Chem. 2008;283:13736–13744. doi: 10.1074/jbc.M709062200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, et al. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Chen X. The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene. 2002;21:7195–7204. doi: 10.1038/sj.onc.1205862. [DOI] [PubMed] [Google Scholar]
- 16.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 17.Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23:74–79. doi: 10.1159/000093497. [DOI] [PubMed] [Google Scholar]
- 18.Bloomston M, Ellison EC, Muscarella P, Al-Saif O, Martin EW, Melvin WS, et al. Stromal osteonectin overexpression is associated with poor outcome in patients with ampullary cancer. Ann Surg Oncol. 2007;14:211–217. doi: 10.1245/s10434-006-9128-3. [DOI] [PubMed] [Google Scholar]
- 19.Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, Baffa R, et al. Loss of WWOX expression in gastric carcinoma. Clin Cancer Res. 2004;10:3053–3058. doi: 10.1158/1078-0432.ccr-03-0594. [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Chen XP, Li WL, Xia J, Du H, Tang WB, et al. Decreased fragile histidine triad expression in colorectal cancer and its association with apoptosis inhibition. World J Gastroenterol. 2007;13:1018–1026. doi: 10.3748/wjg.v13.i7.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X, Li L, Gao Y, Zhang J, Ying J, Xiao T, et al. Fhit protein expression in lung cancer studied by high-throughput tissue microarray. Bull Cancer. 2007;94:E8–E11. [PubMed] [Google Scholar]
- 22.Terry G, Ho L, Londesborough P, Duggan C, Hanby A, Cuzick J. The expression of FHIT, PCNA and EGFR in benign and malignant breast lesions. Br J Cancer. 2007;96:110–117. doi: 10.1038/sj.bjc.6603512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, et al. Loss of FHIT function in lung cancer and pre-invasive bronchial lesions. Cancer Res. 1998;58:5032–5037. [PubMed] [Google Scholar]
- 24.Simon B, Bartsch D, Barth P, Prasnikar N, Münch K, Blum A, et al. Frequent abnormalities of the putative tumor suppressor gene FHIT at 3p14.2 in pancreatic carcinoma cell lines. Cancer Res. 1998;58:1583–1587. [PubMed] [Google Scholar]
- 25.Tsujiuchi T, Sasaki Y, Kubozoe T, Konishi Y, Tsutsumi M. Alterations in the Fhit gene in pancreatic duct adenocarcinomas induced by N-nitrosobis(2-oxopropyl)amine in hamsters. Mol Carcinog. 2003;36:60–66. doi: 10.1002/mc.10099. [DOI] [PubMed] [Google Scholar]
- 26.Dumon KR, Ishii H, Vecchione A, Trapasso F, Baldassarre G, Chakrani F, et al. Fragile histidine triad expression delays tumor development and induces apoptosis in human pancreatic cancer. Cancer Res. 2001;61:4827–4836. [PubMed] [Google Scholar]
- 27.Koda M, Yashima K, Kawaguchi K, Andachi H, Hosoda A, Shiota G, et al. Expression of Fhit, Mlh1, and P53 protein in human gallbladder carcinoma. Cancer Lett. 2003;199:131–138. doi: 10.1016/s0304-3835(03)00385-9. [DOI] [PubMed] [Google Scholar]
- 28.Riquelme E, Tang M, Baez S, Diaz A, Pruyas M, Wistuba, et al. Frequent epigenetic inactivation of chromosome 3p candidate tumor suppressor genes in gallbladder carcinoma. Cancer Lett. 2007;250:100–106. doi: 10.1016/j.canlet.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Tao SF, Zheng YX. Prognostic significance of vascular endothelial growth factor expression and microvessel density in carcinoma of ampulla of Vater. Hepatogastroenterology. 2006;53:45–50. [PubMed] [Google Scholar]
- 30.Fong D, Steurer M, Obrist P, et al. Ep-CAM expression in pancreatic and ampullary carcinomas: frequency and prognostic relevance. J Clin Pathol. 2006;61:31–35. doi: 10.1136/jcp.2006.037333. [DOI] [PubMed] [Google Scholar]
- 31.Iacono C, Verlato G, Zamboni G, Scarpa A, Montresor E, Capelli P, et al. Adenocarcinoma of the ampulla of Vater: T-Stage, chromosome 17p allelic loss, and extended pancreaticoduodenectomy are relevant prognostic factors. J Gastrointest Surg. 2007;11:578–588. doi: 10.1007/s11605-007-0136-9. [DOI] [PubMed] [Google Scholar]
- 32.Lucito R, Suresh S, Walter K, Pandey A, Lakshmi B, Krasnitz A, et al. Copy-number variants in patients with a strong family history of pancreatic cancer. Cancer Biol Ther. 2007;6:1592–1599. doi: 10.4161/cbt.6.10.4725. [DOI] [PubMed] [Google Scholar]
- 33.Guler G, Iliopoulos D, Guler N, Himmetoglu C, Hayran M, Huebner K. Wwox and Ap2gamma expression levels predict tamoxifen response. Clin Cancer Res. 2007;13:6115–6121. doi: 10.1158/1078-0432.CCR-07-1282. [DOI] [PubMed] [Google Scholar]
- 34.Aqeilan RI, Croce CM. WWOX in biological control and tumorigenesis. J Cell Physiol. 2007;212:307–310. doi: 10.1002/jcp.21099. [DOI] [PubMed] [Google Scholar]
- 35.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med. 2007;13:12–22. doi: 10.1016/j.molmed.2006.11.006. [DOI] [PubMed] [Google Scholar]