Abstract
Background
Most studies investigating the neurobiology of depression and suicide have focused on the serotonergic system. While it seems clear that serotonergic alterations play a role in the pathogenesis of these major public health problems, dysfunction in additional neurotransmitter systems and other molecular alterations may also be implicated. Microarray expression studies are excellent screening tools to generate hypotheses about additional molecular processes that may be at play. In this study we investigated brain regions that are known to be implicated in the neurobiology of suicide and major depression are likely to represent valid global molecular alterations.
Methodology/Principal Findings
We performed gene expression analysis using the HG-U133AB chipset in 17 cortical and subcortical brain regions from suicides with and without major depression and controls. Total mRNA for microarray analysis was obtained from 663 brain samples isolated from 39 male subjects, including 26 suicide cases and 13 controls diagnosed by means of psychological autopsies. Independent brain samples from 34 subjects and animal studies were used to control for the potential confounding effects of comorbidity with alcohol. Using a Gene Ontology analysis as our starting point, we identified molecular pathways that may be involved in depression and suicide, and performed follow-up analyses on these possible targets. Methodology included gene expression measures from microarrays, Gene Score Resampling for global ontological profiling, and semi-quantitative RT-PCR. We observed the highest number of suicide specific alterations in prefrontal cortical areas and hippocampus. Our results revealed alterations of synaptic neurotransmission and intracellular signaling. Among these, Glutamatergic (GLU) and GABAergic related genes were globally altered. Semi-quantitative RT-PCR results investigating expression of GLU and GABA receptor subunit genes were consistent with microarray data.
Conclusions/Significance
The observed results represent the first overview of global expression changes in brains of suicide victims with and without major depression and suggest a global brain alteration of GLU and GABA receptor subunit genes in these conditions.
Introduction
Suicide accounts for almost 2% of the world's deaths, and in most developed countries it is the leading cause of death for males younger than 40 years of age [1]. Suicide is caused by a set of complex conditions and is frequently, but not exclusively, associated with depressive disorders. Although it is clear that these conditions are mediated by specific neurobiological processes [2], [3], the precise molecular alterations and the brain circuits involved in suicide and major depression remain largely unknown.
The suicide brain is believed to have a complex pattern of neurochemical alterations involving several neurotransmitter systems and different brain regions [4]. While most of the attention to date has focused on the possible dysregulation of the serotonergic system, and to a lesser extent, the noradrenergic neurotransmitter system [5]–[11], there is also evidence implicating other neurotransmitters, such as the dopaminergic [12]–[15], polyaminergic [16], glutamatergic [17]–[20] and GABAergic systems [4], [5], [21]–[23]. In addition, several studies have also investigated the role of signal transduction and other molecular systems [24]–[27]. Imaging studies of subjects with major depression and/or suicidal behavior using functional magnetic resonance and positron emission tomography have pointed to possible dysfunction of prefrontal neuronal circuits and subcortical areas of the brain, particularly some areas of the limbic system [28]–[34]. The complexity of neurotransmitter systems interacting in many distinct neuroanatomical regions underlines the need of a more comprehensive and inclusive approach monitoring alterations in different regions of the brain.
Microarray technology offers the possibility of parallel monitoring expression levels of several thousands to virtually all genes based on the hybridization of nucleotide probes mounted on high density arrays to a target nucleotide sequence [35], [36]. Recently this technology was implemented in psychiatry to study gene expression changes in postmortem brain tissue from psychiatric patients (for a review see [37]) and from suicide completers [16], [38], [39].
One of the major problems of experiments using dense microarrays is the level of multiple comparisons leading to false positive results. While different statistical approaches exist to correct for type I errors [40]–[42], independent replication, both internal and external, is the method of choice to determine the accuracy of results [43]. We hypothesized that biological processes that are globally altered across different brain regions believed to be implicated in the neurobiology of suicide and major depression are likely to represent valid global molecular alterations. Therefore, in this study, we conducted a global gene expression survey in 17 cortical and subcortical brain areas of male suicides with and without major depression versus matched psychiatrically normal controls aiming at the identification of molecular pathways that are differentially expressed, consistently, across those brain regions.
Methods
Subjects and diagnostic procedures
Quebec Suicide Brain Bank
Brain tissue was obtained from the Quebec Suicide Brain Bank. All samples used in the present study were from male subjects of French-Canadian origin, a homogeneous population with a well-known founder effect [44]. Cases and controls were group-matched for age and post-mortem interval. To be included in this study, suicides and controls had to die suddenly, with no medical or paramedic intervention, and with no prolonged agonal period. Brains were dissected at 4°C and snap-frozen in liquid nitrogen before storage at -80°C. Brain tissue was dissected and Brodmann areas (BA) identified in accordance with standard neuroanatomical definitions [45]. The anterior and posterior cingulate corresponded to the most anterior and the most posterior parts of the cingulate gyrus. In all cases, 1 cm3 human tissue blocks were paraffin-embedded, cryostat-sectioned, slide-mounted, and examined for any signs of disease by two independent pathologists in at least 3 different brain regions. No cases were excluded on this basis. This study was approved by our IRB and signed informed consent was obtained from next of kin.
All suicide and control subjects were psychiatrically diagnosed by means of psychological autopsies, which is a validated method to reconstruct psychiatric history by means of extensive proxy-based interviews, as outlined elsewhere [46]. In total, we analyzed 663 brain samples isolated from 39 subjects throughout the 17 regions, including suicides who died during an episode of major depression (SMD; N = 16); suicide victims with no history of major depression (S; N = 10); and matched psychiatrically normal controls (C; N = 13) who died suddenly from causes other than suicide and had no history of suicidal behavior. No other mood disorders were included in the present study. The vast majority of suicide completers from both the S and the SMD groups died by hanging. This is the most common method of suicide in Canada [46]. Controls died suddenly, without medical intervention by either accidents or myocardial infarctions. While this represents the total sample used in this study, there was some variability between regions following outlier exclusion (see below).
Controlling for alcohol confounding effects
To exclude the possible effect of alcohol on our positive findings, we followed up these results in an independent sample obtained from the University of California, Irvine (UCI) Brain Bank. This sample consisted of brain tissue from 13 male alcohol abusers and 21 controls, both groups psychiatrically normal otherwise. We investigated the dorsolateral prefrontal cortex (BA 9–46), a brain region that has been implicated in the etiology of alcoholism [47]. All subjects were clinically characterized by means of psychological autopsies and died suddenly without prolonged agonal state as described elsewhere [18]. All of the cases and none of the controls from the UCI sample had 6-month histories of alcohol abuse. Control for potential confounding effect of alcohol was also carried out by means of animal experiments as described below.
RNA quality control and microarray experiments
All 663 RNA samples used in this study had a minimum A260/A280 ratio of>1.9 (mean = 2.03±0.14). The samples were further checked for evidence of degradation and integrity. Samples had a minimum 28S/18S ratio>1.6 and an average RIN of 7.14±0.85 (2100-Bioanalyzer, Agilent Technologies).
We used the HG-U133AB chipset, containing around 45,000 probe sets derived from approximately 33,000 human genes (http://www.affymetrix.com). Sample preparation and processing, hybridization to the Human Genome U133 Set, and normalization were performed as described in the Affymetrix GeneChip Expression Analysis Manual (Affymetrix, Santa Clara, CA) in collaboration with Gene Logic Inc (Gaithersburg, MD). The GeneChip IVT Express and the GeneChip® Hybridization, Wash, and Stain kits from Affymetrix were used for first and second cDNA synthesis, IVT/labeling and purification of aRNA, fragmentation and purification. GeneChip analysis was also performed based on the Affymetrix GeneChip Manual, with Microarray Analysis Suite (MAS) 5.1, Data Mining Tool (DMT) 2.0, and Microarray Database software. All of the genes represented on the GeneChip were globally normalized and scaled to a signal intensity of 100. Description of the Affymetrix normalization is available at the following site (http://www.affymetrix.com/support/technical/technotes/statistical_reference_guide.pdf).
Expression data was analyzed using Genesis 2.0 (GeneLogic Inc, Gaithersburg, MD) and AVADIS (Strand Genomics, Redwood City, CA). Several RNA integrity measures, in addition to 28S/18S ratios and RIN numbers, were used in this study to detect samples with poor RNA quality before final analysis: noise (RawQ), consistent number of genes detected as present across arrays, consistent scale factor, and consistent β-actin and GAPDH 5′/3′ signal ratios. Arrays with a significant deviation from the average RawQ, scale factor and 5′/3′ ratios were excluded. Problematic arrays were also identified using principal component analysis (PCA). Outlier subjects/arrays were excluded on a region specific basis, without any subject being excluded from all the regions. The data from this manuscript is available upon request.
Semi-quantitative RT-PCR
For technical validation of differentially expressed genes, we performed semi-quantitative RT-PCR using RNA extracted from additional samples that were collected in each brain region from tissue adjacent to that used in the microarray expression study. All the subjects that passed quality control were also used in these experiments. Reverse transcription was performed in a total volume of 40 µl with 2 µg of total mRNA using M-MLV reverse transcriptase (Gibco, Burlington, Ontario) and oligo(dT)16 primers. PCR amplification was carried out using the Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA), to determine the log linear phase of the amplification and to perform the semi-quantitative PCR. mRNA-specific primers, were designed using Primer3 (www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) and their sequence is available upon request. Products were visualized using ethidium bromide staining after electrophoresis in a 3% agarose gel. Images were digitalized and analyzed using Gene Tools (Syngene, Cambridge). Experiments were carried in parallel in triplicate and β-actin was used as an internal control gene.
Statistical analysis
The Microarray Suite software 5.1 (MAS 5.1) uses an algorithm that associates P-values to indicate statistical significance for gene expression detection and assign a Present, Marginal or Absent call. For each brain area, the list of genes were filtered prior to analysis such that only genes present (according to MAS 5.1) in at least 75 % of the subjects in at least one of the groups were included in our analyses. On average, 14,777 genes were analyzed per region across the 17 regions analyzed.
Gene expression values were floored to 1 and then log2-transformed. ANCOVAs were initially performed for each gene to identify statistically significant gene expression changes between the three groups, with substance abuse/dependence as a covariate. Statistically significant genes according to the ANCOVA were then subjected to a post-hoc t-test and fold-change analysis (FC) in order to identify pair-wise differences between the suicides with major depression (SMD), the suicides without major depression (S), and the controls (C). For a gene to be considered as differentially expressed, it had to have an ANCOVA P-value of less than or equal to 0.01 and a fold change of at least a 1.3 fold change in either direction. Post-hoc analyses were carried out using the Fisher protected LSD test with a P-value set at 0.01.
Cluster analysis was performed using average-linkage hierarchical cluster analysis with a correlation metric. Both expression patterns in individuals and genes were clustered. Principal component analysis (PCA) was performed based on the initial gene sets and on the selected genes (according to our significance criteria).
Functional ontological profiling of the expression changes was performed across all 17 regions using the Gene Score Resampling (GSR) method implemented in the ErmineJ software (version 2.1.8, Columbia University, NY) that examines the distributions of scores (FC or P-values) across the whole array [48]. This method compares the number of genes in a class ontology that show significant differential expression with the expected number of genes in that same class under the null hypothesis [48], eliminating the risk of finding false over-represented categories due to over-representation on the microarray chip. The parameters used were the following: Maximum gene set size: 300; Minimum gene set size: 5; with the mean of replicates, 10,000 iterations and full resampling. The rank and P-value computed by ErmineJ were used to calculate the most overrepresented ontologies across all regions. The distribution pattern of the ErmineJ calculated P-values in the different regions of the brain was examined by hierarchical clustering using AVADIS, with the normalized negative log of the P-values as the input. Further annotations were conducted using the Database for Annotation, Visualization and Integrated Discovery (DAVID) [49].
Animal experiments
Adult male Sprague Dawley rats (Charles River, St. Constant, Québec) housed individually in clear Plexiglas cages (46 cm×18 cm×30 cm) on a 12-hr reverse-light cycle with food and water available ad libitum were used to study the effect of alcohol consumption on selected genes. All procedures were conducted in accordance with guidelines established by the Canadian Council on Animal Care.
For the acute ethanol (EtOH) administration, rats received a single injection of either vehicle (n = 5; 1 ml/kg ip) or EtOH (n = 5; 2.5 g/kg EtOH ip; 15% v/v EtOH in 0.9% saline) [50], [51]. The prefrontal cortex (PFC) was quickly dissected, flash frozen in isopentene and stored at −80°C until further analysis. For the chronic ethanol administration, food and water consumption were monitored for 3 days prior to treatment to ensure no differences in baseline consumption existed between the treatment groups. Rats were randomly assigned to one of 3 treatment conditions: water control (n = 5), sucrose control (n = 5; 10% sucrose solution) or EtOH (n = 5; 15% EtOH in a 10% sucrose solution). Once rats in the EtOH group readily drank the 10% sucrose solution (for 1 day) they were gradually habituated to the 15% EtOH solution [52] . EtOH rats received 5% EtOH in 10 % sucrose for 1 day, followed by 10% EtOH in 10% sucrose for 2 days. The solution was then changed to the 15% EtOH in 10% sucrose. Chronic EtOH treatment persisted for 28 days once the rats had access to the 15% EtOH solution. Twenty-nine days after the 15% EtOH treatment began, rats were sacrificed prior to lights off. Brains were removed and PFC was quickly dissected, flash frozen in isopentane, and stored at −80°C until subsequent analysis.
Results
Global analysis
Demographic and clinical characteristics of the subjects included in this study are shown in Table 1. No significant differences were observed between the groups for different demographic measures such as age (mean±sd: C = 35±11; S = 34±9; SMD = 37±13;), post-mortem interval (mean±sd: C = 24±6; S = 29±15; SMD = 25±7), or brain tissue pH (mean±standard deviation: C = 6.44±0.26; S = 6.32±0.27; SMD = 6.55±0.32). Furthermore, no significant correlation was observed in our sample as a whole between quality control parameters such as noise (RawQ), number of genes detected as present across arrays, scale factor, β-actin and GAPDH 5′/3′ (data not shown). This suggests that RNA quality from our tissue was acceptable, probably reflecting our brain recruitment procedures, which are limited to sudden death without medical intervention, prolonged agonal periods or extended PMI. All subjects in this study underwent toxicological screens and we detected only one subject with an SSRI in his blood, suggesting that medication is not a confounding factor in this study (Table 1).
Table 1. Demographic, clinical and toxicological characteristics of the subjects included in the study.
| Group | Age | PMI | Cause of death | DSM-IV (six months diagnosis) | Toxicology screening |
| C | 51 | 15 | Motor vehicle accident | Alcohol dependence | Alcohol |
| C | 31 | 24 | Cardiac arrest | Alcohol dependence | |
| C | 19 | 32 | Motor vehicle accident | ||
| C | 47 | 12 | Cardiac arrest | Alcohol abuse | |
| C | 30 | 30 | Cardiac arrest | ||
| C | 28 | 27 | Motor vehicle accident | ||
| C | 41 | 24 | Myocardial Infarction | ||
| C | 31 | 29.5 | Motor vehicle accident | ||
| C | 46 | 19.5 | Myocardial Infarction | ||
| C | 21 | 24 | Cardiac arrest | ||
| C | 27 | 20.5 | Cardiac arrest | ||
| C | 32 | 26.5 | Cardiac arrest | Cannabis abuse | |
| C | 55 | 24 | Motor vehicle accident | ||
| S | 38 | 23 | Hanging | Alcohol dependence, cocaine dependence | Alcohol |
| S | 21 | 21 | Asphyxiation | OCD, Alcohol dependence | Alcohol |
| S | 31 | 32.5 | Hanging | ||
| S | 29 | 26.5 | Hanging | ||
| S | 33 | 18 | Hanging | ||
| S | 26 | 69 | Hanging | ||
| S | 30 | 27 | Stabbing | Paranoid schizophrenia | |
| S | 36 | 25 | Hanging | ||
| S | 51 | 21 | Self inflicted gun shot | Alcohol dependence | |
| S | 42 | 27 | Carbon monoxide | ||
| SMD | 28 | 20 | Hanging | MDD, alcohol dependence | Alcohol |
| SMD | 22 | 11.5 | Hanging | MDD, alcohol dependence | Alcohol, cocaine |
| SMD | 53 | 14 | Carbon monoxide | MDD | |
| SMD | 26 | 34 | Hanging | MDD | Cocaine |
| SMD | 40 | 23 | Hanging | MDD, alcohol dependence | |
| SMD | 19 | 29.5 | Hanging | MDD | |
| SMD | 53 | 29 | Hanging | MDD, alcohol dependence | |
| SMD | 42 | 21 | Drowning | MDD | SSRI |
| SMD | 45 | 20.5 | Self inflicted gun shot | MDD, pathological gambling | |
| SMD | 35 | 31 | Hanging | MDD, alcohol dependence | |
| SMD | 39 | 25.5 | Hanging | MDD | |
| SMD | 49 | 32 | Hanging | MDD, alcohol abuse | |
| SMD | 40 | 22 | Hanging | MDD | |
| SMD | 53 | 33.5 | Hanging | MDD | |
| SMD | 18 | 27 | Carbon monoxide | MDD | |
| SMD | 22 | 20 | Hanging | MDD |
C = control, S = suicide, SMD = suicide with major depression, MDD = major depressive disorder, SSRI = Selective serotonin reuptake inhibitor.
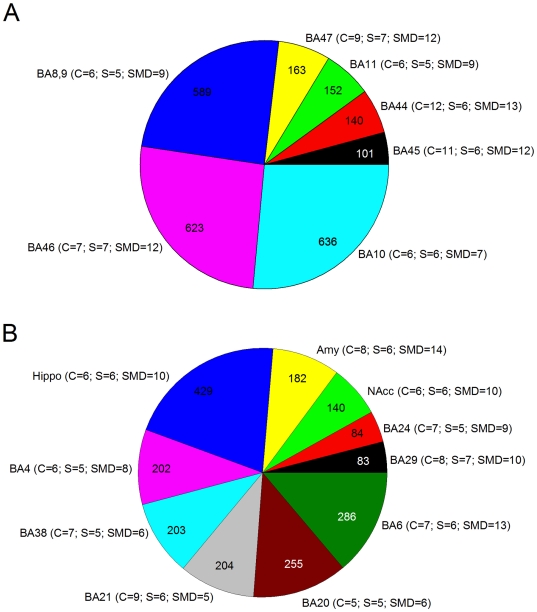
Overall, 251,206 probe sets passed the initial filtering criteria and were included in the analysis across the 17 regions with an average of around 15,000 probe sets per region. A summary of the analyzed and the differentially expressed probe sets per region and per group comparison, controlling for the possible effect of substance abuse/dependence, are shown in Table 2. Figure 1 provides the distribution of the total number of differentially expressed genes in prefrontal and subcortical brain areas. The region with the least probe sets analyzed was BA10 with 11,935 and the one with the most was BA45 with 15,886 probe sets. A total of 5,868 probe sets were significantly altered with the ANCOVA at the P≤0.01 level. Of these, 4,472 probe sets were differentially expressed across the regions after the Fisher protected LSD tests and fold-change (FC) filtering. These 4,472 probe sets were annotated to 3320 unique genes using DAVID. There was substantial variation between regions in terms of total number of differentially expressed genes, ranging from 83 in BA29 to 636 in BA10. In addition to BA10, the region with the second largest number of differentially expressed genes was BA46 with 626 genes. BA46 and BA10 are two prefrontal cortex regions which are anatomically close and have previously been associated with both suicidal behaviors and major depression. On the other hand, two limbic regions located in the cingulate cortex, BA24 and BA29, had the least number of differentially expressed genes, 84 and 83 respectively.
Table 2. Summary of the differential expression analysis in 17 brain areas of controls and suicides with and without major depression.
| Brain Region | Genes analyzed | Genes significant | C-SMD | C-S | SMD-S |
| BA4 (C = 6; S = 5; SMD = 8) | 14632 | 202 | 128 (66 up; 62 down) | 55 (28 up; 27 down) | 62 (35 up; 27 down) |
| BA6 (C = 7; S = 6; SMD = 13) | 15266 | 286 | 216 (105 up; 111 down) | 76 (31 up; 45 down) | 46 (20 up; 26 down) |
| BA8,9 (C = 6; S = 5; SMD = 9) | 14854 | 589 | 73 (33 up; 40 down) | 302 (71 up; 231 down) | 411 (147 up; 264 down) |
| BA10 (C = 6; S = 6; SMD = 7) | 11935 | 636 | 604 (436 up; 168 down) | 41 (30 up; 11 down) | 69 (31 up; 38 down) |
| BA11 (C = 6; S = 5; SMD = 9) | 14410 | 152 | 85 (19 up; 66 down) | 33 (11 up; 22 down) | 77 (50 up; 27 down) |
| BA20 (C = 5; S = 5; SMD = 6) | 13944 | 255 | 77 (36 up; 41 down) | 68 (21 up; 47 down) | 173 (50 up; 123 down) |
| BA21 (C = 9; S = 6; SMD = 5) | 14129 | 204 | 161 (42 up; 119 down) | 20 (8 up; 12 down) | 64 (37 up; 27 down) |
| BA38 (C = 7; S = 5; SMD = 6) | 14395 | 182 | 65 (37 up; 28 down) | 102 (58 up; 44 down) | 64 (16 up; 48 down) |
| BA24 (C = 7; S = 5; SMD = 9) | 15243 | 84 | 52 (27 up; 25 down) | 20 (5 up; 15 down) | 27 (15 up; 12 down) |
| BA29 (C = 8; S = 7; SMD = 10) | 15032 | 83 | 19 (7 up; 12 down) | 40 (13 up; 27 down) | 40 (24 up; 16 down) |
| Amy (C = 8; S = 6; SMD = 14) | 15007 | 153 | 95 (56 up; 39 down) | 47 (22 up; 25 down) | 35 (25 up; 10 down) |
| Hippo (C = 6; S = 6; SMD = 10) | 14495 | 426 | 34 (16 up; 18 down) | 118 (60 up; 58 down) | 359 (196 up; 163 down) |
| NAcc (C = 6; S = 6; SMD = 10) | 15232 | 140 | 22 (8 up; 14 down) | 60 (10 up; 50 down) | 91 (11 up; 80 down) |
| BA44 (C = 12; S = 6; SMD = 13) | 15788 | 140 | 88 (32 up; 56 down) | 24 (15 up; 9 down) | 48 (30 up; 18 down) |
| BA45 (C = 11; S = 6; SMD = 12) | 15886 | 101 | 60 (13 up; 47 down) | 21 (8 up; 13 down) | 27 (17 up; 10 down) |
| BA46 (C = 7; S = 7; SMD = 12) | 15655 | 622 | 140 (37 up; 103 down) | 192 (148 up; 44 down) | 470 (373 up; 97 down) |
| BA47 (C = 9; S = 7; SMD = 12) | 15303 | 163 | 102 (48 up; 54 down) | 29 (13 up; 16 down) | 51 (29 up; 22 down) |
| Total | 251206 | 4472 |
C = control, S = suicide, SMD = suicide with major depression, Amy = amygdala, Hippo = hippocampus, NAcc = nucleus accumbens. Information on Brodmann areas is provided elsewhere93–94. The number of genes analyzed corresponds to the number of genes considered as “Present” by the detection algorithm (MAS 5.1) in at least 75% of subjects in at least one of the groups. The number of genes reported as significant is that obtained in an ANCOVA model which included substance abuse/dependence as a covariate.
Figure 1. Pie charts representing the distribution of the total number of differentially expressed genes in (A) prefrontal cortical areas and in (B) other cortical and subcortical brain areas.
The number of chips per group that passed quality control assessment is also given.
Functional profiling
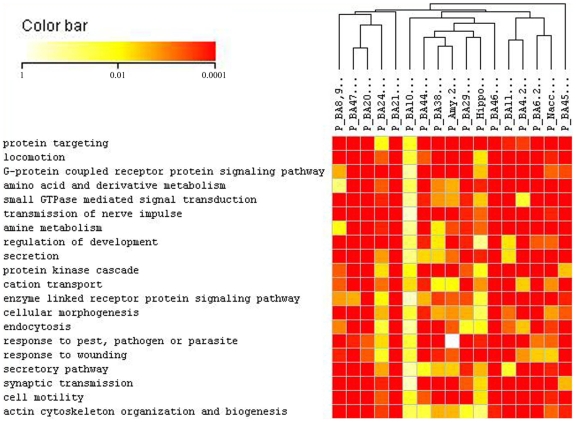
In order to identify altered functional pathways across all the regions investigated in this study, we initially used ErmineJ [48] to generate a list of overrepresented gene ontologies in each of the 17 brain regions independently and then the resulting significant ontologies were compiled to reflect the overlap of global ontologies. Subsequently, hierarchical clustering analyses were carried out to identify those biological processes that were commonly altered across all brain regions, Figure 2 shows the 20 top overrepresented ontologies. We then focused on the 10 most commonly overrepresented ontologies based on rankings and P-values in the 17 brain areas from the GSR analysis in ErmineJ. In order, from most to least commonly represented, these were signal transduction, intracellular signaling cascade, cell organization and biogenesis, protein localization, protein transport, establishment of protein localization, transmission of nerve impulse, small GTPase mediated signal transduction, synaptic transmission and vesicle-mediated transport. The corresponding probe sets belonging to these 10 globally overrepresented ontologies were further annotated using DAVID, resulting in the identification of 568 unique genes. As shown in Table 3, the majority of these genes corresponded to genes implicated in cell communication processes and related subcategories of functions such as intracellular signaling cascade, signal transduction, transmission of nerve impulse, and more specifically, synaptic transmission.
Figure 2. Clustered image map (CIM) of the hierarchical cluster analysis of the distribution pattern of ErmineJ calculated P-values of the 20 top overrepresented ontologies across all the regions studied.
Both ontological categories and the regions were clustered. The color and intensity indicate level of significance: red spectrum colors indicate very highly significant gene ontologies (0.001), yellow colors indicate highly significant gene ontologies (0.01) and white colors represent no significance.
Table 3. The 568 unique genes identified in our database of differentially expressed genes were regrouped using DAVID according to their function.
| Category | Term | Count | % | P-value |
| Biological Process | signal transduction | 237 | 41.73% | 2.57E-37 |
| Biological Process | intracellular signaling cascade | 138 | 24.30% | 2.80E-42 |
| Biological Process | cell organization and biogenesis | 132 | 23.24% | 1.38E-24 |
| Biological Process | protein localization | 79 | 13.91% | 2.03E-25 |
| Biological Process | protein transport | 77 | 13.56% | 8.29E-26 |
| Biological Process | establishment of protein localization | 77 | 13.56% | 6.87E-25 |
| Biological Process | transmission of nerve impulse | 61 | 10.98% | 6.73E-25 |
| Biological Process | small GTPase mediated signal transduction | 59 | 10.39% | 1.54E-29 |
| Biological Process | synaptic transmission | 57 | 10.03% | 2.50E-22 |
| Biological Process | vesicle-mediated transport | 55 | 9.68% | 3.12E-20 |
As intracellular signaling cascade, signal transduction and transmission of nerve impulse are parent nodes related to synaptic transmission, a more specialized molecular function that is of particular interest to the neurobiological investigation of major depression and suicide, we explored more specifically the genes related to synaptic transmission. A total of 57 genes corresponded to this category (Table 4) and consisted of several pre-synaptic proteins (SYN2, SYPL1, SNAP25, SYT1, SYT5, SNPH) and signal transduction genes such as the mitogen-activated protein kinase 1 (MAPK1) and the 2′,3′-cyclic nucleotide 3′ phosphodiesterase (CNP). However, it was remarkable that a large proportion of these 57 genes (22 out of 57 or 38.6%) corresponded to genes implicated in GABAergic or glutamatergic neurotransmission or in the transport of these neurotransmitters (Table 4). For the following analyses, we then also opted to further explore GABAergic and glutamatergic genes, because of prior reports to their implication in both major depression [22], [23], [53]–[55] and in suicide [17], [19], [20], [55], [56].
Table 4. Differentially expressed genes directly implicated in synaptic transmission as determined using DAVID (2006).
| Name | Symbol | Cytoband | Entrez Gene |
| 2′,3′-cyclic nucleotide 3′ phosphodiesterase | CNP | 17q21 | 1267 |
| 4-aminobutyrate aminotransferase | ABAT | 16p13.2 | 18 |
| 5-hydroxytryptamine (serotonin) receptor 2a | HTR2A | 13q14–q21 | 3356 |
| adenylate cyclase activating polypeptide 1 (pituitary) receptor type i | ADCYAP1R1 | 7p14 | 117 |
| amphiphysin (stiff-man syndrome with breast cancer 128 kda autoantigen) | AMPH | 7p14–p13 | 273 |
| apolipoprotein e | TOMM40 | 19q13 | 10452 |
| cocaine- and amphetamine-regulated transcript | CART | 5q13.2 | 9607 |
| cortistatin | APITD1 | 1p36.22 | 378708 |
| discs, large (drosophila) homolog-associated protein 1 | DLGAP1 | 18p11.3 | 9229 |
| double c2-like domains, alpha | DOC2A | 16p11.2 | 8448 |
| drebrin 1 | DBN1 | 5q35.3 | 1627 |
| dystrobrevin, alpha | DTNA | 18q12 | 1837 |
| gaba(a) receptor-associated protein like 1 | GABARAPL1 | 12p13.2 | 23710 |
| gamma-aminobutyric acid (gaba) a receptor, alpha 1 | GABRA1 | 5q34–q35 | 2554 |
| gamma-aminobutyric acid (gaba) a receptor, alpha 4 | GABRA4 | 4p12 | 2557 |
| gamma-aminobutyric acid (gaba) a receptor, alpha 5 | GABRA5 | 15q11.2–q12 | 2558 |
| gamma-aminobutyric acid (gaba) a receptor, beta 1 | GABRB1 | 4p12 | 2560 |
| gamma-aminobutyric acid (gaba) a receptor, delta | GABRD | 1p|1p36.3 | 2563 |
| gamma-aminobutyric acid (GABA) A receptor, gamma 1 | GABRG1 | 4p12 | 2565 |
| gamma-aminobutyric acid (gaba) a receptor, gamma 1 | GRIA2 | 4q32–q33 | 2891 |
| gamma-aminobutyric acid (gaba) a receptor, gamma 2 | GABRG2 | 5q31.1–q33.1 | 2566 |
| gamma-aminobutyric acid (gaba) b receptor, 2 | GABBR2 | 9q22.1–q22.3 | 9568 |
| gamma-aminobutyric acid (gaba) receptor, rho 1 | GABRR1 | 6q14–q21|6q13–q16.3 | 2569 |
| glutamate dehydrogenase 1 | GLUD1 | 10q23.3 | 2746 |
| glutamate receptor, ionotrophic, ampa 3 | GRIA3 | Xq25–q26 | 2892 |
| glutamate receptor, ionotropic, ampa 1 | GRIA1 | 5q33|5q31.1 | 2890 |
| glutamate receptor, ionotropic, ampa 2 | GRIA2 | 4q32–q33 | 2891 |
| glutamate receptor, ionotropic, kainate 1 | GRIK1 | 21q22.11 | 2897 |
| glutamate receptor, ionotropic, n-methyl d-aspartate 2a | GRIN2A | 16p13.2 | 2903 |
| glutamate receptor, metabotropic 3 | GRM3 | 7q21.1–q21.2 | 2913 |
| glutamate-ammonia ligase (glutamine synthetase) | GLUL | 1q31 | 2752 |
| gtp cyclohydrolase 1 (dopa-responsive dystonia) | GCH1 | 14q22.1–q22.2 | 2643 |
| mitogen-activated protein kinase 1 | MAPK1 | 22q11.2|22q11.21 | 5594 |
| myelin basic protein | MBP | 18q23 | 4155 |
| myelin oligodendrocyte glycoprotein | MOG | 6p22.1 | 4340 |
| nad(p)h dehydrogenase, quinone 1 | NQO1 | 16q22.1 | 1728 |
| neuronal pentraxin ii | NPTX2 | 7q21.3–q22.1 | 4885 |
| neuropeptide y | NPY | 7p15.1 | 4852 |
| pallidin homolog (mouse) | PLDN | 15q21.1 | 26258 |
| peripheral myelin protein 22 | PMP22 | 17p12–p11.2 | 5376 |
| phosphatidylinositol 4-kinase, catalytic, alpha polypeptide | PIK4CA | 22q11.21 | 5297 |
| piccolo (presynaptic cytomatrix protein) | PCLO | 7q11.23–q21.3 | 27445 |
| potassium large conductance calcium-activated channel, subfamily m, beta member 4 | KCNMB4 | 12q | 27345 |
| potassium voltage-gated channel, kqt-like subfamily, member 2 | KCNQ2 | 20q13.3 | 3785 |
| rab14, member ras oncogene family | RAB14 | 9q32–q34.11 | 51552 |
| s100 calcium binding protein, beta (neural) | S100B | 21q22.3 | 6285 |
| sodium channel, voltage-gated, type x, alpha | SCN10A | 3p22–p21 | 6336 |
| solute carrier family 1 (glial high affinity glutamate transporter), member 2 | SLC1A2 | 11p13–p12 | 6506 |
| solute carrier family 1 (glial high affinity glutamate transporter), member 3 | SLC1A3 | 5p13 | 6507 |
| solute carrier family 6 (neurotransmitter transporter, creatine), member 8 | SLC6A8 | Xq28 | 6535 |
| solute carrier family 6 (neurotransmitter transporter, gaba), member 1 | SLC6A1 | 3p25–p24 | 6529 |
| synapsin ii | SYN2 | 3p25 | 6854 |
| synaptophysin-like 1 | SYPL1 | 7q22.2 | 6856 |
| synaptosomal-associated protein, 25kda | SNAP25 | 20p12–p11.2 | 6616 |
| synaptotagmin i | SYT1 | 12cen–q21 | 6857 |
| synaptotagmin v | SYT5 | 19q|11p | 6861 |
| syntaphilin | SNPH | 20p13 | 9751 |
Pathways globally differentially expressed
In order to specifically explore GABAergic and glutamatergic genes that were differentially expressed across the different brain regions, we interrogated the list of 5,868 differentially expressed genes using the probe sets identified and annotated using DAVID.
GABAergic genes
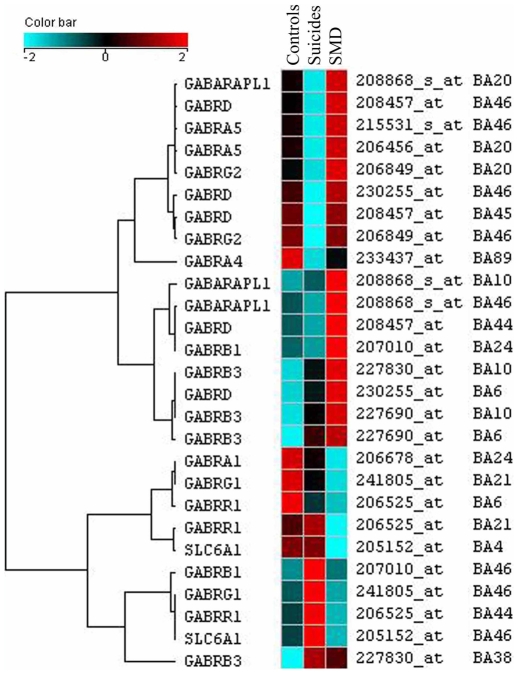
A total of 27 GABAergic-related probe sets were differentially expressed across the regions, many corresponding in fact to probe sets for the same genes as graphically represented in Figure 3. For instance, one gene, the Gamma-aminobutyric acid (GABA) A receptor, delta (GABRD) gene was differentially expressed in BA6, BA44, BA45, BA46 and the GABA(A) receptor-associated protein like 1 gene (GABARAPL1) was differentially expressed in BA10, BA20 and BA46 (Figure 3). Part of the ventrolateral prefrontal cortex, BA46 was of particular interest with a total of six GABAergic genes differentially expressed (GABARAPL1, GABRA5, GABRB1, GABRD, GABRG1, GABRG2). Also noteworthy, the majority of the differentially expressed GABAergic genes (19 out of 27) corresponded to different subunits of the GABA(A) receptor, particularly the alpha, beta, delta, gamma and rho subunits. As seen in Figure 3, a clear pattern of dysregulation was observed in terms of genes and regions implicated with a majority of GABAergic genes being up-regulated (red) among the suicides with major depression. For instance, in the hippocampus, all differentially expressed GABAergic genes were clearly up-regulated in suicides with major depression (GABARAPL1, GABARA4 and GABARB1) and with low expression among suicides without history of depressive disorders, suggesting a depression specific effect. Also seen in Figure 3, a total of 10 GABA(A) receptor beta probe sets were differentially expressed and were generally up-regulated among the depressed suicides. The same was observed for the GABA(A) receptor–associated protein like 1 (GABARAPL1) which was up-regulated in the depressed suicide group in BA10, BA20, BA46 and hippocampus. In summary, a striking number of probe sets corresponding to GABA(A) receptors or GABA(A) receptor-associated binding protein were altered between the three groups, with the majority being up-regulated among the suicides with major depression and having lower expression levels among the suicides without major depression or the controls, suggesting their role in molecular processes that may be more specific to the pathophysiology of major depressive disorder.
Figure 3. Clustered image map (CIM) of the hierarchical cluster analysis of the GABAergic differentially expressed subunit genes across the 17 regions investigated.
The color and intensity indicate direction and level of change: blue spectrum colors indicate down-regulated expression, while red spectrum colors indicate up-regulated expression.
Glutamatergic genes
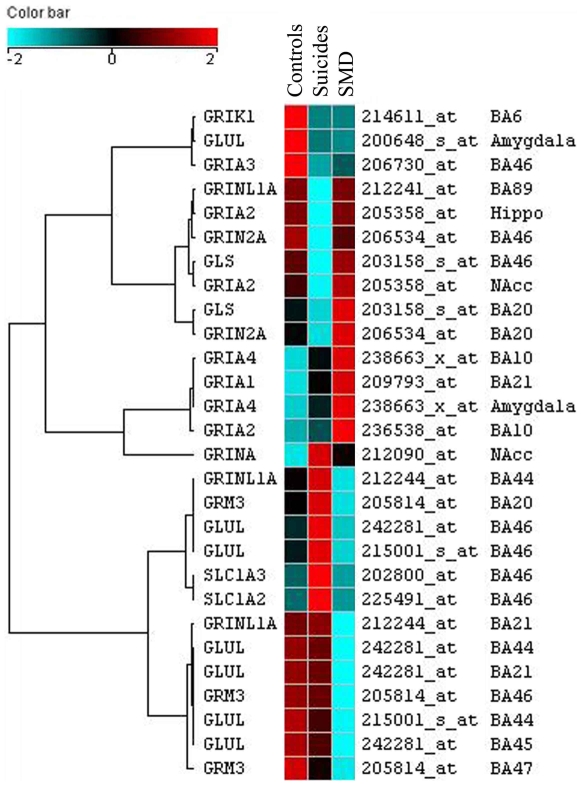
A total of 28 probe sets corresponding to genes implicated in glutamatergic neurotransmission were differentially expressed across the regions. A graphical representation of the gene expression changes between the three groups is shown in Figure 4. A good proportion of these probe sets (7) corresponded to the Glutamate-ammonia ligase (glutamine synthase) gene (GLUL) that codes for an enzyme implicated in glutamate recycling. GLUL probe sets were found consistently down-regulated among the depressed suicides in the prefrontal cortex (BA44 BA45, BA46) and the amygdala (Figure 4). Also of particular interest, 7 out of 28 probe sets (Figure 4) correspond to one of four subtypes (AMPA1, AMPA2, AMPA3, AMPA4) of the glutamate AMPA receptor that was differentially expressed in several brain cortical (BA10, BA21, BA46) and subcortical areas (hippocampus, nucleus accumbens, amygdala). A majority of the glutamatergic related probe sets correspond to ionotropic NMDA receptor subunits (GRINA, GRIN2A, GRINL1A) and AMPA (GRIA3,GRIA4, GRIA1, GRIA2) receptors with the later being consistently up-regulated among the suicides with major depression versus the controls or the suicides without history of major depression (Figure 4). Also noteworthy, the glutamate receptor metabotropic 3 (GRM3) was consistently down-regulated among the suicides with and without major depression in two areas of the prefrontal cortex BA46 and BA47 and in two areas of the parietal cortex BA38 and BA20 (Figure 4). In summary, we observed a global up-regulation of AMPA receptors and a global down-regulation of the GRM3 receptor and the glutamine synthase (GLUL) genes expression in the suicide with major depression group.
Figure 4. Clustered image map (CIM) of the hierarchical cluster analysis of the glutamatergic system differentially expressed genes across the 17 regions investigated.
The color and intensity indicate direction and level of change: blue spectrum colors indicate down-regulated expression, while red spectrum colors indicate.
Validation of microarray gene expression results
The differential expression of GABAergic and glutamatergic genes was further confirmed by means of semi-quantitative RT-PCR in additional samples from the same individuals that were immediately adjacent to those used for the microarray assays. We investigated only the brain areas where differential expression was observed in the microarray experiments (Table 5). Out of the 16 genes/regions tested, 15 showed the same expression direction (up-/-down regulation) in comparisons between groups as those observed in the microarray experiments, and 12 were also significantly differentially expressed in the semi-quantitative RT-PCR experiments (Table 5).
Table 5. Confirmation of the microarray results involving critical GABAergic and glutamatergic genes in major depression and suicide using independent adjacent samples from the same subjects/areas by semi-quantitative RT-PCR (SemiQ RT-PCR).
| SemiQ RT-PCR (Mean) | Affymetrix (Mean) | ||||||||
| Gene | Region | Control | Suicide | SMD | P | Control | Suicide | SMD | P |
| GABARAPL1 | BA46 | 135.35 | 102.88 | 171.73 | 0.13 | 197.566 | 181.244 | 261.768 | 0.002 |
| GABARD | BA45 | 70.96 | 49.53 | 69.86 | 0.04 | 265.784 | 190.645 | 273.365 | 0.002 |
| GABARD | BA46 | 196.79 | 92.43 | 109.35 | 0.04 | 405.741 | 300.856 | 443.493 | 0.003 |
| GABARG1 | BA21 | 64.17 | 85.35 | 61.89 | 0.13 | 262.739 | 215.787 | 170.116 | 0.002 |
| GABARG1 | BA46 | 35.92 | 46.04 | 31.30 | 0.02 | 120.571 | 174.447 | 107.812 | 0.000 |
| GABRG2 | BA46 | 52.95 | 36.15 | 48.91 | 0.04 | 416.956 | 267.694 | 418.698 | 0.007 |
| GABRR1 | BA44 | 195.05 | 376.23 | 138.60 | 0.01 | 23.6967 | 35.345 | 19.1808 | 0.007 |
| GLS | BA46 | 56.56 | 48.36 | 56.43 | 0.12 | 231.561 | 163.459 | 243.658 | 0.002 |
| GLUL | Amy | 93.46 | 76.52 | 68.49 | 0.02 | 789.678 | 438.535 | 422.579 | 0.002 |
| GLUL | BA21 | 131.67 | 118.04 | 107.04 | 0.08 | 463.524 | 442.932 | 265.696 | 0.002 |
| GLUL | BA45 | 142.64 | 128.99 | 108.66 | 0.02 | 338.71 | 307.803 | 197.303 | 0.002 |
| GLUL | BA46 | 128.30 | 184.69 | 87.91 | 0.01 | 251.503 | 361.113 | 190.146 | 0.001 |
| GRIA1 | BA21 | 71.30 | 67.63 | 142.03 | 0.04 | 118.831 | 150.885 | 180.902 | 0.004 |
| GRIA3 | BA46 | 82.77 | 48.63 | 51.32 | 0.05 | 132.681 | 93.5443 | 100.471 | 0.006 |
| GRM3 | BA46 | 70.75 | 52.57 | 38.63 | 0.02 | 286.41 | 281.21 | 241.308 | 0.004 |
| SLC6A1 | BA4 | 150.61 | 156.09 | 140.69 | 0.58 | 648.13 | 648.05 | 478.61 | 0.002 |
Mean values per group are shown for the SemiQ RT-PCR experiment as well as for the MAS 5.1 normalized Affymetrix gene expression experiment.
Controlling for potential confounding effect of alcohol
The possible effect of alcohol on gene expression was a potential confounder of our results, considering that a comorbid diagnosis of alcohol abuse/dependence was present in some of the subjects in the three groups. To exclude the potential confounding effect of alcohol on our results, we first conducted analyses of covariance (Table S1) investigating genes associated with substance use across all the brain regions. None of the GABAergic or glutamatergic genes differentially expressed in suicides with or without major depression were associated to substance use.
We also investigated whether or not these GABAergic or glutamatergic genes were associated with alcohol in an independent gene expression dataset from the UCI Brain Bank. To this end, we compared samples from the dorsolateral prefrontal cortex of 13 alcohol abusers to samples from the same brain region of 21 normal controls using the Affymetrix HG-U133 Plus 2 microarrays. Results are presented in Table S2 and suggest that none of the GABAergic or glutamatergic genes found to be globally differentially expressed in depression and suicide were significantly differentially expressed in alcoholics.
Finally, we studied the long- (one month) and short-term (5 days) effects of alcohol on GABARD, GABRG2, GLS, GLUL, GRIA1, GRIA3, GABARG1, GRM3, GABARAPL1, SLC6A1, and GABRR1 gene expression by semi-quantitative RT-PCR in rat prefrontal cortex. We found no significant differences for these genes in either chronic or acute alcohol treatments (Table S3).
Discussion
In this study, gene expression was investigated using genome-wide microarrays in 17 brain areas thought to be involved in the neurobiology of suicide and major depression, comparing suicides with and without major depression to psychiatrically normal controls. This is, to our knowledge, the first large-scale brain expression study aiming at identifying global brain alterations associated with suicide and major depression. The extent of the expression changes varied considerably between the diverse brain areas investigated, with certain areas, such as those that comprise the prefrontal cortex and hippocampus, accounting for the majority of expression changes. This is consistent with what one would expect according to neuroanatomical studies of depression and suicide and is also consistent with previous studies looking at discrete brain regions [4], [57]–[63]. The functional analysis using gene ontologies also revealed that an over-representation of genes involved in cell communication processes were globally altered. More specifically, among genes involved in synaptic transmission, a striking number of GABAergic receptor subunit genes were generally up-regulated among the suicides with major depression, but showed lower expression levels among the 2 other groups. We also observed for the suicide with major depression group a general up-regulation of AMPA receptors subunit genes and a global down-regulation of GRM3 receptors and glutamine synthase (GLUL) gene expression. Our study suggests the presence of consistent alterations of several genes coding for components of the same pathways across different brain regions.
The HG-133AB chipset contains around 44,000 probe sets many of which may not be expressed at biologically significant or detectable levels. Accordingly, Jongeneel et al. estimated that between 10 to 15 thousand transcripts are actually expressed in several types of human cell lines [64]. For that reason and in order to reduce the multiplicity problem, we used a combination of filtering methods in order to include in our analysis only transcripts that were actually expressed and reliably detectable. This approach efficiently allows to significantly reduce the total number of analyzed probe sets without notably decreasing the number of truly positive genes [65]. This resulted in an average of around 15,000 probe sets analyzed per region Second, in order to control for type I errors, we also used a combination of stringent P-value thresholds (≤0.01 both at the ANOVA and post-hoc test), as well as a fold change of at least 1.3 in either direction. Most importantly, by focusing on results that replicate across several different brain regions, which constitute partially independent experiments, we are likely to have significantly reduced the occurrence of type I errors in our study.
The current approach led to the identification of 4,472 differentially expressed probe sets over the 17 brain regions (Table 2). As expected, and in accordance with the neuroanatomical and post-mortem biomarkers literature, three prefrontal cortex areas, BA8,9, BA10, BA46, and the hippocampus, had the highest number of differentially expressed probe sets, thus confirming the implication of these regions in the pathophysiology of suicide and major depression (Table 2 and Figure 1). These four areas have been well characterized and have been previously shown to be implicated in suicidal behaviors and depression in numerous post-mortem studies [4], [66]–[78]. In vivo, neuroimaging studies have also pointed to alterations in the prefrontal cortex and in the hippocampus in patients suffering from major depression [29], [59], [61], [79]–[81]. Our study provides on a genomic scale, potential molecular targets that may account for those alterations in the brains of suicide victims with and without major depression.
Functional analysis using gene ontologies [82] was performed across the 17 regions using a new tool (ErmineJ) that efficiently addresses many of the limitations and problems of the initial gene ontology tools [83], by implementing more comprehensive algorithms and the possibility of performing analyses in parallel. This global functional ontological profiling revealed specific ontological categories commonly overrepresented in all the regions investigated in this study, and further investigation showed that an important proportion of genes belonged to cell-communication processes. Among these, a remarkable number of probe sets corresponded to genes coding for various molecular units of the GABAergic and glutamatergic neurotransmitter systems.
L-glutamic acid (glutamate) and GABA are respectively the main excitatory and inhibitory neurotransmitters in the central nervous system [84]. Growing evidence has supported alterations in both of these neurotransmitter systems in major depression [22], [23], [53]–[55] and suicide [17], [19], [20], [55], [56]. Sanacora et al. [21] using a magnetic resonance spectroscopy protocol observed elevated levels of glutamate and lower levels of GABA in the occipital cortex of subjects diagnosed with major depression. Furthermore, Hasler et al. [85] demonstrated that abnormal reductions in glutamate/glutamine and GABA concentrations are present in the prefrontal cortex of unmedicated depressed patients. Our results are also in concordance with those of Choudary et al. [18], who performed a gene expression study in the cingulate and prefrontal cortex brain areas of suicides and depressed suicides using one of the chips (HG-U133A) of the microarray set used in our study. Interestingly, their results point to similar alterations in glutamate recycling (glutamine synthase, GLUL), glutamate receptors (GRIA1, GRIA3, GRIK1, GRM3) and GABA receptors (GABARB3, GABRD, GABARG2) in depressed suicides versus controls. Also, recently, Merali et al. [55] observed altered levels of GABA(A) receptor subunits (α1, α3, α4 and δ) in the BA10 of depressed suicide victims versus non-depressed controls.
GRIA3, which was also confirmed to be differentially expressed by SemiQ RT-PCR (Table 5), is of particular interest in suicide as it was significantly down-regulated in the prefrontal cortex in both suicide groups (BA46, Figure 4), with and without major depression, suggesting an implication in suicide irrespective of the presence of major depression. This result is particularly important in the light of the recent observation by Laje et al. [86] that genetic variation at the GRIA3 gene seems to be associated with suicidal ideation during citalopram therapy and suggests that expression changes in this gene may also confer susceptibility to suicide and suicidal ideation in antidepressant treated patients.
Glia and astroglia in particular are responsible for the uptake,via the glial glutamate transporter (EAAT2) and metabolism and recycling, via glutamine synthase (GLUL) of glutamate [87]. Glutamine synthase is responsible for the recycling of glutamate by its conversion into glutamine, which is then released by the astrocytes and taken up at the synaptic terminals where it can be reconverted into glutamate or GABA [87]. Glutamine synthase was down-regulated in several prefrontal and parietal areas of brains of suicides with major depression, but not in suicides without major depression suggesting a depression specific dysregulation of glutamate recycling probably leading to altered glutamatergic and/or GABAergic neurotransmission. At the same time the majority of ionotropic glutamatergic receptors differentially expressed were up-regulated in these brain regions in depressed suicides, reinforcing the idea of a substantial alteration of glutamatergic neurotransmission in this group. Given the importance of some of these molecules in glial metabolism, and the growing evidence pointing to astroglial alterations in major depression [87], future studies should investigate cell specific changes in gene expression by means of laser capture microdissection in the brains of depressed suicides.
This hypothesis, if true, is in agreement with the observation that a single dose of Ketamine, an NMDA antagonist, is sufficient to produce a rapid and long lasting antidepressant effect [88]. Glutamate seems to mediate stress-induced neuronal atrophy in the hippocampus [89]. In addition, although not always consistent, there are different lines of evidence, comprising peripheral studies [90], postmortem brain studies [20], and in-vivo imaging studies [91] reporting glutamatergic dysfunction in major depression. Interestingly, glutamatergic neurotransmission is closely controlled by intracellular levels of polyamines, spermine and spermidine being specific modulators of NMDA and AMPA receptors activity [92]–[97]. Polyamines, and more specifically SSAT, the rate limiting enzyme in the catabolism of polyamines, were associated with suicide and depression in a previous study by our group [16]. Polyamines modulate GABAergic and glutamatergic neurotransmission, genes of those systems as well as SSAT were also found to be altered in the present study. In light of these observations, it is important to consider the polyamine-glutamatergic systems as a possible target for future strategies for the treatment of major depression.
Serotonergic and adrenergic dysfunction has been implicated in suicide [5], [70] , yet we did not detect a significant representation of genes coding for components of these neurotransmitter systems in our differential expression analyses. This result is in accordance with all other microarryay experiments performed to date using suicide brains, where no serotonergic genes have been detected as differentially expressed [16], [38].
While our microarray experiment sampled multiple brain regions making our analysis global in nature, although enriched for frontal cortical regions due to the implication of these regions in depression and suicide, not all differentially expressed GABAergic and glutamatergic genes were differentially expressed across all regions. In general, we observed a particular probe set as differentially expressed across 2–3 regions, many of which did not overlap with other probe sets identified as differentially expressed. Still, the consistency of the dysregulation in the GABA-glutamate gene systems was striking.
Even though possible limitations regarding pre- and post-mortem factors, such as agonal period, alcohol abuse/dependence and post mortem interval were experimentally controlled for in this study, the conclusions presented here are to be taken with caution and need to be confirmed in an independent and larger sample. Our study design does not allow to clearly differentiating the alterations solely related to suicide from those specific to major depression. This would be possible to resolve only by including a group of matching patients with major depression who did not die by suicide, but such a group would be too difficult to obtain due to the demographic characteristics of suicide victims and depressed patients. Nevertheless, our results are interesting as they shed light into the molecular alterations simultaneously taking place in several important brain regions of individuals with and without major depressive disorder at the moment of their suicide. Another limitation of this study is the choice of 1.3 as a fold-change cut-off. While this allowed us to focus on more robust effects, it prevented us from detecting more subtle changes in gene expression that may be at play.
In conclusion, this is, to our knowledge, the first study attempting to determine global brain expression changes taking place in the brain of suicide victims with and without major depression. We observed global changes in genes implicated in synaptic transmission, and more specifically, in genes involved in GABAergic (inhibitory) and glutamatergic (excitatory) neurotransmission. Further studies are warranted in order to examine in detail the cellular origin of the alterations observed in our analyses, to validate the observed changes using complementary approaches and to investigate possible genetic factors related to the observed alterations.
Supporting Information
Genes differentially expressed after an ANCOVA analysis between the three groups with substance history or presence in the toxicological screening as a covariate. The 17 regions were analyzed, only genes associated with substance use are shown the significant genes at the P<0.01 level.
(0.07 MB DOC)
The effect of substances on the expression of glutamatergic and GABAergic genes.
(0.04 MB DOC)
Q-RT-PCR results from acute (N = 10) and chronic (N = 15) alcohol experiments in rats.
(0.03 MB DOC)
Glutamatergic and gabaergic raw data
(0.61 MB XLS)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded in part by Canadian Institute of Health Research (CIHR) grants MOP#53321 and MOP#79253. In addition, microarray studies in this study were carried out as part of a collaboration with Gene Logic Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. World Health Report 2000. Health Systems: Improving Performance 2000 [Google Scholar]
- 2.Blumenthal SJ, Kupfer DJ. Washington, DC: The Americal Psychiatric Press Inc; 1990. Suicide over the life cycle: risk factors, assessment, and treatment of suicidal patients. [Google Scholar]
- 3.Turecki G. Suicidal Behavior: Is There a Genetic Predisposition? Bipolar Disorders. 2001;3:335–349. doi: 10.1034/j.1399-5618.2001.30608.x. [DOI] [PubMed] [Google Scholar]
- 4.Gross-Isseroff R, Biegon A, Voet H, Weizman A. The suicide brain: a review of postmortem receptor/transporter binding studies. Neurosci Biobehav Rev. 1998;22:653–661. doi: 10.1016/s0149-7634(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 5.Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacol. 2001;24:467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 6.Bourne HR, Bunney WE, Jr, Colburn RW, Davis JM, Davis JN, et al. Noradrenaline, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid in hindbrains of suicidal patients. Lancet. 1968;2:805–808. doi: 10.1016/s0140-6736(68)92459-8. [DOI] [PubMed] [Google Scholar]
- 7.Meana JJ, Barturen F, Garcia-Sevilla JA. Alpha 2-adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. Biol Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- 8.Ordway GA. Pathophysiology of the locus coeruleus in suicide. Ann N Y Acad Sci. 1997;836:233–252. doi: 10.1111/j.1749-6632.1997.tb52363.x. [DOI] [PubMed] [Google Scholar]
- 9.Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy A, Pickar D, De Jong J, Karoum F, Linnoila M. Suicidal behavior in depression: relationship to noradrenergic function. Biol Psychiatry. 1989;25:341–350. doi: 10.1016/0006-3223(89)90181-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, et al. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist J, Traskman-Bendz L, Lindstrom MB, Ekman R. Suicide-attempters having immunoglobulin G with affinity for dopamine in cerebrospinal fluid. Eur Neuropsychopharmacol. 2002;12:153–158. doi: 10.1016/s0924-977x(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 13.Bowden C, Theodorou AE, Cheetham SC, Lowther S, Katona CLE, et al. Dopamine D1 and D2 receptor binding sites in brain samples from depressed suicides and controls. Brain Res. 1997;752:227–233. doi: 10.1016/s0006-8993(96)01460-6. [DOI] [PubMed] [Google Scholar]
- 14.Pare CMB, Yeung DPH, Price K, Stacey RS. 5-hydroxytrytamine, noradrenaline, and dopamine in brainstem, hypoythalamus, and caudate nucleus of controls and patients committing suicide by coal-gas poisining. Lancet. 1969;2:133–135. doi: 10.1016/s0140-6736(69)92442-8. [DOI] [PubMed] [Google Scholar]
- 15.Zalsman G, Frisch A, Lewis R, Michaelovsky E, Hermesh H, et al. DRD4 receptor gene exon III polymorphism in inpatient suicidal adolescents. J Neural Transm. 2004;111:1593–1603. doi: 10.1007/s00702-004-0182-3. [DOI] [PubMed] [Google Scholar]
- 16.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Cheetham SC, Crompton MR, Katona CL, Parker SJ, Horton RW. Brain GABAA/benzodiazepine binding sites and glutamic acid decarboxylase activity in depressed suicide victims. Brain Res. 1988;460:114–123. doi: 10.1016/0006-8993(88)91211-5. [DOI] [PubMed] [Google Scholar]
- 18.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holemans S, De Paermentier F, Horton RW, Crompton MR, Katona CL, et al. NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res. 1993;616:138–143. doi: 10.1016/0006-8993(93)90202-x. [DOI] [PubMed] [Google Scholar]
- 20.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 21.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 22.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 23.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–37, 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 24.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 25.Manji HK. G proteins: implications for psychiatry. Am J Psychiatry. 1992;149:746–760. doi: 10.1176/ajp.149.6.746. [DOI] [PubMed] [Google Scholar]
- 26.Garlow S, Musselman D, Nemeroff C. Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of Mental Illness. Neurobiology of Mental Illness. 1999.
- 27.Nemeroff CB. Recent advances in the neurobiology of depression. Psychopharmacol Bull. 2002;36(Suppl 2):6–23. [PubMed] [Google Scholar]
- 28.Soares JC, Mann JJ. The anatomy of mood disorders- review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 31.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 32.Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur Psychiatry. 2002;17(Suppl 3):300–305. doi: 10.1016/s0924-9338(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 33.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 34.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 36.Lockhart D, Dong H, Byrne M, Folletie MT, Gallo MV, et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nature Biotechnology. 1996;1438:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- 37.Bunney WE, Bunney BG, Vawter MP, Tomita H, Li J, et al. Microarray technology: a review of new strategies to discover candidate vulnerability genes in psychiatric disorders. Am J Psychiatry. 2003;160:657–666. doi: 10.1176/appi.ajp.160.4.657. [DOI] [PubMed] [Google Scholar]
- 38.Sibille E, Arango V, Galfalvy HC, Pavlidis P, Erraji-Benchekroun L, et al. Gene expression profiling of depression and suicide in human prefrontal cortex. Neuropsychopharmacol. 2004;29:351–361. doi: 10.1038/sj.npp.1300335. [DOI] [PubMed] [Google Scholar]
- 39.Gwadry FG, Sequeira A, Hoke G, Ffrench-Mullen JM, Turecki G. Molecular characterization of suicide by microarray analysis. Am J Med Genet C Semin Med Genet. 2005;133:48–56. doi: 10.1002/ajmg.c.30046. [DOI] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 41.Pawitan Y, Murthy KR, Michiels S, Ploner A. Bias in the estimation of false discovery rate in microarray studies. Bioinformatics. 2005;21:3865–3872. doi: 10.1093/bioinformatics/bti626. [DOI] [PubMed] [Google Scholar]
- 42.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 43.Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Genet. 2006;7:55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- 44.Simard LR, Prescott G, Rochette C, Morgan K, Lemieux B, et al. Linkage disequilibrium analysis of childhood-onset spinal muscular atrophy (SMA) in the French-Canadian population. Human Molecular Genetics. 1994;3:459–463. doi: 10.1093/hmg/3.3.459. [DOI] [PubMed] [Google Scholar]
- 45.DeArmond, Stephen J, Fusco, Madeline M, Dewey, et al. Structure of the Human Brain: A Photographic Atlas. Newyork: Oxford University Press; 1989. [Google Scholar]
- 46.Kim C, Therrien N, Riopel G, Seguin M, Chawky N, et al. Familial aggregation of suicidal behaviour: a family study of male suicide victims from the general population. Am J Psychiatry In press. 2004 doi: 10.1176/appi.ajp.162.5.1017. [DOI] [PubMed] [Google Scholar]
- 47.George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. yoa9468 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Lee HK, Braynen W, Keshav K, Pavlidis P. ErmineJ: tool for functional analysis of gene expression data sets. BMC Bioinformatics. 2005;6:269. doi: 10.1186/1471-2105-6-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- 50.Schulz R, Wuster M, Duka T, Herz A. Acute and chronic ethanol treatment changes endorphin levels in brain and pituitary. Psychopharmacology (Berl) 1980;68:221–227. doi: 10.1007/BF00428107. [DOI] [PubMed] [Google Scholar]
- 51.Seizinger BR, Bovermann K, Maysinger D, Hollt V, Herz A. Differential effects of acute and chronic ethanol treatment on particular opioid peptide systems in discrete regions of rat brain and pituitary. Pharmacol Biochem Behav. 1983;18(Suppl 1):361–369. doi: 10.1016/0091-3057(83)90200-9. [DOI] [PubMed] [Google Scholar]
- 52.Samson HH, Files FJ, Denning C. Chronic ethanol self-administration in a continuous-access operant situation: the use of a sucrose/ethanol solution to increase daily ethanol intake. Alcohol. 1999;19:151–155. doi: 10.1016/s0741-8329(99)00032-4. S0741832999000324 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Stewart CA, Reid IC. Antidepressant mechanisms: functional and molecular correlates of excitatory amino acid neurotransmission. Mol Psychiatry. 2002;7(Suppl 1):S15–S22. doi: 10.1038/sj.mp.4001014. [DOI] [PubMed] [Google Scholar]
- 54.Cryan JF, Kaupmann K. Don't worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, et al. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 57.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 58.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 59.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 60.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 61.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 62.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 63.Fujita M, Charney DS, Innis RB. Imaging serotonergic neurotransmission in depression: hippocampal pathophysiology may mirror global brain alterations. Biol Psychiatry. 2000;48:801–812. doi: 10.1016/s0006-3223(00)00960-4. [DOI] [PubMed] [Google Scholar]
- 64.Jongeneel CV, Iseli C, Stevenson BJ, Riggins GJ, Lal A, et al. Comprehensive sampling of gene expression in human cell lines with massively parallel signature sequencing. Proc Natl Acad Sci U S A. 2003;100:4702–4705. doi: 10.1073/pnas.0831040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClintick JN, Edenberg HJ. Effects of filtering by Present call on analysis of microarray experiments. BMC Bioinformatics. 2006;7:49. doi: 10.1186/1471-2105-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 67.Mann JJ. The neurobiology of suicide. Nature Medicine. 1998;4:25–30. doi: 10.1038/nm0198-025. [DOI] [PubMed] [Google Scholar]
- 68.Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Arch Gen Psychiatry. 1990;47:1029–1034. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- 69.Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, et al. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 70.Arango V, Ernsberger P, Sved AF, Mann JJ. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res. 1993;630:271–282. doi: 10.1016/0006-8993(93)90666-b. [DOI] [PubMed] [Google Scholar]
- 71.Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, et al. Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry. 2004;55:234–243. doi: 10.1016/j.biopsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Turecki G, Briere R, Dewar K, Antonetti T, Lesage AD, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 73.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, et al. mRNA and protein expression of selective alpha subunits of G proteins are abnormal in prefrontal cortex of suicide victims. Neuropsychopharmacol. 2002;27:499–517. doi: 10.1016/S0893-133X(02)00335-4. [DOI] [PubMed] [Google Scholar]
- 74.Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacol. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 75.Meana JJ, Garcia-Sevilla JA. Increased alpha 2-adrenoceptor density in the frontal cortex of depressed suicide victims. J Neur Transm. 1987;70:377–381. doi: 10.1007/BF01253612. [DOI] [PubMed] [Google Scholar]
- 76.Dwivedi Y, Rizavi HS, Conley RR, Pandey GN. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- 77.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Arch Gen Psychiatry. 2004;61:685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- 78.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, et al. Brain Region Specific Alterations in the Protein and mRNA Levels of Protein Kinase A Subunits in the Post-Mortem Brain of Teenage Suicide Victims. Neuropsychopharmacol. 2005 doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- 79.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 81.Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, et al. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- 82.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khatri P, Draghici S. Ontological analysis of gene expression data: current tools, limitations, and open problems. Bioinformatics. 2005;21:3587–3595. doi: 10.1093/bioinformatics/bti565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- 85.Hasler G, van d V, Tumonis T, Meyers N, Shen J, et al. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 86.Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 87.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 89.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 90.Berk M, Plein H, Ferreira D. Platelet glutamate receptor supersensitivity in major depressive disorder. Clin Neuropharmacol. 2001;24:129–132. doi: 10.1097/00002826-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, et al. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 92.Williams K. Interactions of polyamines with ion channels. Biochem J . 1997;325 (Pt 2):289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romano C, Williams K, Molinoff PB. Polyamines modulate the binding of [3H]MK-801 to the solubilized N-Methyl-D-Aspartate receptor. J Neurochem. 1991;57:811–818. doi: 10.1111/j.1471-4159.1991.tb08223.x. [DOI] [PubMed] [Google Scholar]
- 94.DiScenna PG, Ferchmin PA, Eterovic VA, Teyler TJ. Spermine depresses NMDA, K/AMPA and GABAA-mediated synaptic transmission in the rat hippocampal slice preparation. Brain Res. 1994;647:353–356. doi: 10.1016/0006-8993(94)91335-8. [DOI] [PubMed] [Google Scholar]
- 95.Pellegrini-Giampietro DE. An activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003;26:9–11. doi: 10.1016/s0166-2236(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 96.Ragnarsson L, Mortensen M, Dodd PR, Lewis RJ. Spermine modulation of the glutamate(NMDA) receptor is differentially responsive to conantokins in normal and Alzheimer's disease human cerebral cortex. J Neurochem. 2002;81:765–779. doi: 10.1046/j.1471-4159.2002.00872.x. [DOI] [PubMed] [Google Scholar]
- 97.Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes differentially expressed after an ANCOVA analysis between the three groups with substance history or presence in the toxicological screening as a covariate. The 17 regions were analyzed, only genes associated with substance use are shown the significant genes at the P<0.01 level.
(0.07 MB DOC)
The effect of substances on the expression of glutamatergic and GABAergic genes.
(0.04 MB DOC)
Q-RT-PCR results from acute (N = 10) and chronic (N = 15) alcohol experiments in rats.
(0.03 MB DOC)
Glutamatergic and gabaergic raw data
(0.61 MB XLS)