Abstract
The attenuated strain of Mycobacterium bovis known as bacille Calmette-Guérin (BCG) has been widely used as a vaccine for prevention of disease by Mycobacterium tuberculosis, but with relatively little evidence of success. Recent studies suggest that the failure of BCG may be due to its retention of immune evasion mechanisms that delay or prevent the priming of robust protective cell-mediated immunity. Here we describe an approach to enhance the immunogenicity of BCG by incorporating glycolipid activators of CD1d–restricted Natural Killer T cells (NKT cells), a conserved T cell subset with the potential to augment many types of immune responses. A method was developed for stably incorporating two forms of the NKT cell activator α-galactosylceramide (αGalCer) into live BCG organisms, and the impact of this on stimulation of T cell responses and protective anti-mycobacterial immunity was evaluated. We found that live BCG containing relatively small amounts of incorporated αGalCer retained the ability to robustly activate NKT cells. Compared to immunization with unmodified BCG, the glycolipid-modified BCG stimulated increased maturation of dendritic cells and markedly augmented the priming of antigen-specific CD8+ T cells responses. These effects were correlated with improved protective effects of vaccination in mice challenged with virulent M. tuberculosis. These results support the view that mycobacteria possess mechanisms to avoid stimulation of CD8+ T cell responses, and that such responses contribute significantly to protective immunity against these pathogens. Our findings raise the possibility of a simple modification of BCG that could yield a more effective vaccine for control of tuberculosis.
Introduction
Mycobacterium tuberculosis remains one of the major causes of global morbidity and mortality from infectious disease. Currently, it is estimated that a third of the people on the planet are infected with this organism, with the majority of these representing latent infections with the potential for reactivation leading to active disease and transmission (1). Approximately 2 million people die from tuberculosis (TB) every year, making it the second highest cause of death from an infectious disease worldwide after HIV/AIDS (2,3). The only currently available vaccine for prevention of TB, the M. bovis strain bacille Calmette-Guérin (BCG), has had little impact on halting the progression of the global TB epidemic (3). A wide range of epidemiologic studies demonstrate that vaccination with BCG provides partial protection against TB in newborns, but has minimal or no efficacy in preventing latent TB or reactivation of pulmonary disease in adults (4). In addition the growing prevalence of TB and the emergence of extensively drug resistant (XDR) strains of M. tuberculosis, has stimulated substantial efforts to develop better vaccines for TB by either improving or replacing BCG (5–7). These efforts have focused largely on enhancing the priming and maintenance of T cell responses against mycobacterial antigens, which are believed to be central to protective immunity against M. tuberculosis.
Studies in animal models and observations in human subjects demonstrate the importance of antigen-specific T cell responses in controlling infections with M. tuberculosis (8). Experiments in mice with specific gene deletions support a major role for MHC class II-restricted CD4+ T cells in the control of experimental TB (9). CD4+ T cells may exert their protective function in TB largely through the secretion of TH1 type cytokines including interferon-γ (IFNγ) and other factors that stimulate the bactericidal activities of M. tuberculosis infected macrophages and dendritic cells. In addition, recent data indicate a potential role for CD4+ IL-17-producing (TH17) T cells in vaccine-induced immunity to M. tuberculosis (10). MHC class I-restricted CD8+ T cells also contribute to protective immunity in TB, although most studies have indicated that they play a less prominent role than CD4+ T cells in acute and chronic M. tuberculosis infection (8,11). Recent work indicates that M. tuberculosis possesses multiple mechanisms to delay or prevent the priming of both MHC class I and class II restricted T cell responses (5,12,13). Similar immune evasion properties are also likely to be operational in BCG, providing an explanation for the failure of this attenuated mycobacterium to provide strong protective immunity against M. tuberculosis. The development of methods to overcome the immune evasion properties inherent in live attenuated vaccines such as BCG is thus of central relevance to the creation of more effective vaccines for prevention of TB.
Natural Killer T cells (NKT cells) are an unconventional T cell subset that has the potential when activated to augment many types of immune responses, and may thus offer a potential strategy for augmenting the immunogenicity of vaccines such as BCG. Among the defined subsets of NKT cells, the most abundant and well-studied are those with an invariant TCRα chain and are known as type 1 or invariant NKT cells (iNKT cells). These T cells recognize glycolipid antigens presented by the nonpolymorphic MHC class I-like CD1d molecule, and display a wide variety of regulatory and effector activities upon activation (14,15). A few natural lipid and glycolipid antigens recognized by iNKT cells have been described, but much more extensive studies have been carried out using synthetic glycolipid ligands that mimic natural CD1d–presented antigens. The prototype and most widely analyzed iNKT cell agonist is a synthetic form of α-galactosylceramide with a saturated 26 carbon acyl chain and a C18 phytosphingosine base (referred to here as αGalCer; Figure 1A) (16). A single injection of αGalCer into mice activates the entire population of iNKT cells, directly stimulating the rapid release of multiple cytokines and also secondary activation of NK cells, dendritic cells, B cells, and conventional T cells (17–19). Activation of iNKT cells in vivo by injections of αGalCer has beneficial effects in a wide variety of mouse infection and tumor models, including chronic infections with M. tuberculosis and other highly persistent pathogens (20,21).
Figure 1. Stable incorporation of aGalCer into live M. bovis BCG.
(A) Structures of the iNKT cell activating glycolipids αGalCer and α-C-GalCer. The 14C-labeled form of αGalCer used in this study was identical to that illustrated except that the N-linked acyl chain was 6 carbons shorter (i.e., C20). (B) Solubility of 14C-aGalCer in PBS containing 0.05% Tween 80 or 0.05% tyloxapol, compared to a 2:1 mixture of chloroform and methanol (C:M 2:1) or petroleum ether. The amount of 14C-aGalCer indicated on the X-axis was added to glass vials in C:M 2:1 and the solvent completely evaporated. Y-axis indicates CPM recovered with each solvent when used to redissolve the glycolipid, as determined by β-scintillation counting of an aliquot of the resulting solutions. (C) Growth rate of BCG cultures (monitored by optical density at 600 nm (OD600)) in protein-free Middlebrook 7H9 medium with 0.05% tyloxapol. (D) To determine the optimal concentration of αGalCer for incorporation into live BCG, the bacteria were grown in Middlebrook 7H9 medium with 0.05% tyloxapol containing a range of concentrations of 14C-αGalCer. After growth for 6 days to an OD600 of ~0.8, cultures were harvested and αGalCer incorporation was determined by β-scintillation counting of extensively washed bacteria (% incorporated indicates CPM in the washed bacterial pellet divided by total CPM of 14C-αGalCer added to the culture initially X 100). (E) αGalCer remained intact following incorporation into live BCG. Autoradiograph of a TLC plate is shown. 14C-containing lipids extracted from BCG grown for 8 days (OD600 = 0.7) with 16 µg/ml 14C-αGalCer in Middlebrook 7H9 medium with 0.05% tyloxapol (lane 2) had identical mobility as the 14C-αGalCer reference standard (lane 1). Quantitation of the autoradiographic signals from the TLC plate confirmed that approximately 25% of the radiolabeled glycolipid added to the original culture was stably incorporated into the bacteria as intact αGalCer.
Among the many chemically modified forms of αGalCer, a synthetic analogue containing a C-glycoside in place of the usual O-glycosidic bond (α-C-GalCer; Figure 1A) has been shown to have superior activity compared to αGalCer for enhancing TH1-type immunity. In direct comparisons with αGalCer, injection of mice with the α-C-GalCer analogue has been shown to produce better immune responses against tumors and improved resolution of infections with malaria parasites (22). Like αGalCer and to an even greater extent, the α-C-GalCer analogue induces markedly increased and sustained levels of IFNγ and IL-12p70 in mice, which are two of the cytokines that are well established to be essential for control of TB in mice and humans (23). Based on these observations we hypothesized that iNKT cell activation with these glycolipids, in conjunction with priming of T cells against mycobacterial vaccine antigens, could lead to improved adaptive responses against virulent mycobacteria.
In this study, we have developed an approach for stably incorporating αGalCer and its analogue α-C-GalCer into live BCG organisms, and have evaluated the impact of this on the stimulation of T cell responses and protective immunity. We found that live BCG containing relatively small amounts of incorporated αGalCer retained the ability to robustly activate iNKT cells. Compared to immunization with unmodified BCG, the glycolipid-modified BCG stimulated increased maturation of dendritic cells and markedly augmented the priming of antigen-specific CD8+ T cells responses. A single intradermal immunization of mice with the glycolipid-modified BCG also provided a significantly higher level of protective immunity than standard BCG vaccination against an aerosol challenge with M. tuberculosis strain H37Rv. These results provide the basis for a simple modification of BCG that could overcome the CD8+ T cell priming defect inherent in this vaccine, and potentially lead to a more effective vaccine for prevention and control of M. tuberculosis infections.
Materials and Methods
Mice
Six to 8 week old female wild type C57BL/6 and BALB/c mice and B6.PL (Thy1.1+) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). CD1D−/− mice (C57BL/6 background) were provided by M. Exley and S. Balk (Harvard Medical School, Boston, MA) (24). Jα18−/− mice (C57BL/6 background) were a gift from M. Taniguchi and T. Nakayama (Chiba University, Japan) (25). C57BL/6 Rag-1−/− OT-1 TCR-transgenic mice were obtained from the National Institute of Allergy and Infectious Diseases [NIAID] Exchange Program at Taconic Farms (Germantown, NY). C57BL/6 background p25TCR-Tg/Rag-1−/− mice were described previously (26), and were bred in our facility from founders provided by Dr. Joel Ernst (New York University School of Medicine, New York, NY). All mice were maintained in specific pathogen-free conditions, and were transferred to biosafety level 3 conditions following infection with M. tuberculosis. All procedures involving the use of animals were in compliance with protocols approved by the Einstein Institutional Animal Use and Biosafety Committees.
Glycolipids
αGalCer was synthesized as previously described (27). The 14C-labeled α-GalCer was synthesized using the same method except that [1-14C]-arachidonic acid (Sigma Aldrich) was used in place of the C26:0 fatty acid to provide αGalCer-[1-14C] C20:4 as an intermediate, which was then hydrogenated to yield αGalCer-[1-14C] C20:0 (specific activity 183 µCi/mg). The use of 14C-αGalCer-C20:0 for these studies, although not optimal, was necessary because of technical limitations that have so far precluded synthesis of a radiolabeled form with longer N-acyl chains (e.g., C26:0) that are present in the most immunologically active forms of αGalCer. The α-C-GalCer was obtained from the NIH Tetramer Core Facility (http://www.niaid.nih.gov/reposit/tetramer/overview.html). Both glycolipids were stored as solvent-free aliquots in glass vials at −20°C, and were reconstituted either in 100% DMSO at 100 µM for in vitro studies, or in aqueous vehicle consisting of PBS with 0.05% Tween-20 and 0.1% DMSO at 500 µM for in vivo studies.
Bacterial strains
M. bovis BCG (Danish strain) was obtained from Statens Serum Institute (Copenhagen, Denmark) and the recombinant BCG-OVA strain (derived from BCG-Pasteur transformed with plasmid pMO230 encoding codons 230–359 of chicken ovalbumin fused to the Antigen 85B signal sequence) was a gift of Dr. Subash Sad (National Research Council-Institute for Biological Sciences, Ottawa, Ontario, Canada). These strains were grown in protein-free Middlebrook 7H9 medium (M7H9; Difco Laboratories) with 0.05% tyloxapol (Sigma-Aldrich). Virulent M. tuberculosis strain H37Rv (obtained from Trudeau Institute), was grown in M7H9 supplemented with the oleic acid-albumin-dextrose complex (OADC; Difco Laboratories). Bacterial CFU titers were determined by plating tissue homogenates or aliquots of bacterial suspension on Middlebrook 7H11 agar plates containing OADC and 2 µg/ml of thiophene-2-carboxylic acid hydrazide (TCH, Sigma-Aldrich) to inhibit growth of residual BCG from immunized mice.
Incorporation of glycolipids into live BCG
Unlabeled αGalCer or α-C-GalCer or 14C-labeled αGalCer were solubilized at 200 µg/ml in glass vials with protein-free M7H9 medium containing 0.5% tyloxapol by sonicating for 5 minutes, heating at 80° C for 2 minutes and vortexing for one minute. The solubilized glycolipid was immediately diluted in protein-free M7H9 to give the required concentration of glycolipid and a final concentration of 0.05% tyloxapol. M. bovis BCG (mid log phase culture, OD600 0.5–0.8) was inoculated (200 µl into 10 ml of M7H9 with 0.05% tyloxapol plus glycolipid) and grown to mid log phase (OD600 0.5 to 1.0) which generally required 7–10 days. The bacteria were harvested by centrifugation, washed thoroughly with PBS + 0.05% tyloxapol, and resuspended in PBS + 0.05% tyloxapol for injection or use in cell culture experiments. In the case of cultures grown with 14C-labeled αGalCer, bacteria were washed thoroughly with PBS + 0.05% tyloxapol, dried and lipid incorporation was assessed by β-scintillation counting.
Thin-layer chromatography
For analysis of extractable cell wall lipids, M. bovis BCG was grown with 14C-labeled αGalCer to an OD600 of 0.5. The cell pellet was washed and incubated overnight in a mixture of chloroform/methanol (2:1), following which the solvent was collected and dried under a stream of nitrogen. The extracted lipids were resuspended in chloroform and spotted onto silica TLC plates (Alltech Associates, Deerfield, IL) for separation using a solvent mixture composed of chloroform, methanol and water (60:16:2). After running solvent front to within ~1 cm of the top, the plates were dried and analyzed by autoradiography using a 24 hour exposure to X-ray film.
Primary cell suspensions and cell lines
Bone marrow derived dendritic cells (BMDC) from C57BL/6 and BALB/c mice were prepared based on a published protocol (28). The Vα14i NKT hybridoma DN3A4-1.2 was provided by M. Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA). Spleen cell suspensions were prepared by gently forcing spleen tissue through a 70 µM cell strainer. Liver mononuclear cells were isolated by treating the liver tissue with Liberase Blendzyme 2 (Roche Applied Science) and using a density gradient of 45% and 67.5% percoll. Human monocyte-derived DCs were prepared by treatment of normal random donor blood monocytes with GM-CSF and IL-4 as previously described (29). Human CD4−CD8− iNKT cell clone HDE3 was established from normal donor blood and cultivated using previously described methods (30).
In vitro assays of iNKT cell activation
For mouse iNKT hybridoma assay, BMDC were infected with BCG, αGalCer/BCG or α-C-GalCer/BCG at a multiplicity of infection (MOI) of 10:1 for 2 hours at 37°C, followed by washing to remove extracellular bacteria, and cultured at numbers indicated with mouse iNKT hybridoma DN3A4-1.2 (5 × 104 cells) for 16 hrs. Supernatants were assayed for IL-2 by ELISA. For splenocyte or hepatic mononuclear cell stimulations, splenocytes were plated at 5 × 105 cells per well or liver mononuclear cells were plated at 4 × 105 cells per well with the indicated numbers of C57BL/6 BMDCs infected at MOI of 10:1 with BCG, αGalCer/BCG or α-C-GalCer/BCG. After 48 h supernatant levels of IL-4 and IFNγ were measured by ELISA using capture and biotinylated detection antibody pairs (BD Biosciences). For human iNKT cell clone activation, human monocyte-derived dendritic cells were infected with live BCG with or without glycolipid incorporation at an MOI of 5:1 and incubated at various numbers with human CD4−CD8− iNKT cell clone HDE3 (5 × 104 cells per well). Supernatant samples were harvested after 24 hours and assayed by capture ELISA using antibody pairs for IFNγ, TNFα ( both from Pierce/ThermoScientific) and IL-13 (BD Biosciences).
In vivo assay of iNKT cell activation
Mice were given i.p. injections of inert vehicle (PBS + 0.01% Tween 20 + 0.1% DMSO, or PBS + 0.05% tyloxapol), free glycolipids (αGalCer or α-C-GalCer, 4 µg in 0.2 ml vehicle), or BCG suspensions (unmodified or glycolipid-modified BCG at 5×106 CFU/mouse in 0.2 ml PBS + 0.05% tyloxapol). Sera were assayed at the indicated times for IL-4, IL-12p70, and IFNγ by capture ELISA as previously described (27).
Multiparameter FACS analyses
All experiments requiring analysis of more than four fluorescence parameters were performed using an LSR II flow cytometer (BD Biosciences). Aggregates were excluded by gating on cells with equivalent FSC-H/FSC-A profiles, and dead cells were excluded by gating on Blue Live/Dead viability dye (BluVID) (Molecular Probes, Eugene, OR) negative cells. Data analysis was performed using FlowJo software (TreeStar, Inc., Ashland, OR).
For analysis of in vivo DC maturation, wild type C57BL/6 or CD1d−/− mice were injected i.p. with unmodified or glycolipid-modified BCG (5×106 CFU/mouse in 0.2 ml PBS + 0.05% tyloxapol). Splenocytes and hepatic mononuclear cells were harvested 40 hours later and stained with BluVID. The cells were then blocked with rat anti-mouse FcγII/III clone 2.4G2 in PBS + 2% FCS and labeled with monoclonal antibodies against CD11c and CD45R/B220 (PE-Cy7 and APC-Cy7 conjugates; BD Biosciences). Biotin-labeled mAbs against one of the following were also added to replicate samples: CD80, CD86, MHC class II and CD70 (eBiosciences, San Diego, CA). After incubation with Streptavidin- Alexa Fluor® 405 (InVitrogen), cells were fixed with 0.5% paraformaldehyde and analyzed. DCs were identified by gating on CD11chi B220neg cells.
For analysis of multifunctional CD4+ T cells (MFT cells), splenocytes and lung mononuclear cells were prepared from animals vaccinated intradermally 2 months previously and restimulated with 20 µg/ml total sonicate of M. tuberculosis H37Rv or 5 µg/ml of Ag85B peptide p25 plus 1 µg/ml soluble anti-CD28 mAb (eBiosciences). After incubation (37°C, 5% CO2) for 2 hrs, 10 µg/ml Brefeldin-A (Sigma-Aldrich) was added for an additional 4 hrs. Cells were labeled with BluVID, blocked with mAb 2.4G2 and stained with anti-CD3e–FITC, anti-CD4-APC-Cy7, and anti-CD8αPacific Blue (BD Biosciences). Cells were fixed with 2% paraformaldehyde, washed with permeabilization buffer (PBS with 1 mM Ca2+, 1 mM Mg2+, 1 mM HEPES, 2% FCS and 0.1% saponin), and then blocked in permeabilization buffer + 10% normal mouse serum (Jackson Immunoresearch). The following mAbs were then added: anti-IL-2-APC, anti-IFNγ-Alexa Fluor® 700 and anti-TNFα-PE-Cy7 (BD Biosciences).
For analysis of CD4+ regulatory T cells (Treg), single cell suspensions were stained with BluVID, followed by blocking with 2.4G2 and surface staining with anti-CD3e–FITC, anti-CD4-APC-Cy7 and anti-CD25-PE (BD Biosciences). Cells were then prepared for intracellular staining using the FoxP3 staining kit (eBiosciences) according to the manufacturer’s instructions, and stained with anti-FoxP3-Alexa Fluor® 647 (eBiosciences).
IFNγ ELISPOT assay
Splenic T cells were purified using the Dynal Mouse T Cell Negative Isolation Kit (Invitrogen). The separated T cells were cultured in ELISPOT plates (Millipore, Danvers, MA) coated with IFNγ capture antibody (clone R4.6A2; BD Biosciences). Purified T cells (1 × 105/well) were added to the plate in complete medium (0.1 ml/well) with splenocytes from a naïve mouse (9 × 105/well) and the antigenic peptides (5 µg/ml) for 24 hours at 37°C. After removal of cells, plates were washed with PBS followed by PBS with 0.05% Tween 20 (PBST). Biotinylated anti-IFNγ detection antibody (clone 4S.B3; BD Biosciences) was added for 2 hours at 37°C, followed by washing with PBST. Streptavidin–alkaline phosphatase (Sigma-Aldrich) was added to the plates for 1 hour (37°C), followed by washing and addition of BCIP/NBT substrate (Sigma-Aldrich). The reaction was stopped by washing the wells with water, and spots were counted using an ELISPOT reader (Autoimmun Diagnostika, Strassberg, Germany).
Assessment of in vivo T cell proliferation by CFSE dilution
Donor splenocytes were isolated from p25TCR-Tg/Rag-1−/− mice (for CD4+ T cell analysis) or from OT-1 TCR-transgenic Rag-1−/− mice (for CD8+ T cell analysis) and labeled with 10 µM CFSE . Recipient B6.PL (Thy1.1+) mice received 5 × 106 labeled cells via the lateral tail vein and were then vaccinated subcutaneously with 5 × 106 CFU of glycolipid-modified or unmodified BCG or BCG-OVA as indicated, or PBS + 0.05% tyloxapol as negative control. Splenocytes were harvested 5–7 days later, stained with anti-Thy1.2-APC, anti-B220-PerCP and either anti-CD8-PE or anti-CD4-PE antibodies (BD Biosciences), and analyzed using a FACSCalibur flow cytometer (BD Biosciences). CFSE fluorescence of the transferred population was determined by gating on the B220neg CD8+ or CD4+ lymphocytes expressing Thy1.2.
Vaccination and challenge studies
Wild type C57BL/6 mice or iNKT cell deficient CD1d−/− or Jα18−/− mice were vaccinated intradermally with either unmodified or glycolipid-modified BCG (5×106 CFU/mouse in 0.2 ml PBS + 0.05% tyloxapol). Aerogenic challenge was done 2 months later using a whole-body exposure aerosol chamber (Mechanical Engineering Workshop, Madison, WI) custom fitted to a class III biosafety cabinet (Baker Co., Sanford, ME) to deliver 50–100 CFU per animal of virulent M. tuberculosis (strain H37Rv). Mice were sacrificed at 3 and 6 weeks after challenge. Lungs and spleens of individual mice were aseptically removed , homogenized using a Seward Stomacher 80 blender (Tekmar, Cincinnati, OH) and plated on Middlebrook 7H11 agar. Lung tissues were processed for histopathology using standard paraffin fixation, sectioning and H&E staining.
Statistical analyses
GraphPad Prism 5.0 software was used for statistical analyses. One-way ANOVA and unpaired Student's t test was used to compare the experimental groups. The Bonferroni multiple-comparison test (for 6 or fewer groups) or the Tukey multiple-comparison test (for more than 6 groups) were used to generate p values for selected pairwise comparisons, and p values of less than 0.05 were considered significant.
Results
Stable incorporation of αGalCer into live BCG
Previous studies on the use of αGalCer as an adjuvant for enhancing T cell responses to protein antigens have shown the importance of administering the glycolipid at the same site as the antigen (31). This is likely to reflect the need to activate iNKT cells at the surface of the same APCs that are actively engaged in the processing and presentation of the antigen in order to achieve a strong adjuvant effect. In order to optimize the delivery of αGalCer to the same APCs that become infected by BCG during vaccination, we developed an approach that enabled the glycolipid to be physically incorporated into the cell wall or membranes of the bacterium. Mycobacteria such as BCG are surrounded by an outer membrane-like structure composed of mycolic acids and other extremely hydrophobic lipid molecules, and studies have demonstrated that exogenous lipids can be incorporated into this structure without affecting viability of the bacteria (32). Although such lipid transfer in mycobacteria has generally been done using the organic solvent petroleum ether, we found that the limited solubility of αGalCer in this solvent prevented the use of that method (Figure 1B). We therefore examined the solubility of αGalCer in aqueous solutions of the detergents Tween 80 and tyloxapol, which are routinely used in mycobacterial culture media and are known to be compatible with the growth of BCG.
Using a 14C-labeled preparation of a biologically active C20 N-acyl form of αGalCer (14C-αGalCer-C20:0) we observed excellent solubilization of the glycolipid in PBS containing 0.05% tyloxapol, which was comparable to solubility in a 2:1 choroform:methanol mixture and clearly superior to PBS containing 0.05% Tween 80 (Figure 1B). The growth of BCG in liquid medium containing 0.05% tyloxapol was unaffected by the addition of αGalCer or α-C-GalCer, indicating that these glycolipids did not have toxic effects on the bacteria (Figure 1C). The colony counts obtained by plating BCG on standard growth media were also unaffected by their growth in medium containing αGalCer (data not shown). Growth of BCG in protein free medium with 0.05% tyloxapol and various concentrations of 14C-labeled αGalCer demonstrated that greater than 20% of the added radiolabel became stably incorporated into the bacteria (i.e., not removed by repeated washing of bacterial pellet with PBS + 0.05% tyloxapol) when the glycolipid was initially added at a concentration of 16 µg/ml or greater (peak incorporation of 28% at 32 µg/ml) (Figure 1D). Lipids were re-extracted BCG grown in medium containing 16 µg/ml of 14C-labelled αGalCer and analyzed by thin layer chromatography (TLC), which revealed that the majority of the incorporated glycolipid migrated identically to the standard preparation of αGalCer and was therefore chemically intact (Figure 1E).
Biologic activity of glycolipids incorporated into live BCG
For the initial assessment of the biological activity of iNKT cell activating glycolipids incorporated into live BCG, we used a standard iNKT cell hybridoma stimulation assay (27). BCG grown in medium containing 20 µg/ml of αGalCer (αGalCer/BCG) or its related α-C-GalCer (α-C-GalCer/BCG) were prepared, and the extensively washed bacteria were used to infect cultures of mouse bone marrow-derived DCs. The DCs infected with glycolipid-modified BCG preparations strongly stimulated IL-2 production by iNKT cell hybridoma cells in comparison to DCs infected with unmodified BCG, indicating that the incorporated glycolipids could be released during intracellular growth or processing of the bacteria and presented by CD1d molecules (Figure 2A). We observed similar activity when iNKT cell hybridoma cells were activated by holding the number of infected BMDC constant but varying the MOI of BCG or glycolipid-modified BCG (Supplemental Figure 1). Strong cytokine responses were also observed when BMDCs infected with αGalCer/BCG were co-cultured with mouse splenocytes, with significant levels of IFNγ and IL-4 accumulating in the culture supernatant after 48 hours as is typical for primary iNKT cell responses (Figure 2B). Interestingly, with splenocytes as the source of the responding iNKT cells, we did not observe any cytokine production using α-C-GalCer/BCG. However, with liver mononuclear cells which are highly enriched in iNKT cells, we were able to observe a significant IFNγ response to α-C-GalCer modified BCG without detectable IL-4 (Figure 2C). These findings were consistent with the limited activity of α-C-GalCer that has been generally observed in cell culture assays (M. Venkataswamy and S. Porcelli, unpublished data), which contrasts strongly with the marked in vivo activity of this glycolipid in mice. In addition, the absence of detectable IL-4 in the in vitro response of hepatic mononuclear cells to α-C-GalCer/BCG suggested a strongly TH1-biased iNKT cell response, which is consistent with in vivo studies using this analogue in mice (22).
Figure 2. Retention of biologic activity of αGalCer incorporated into BCG.
(A) Supernatant levels of IL-2 produced by mouse iNKT cell hybridoma DN3A4-1.2 following culture for 16 hrs with the indicated numbers of autologous BMDC infected at MOI of 10:1 with BCG, or with BCG that was modified by incorporation of either αGalCer (αGalCer/BCG) or α-C-GalCer (α-C-GalCer/BCG). (B) Supernatant levels of IFNγ (left) and IL-4 (right) of naïve C57BL/6 mouse splenocytes (5 × 105) following culture for 48 hrs with autologous BMDC infected at MOI of 10:1 with BCG, αGalCer/BCG or α-C-GalCer/BCG. (C) Hepatic mononuclear cells (4 × 105) from naïve C57BL/6 mice were used as responders, and cultured with autologous BMDC (1 × 105) infected at MOI of 10:1 with the indicated bacteria. Supernatant levels of IFNγ (top) and IL-4 (bottom) were measured after 48 hours. (D) Supernatant levels of IFNγ, TNFα and IL-13 produced by human iNKT cell clone HDE3 following 24 hours of culture with heterologous human monocyte-derived DC infected with BCG, αGalCer/BCG or α-C-GalCer/BCG. (E) In vivo activity of αGalCer incorporated into live BCG. At time 0 hrs, C57BL/6 received a single i.p. injection of either free αGalCer (4.8 nmol in aqueous vehicle), 5 × 106 CFU of unmodified BCG or 5 × 106 CFU of live BCG modified by incorporation of αGalCer (αGalCer/BCG). Serum samples were obtained at 2, 8, 12 and 24 hours post injection, and serum levels of IFNγ, IL-12p70 and IL-4 were determined. For panels A–E, means of triplicate values and standard deviations are shown, and all results shown are representative of at least two independent experiments.
We also tested the activity of αGalCer/BCG or α-C-GalCer/BCG for stimulation of a human iNKT cell clone in cultures with infected monocyte-derived human dendritic cells. This demonstrated that αGalCer/BCG was strongly stimulatory toward human iNKT cells, and activated their secretion of IFNγ, TNFα and IL-13 at relative levels comparable to those obtained with addition of free αGalCer (Figure 2D, and additional data not shown). These results were consistent with the known conservation of many of the features of iNKT cell responses between humans and mice (33). Similar to the mouse splenocyte cultures, no activity for α-C-GalCer/BCG was observed in this in vitro human iNKT cell culture system. Although the question of whether or how strongly human iNKT cells can respond to α-C-GalCer has not been clearly resolved, one previous study has reported that cultured human iNKT cells can be activated by this glycolipid (34). By analogy with the observations in the mouse system, it is very possible that α-C-GalCer could be a strongly activating ligand for human iNKT cells in vivo, but for reasons that remain unclear this activity is difficult to reveal using available in vitro assays.
Injection of αGalCer into mice by intravenous or intraperitoneal routes is known to induce a robust systemic response that is manifested by easily detectable levels of serum cytokines (27). To determine the in vivo activity of the glycolipids incorporated stably into live BCG, we measured serum cytokine levels at various time points after i.p. injection of αGalCer/BCG into C57BL/6 mice. αGalCer/BCG was clearly active in vivo, and induced low but detectable serum levels of IFNγ, IL-12 and IL-4 within 6–12 hours (Figure 2E). Intraperitoneal injection of unmodified BCG induced low levels of serum IL-12p70, and no detectable IFNγ or IL-4 over a 48 hour period, whereas a single injection of free αGalCer rapidly induced all three cytokines as previously described (35). Serum cytokines were not detected in similar experiments carried out using CD1d−/− or Jα18−/− knockout mice, which are both completely deficient in iNKT cells (data not shown). Interestingly, although injection of free α-C-GalCer gave strong and sustained serum IFNγ levels with no detectable IL-4 as previously reported (22), we detected only minimal augmentation of serum IFNγ at 24 hours with i.p. injection of BCG modified by incorporation of α-C-GalCer (data not shown).
Since αGalCer and α-C-GalCer have been reported to induce differentiation and maturation of dendritic cells, we also assessed expression of MHC class II and costimulatory molecules on the CD11c+ cells in the spleens and livers of C57BL/6 mice that were injected i.p with αGalCer/ BCG or α-C-GalCer/BCG. In the spleen (Figure 3A), neither unmodified nor glycolipid-modified BCG had a significant effect on the surface levels of MHC class II molecules. However, the costimulatory molecules CD80, CD86 and CD70, while only slightly increased on CD11c+ cells by unmodified BCG, showed pronounced induction with αGalCer/BCG and also to a lesser extent with α-C-GalCer/BCG. In the liver mononuclear cell fraction (Figure 3B), marked increases of MHC class II were observed with both αGalCer/BCG and α-C-GalCer/BCG, as well as increases in CD80, CD86 and CD70. In all cases, these effects were greater than those observed with unmodified BCG. We also verified that these effects depended on iNKT cell activation, since they did not occur in parallel experiments conducted in CD1D−/− knockout mice which lack iNKT cells (data not shown). These results strongly suggested that the enhancement of DC maturation was an important consequence resulting from incorporation of iNKT cell agonist glycolipids into BCG.
Figure 3. Enhancement of dendritic cell maturation by glycolipid-modified BCG.
(A) FACS analysis of indicated markers on CD11c+ cells from spleens of C57BL/6 mice injected i.p. 40 hrs earlier with inert vehicle or with 5 × 106 CFU of live BCG or glycolipid-modified BCG as indicated. The open histograms in the top row indicate staining with isotype matched control antibodies, which gave similar profiles for all four splenocyte preparations. Shaded histograms indicate staining with mAbs specific for MHC class II (I-Ab), CD80, CD86 or CD70 as indicated at the top of each column. The bar graph to the right shows calculated fold increase in median fluorescence intensities for each molecule on CD11c+ cells from spleens of mice immunized with glycolipid-modified BCG compared to mice receiving unmodified BCG. (B) Same analysis as in (A) except for CD11c+ cells in hepatic mononuclear cell suspensions obtained from similarly immunized mice. Results shown are representative of two independent experiments.
Influence of glycolipid incorporation on antigen specific T cell priming
Given the known effects of free αGalCer on the priming of antigen specific T cells, and the effects observed on DC maturation with glycolipid-modified BCG, we assessed the impact of incorporating iNKT cell agonist glycolipids into BCG on the priming of both CD4+ and CD8+ T cells. Surprisingly, using a variety of different methods to assess CD4+ T cell responses against an immunodominant secreted mycobacterial antigen (peptide 25 of Antigen 85B, or p25), we were unable to detect any change in CD4+ responses with incorporation of either αGalCer or α-C-GalCer into BCG (Figure 4). This included the initial priming of naïve T cells specific for the p25 epitope as assessed by the cell division of adoptively transferred p25-specific TCR transgenic T cells during the first week of immunization (Figure 4A). Analysis of this response using lower doses of BCG (104 CFU) also failed to reveal any augmentation of the response with glycolipid incorporation (Supplemental Figure 2A). Similarly, analysis by IFNγ ELISPOT assay of responses against p25 at 3 weeks or 8 weeks (Figure 4B) after immunization revealed uniformly strong responses in animals that received unmodified or glycolipid-modified BCG. Multiparameter FACS analyses revealed no differences in the percentages of CD4+ T cells that simultaneously produced IFNγ, IL-2 and TNFα (multifunctional or polyfunctional T cells (36)), or in the levels of FoxP3+ and CD25+ regulatory T cells (Treg (37)) in the spleen (Figure 4C and Supplemental Figure 2B) or lungs (data not shown) of animals immunized with the various BCG preparations, indicating that CD4+ T cell differentiation proceeded similarly in mice immunized with unmodified and glycolipid-modified BCG.
Figure 4. Glycolipid incorporation did not alter CD4+ T cell priming or memory responses induced by BCG.
(A) Proliferation of transferred p25-specific T cells was observed at similar levels in mice infected with BCG, αGalCer/BCG or α-C-GalCer/BCG BCG. Thy1.1+ B6.PL mice were injected i.v. with CFSE-labeled Thy1.2+ splenocytes from p25TCR-Tg/Rag-1−/− mice, followed by subcutaneous injection either with saline (control), or with 5 × 106 CFU of live bacilli (unmodified BCG, αGalCer/BCG or α-C-GalCer/BCG as indicated). Mice were sacrificed after 7 days, and splenocytes were analyzed by FACS. Dot plots of splenocytes gated for CD4+ and B220− staining are shown for representative mice, and the Thy1.2+ CFSE-labeled populations are indicated in each case within the dashed rectangles. The bar graph on the right indicates the percentage of CD4+ Thy1.2+ cells which retained bright (> FL1 channel 102) CFSE staining. Each bar indicates mean and standard deviation for groups of 3 mice. (B) IFNγ ELISPOT was used to measure recall responses of endogenous CD4+ T cells specific for p25 in spleen cell suspensions from C57BL/6 mice injected intradermally 3 or 8 weeks previously with saline (control) or with 5 × 106 CFU of BCG, αGalCer/ BCG or α-C-GalCer/BCG. All groups receiving unmodified or modified BCG responded similarly. (C) Multiparameter FACS with intracellular staining for cytokines, FoxP3 and CD25 was used to assess the frequencies of multifunctional and regulatory CD4+ T cells (Treg) in cultured splenocytes from animals immunized 8 weeks previously as in (B) and restimulated in vitro with M. tuberculosis (strain H37Rv) sonicate plus anti-CD28 mAb. The graphs show the percentages of total CD4+ T cells simultaneously producing IFNγ, IL-2 and TNFα (multifunctional T cells, left), and the percentages expressing both FoxP3 and CD25 (Treg, right). No significant differences were observed between the groups immunized with BCG versus glycolipid-modified BCG preparations in any of these studies (p > 0.05, ANOVA). All results shown are representative of at least two independent experiments.
In marked contrast to our findings on CD4+ T cell responses, evaluation of antigen specific CD8+ T cell priming and recall responses revealed a dramatic effect of the glycolipid incorporation. To facilitate analysis of this component of the T cell response, we took advantage of a recombinant BCG expressing a fragment of ovalbumin (OVA) containing the immunodominant H-2Kb-presented OVA257–264 epitope (SIINFEKL) (38). To directly observe the priming of MHC class I–restricted CD8+ T cells in the context of vaccination, we employed adoptive transfer of CFSE-labeled naive T cells from SIINFEKL/H-2Kb–reactive TCR-transgenic OT-I mice (5). Thy1.1+ B6.PL mice were injected with CFSE-labeled Thy1.2+ splenocytes from OT-I mice, followed by vaccination either with BCG-OVA, αGalCer/BCG-OVA or α-C-GalCer/BCG-OVA. CD8+ T cell activation and proliferation were assessed by dilution of CFSE in the transferred population at 5–7 days after infection (Figure 5A). Only minimal activation and proliferation of transferred OT-I T cells was observed in mice infected with BCG-OVA, as approximately 80% of the remaining Thy1.2+ cells had CFSE levels that indicated a failure to undergo division. In contrast, αGalCer/BCG-OVA or α-C-GalCer/BCG-OVA infection induced a massive increase in proliferation of transferred T cells. Nearly all detectable Thy1.2+ T cells had diluted their CFSE staining to backgrounds levels, and the size of the Thy1.2+ population had increased substantially indicating survival and accumulation of the proliferating CD8+ T cells. Similar results were observed for cells from the spleen (Figure 5A) and from the draining lymph nodes (Supplemental Figure 3).
Figure 5. Marked enhancement of CD8+ T cell responses by glycolipid-modified BCG.
(A) Thy1.1+ B6.PL mice were injected i.v. with CFSE-labeled Thy1.2+ OT-I splenocytes, and infected with the indicated bacteria. CD8+ T cell activation was assessed 7 days after infection by CFSE dilution. Dot plots of splenocytes gated for CD8+ and B220− staining are shown for representative mice, and the Thy1.2+ CFSE-labeled populations are indicated in each case within the dashed rectangles. The bar graph on the right indicates the percentage of CD8+ Thy1.2+ cells which retained bright (> FL1 channel 102) CFSE staining. Each bar indicates the means and standard deviations for groups of 3 mice. (B) ELISPOT assay for IFNγ producing CD8+ T cells specific for the H-2Kb restricted OVA peptide (SIINFEKL) in splenocytes of C57BL/6 mice immunized 3 or 8 weeks previously with saline (control), or with unmodified or glycolipid-modified BCG as indicated. (C) ELISPOT assay for IFNγ producing CD8+ T cells specific for H-2Kd restricted TB10.3/10.4 peptide (GYAGTLQSL) in splenocytes of BALB/c mice immunized 2 weeks previously with saline (control) or with unmodified BCG or αGalCer/BCG as indicated. (D) ELISPOT analysis for IFNγ producing CD8+ T cells specific for H-2Kb presented TB10.3/10.4 epitope (QIMYNPAM) in splenocytes of C57BL/6 mice at 3 weeks post-immunization with unmodified BCG or glycolipid-modified BCG as indicated. Bars indicate responses for animals immunized with BCG with glycolipids physically incorporated (Inc, solid bars), with unmodified BCG plus glycolipids (0.1 µg) injected at a separate site on the opposite flank (Sep; diagonally hatched bars), or with unmodified BCG mixed with glycolipids (0.1 µg) immediately before administration and injected together into the same site (Mix; horizontally hatched bars). (E) IFNγ ELISPOT assay showing CD8+ T cells responses specific for H-2Kb presented TB10.3/10.4 epitope (QIMYNPAM) in spleen cell suspensions from CD1d deficient (CD1D−/−) mice injected intraperitoneally 2 weeks previously with saline (control), or with unmodified or glycolipid-modified BCG as indicated.* p < 0.001; ***, p < 0.0001 (one way ANOVA, Tukey post-hoc test). The vaccine dose was 5 × 106 CFUs for all experiments shown in panels A–E. Results shown are representative of three (A,B) or two separate (C,D,E) independent experiments.
To analyze recall responses of endogenous CD8+ T cells, C57BL/6 mice were vaccinated with unmodified or glycolipid-modified BCG-OVA and analyzed after 3 or 8 weeks for SIINFEKL responsive CD8+ T cells in the spleen by IFNγ ELISPOT (Figure 5B). This revealed significantly enhanced responses to the SIINFEKL peptide in mice which had received α-GalCer/BCG-OVA or α-C-GalCer/BCG-OVA compared to BCG-OVA without glycolipid incorporation. To confirm this finding using a natural epitope of BCG, we analyzed IFNγ ELISPOT responses to an H-2Kd presented epitope (GYAGTLQSL) shared by the endogenous mycobacterial antigens TB10.3 and TB10.4 (TB10.3/10.4). Responses to this peptide were also significantly enhanced in BALB/c mice vaccinated 2 weeks previously with αGalCer/BCG compared to unmodified BCG (Figure 5C). Taken together, these results provided strong evidence that mycobacterial antigen-specific CD8+ T cell responses were significantly accelerated and enhanced by incorporation of αGalCer or α-C-GalCer into live BCG.
Experiments were also carried out to address the question of whether the effect of αGalCer or α-C-GalCer on enhancing CD8+ T cell responses required the physical association of the glycolipids with the immunizing bacteria. The effects on CD8+ T cell priming of free glycolipids injected at a separate site from unmodified BCG, or of injection of the two components at the same site by mixing the free glycolipid with the bacteria immediately before injection, were compared to administration of the glycolipid-modified BCG (Figure 5D). This revealed that only direct physical incorporation of the glycolipids into live BCG organisms was able to induce significantly improved CD8+ T cell priming against an H-2Kb presented TB10.3/10.4 epitope (QIMYNPAM) (39). Comparable findings were also obtained using BCG-OVA with analysis of the H-2Kb presented SIINFEKL epitope in C57BL/6 mice (data not shown). The difference between directly incorporated versus free αGalCer or α-C-GalCer was apparent with a dose of 0.1 µg of glycolipid (which was approximately equivalent to the amount of glycolipid delivered when incorporated into the inoculum of 5 × 106 CFU of modified BCG organisms) (Figure 5D). Similar results were obtained when up to 40 fold higher amounts (4 µg) of the free glycolipids were injected without incorporation into the bacilli at the same or separate sites (data not shown). Enhancement of the CD8 T cell responses to the TB10.3/10.4 epitope (QIMYNPAM) was not observed in CD1D−/− mice (C57BL6 background) by ELISPOT assay for glycolipid modified BCG, indicating that the adjuvant effect of the glycolipids was CD1d–dependant (Figure 5E).
Enhanced vaccine efficacy of glycolipid-modified BCG
Immunization and challenge studies were performed to determine whether the enhanced CD8+ T cell priming associated with αGalCer/BCG or α-C-GalCer/BCG could improve protective immunity induced by BCG vaccination. C57BL/6 mice that were either naïve or immunized 2 months earlier by intradermal inoculation with live BCG, αGalCer/ BCG or α-C-GalCer/BCG were challenged by low-dose aerosol infection with virulent M. tuberculosis H37Rv, and CFU counts in tissues were determined at 3 and 6 weeks post-challenge. In naive mice, substantial growth in the lungs and dissemination to spleens were detected at 3 and 6 weeks after challenge. However, vaccination with BCG, αGalCer/BCG or the α-C-GalCer/BCG considerably reduced M. tuberculosis bacterial loads in both lungs and spleens of aerosol-challenged mice as compared with naive controls (Figures 6A and 6B). Interestingly α-C-GalCer/BCG vaccination protected significantly better than BCG, at the 3 week time point, in both lungs and spleen. Immunization with α-C-GalCer/BCG also showed a more prolonged effect on control of M. tuberculosis infection compared with BCG immunization, with reductions in CFU in both the organs at 6 weeks after challenge. Similar trends toward enhanced protection were observed with αGalCer/BCG immunization, although this was clearly less pronounced than with α-C-GalCer/BCG and achieved statistical significance only at the 6 week time point in lung. Histopathological examination of the lungs from mice immunized with either BCG, αGalCer/BCG or α-C-GalCer/BCG showed relatively mild inflammation with small and compact lymphocyte-rich granulomas, compared with naive mice, which had extensive, poorly organized granulomatous lesions (Figure 6C, top row). A trend toward greater lymphocyte predominance of the lesions was apparent in areas of granulomatous inflammation of the lungs in αGalCer/BCG or α-C-GalCer/BCG immunized mice compared to naïve or unmodified BCG immunized mice (Figure 6C, bottom row).
Figure 6. Improved vaccine efficacy against M. tuberculosis challenge with glycolipid-modified BCG.
C57BL/6 mice were vaccinated intradermally with saline (naïve, open bars), with 5 × 106 unmodified BCG (diagonally hatched bars) or 5 × 106 glycolipid-modified BCG (αGalCer/BCG, solid bars; α-C-GalCer/BCG, cross hatched bars). Two months later, animals were challenged by aerosol infection with 50–100 CFU of virulent M. tuberculosis (strain H37Rv). Bars show means and SD for CFU of M. tuberculosis in lungs (A) and spleens (B) at 3 and 6 weeks after challenge for groups of 7 mice. π, p < 0.05 (unpaired t test); *, p < 0.05; **, p < 0.007; ***, p < 0.0003 (one way ANOVA, Tukey post-hoc test). Results shown are representative of two independent experiments. (C) Lungs of mice vaccinated and challenged with virulent M. tuberculosis were examined histologically at 6 weeks after challenge. Scale bars: top, 0.5 mm; bottom, 50 µm.
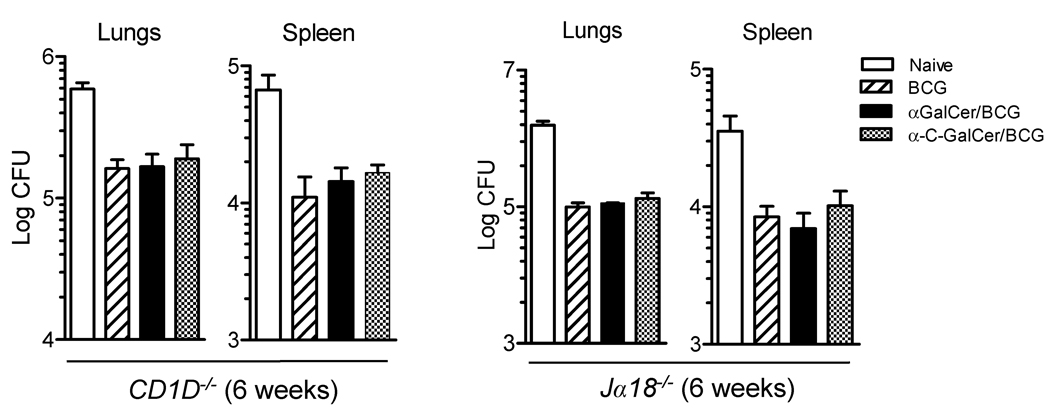
In order to demonstrate that the adjuvant activity provided by the two glycolipids was due to and dependent on the specific activation of iNKT cells, we set up immunization and challenge experiments with CD1D−/− and Jα18−/− knockout mice, both of which are completely deficient in iNKT cells but have normally functioning innate and adaptive immune systems in most other respects(25,40). There was no difference in protection between BCG immunized and the glycolipid-modified BCG immunized CD1D−/− or Jα18−/− mice (Figure 7), demonstrating that iNKT cells were essential for enhanced protection induced in wild type mice by immunization with αGalCer/BCG or α-C-GalCer/BCG compared to unmodified BCG.
Figure 7. Enhanced vaccine efficacy of glycolipid-modified BCG required iNKT cells.
Mice lacking iNKT cells (CD1d−/− or Jα18−/− mice, C57BL/6 background) were vaccinated intradermally with saline (naïve, open bars), with 5 × 106 unmodified BCG (diagonally hatched bars) or 5 × 106 glycolipid-modified BCG (αGalCer/BCG, solid bars; α-C-GalCer/BCG, cross hatched bars). Two months later, animals were challenged by aerosol infection with 50–100 CFU of virulent M. tuberculosis (strain H37Rv). Bars show means and SD for CFU of M. tuberculosis in lungs and spleens 6 weeks after challenge for groups of 4 mice. No significant differences were observed between groups vaccinated with unmodified or glycolipid-modified BCG (p > 0.05, one way ANOVA).
Discussion
By developing a simple approach for incorporating synthetic α-galactosylceramides stably but reversibly into live mycobacteria, we describe a novel method that enables the activation of CD1d–restricted iNKT cells to be tightly coupled with the presentation of bacterial antigens during immunization with M. bovis BCG. This simple modification of BCG led to several marked changes in the immunological effects of the vaccine. Although our studies have not resolved the exact site or sites in the bacteria in which the glycolipids become localized, we speculate that they are likely to be inserted into the lipid rich outer membrane of the cell wall. Other possibilities include stable intercalation into the plasma membrane, or possibly partitioning into cytosolic lipid storage bodies of the bacteria (41). We observed that glycolipid-modified but not unmodified BCG induced a rapid and transient production of cytokines including IL-12p70 and IFNγ, which was consistent with the known effects of in vivo activation of iNKT cells by αGalCer. Dendritic cell maturation was enhanced by the incorporation of αGalCer into BCG, and most notably, CD8+ T cell responses against mycobacterial protein antigens were significantly augmented. These immunological effects were correlated with a significant improvement in the ability of glycolipid-modified BCG to stimulate protective immunity in a mouse model of vaccination followed by challenge with virulent M. tuberculosis.
Some microorganisms are known to contain glycolipids that are recognized as CD1d–presented antigens and have the capacity to directly activate iNKT cells during infection. This has been most clearly demonstrated for a group of Gram negative bacteria known as sphingomonads (genus Sphingomonas or Novosphingobium), in which the major outer membrane glycolipids are α-glycosylceramides that strongly activate iNKT cells (42,43). Similarly, the spirochete Borrelia burgdorferi which causes Lyme disease has been shown to possess α-glycosylated glycerophospholipids which likewise function as CD1d–presented antigens for iNKT cells (44). For organisms such as these which possess potent natural activators of iNKT cells, it is likely that the responses of this specialized T cell population are a prominent part of the initial immune response to infection and play a significant role in host defense. In contrast, in the case of mycobacteria such as BCG it appears that strong endogenous iNKT cell activating glycolipids are absent or not accessible, which prompted us to assess the possibility of increasing the immunogenicity of BCG by incorporation of synthetic αGalCer. Previous work provides a strong rationale to the approach of using iNKT cell activating glycolipids such as αGalCer as adjuvants for both cellular and humoral adaptive immune responses (45). Initial proof of concept studies showed that intravenous administration of soluble protein antigen (ovalbumin) together with αGalCer augmented both CD4+ and CD8+ antigen-specific T cell responses in mice (31). Subsequent studies extended this approach to include the use of αGalCer to enhance adaptive immune responses against tumors or a variety of other antigenic challenges (46). These have included the use of αGalCer in conjunction with a variety of types of vaccines against several pathogens, including malaria parasites (47), influenza virus (48), HIV (49) or Leishmania major (50). Our findings reported in the current study are the first to our knowledge to evaluate the adjuvant role of αGalCer or its analogue α-C-GalCer in the context of a vaccine against tuberculosis.
Although our studies indicated that unmodified BCG lacked the ability to rapidly and strongly stimulate iNKT cells, there is evidence that iNKT cells are able to sense and respond to mycobacterial infections. One previous study identified a glycolipid from BCG, phosphatidylinositol tetramannoside (PIM4), that binds to CD1d, although in vitro assays suggest that it may be recognized by at most a small subset of iNKT cells (51). In addition, mechanisms independent of specific bacterial antigen recognition could also lead to activation of iNKT cells during mycobacterial infections, as this is known to occur as a result of self-reactivity of iNKT cells coupled with the influence of inflammatory cytokines that may be prominently released during infection (52). In fact, iNKT cells in mouse splenocytes become activated when cultured with M. tuberculosis infected macrophages (53), and intravenous infection of mice with BCG has been shown to cause activation and expansion of iNKT cells in spleen and liver within one week of infection (54). However, our results indicated that iNKT cell activation by unmodified BCG is likely to be extremely weak or delayed, as we observed little or no cytokine production following a 16 hour exposure of iNKT cell hybridoma cells to BCG-infected DC, or with naive splenocytes following 48 hours of culture with BCG-infected DCs. This stands in marked contrast to the results obtained with αGalCer-modified BCG, which under the same experimental conditions gave rapid and robust cytokine production.
While the direct effect of αGalCer incorporation was to endow BCG with the capacity to rapidly and powerfully activate iNKT cells, it was most likely the indirect secondary effects of iNKT cell activation that accounted for the improvement in vaccine efficacy against subsequent M. tuberculosis challenge. Multiple previous studies have generally led to the conclusion that iNKT cells exert relatively little if any effect on the course of tuberculosis in mice, since the outcome of M. tuberculosis infection is not significantly altered in CD1d−/− or Jα18−/− mice that completely lack iNKT cells (55–58). On the other hand, strong activation of iNKT cells by administering αGalCer during established tuberculosis in mice (59), or augmentation of iNKT cells by adoptive transfer at the time of M. tuberculosis infection (53), are both associated with improved control of infection with reduced bacterial burden. Such effects are unlikely to account for the enhanced protective immunity we observed in our vaccination and challenge studies, since the M. tuberculosis challenge was not administered until 2 months after the initial exposure to glycolipid-modified BCG. Long term memory which could account for the vaccine induced protection has generally not been observed with iNKT cells, which typically undergo depletion and induction of anergy following activation, with recovery to their baseline state over a period of several weeks (54,60). Thus, within the context of the vaccination protocol used in our studies, the beneficial effect of αGalCer incorporation into BCG most likely resulted from effects on priming and differentiation of memory T cells specific for mycobacterial peptide antigens, which represent the major known mediators of protective vaccine-induced adaptive immunity to M. tuberculosis (9).
A striking result in our analysis was the impact of glycolipid incorporation on the initial priming of CD8+ T cells, and the subsequent augmentation of recall responses by this population. Our previous work has highlighted the ability of M. tuberculosis to actively inhibit and delay antigen presentation via MHC class I to CD8+ T cells (5), and further experiments indicate that this immune evasion strategy is also retained in BCG (Figure 5, and J. Hinchey and S. A. Porcelli, unpublished data). This striking effect on CD8+ T cell responses stood in marked contrast to our findings on CD4+ T cells, which appeared to be unaffected by the incorporation of iNKT cell activating glycolipids into BCG. Although the mechanism responsible for the enhanced CD8+ priming and memory T cell generation will require further analysis, one intriguing possibility is that the activation of iNKT cells could enhance MHC class I restricted cross presentation via the "detour pathway" which involves uptake of apoptotic mycobacteria infected macrophages by activated dendritic cells (61). The glycolipid adjuvants used in our study are known to rapidly induce high levels of IFNγ in mice, which is associated with the development of strong CD8+ T cell responses, regulates subsequent CD8+ T cell contraction and affects the formation of CD8+ T cell memory (62).
The enhanced CD11c+ dendritic cell maturation observed with glycolipid-modified BCG, which most likely reflected at least in part the increased expression of CD40L on activated iNKT cells and its interaction with CD40 on DCs (31), could also promote CD8+ T cell priming via the detour pathway or by other mechanisms of antigen cross-presentation. It is pertinent to note that the increased expression of maturation and co-stimulatory markers was delayed and reduced in magnitude in mice given unmodified BCG compared to those immunized with glycolipid-modified BCG. These differences would be expected to influence the strength and quality of T cell responses against mycobacterial antigens. Among the surface molecules that we observed to be increased by immunization with glycolipid-modified BCG was CD70, which has been identified as a key costimulatory factor expressed by a CD205+ DC subset in mice that plays a predominant role in antigen cross-presentation to MHC class I restricted CD8+ T cells (63,64). Consistent with a possible role for CD70 in the enhanced priming of CD8+ T cells that we observed, a recent study found that CD70 expression on dendritic cells is essential for mediating the adjuvant effects of αGalCer on conventional CD8+ T cells (65).
The direct comparison between αGalCer and its TH1-biasing analogue α-C-GalCer also proved interesting and revealing in our study. Although we observed a surprising lack of rapid cytokine secretion in response to α-C-GalCer-modified BCG both in vitro and in vivo (Figure 2), this formulation showed largely similar efficiency to conventional αGalCer-modified BCG for inducing DC maturation and in augmenting CD8+ T cell priming in vivo (Figure 3 and Figure 5). Most notably, in vaccination and challenge studies we found BCG modified with α-C-GalCer to be superior at inducing anti-mycobacterial protective immunity (Figure 6). The improvement in protective efficacy of BCG modified with α-C-GalCer both in the lungs and spleen is potentially attributable to the sustained IFNγ production and reduction of IL-4 levels associated with this analogue of αGalCer (22). Although the mechanism for the altered cytokine response to α-C-GalCer remains unresolved, we speculate based on recent studies of the mechanisms controlling the fine tuning of iNKT cell cytokine responses to αGalCer analogues that the increased hydrophobicity of this compound may be a key factor in producing its unique activities (66). Conversely, the lack of enhanced protection in the spleens of mice immunized with αGalCer/BCG may have reflected a relative TH2 polarization resulting from the IL-4 production that is associated with administration of αGalCer. Our studies have not yet determined the reasons for the apparent disparity between the direct stimulation of iNKT cell cytokine production and the other more indirect effects of iNKT cell activation (DC maturation, CD8+ T cell priming) with α-C-GalCer. However, these observations raise important considerations about which assays should be used to determine optimal formulations for vaccines containing iNKT cell dependent adjuvants.
Most of the earlier studies on the adjuvant effect of αGalCer with vaccines against various infectious diseases have emphasized the importance of co-administration of this glycolipid with the respective vaccine in order to harness its adjuvant activity (47,49,50,67). We found this to be extremely important in the case of BCG, since our findings showed that simple co-injection of αGalCer or α-C-GalCer did not enhance CD8+ T cell responses, and this effect could only be achieved by the direct physical incorporation of the glycolipids into BCG (Figure 5D). This implies a requirement for tight linkage of the location or timing of iNKT cell activation with bacterial protein antigen processing and presentation in order to obtain the desired adjuvant effect. Our approach achieves this by guaranteeing the simultaneous delivery of the glycolipid and live BCG organisms into the same phagocytic cells. The direct incorporation approach also has important practical advantages including simplicity and ease of administration, and the possibility of dose-sparing effects that could reduce production costs and minimize unwanted systemic side effects of a modified BCG vaccine. This dose limiting effect may also mitigate the potential of αGalCer to cause depletion or anergy of iNKT cells, although this possibility has not yet been analyzed directly. Future studies to combine the current approach with additional modifications of BCG, including incorporation of compounds known to exert enhancing effects on CD4+ T cell responses such as CpG oligodeoxynucleotides (23), could yield further improvements and lead to much needed advances in vaccines to combat the growing global epidemic of tuberculosis.
Supplementary Material
Acknowledgements
We thank Dr. Rani Sellers for assistance in analysis of histopathology. John Kim, Mei Chen, Weijun Liu and Bing Chen provided expert technical assistance with animal vaccine and challenge studies.
Grant Support: This work was supported by NIH/NIAID grants AI45889 (to SAP) and AI063537 (PO1 to SAP, WRJ and JC). Core resources that facilitated flow cytometry and histopathology studies were supported by the Einstein Center for AIDS Research (AI 051519) and the Einstein Cancer Center (CA 13330). SAP and WRJ were also supported by funding from the AERAS Global TB Vaccine Foundation and the Collaboration for AIDS Vaccine Discovery of the Bill and Melinda Gates Foundation. GSB acknowledges support from a Personal Research Chair from Mr. James Bardrick, a Royal Society Wolfson Research Merit Award, a former Lister Institute-Jenner Research Fellowship, the Medical Research Council and The Wellcome Trust (084923/B/08/Z).
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. Journal of Clinical Investigation. 2008;118:1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008;372:164–175. doi: 10.1016/S0140-6736(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P. Tuberculosis vaccines - an update. Nature Reviews Microbiology. 2007;5:484–U16. doi: 10.1038/nrmicro1703. [DOI] [PubMed] [Google Scholar]
- 5.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, Morris SL, Jacobs WR, Jr, Porcelli SA. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J.Clin.Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic' S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc.Natl.Acad.Sci.U.S.A. 2000;97:13853–13858. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser EA, Mann P, Goosmann C, Bandermann S, Smith D, Bancroft GJ, Reyrat JM, van D, Soolingen B, Raupach B, Kaufmann SH. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J.Clin.Invest. 2005;115:2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. Journal of Experimental Medicine. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn JL, Chan J. Immunology of tuberculosis. Annu.Rev.Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat.Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 11.Pinxteren van, L. A., Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur.J.Immunol. 2000;30:3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J.Exp.Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J.Exp.Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu.Rev.Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 15.Bricard G, Porcelli SA. Antigen presentation by CD1 molecules and the generation of lipid-specific T cell immunity. Cell Mol.Life Sci. 2007;64:1824–1840. doi: 10.1007/s00018-007-7007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d–restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nakui M, Sekimoto M, Koda T. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d–expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int.Immunol. 2000;12:987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Kaer LVan, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J.Exp.Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J.Exp.Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behar SM, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr.Top.Microbiol.Immunol. 2007;314:215–250. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- 21.Yu KOA, Porcelli SA. The diverse functions of CD1d–restricted NKT cells and their potential for immunotherapy. Immunology Letters. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Schmieg J, Yang GL, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. Journal of Experimental Medicine. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freidag BL, Melton GB, Collins F, Klinman DM, Cheever A, Stobie L, Suen W, Seder RA. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect.Immun. 2000;68:2948–2953. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SM, Carter QL, Garcia J, Wang C, Patten K, Stills HF, Alt FW, Snapper SB, Balk SP. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui JQ, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for V(α)14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 26.Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, Tokunaga T, Xu W, Kariyone A, Saito T, Kitamura T, Maxwell G, Takaki S, Takatsu K. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int.Immunol. 2004;16:1691–1699. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 27.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Modulation of CD1d–restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc.Natl.Acad.Sci.U.S.A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J.Immunol.Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 29.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, Ndonye R, Howell AR, Santamaria P, Besra GS, DiLorenzo TP, Porcelli SA. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J Immunol. 2007;178:1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 30.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J.Exp.Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J.Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 32.Rao V, Fujiwara N, Porcelli SA, Glickman MS. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J.Exp.Med. 2005;201:535–543. doi: 10.1084/jem.20041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d–mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J.Exp.Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu X, Song L, Metelitsa LS, Bittman R. Synthesis and evaluation of an α-C-Galactosylceramide analogue that induces Th1-biased responses in human natural killer T cells. Chembiochem. 2006;7:1750–1756. doi: 10.1002/cbic.200600197. [DOI] [PubMed] [Google Scholar]
- 35.Forestier C, Molano A, Im JS, Dutronc Y, Diamond B, Davidson A, Illarionov PA, Besra GS, Porcelli SA. Expansion and hyperactivity of CD1d–restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 2005;175:763–770. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- 36.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat.Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 37.Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J.Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 38.Russell MS, Iskandar M, Mykytczuk OL, Nash JH, Krishnan L, Sad S. A reduced antigen load in vivo, rather than weak inflammation, causes a substantial delay in CD8+ T cell priming against Mycobacterium bovis (bacillus Calmette-Guerin) J.Immunol. 2007;179:211–220. doi: 10.4049/jimmunol.179.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J.Immunol. 2007;179:3973–3981. doi: 10.4049/jimmunol.179.6.3973. [DOI] [PubMed] [Google Scholar]
- 40.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 41.Waltermann M, Hinz A, Robenek H, Troyer D, Reichelt R, Malkus U, Galla HJ, Kalscheuer R, Stoveken T, von Landenberg P, Steinbuchel A. Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Molecular Microbiology. 2005;55:750–763. doi: 10.1111/j.1365-2958.2004.04441.x. [DOI] [PubMed] [Google Scholar]
- 42.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 43.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 44.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat.Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 45.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat.Rev.Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 46.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, Mathew B, Schmidt RR, Lunt SJ, Williams KJ, Stratford IJ, Harris AL, Cerundolo V. Utilizing the adjuvant properties of CD1d–dependent NK T cells in T cell-mediated immunotherapy. J.Clin.Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Aseguinolaza G, Kaer LVan, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J.Exp.Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Youn HJ, Ko SY, Lee KA, Ko HJ, Lee YS, Fujihashi K, Boyaka PN, Kim SH, Horimoto T, Kweon MN, Kang CY. A single intranasal immunization with inactivated influenza virus and α-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25:5189–5198. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 49.Huang YX, Chen A, Li XM, Chen ZW, Zhang WY, Song Y, Gurner D, Gardiner D, Basu S, Ho DD, Tsuji M. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, α-galactosylceramide. Vaccine. 2008;26:1807–1816. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, Yamamura T, McMahon-Pratt D. Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. European Journal of Immunology. 2008;38:706–719. doi: 10.1002/eji.200737660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, Schaible UE. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d–restricted T cells. Proc.Natl.Acad.Sci.U.S.A. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d–restricted natural killer T cell activation during microbial infection. Nat.Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 53.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-γ, and kill intracellular bacteria. PLoS Pathog. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. Journal of Immunology. 2008;181:2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- 55.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J.Exp.Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sousa AO, Mazzaccaro RJ, Russell RG, Lee FK, Turner OC, Hong S, Kaer LVan, Bloom BR. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc.Natl.Acad.Sci.U.S.A. 2000;97:4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, Nakayama T, Taniguchi M, Saito A. Minimal contribution of Vα14 natural killer T cells to Th1 response and host resistance against mycobacterial infection in mice. Microbiol.Immunol. 2002;46:207–210. doi: 10.1111/j.1348-0421.2002.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 58.Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T, Taniguchi M. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis (Edinb.) 2002;82:97–104. doi: 10.1054/tube.2002.0331. [DOI] [PubMed] [Google Scholar]
- 59.Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infection and Immunity. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan YF, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: Costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. Journal of Immunology. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winau F, Weber S, Sad S, Diego Jde, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-γ signaling dramatically enhances CD4+ and CD8+ T cell memory. J.Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 63.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 64.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J.Exp.Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taraban VY, Martin S, Attfield KE, Glennie MJ, Elliott T, Elewaut D, Van Calenbergh S, Linclau B, Shamkhani AAl. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J.Immunol. 2008;180:4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 66.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, Ndonye RM, Howell AR, Yuan W, Cresswell P, Chang Y, Illarionov PA, Besra GS, Porcelli SA. Kinetics and cellular site of glycolipid loading control the outcome of Natural Killer T cell activation. Immunity. 2009 doi: 10.1016/j.immuni.2009.03.022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enomoto N, Nagata T, Suda T, Uchijima M, Nakamura Y, Chida K, Nakamura H, Koide Y. Immunization with dendritic cells loaded with α-galactosylceramide at priming phase, but not at boosting phase, enhances cytotoxic T lymphocyte activity against infection by intracellular bacteria. FEMS Immunol.Med.Microbiol. 2007;51:350–362. doi: 10.1111/j.1574-695X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.