Abstract
Background
The intervertebral disc (IVD) is the largest avascular structure in human body. Transport of small molecules in IVD is mainly through diffusion from the endplates and the peripheral blood vessels surrounding IVD. Studies have investigated the structure, chemical components and water content in IVD, but to our knowledge no study has investigated the effect of mechanical loading on oxygen transport in IVD. The objective of this study was to determine the stain-dependent behavior of oxygen diffusivity in IVD tissue.
Method of Approach
A one-dimensional steady-state diffusion experiment was designed and performed to determine the oxygen diffusivity in bovine annulus fibrosus (AF). The oxygen diffusivity was calculated using equation derived from Fick’s law. A total of 20 AF specimens (d=6 mm, h~0.5 mm) from bovine coccygeal IVD were used to determine oxygen diffusivity at three levels of compressive strain.
Results
The average oxygen diffusivity (mean ± SD) of bovine AF in the axial direction was 1.43 ± 0.242×10−5 cm2/s (n=20) at 4.68 ± 1.67% compressive strain level, 1.05 ± 0.282×10−5 cm2/s (n=20) at 14.2 ± 1.50% strain level, and 7.71 ± 1.63×10−6 cm2/s (n=20) at 23.7±1.34% strain level. There was a significant decrease in oxygen diffusivity with increasing level of compressive strain (ANOVA, p<0.05).
Conclusions
Oxygen diffusivity of bovine AF in the axial direction has been determined. The mechanical loading has a significant effect on oxygen transport in IVD tissues. This study is important in understanding nutritional transport in IVD tissues and related disc degeneration.
Keywords: compression, nutrition, transport, intervertebral disc, diffusion coefficient, spine
Introduction
Statistics show that low back pain affects up to 75% of the adult population during some time in their lives. Studies aimed at quantifying the effects of low back pain on productivity and profitability have estimated the combined cost of back pain-related medical care and disability compensation to reach upwards of billions of dollars annually in the U.S. alone [1]. While the exact cause of low back pain is still poorly understood, scientists and physicians have reached a popular assumption that its cause can be primarily traced to the degeneration of the intervertebral disc [2–5].
The intervertebral disc, or IVD, is the largest avascular structure in the human body. Due to its avascular feature, nutrition supply into IVD is mainly through the diffusion of small solutes from the peripheral blood vessels [6–8]. Poor nutrition is believed to be an important factor leading to the onset of disc degeneration [9–13]. While many studies have aimed at analyzing the effects of mechanical loading on water content, chemical composition, and nutritional levels in the IVD [10,12,14], to our knowledge, no study has investigated the effect of mechanical compression on oxygen diffusivity in the IVD tissue. Therefore, the objective of this study was to investigate the effects of mechanical loading on oxygen transport in IVD tissue by determining oxygen diffusivity in bovine annulus fibrosus (AF) under 3 levels of compressive strain (5%, 15%, and 25%).
Materials and methods
Previous studies have shown that the composition, mechanical properties, and synthesis rates of bovine coccygeal discs are similar to those for human IVD [15,16]. In order to meet our objective of investigating the strain-dependent behavior of oxygen diffusivity using a tissue similar to human IVD, bovine coccygeal IVD were used in this study as they are easily obtainable at a low cost. A total of three fresh bovine tails (~6 months old) were obtained from a local supermarket. After carefully removing the excess tissue and ligaments surrounding the discs, a total of five coccygeal IVD (S2–3 and S3–4) were harvested. A total of 20 axial AF samples were prepared using a corneal trephine (Biomedical Research Instruments, Inc., Silver Spring, MD) and sledge microtome (Model SM2400, Leica Instruments, Nussloch, Germany) with freezing stage (Model BFS-30, Physitemp Instruments, Inc., Clifton, NJ). The cylindrical samples were 6 mm in diameter and approximately 0.5mm in thickness. Note that the thickness of each specimen was measured using a custom-designed current sensing micrometer (accuracy: ±3 µm) [17], and used for calculating the level of compressive strain (i.e., engineering strain) in the testing chamber. Each of the 20 specimens was tested to measure oxygen diffusivity at 3 different levels of nominal compressive strain (5%, 15%, and 25%).
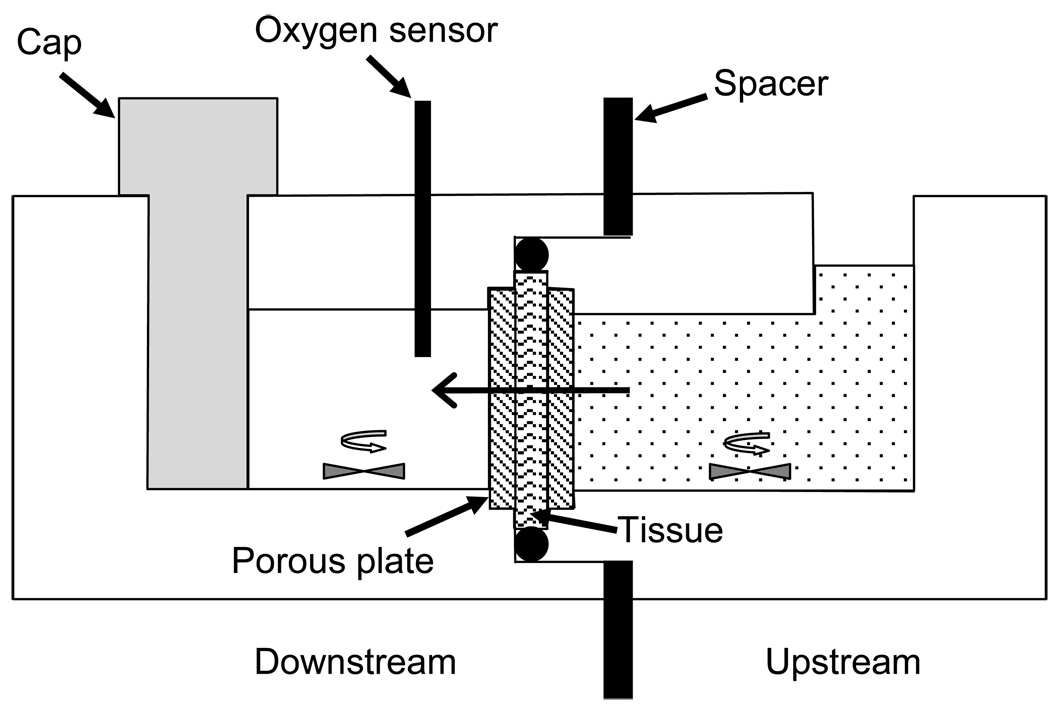
In order to accurately measure the strain-independent diffusivity of oxygen in IVD tissue, an acrylic diffusion chamber was custom-designed and built (Figure 1). The diffusion chamber consisted of two compartments divided by a specimen holder located in the middle. Internal volumes of upstream and downstream compartments were 0.24 ml and 0.2 ml, respectively. Prior to the start of the experiment, deoxygenated phosphate buffered solution (PBS) was prepared by introducing nitrogen gas into 2ml PBS solution for one hour [18].
Figure 1.
Schematic of the custom-designed diffusion apparatus. Oxygen diffusion occurs from upstream chamber (right), across the tissue specimen, and into the downstream chamber (left), where the oxygen concentration is measured using an oxygen sensor.
The specimen was compressed between two porous plates with 50–90µm pore size and 50% porosity, and sealed with an o-ring. The amount of compression was controlled by the size of the spacer. The AF specimen was first confined to 5% nominal compressive strain, and the downstream compartment was filled with the deoxygenated PBS solution while the upstream compartment was filled with air-saturated PBS. To maintain a constant oxygen concentration upstream, the PBS solution in it was replaced periodically (about every 2 minutes) with fresh solution. Real-time oxygen concentration downstream was recorded using an oxygen sensor system (Ocean Optics Inc., Dunedin, FL).
From Fick’s Law and conservation of mass, we can derive the following differential equation:
| (1) |
where D is the diffusivity, Cup is the oxygen concentration in the upstream compartment, Cdown is the concentration of oxygen in the downstream compartment, Vdown is the volume of solution in the downstream compartment, A is the diffusion area, and h is the thickness of the sample after compression. K is the partition coefficient; in this study, it was assumed to be unity since oxygen is a small molecule. In arriving to Eq. (1), a linear distribution of concentration within the specimen has been assumed. The oxygen diffusivity may be calculated by [19,20]:
| (2) |
where Cdown(to) and Cdown(t) are the concentrations of oxygen in the downstream chamber at time to (initial time) and t, respectively. To ensure that concentration distribution within the tissue is linear, oxygen diffusivity was calculated from the measured data using the final 15 minutes of the experiment (i.e, to = 45 minutes and t − to = 15 minutes). The experiment was repeated for 15% and 25% (nominal) compressive strains.
Results
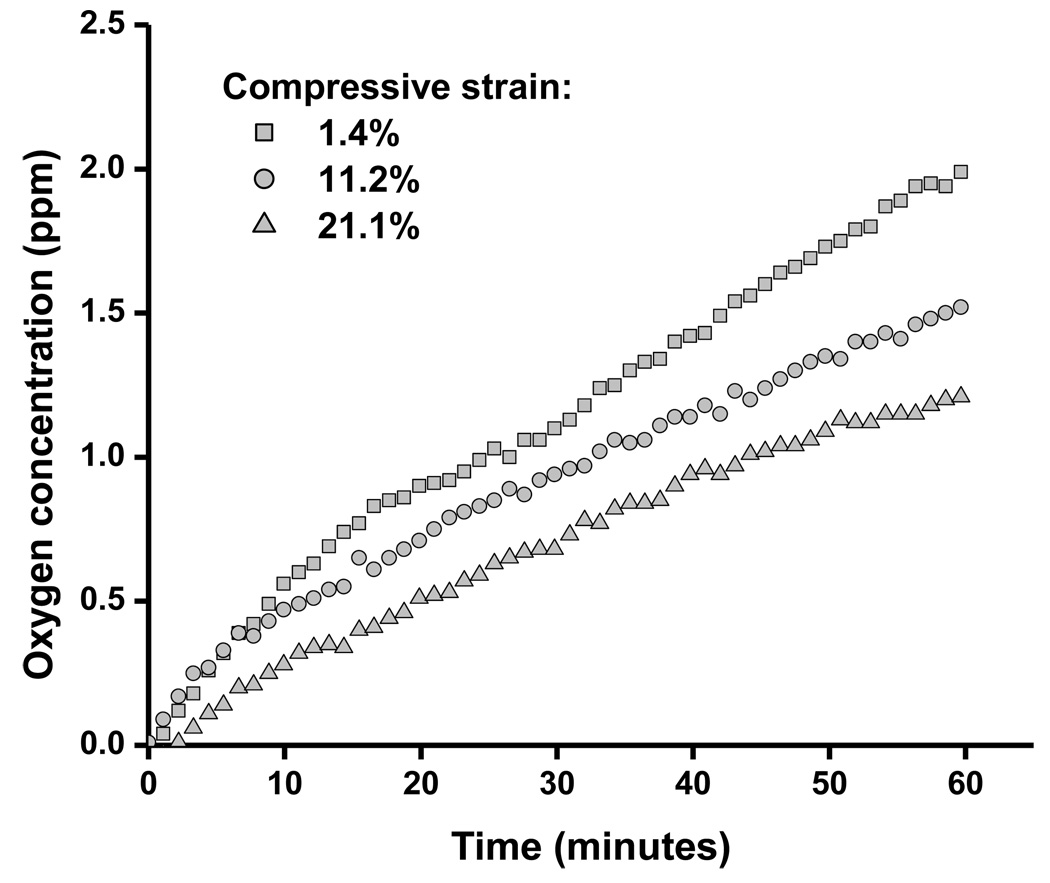
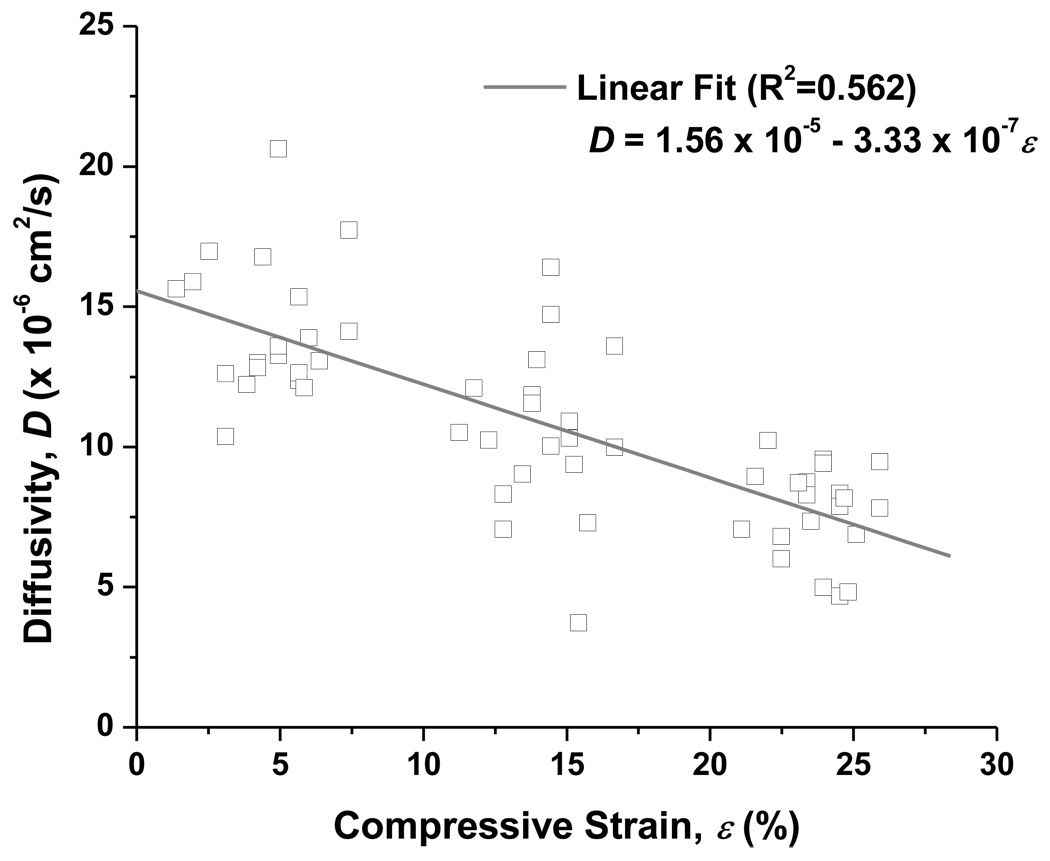
A sample of raw experimental data showing the change in the oxygen concentration in the downstream chamber with elapsed time is shown in Figure 2. Our results, shown in Figure 3, indicate that the oxygen diffusivity in AF decreased with increasing compressive strain. To estimate the value of diffusivity at zero-strain, a linear regression line was used to fit the experiment data. It was found that the oxygen diffusivity in AF at 0% compression was 1.56 ×10−5 cm2/s (Figure 3). The average oxygen diffusivity (mean ± SD) of bovine AF in the axial direction was 1.43 ± 0.242 ×10−5 cm2/s (n=20) at 4.68 ± 1.67% compressive strain level, 1.05 ± 0.282 ×10−5 cm2/s (n=20) at 14.2 ± 1.50% strain level, and 7.71 ± 1.63 ×10−6 cm2/s (n=20) at 23.7 ± 1.34% strain level. Measurements were carried out at room temperature (22.2°C ± 0.45°C). The mean height of the specimens under zero compression condition was 0.525 ± 0.009 mm.
Figure 2.
An example of raw experimental data showing the change in the oxygen concentration in the downstream chamber with elapsed time. For this particular specimen, the actual levels of compression were 1.4%, 11.2%, and 21.1%. The diffusivity was calculated using Eq. (2). Note the decrease in slope with increasing compressive strain, indicating the strain-dependent behavior of oxygen diffusivity.
Figure 3.
Variation in diffusivity of oxygen with applied strain at room temperature (22.2°C ± 0.45°C). A linear regression (R2 = 0.562, n=60) was used to estimate the diffusivity at zero stain. In the linear regression, D is the diffusivity and ε is the applied compression (%). From this, the oxygen diffusivity in bovine AF at 0% compression (i.e., ε = 0) was determined to be 1.56 × 10−5 cm2/s.
A one-way analysis of variance (ANOVA) test was used to analyze the data and showed that oxygen diffusivity was significantly affected by the level of compression (P<0.05). A Student-Newman-Keuls post-hoc test showed a significant difference between oxygen diffusivity in compressive strain group (P<0.05).
Discussion
There are few studies in the literature that have focused on oxygen diffusivity of IVD and cartilage tissues [19,21–23]. Previous studies have reported the strain-dependent diffusivity behavior of different solutes in articular cartilage and IVD tissues [20,24–29] but no study has investigated the stain-dependent behavior of oxygen diffusivity in IVD.
Our previous study [31] showed that the diffusivity (D) of small and macro molecules in gel and cartilaginous tissue could be estimated by the following model:
| (3) |
where rs is the solute Stokes radius, κ is the tissue Darcy permeability, α and β are two positive parameters that depend on the structure of the tissue, and Do is the solute diffusivity in water at the same temperature. While the values of both D and Do are sensitive to temperature, the value of the relative diffusivity (D/Do) should not be, assuming the structure and composition of the tissue do not change with temperature. One previous study showed that the relative oxygen diffusivity (D/Do) for porcine AF was between 0.3 to 0.6 [22], and the other studies showed that the relative oxygen diffusivities for bovine and avian articular cartilage were ~0.71 (~2.2×10−5 cm2/s in tissue at 37°C) and ~0.66 (~2.0×10−5 cm2/s in tissue at 35°C), respectively [19,21]. Using the value (2.2×10−5 cm2/s) of oxygen diffusivity in water at 22°C [30], and our experimental data, it was found that the relative oxygen diffusivity for AF at zero compression was 0.71, which is in agreement with the result reported for bovine articular cartilage [19].
The results from our experiment showed that oxygen diffusivity decreased as the compressive strain increased. IVD tissue may be considered as a porous material with various sizes of pores, which are filled with water. The overall porosity of tissue can be measured by the value of water volume fraction. The average size of the pores in the tissue can be estimated by the square root of its Darcy permeability [31,32], which is at nanometer scale for cartilaginous tissues [32]. For these tissues, the Darcy permeability (or average pore size) is related to the volume fraction of water (or water content) (e.g., [32]). When a tissue is compressed, fluid exudation leads to a reduction in tissue water content. For bovine coccygeal AF, our previous study showed that water content was 76% at zero compression, and was estimated to decrease to 75%, 72%, and 68% under 5%, 15%, and 25% compressive strains, respectively [20]. A decrease in the water content of IVD tissue results in decreased pore size of the tissue [10,12,14,20,29,33–36]. Because the main factor governing the relative diffusivity in cartilaginous tissues is the ratio of solute size to the pore size of the tissue [31,37], see Eq. (3), compression would contribute to a decrease in the diffusivity of oxygen in the tissue. Recent studies have also shown the existence of microtubes, which are small (~10 µm in diameter) tubular structures that extend along the direction of collagen fiber bundles, in bovine and murine AF [20,36,38]. These microtubes have been suggested as a pathway for solute transport through AF tissue [20,38]. We believe that, when compression is applied to the tissue, tissue compaction may also cause a reduction in the size of the microtubes which, in turn, leads to a decrease in the solute diffusivity in the tissue. Therefore, the strain-dependence of oxygen diffusivity in bovine AF can likely be attributed to the decreased tissue porosity and pore size, at both the nano- and micro-levels, caused by compression.
The current study only investigated the strain-dependent oxygen diffusivity in the axial direction in AF tissue. Previous studies have shown that transport in IVD tissues is anisotropic (i.e., direction-dependent) [20,28,38–40]. For instance, our earlier studies have shown that, for bovine AF tissue, diffusivity in the axial direction is approximately 1.5 times that in the radial direction [20,38]. Therefore, further investigation into the effects of compression on oxygen diffusivity in other directions (e.g., radial and circumferential) of AF, similar to our previous study on strain-dependent glucose diffusivity in bovine AF [20], is needed to better understand the anisotropy of oxygen transport in IVD. This information is important to the development of numerical models for nutritional transport in IVD tissues.
In summary, this study measured the oxygen diffusivity of bovine AF in the axial direction, and was the first study to report stain-dependent oxygen diffusivity in AF. Results in the current study show that mechanical loading affects the oxygen diffusivity in bovine IVD tissue, decreasing as the compression increases. The findings from this study provide an additional insight into the nutrition transport in IVD under mechanical loading and highlight the importance of approaching and understanding mechanisms of nutrient transport in IVD tissue.
Acknowledgements
This study was supported by a grant from the NIAMS of the NIH (AR050609). The authors also wish to thank Mr. Andre Castillo for his help with apparatus machining.
Contributor Information
T-Y Yuan, Tissue Biomechanics Laboratory, Department of Biomedical Engineering, University of Miami, Coral Gables, FL 33146.
AR Jackson, Tissue Biomechanics Laboratory, Department of Biomedical Engineering, University of Miami, Coral Gables, FL 33146.
C-Y Huang, Stem Cell and Orthopaedic Bioengineering Laboratory, Department of Biomedical Engineering, University of Miami, Coral Gables, FL 33146.
W Y Gu, Tissue Biomechanics Laboratory, Department of Biomedical Engineering, University of Miami, Coral Gables, FL 33146.
References
- 1.NIH. Research on low back pain and common spinal disorders. NIH Guide. 1997;26(16) [Google Scholar]
- 2.Eyre DR, Benya P, Buckwalter J, Caterson B, Heinegard D, Oegema T, Pearce R, Pope M, Urban J. Intervertebral disk: Basic science perspectives. In: Frymoyer JW, Gordon SL, editors. New Perspectives on Low Back Pain. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1989. pp. 147–207. [Google Scholar]
- 3.Kelsey JL, Mundt DF, Golden AL. Epidemiology of low back pain. In: Malcolm JIV, editor. The Lumbar Spine and Back Pain. New York: Churchill Livingstone; 1992. pp. 537–549. [Google Scholar]
- 4.White AA. Biomechanics of lumbar spine and sacroiliac articulation: relevance to idiopathic low back pain. In: White AA, Gordon SL, editors. Symposium on Idiopathic Low Back Pain. St. Louis: CV Mosby Co; 1981. pp. 296–322. [Google Scholar]
- 5.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20(11):1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: an in vivo study. Biorheology. 1978;15(3–4):203–221. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 7.Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- 8.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: Effect of fluid flow on solute transport. Clin Orthop. 1982;170:296–302. [PubMed] [Google Scholar]
- 9.Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970;41(6):589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- 10.Holm S, Nachemson A. Nutritional changes in the canine intervertebral disc after spinal fusion. Clin Orthop. 1982;(169):243–258. [PubMed] [Google Scholar]
- 11.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26(23):2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bibby SR, Fairbank JC, Urban MR, Urban JP. Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine. 2002;27(20):2220–2228. doi: 10.1097/00007632-200210150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2001;30(6):858–864. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 14.Adams MA, Hutton WC. The effect of posture on diffusion into lumbar intervertebral discs. J Anat. 1986;147:121–134. [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima H, Ishihara H, Urban JP, Tsuji H. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res. 1993;11(3):332–338. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- 16.Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliot DM. Comparison of animal discs used in disc research to human lumbar disc. Spine. 2008;33(6):E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 17.Gu WY, Justiz MA. Apparatus for measuring the swelling dependent electrical conductivity of charged hydrated soft tissues. J Biomech Engng. 2002;124:790–793. doi: 10.1115/1.1516571. [DOI] [PubMed] [Google Scholar]
- 18.Bibby SRS, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30(5):487–496. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 19.Malda J, Rouwkema J, Martens DE, Le Comte EP, Kooy FK, Tramper J, van Blitterswijk CA, Riesle J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng. 2004;86(1):9–18. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 20.Jackson AR, Yuan TY, Huang CY, Travascio F, Gu WY. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine. 2008;33(1):1–7. doi: 10.1097/BRS.0b013e31815e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haselgrove JC, Shapiro IM, Silverton SF. Computer modeling of the oxygen supply and demand of cells of the avian growth cartilage. Am J Physiol. 1993;265(2 Pt 1):C497–C506. doi: 10.1152/ajpcell.1993.265.2.C497. [DOI] [PubMed] [Google Scholar]
- 22.O'Hare D, Winlove CP, Parker KH. Electrochemical method for direct measurement of oxygen concentration and diffusivity in the intervertebral disc: electrochemical characterization and tissue-sensor interactions. J Biomed Eng. 1991;13(4):304–312. doi: 10.1016/0141-5425(91)90112-k. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson JV, O'Hare D, Unwin PR, Winlove CP. Quantitative spatially resolved measurements of mass transfer through laryngeal cartilage. Biophys J. 1997;73(5):2771–2781. doi: 10.1016/S0006-3495(97)78306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11(4):465–478. doi: 10.1002/jor.1100110402. [DOI] [PubMed] [Google Scholar]
- 25.Quinn TM, Kocian P, Meister JJ. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys. 2000;384:327–334. doi: 10.1006/abbi.2000.2077. [DOI] [PubMed] [Google Scholar]
- 26.Quinn TM, Morel V, Meister JJ. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J Biomech. 2001;34(11):1463–1469. doi: 10.1016/s0021-9290(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 27.Ngwa W, Geier O, Stallmach F, Naji L, Schiller J, Arnold K. Cation diffusion in cartilage measured by pulsed field gradient NMR. Eur Biophys J. 2002;31(1):73–80. doi: 10.1007/s002490100184. [DOI] [PubMed] [Google Scholar]
- 28.Chiu EJ, Newitt DC, Segal MR, Hu SS, Lotz JC, Majumdar S. Magnetic resonance imaging measurement of relaxation and water diffusion in the human lumbar intervertebral disc under compression in vitro. Spine. 2001;26(19):E437–E444. doi: 10.1097/00007632-200110010-00017. [DOI] [PubMed] [Google Scholar]
- 29.Drew SC, Silva P, Crozier S, Pearcy MJ. A diffusion and T2 relaxation MRI study of the ovine lumbar intervertebral disc under compression in vitro. Phys Med Biol. 2004;49(16):3585–3592. doi: 10.1088/0031-9155/49/16/006. [DOI] [PubMed] [Google Scholar]
- 30.Himmelblau DM. Diffusion of dissolved gases in liquids. Chem Rev. 1964;64:527–550. [Google Scholar]
- 31.Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Annals of Biomedical Engineering. 2004;32:1710–1717. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 32.Gu WY, Yao H, Huang C-Y, Cheung HS. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. J Biomech. 2003;36:593–598. doi: 10.1016/s0021-9290(02)00437-2. [DOI] [PubMed] [Google Scholar]
- 33.Kraemer J, Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine. 1985;10(1):69–71. doi: 10.1097/00007632-198501000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Ohshima H, Tsuji H, Hiarano N, Ishihara H, Katoh Y, Yamada H. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine. 1989;14:1234–1244. doi: 10.1097/00007632-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Adams MA, Hutton WC. The effect of posture on the fluid content of lumbar intervertebral discs. Spine. 1983;8(6):665–671. doi: 10.1097/00007632-198309000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37:1165–1175. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120(1):113–130. [PMC free article] [PubMed] [Google Scholar]
- 38.Travascio F, Gu W. Anisotropic diffusive transport in annulus fibrosus: Experimental determination of the diffusion tensor by FRAP technique. Annals of Biomedical Engineering. 2007;35(10):1739–1748. doi: 10.1007/s10439-007-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson AR, Yao H, Brown MD, Gu WY. Anisotropic ion diffusivity in intervertebral disc: an electrical conductivity approach. Spine. 2006;31:2783–2789. doi: 10.1097/01.brs.0000245842.02717.1b. [DOI] [PubMed] [Google Scholar]
- 40.Hsu EW, Setton LA. Diffusion tensor microscopy of the intervertebral disc annulus fibrosus. Magn Reson Med. 1999;41(5):992–999. doi: 10.1002/(sici)1522-2594(199905)41:5<992::aid-mrm19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]