Abstract
Experiments in two mouse models of thromboinflammatory disease show how neutrophils stick to red blood cells and platelets—leading to reduced blood flow and damage to the microcirculation. Polarized expression of αMβ2 integrins on neutrophils helps set the process in motion (pages 384−391).
The systemic and pulmonary microcirculations are the targets of injury in sepsis and acute lung injury, disorders that are major causes of morbidity and mortality in critically ill individuals. Damage to the smallest blood vessels, including arterioles, capillaries and venules, leads to shock, acute respiratory failure and acute renal failure.
Individuals who have sickle cell disease develop occlusion and hypoxia of the microcirculation when challenged with inflammatory insults1,2. The microcirculation is also the target of injury from the transfusion of blood products that can produce severe damage to the lung, known as transfusion-related acute lung injury (TRALI). TRALI is estimated to occur in approximately 1 in 5,000 blood transfusions, although it is probably more common, with the lower numbers due to underreporting and other factors3.
Mouse models of sickle cell disease and TRALI have provided insights into how damage to the microcirculation occurs in response to inflammation. In two such clinically relevant animal models, Hidalgo et al.4 now provide insight into how neutrophils respond to acute inflammation and contribute to damage. In this issue of Nature Medicine, the authors show how sequestered neutrophils coordinate tissue injury by interacting with the endothelium, circulating red blood cells and platelets4. These interactions are mediated through the actions of endothelial E-selectin, neutrophil E-selectin ligand-1 (ESL-1) and the promiscuous leukocyte integrin αMβ2 (also known as CD11b/CD18, Mac-1 and CR3).
Neutrophils are key sentinel cells of the innate immune system and are the premier cellular responders to acute inflammation. For example, in models of acute lung injury ranging from acid aspiration5 to ischemia-reperfusion6 to TRALI7, depletion of neutrophils before the injury stimulus protects rabbits, rats and mice, respectively, from lung injury. These noninfectious models differ from the most common clinical causes of acute lung injury, pneumonia and sepsis, in which neutrophils are needed to control the infection. Nonetheless, animal work has demonstrated that the proinflammatory response of the neutrophil can lead to an increase in endothelial and epithelial permeability and, in the case of sepsis, shock and global organ injury8. Directing the neutrophil responses to combat acute infections without injuring the host remains a lofty goal of modern medicine.
As intrepid responders to acute inflammation, neutrophils are uniquely equipped to mingle with a variety of cells in the microcirculation. To facilitate delivery of neutrophils in the bloodstream to areas of injury and infection, circulating neutrophils marginate along endothelial cells via endothelial E- and P-selectins and their corresponding neutrophil ligands ESL-1, P-selectin glycoprotein ligand-1 and CD44.
Hidalgo et al.4 provide evidence for neutrophil-platelet or neutrophil–red blood cell interactions in the inflamed systemic microcirculation in the absence of organ injury, findings that imply a possible functional role for the heterotypic aggregates. However, neutrophil-platelet aggregates detected in a mouse model of acid-induced acute lung injury were responsible for organ injury, as blocking these interactions via the P-selectin pathway protected the mice from lung endothelial injury9.
Using elegant in vivo multichannel fluorescent imaging of the systemic circulation, the authors studied how endothelial cells communicate with neutrophils to stimulate heterotypic interactions with red blood cells and platelets4. The authors report that ESL-1 on neutrophils, by binding to E-selectin on endothelium, signals the polarized activation of surface αMβ2 integrin on the neutrophil4.
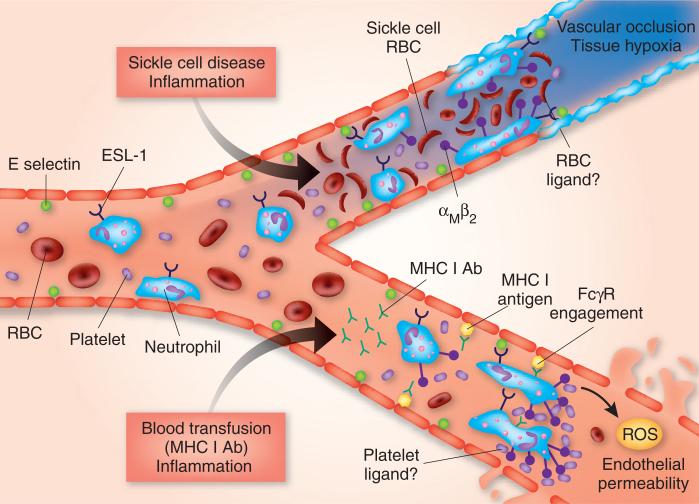
In a mouse model of sickle cell disease, they show that αMβ2 integrin in turn mediates the heterotypic associations of neutrophils with normal and sickle red blood cells4; in the TRALI model, αMβ2 integrin mediates the interaction of neutrophils with platelets. The αMβ2 integrin is constitutively present on the neutrophil surface, but inflammation and ESL-1 signaling activates and polarizes it to the leading edge of crawling neutrophils, where the heterophile aggregates form. In the sickle cell model, these events lead to capture of sickle red blood cells and vascular occlusion, and, in the TRALI model, they lead to the capture of platelets and endothelial injury mediated by reactive oxygen species (Fig. 1).
Figure 1.
Heterotypic interactions of neutrophils in the inflamed microvasculature. With inflammation, E-selectin is expressed in the microvasculature leading to rolling of neutrophils on the endothelium via ESL-1. In the sickle cell disease model (top), inflammation is combined with circulating sickle red blood cells. E-selectin engagement of ESL-1 on neutrophils produces outside-in signaling and clustered activation of αMβ2 integrin on the neutrophil leading edge. Activated αMβ2 integrin binds sickle red blood cells, possibly through complement component 3 deposited on the sickle red blood cell surface—leading to vascular occlusion and tissue hypoxia, which could produce the clinical syndrome of a vaso-occlusive crisis. In the TRALI model (bottom), a two-event model of injury is produced by combining inflammation with a blood transfusion containing major histocompatibility complex class I–specific antibody (MHC I Ab). The transfused antibody binds to endothelial MHC I antigen leading to the capture of neutrophils via FcγR. As in the sickle cell disease model, E-selectin–ESL-1 engagement also polarizes activated αMβ2 integrin at the neutrophil leading edge—resulting in capture of circulating platelets, although the platelet antigen has not been identified. Neutrophils ultimately produce reactive oxygen species (ROS), which leads to endothelial injury, endothelial permeability and extravasation of plasma into the interstitium and air spaces of the lung. RBC, red blood cell.
These findings are noteworthy and clinically relevant for several reasons. They establish a link between E-selectin–induced regional activation of the neutrophil αMβ2 integrin through ESL-1, a ligand previously not known to transduce signals. The results also expand the function of E-selectin to include the induction of distinct signaling events in neutrophils, adding to its classical role in leukocyte rolling. The E-selectin–ESL-1–αMβ2 integrin pathway has the potential for pharmacologic intervention. Because of its importance in both thrombotic and inflammatory experimental models, this pathway also has the potential for broad relevance in microcirculatory diseases.
Future work is needed to identify the specific ligands on red blood cells and platelets that participate in the heterotypic neutrophil interactions. Targeting platelets in thromboinflammatory diseases is potentially an attractive area of investigation, but it must be carefully tested given the role of platelets in maintaining normal systemic and pulmonary vascular permeability10. Also needed is an investigation of the dynamic neutrophil–red blood cell and neutrophil-platelet interactions—a circulatory bed that differs substantially from the systemic microcirculation11. In the lung microcirculation, for example, neutrophils are sequestered by mechanical forces as well as by selectin-mediated mechanisms.
Although pharmacologically disrupting the E-selectin–ESL-1–αMβ2 integrin pathway has the potential to protect against injury to the microcirculation, timing may be key. Lung injury in TRALI, for example, usually manifests maximally within 30−60 minutes after infusion of a blood product and then resolves over the next few days12. With complications of sickle cell disease, such as acute chest syndrome and vaso-occlusive pain crises, microcirculatory injury is dynamic and will challenge attempts to interrupt the pathway. Investigating whether heterotypic neutrophil interactions are important in subacute or resolving inflammation will be a major goal of future investigations.
References
- 1.Bunn HF. N. Engl. J. Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Gladwin MT, Vichinsky E. N. Engl. J. Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 3.Toy P, et al. Crit. Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo A, et al. Nat. Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. J. Clin. Invest. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eppinger MJ, Deeb GM, Bolling SF, Ward PA. Am. J. Pathol. 1997;150:1773–1784. [PMC free article] [PubMed] [Google Scholar]
- 7.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. J. Clin. Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Zarbock A, Singbartl K, Ley K. J. Clin. Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Am. J. Respir. Cell Mol. Biol. 2009;40:123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns AR, Smith CW, Walker DC. Physiol. Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 12.Bux J, Sachs UJ. Br. J. Haematol. 2007;136:788–799. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]