In higher eukaryotes, pteridine cofactors such as tetrahydro-biopterin (BH4) are essential for a range of enzyme-catalysed reactions, including hydroxylation of aromatic amino acids and cleavage of ether lipids by specific monooxygenases, as well as production of nitric oxide by NO synthase [1]. Among the protozoa, it has long been known that trypanosomatids are pteridine auxotrophs that salvage the necessary molecules from the host organism [2]. Although pterins (i.e. naturally occurring pteridines with 2-amino and 4-oxy substitutions on the pteridine ring) are recognised as a growth factor for trypanosomatids, their precise functions in these organisms are not clear, but may include roles in dealing with oxidative stress and in parasite differentiation [3,4]. Interestingly, there is also evidence that Leishmania can synthesise folates from biopterin by a route that differs from the standard folate biosynthetic pathway found in other microorganisms [5]. These observations led us to consider a possible role for pterin metabolism in the apicomplexan parasites.
The conventional route to pterin synthesis, lacking in trypanosomatids, initially involves the conversion of GTP to 7,8-dihydroneopterin triphosphate by GTP cyclohydrolase I (GTPC; EC 3.5.4.16), an enzyme that is present in both P. falciparum [6] and T. gondii (Smith, Hyde and Sims, unpublished data). This product can then be utilised by many microorganisms (but not higher eukaryotes, other than plants) in a biosynthetic pathway leading to tetrahydrofolate and its derivatives, which are also key enzyme cofactors, or it can be acted upon by 6-pyruvoyl-tetrahydropterin synthase (PTPS; EC 4.2.3.12) and sepiapterin reductase (SR; EC 1.1.1.153) to yield BH4.
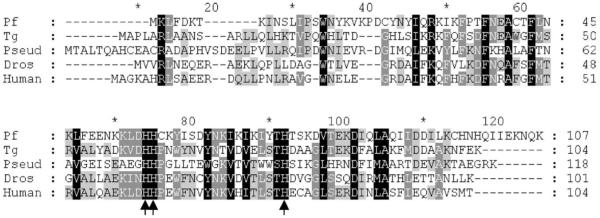
Although much has been learnt about folate biosynthesis and salvage in P. falciparum [7] and to a lesser extent, in T. gondii [8], almost nothing is known about the pterin content of these organisms and any metabolic role such molecular species might play. The only annotated gene in the complete P. falciparum genome sequence hinting at the existence of pterin metabolism is PFF1360w, putatively encoding a PTPS orthologue, although there is no obvious orthologue of SR. However, we discovered an unannotated gene located between PF11_0095 and PF11_0096 whose predicted product bore histidine motifs separated by 16 residues characteristic of pterin-4a-carbinolamine dehydratase (PCD; EC 4.2.1.96) (Fig. 1). PCD is an enzyme that in many organisms is essential for the recycling of the BH4 moiety, which is oxidised to the dihydro- level when acting as a cofactor for amino acid hydroxylations (e.g. Phe to Tyr, Tyr to L-dopa, Trp to 5-hydroxytryptamine) and other reactions. PCD executes a dehydration step, removing as water an –OH group introduced onto the 4a position of the pterin ring in the first step of BH4 utilisation (Fig. 2). This gene may have been missed in the original annotation, as it is very short, with a coding length of 324 bp split by a 142 bp intron between codons 39 and 40. We also found an equivalent gene in the T. gondii database with a 315 bp ORF and a single intron (271 bp) in the same relative position (between codons 44 and 45) as in the P. falciparum gene (Fig. 1).
Fig. 1.
Alignment of pterin-4a-carbinolamine dehydratase (PCD) sequences. Pf, P. falciparum (this work, accession no. DQ223776); Tg, T. gondii (this work, DQ223777); Pseud, Pseudomonas aeruginosa (P43335); Dros, Drosophila melanogaster (AAC25196); Human, Homo sapiens (P80095). Arrows indicate the three conserved His residues involved in binding the pterin ligand [13].
Fig. 2.
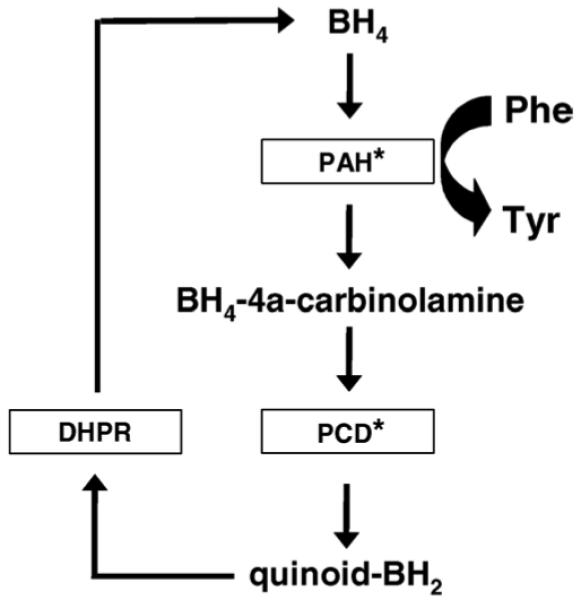
Recycling of tetrahydrobiopterin (BH4) via the pterin-4a-carbinolamine dehydratase (PCD) activity after acting as a cofactor in the hydroxylation of phenylalanine by phenylalanine hydroxylase (PAH). The redox cycle is completed by dihydropterin reductase (DHPR). Asterisks indicate enzymes absent in E. coli but present in P. aeruginosa.
To test the function of a putative PCD gene biochemically is not straightforward as the carbinolamine substrate is not readily available. We therefore took advantage of the fact that phenylalanine hydroxylation systems are relatively rare in prokaryotes to set up a microbiological complementation test in an Escherichia coli tyrosine auxotroph [9]. Thus, E. coli lacks both phenylalanine hydroxylase and PCD genes, but does have a dihydropteridine reductase activity (DHPR; EC 1.6.99.7), which is necessary for completion of a pterin recycling pathway (Fig. 2). However, phenylalanine hydroxylase and PCD genes both occur in certain bacteria including the gamma-proteobacterium Pseudomonas aeruginosa [10]. Thus, when transformed into E. coli, the products of these two genes can combine with the endogenous DHPR to establish a pterin recycling assay with the necessary positive controls.
Our strategy was thus to assay for recycling by the successful synthesis of Tyr from Phe, dependent upon the introduced P. aeruginosa phenylalanine hydroxylase and PCD activities, and then test the putative P. falciparum and T. gondii PCD genes for function by substituting them for the P. aeruginosa PCD gene in the positive control. Without pterin recycling, the synthesis of Tyr is inadequate for viability. After PCR amplification of the P. falciparum and T. gondii genes from cDNA libraries, the products were cloned into pGEM-T or pGEM-T Easy (Promega) in such a way that they were positioned downstream of the lacZ promoter with a stop codon in-frame with the lac gene situated shortly before the PCD ATG start codon. This was to truncate the synthesis of the beta-galactosidase alpha-peptide product. We were thus depending upon translational reinitiation at the start codon of a correctly orientated PCD gene in order to observe activity. To complete the recycling system, the P. aeruginosa phenylalanine hydroxylase gene (phhA) was introduced into the E. coli host (JP2255) on a compatible pACYC177-based plasmid, pJSll [9].
For both P. falciparum and T. gondii, clones were tested for their ability to rescue the E. coli mutant to a degree comparable to that observed with the P. aeruginosa positive control, as judged by growth rate on the minimal medium agar plates containing phenylalanine (Fig. 3A and B). All positive clones were found to contain the putative PCD gene orientated in the sense direction with respect to the lacZ promoter. To further confirm that the activity was not spurious but was a direct result of the genes we had inserted, the T. gondii ORF was reversed in the plasmid while the P. falciparum gene was cut with BstBI and the two-base overhang filled in with Klenow polymerase, causing the PCD reading frame to be disrupted about half-way into the coding sequence. Neither of these constructs was able to rescue the E. coli auxotroph (Fig. 3C and D). We thus conclude that the genes we have identified in P. falciparum and T. gondii indeed encode PCD activity.
Fig. 3.
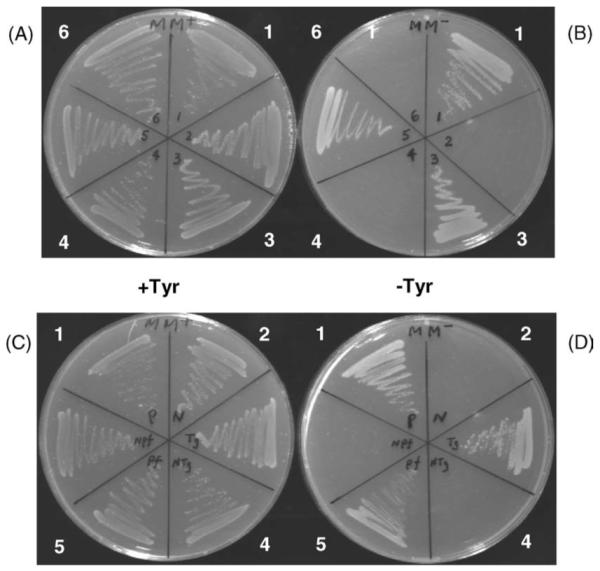
Complementation tests in the E. coli tyrosine auxotroph JP2255 [9] of the putative P. falciparum and T. gondii pcd genes. Panels A and B: sector 1, positive control carrying Pseudomonas phhB plus Pseudomonas phhA genes; 2, negative control carrying Pseudomonas phhB only; 3, T. gondii cDNA clone tg2 plus Pseudomonas phhA; 4, T. gondii cDNA clone tg2 alone; 5, P. falciparum cDNA clone pf3 plus Pseudomonas phhA; 6, P. falciparum cDNA clone pf3 alone. Panels C and D: sectors 1 and 2, controls as above; 3, T. gondii cDNA clone tg2 plus Pseudomonas phhA; 4, T. gondii clone tg2 with inverted insert plus Pseudomonas phhA gene; 5, P. falciparum cDNA clone pf3 plus Pseudomonas phhA gene; 6, P. falciparum clone pf3 with insert frameshifted ca. half-way along the ORF, plus Pseudomonas phhA gene. Note that the Pseudomonas phhB gene (introduced on plasmid pJZ9-4 [9]) encodes PCD. Growth was for 48 h at 37 °C on minimal medium plates supplemented with 10 μg ml−1 streptomycin, 50 μg ml−1 phenylalanine, with (A and C) or without (B and D) 50 μg ml−1 tyrosine.
Our observations point to a new area of metabolism that merits investigation in these apicomplexan organisms. It is not yet clear what role pterins might play in these parasites, although observations made in trypanosomatids are suggestive [4]. As found in E. coli and P. aeruginosa, although both P. falciparum and T. gondii appear to have a gene encoding PTPS, albeit functionally untested, there seems to be no equivalent of an SR-encoding gene whose product could convert 6-pyruvoyltetrahydropterin to BH4, at least in the case of P. falciparum. It has been suggested in the case of the bacterial systems that one or more pterin cofactors other than BH4, such as tetrahydroneopterin, might be involved in the recycling reaction described above [9]. The feasibility of this is reinforced by the fact that chemical studies of the rat PCD enzyme have demonstrated a lack of sensitivity to the nature of the 6-substituent, and to the stereochemistry of this or the 4a-hydroxyl group [11], indicative of a flexible binding pocket. Interestingly, it has also been shown in humans that the PCD protein plays a second role as a transcriptional regulator (known as DCoH) in the nucleus [12], although this may be a function only found in higher organisms. PCD genes appear to be quite widely distributed in bacteria, but the known enzymes that use a pterin cofactor, such as the hydroxylases described above, are much less common. Thus there probably remain to be discovered in bacteria, and possibly in apicomplexans, other enzymes that produce a carbinolamine intermediate requiring recycling via PCD. The identification and functional demonstration of a pterin recycling activity reported here is a first step in exploring the likely importance of pterin metabolism in P. falciparum and T. gondii.
Acknowledgements
We thank Roy A. Jensen (University of Florida) for E. coli strain JP2255 and plasmid pJZ9-4, and the Wellcome Trust, UK (Grant No. 056845) for financial support.
References
- [1].Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:l–16. [PMC free article] [PubMed] [Google Scholar]
- [2].Trager W. Pteridine requirement of hemoflagellate Leishmania tarentolae. J Protozool. 1969;16:372–5. doi: 10.1111/j.1550-7408.1969.tb02284.x. [DOI] [PubMed] [Google Scholar]
- [3].Cunningham ML, Titus RG, Turco SJ, Beverley SM. Regulation of differentiation to the infective stage of the protozoan parasite Leishmania major by tetrahydrobiopterin. Science. 2001;292:285–7. doi: 10.1126/science.1057740. [DOI] [PubMed] [Google Scholar]
- [4].Ouellette M, Drummelsmith J, El Fadili A, Kundig C, Richard D, Roy G. Pterin transport and metabolism in Leishmania and related trypanosomatid parasites. Int J Parasit. 2002;32:385–98. doi: 10.1016/s0020-7519(01)00346-0. [DOI] [PubMed] [Google Scholar]
- [5].Beck JT, Ullman B. Biopterin conversion to reduced folates by Leish-mania donovani promastigotes. Mol Biochem Parasitol. 1991;49:21–8. doi: 10.1016/0166-6851(91)90126-q. [DOI] [PubMed] [Google Scholar]
- [6].Lee CS, Salcedo E, Wang Q, Wang P, Sims PFG, Hyde JE. Characterization of three genes encoding enzymes of the folate biosynthetic pathway in Plasmodium falciparum. Parasitology. 2001;122:l–13. doi: 10.1017/s0031182000006946. [DOI] [PubMed] [Google Scholar]
- [7].Hyde JE. Exploring the folate pathway in Plasmodium falciparum. Acta Trop. 2005;94:191–206. doi: 10.1016/j.actatropica.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pashley TV, Volpe F, Pudney M, Hyde JE, Sims PFG, Delves CJ. Isolation and molecular characterization of the bifunctional hydroxy-methyldihydropterin pyrophosphokinase-dihydropteroate synthase gene from Toxoplasma gondii. Mol Biochem Parasitol. 1997;86:37–47. [PubMed] [Google Scholar]
- [9].Song J, Xia TH, Jensen RA. PhhB, a Pseudomonas aeruginosa homolog of mammalian pterin 4a-carbinolamine dehydratase/DCoH, does not regulate expression of phenylalanine hydroxylase at the transcriptional level. J Bacteriol. 1999;181:2789–96. [Google Scholar]
- [10].Zhao GS, Xia TH, Song J, Jensen RA. Pseudomonas aeruginosa possesses homologs of mammalian phenylalanine hydroxylase and 4-alpha-carbinolamine dehydratase/DCoH as part of a 3-component gene cluster. Proc Natl Acad Sci USA. 1994;91:1366–70. doi: 10.1073/pnas.91.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rebrin I, Bailey SW, Boerth SR, Ardell MD, Ayling JE. Catalytic characterization of 4a-hydroxytetrahydropterin dehydratase. Biochemistry. 1995;34:5801–10. doi: 10.1021/bi00017a011. [DOI] [PubMed] [Google Scholar]
- [12].Citron BA, Davis MD, Milstien S, Gutierrez J, Mendel DB, Crabtree GR, et al. Identity of 4a-carbinolamine dehydratase, a component of the phenylalanine hydroxylation system, and DCoH, a transregulator of homeodomain proteins. Proc Natl Acad Sci USA. 1992;89:11891–4. doi: 10.1073/pnas.89.24.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cronk JD, Endrizzi JA, Alber T. High-resolution structures of the bifunctional enzyme and transcriptional coactivator DCoH and its complex with a product analogue. Protein Sci. 1996;5:1963–72. doi: 10.1002/pro.5560051002. [DOI] [PMC free article] [PubMed] [Google Scholar]