Abstract
X-band and Q-band electron paramagnetic resonance (EPR) spectroscopic techniques were used to investigate the structure and dynamics of cholesterol containing phospholipid bicelles based upon molecular order parameters (Smol), orientational dependent hyperfine splittings and line shape analysis of the corresponding EPR spectra. The nitroxide spin label 3-β-doxyl-5-α-cholestane (cholestane) was incorporated into DMPC/DHPC bicelles to report the alignment of bicelles in the static magnetic field. The influence of cholesterol on aligned phospholipid bicelles in terms of ordering, the ease of alignment, phase transition temperature have been studied comparatively at X-band and Q-band. At a magnetic field of 1.25 T (Q-band), bicelles with 20 mol% cholesterol aligned at a much lower temperature (313 K), when compared to 318 K at a 0.35 T field strength for X-band, showed better hyperfine splitting values (18.29 G at X-band vs. 18.55 G at Q-band for perpendicular alignment and 8.25 G at X-band vs. 7.83 G at Q-band for the parallel alignment at 318 K) and have greater molecular order parameters (0.76 at X-band vs. 0.86 at Q-band at 318 K). Increasing cholesterol content increased the bicelle ordering, the bicelle-alignment temperature and the gel to liquid crystalline phase transition temperature. We observed that Q-band is more effective than X-band for studying aligned bicelles, because it yielded a higher ordered bicelle system for EPR spectroscopic studies.
Keywords: Phospholipids, X-band, Q-band, EPR, cholestane, molecular order parameter, hyperfine splitting
1. Introduction
Cholesterol is an essential component of biological membranes (Yeagle, 1985). The composition of cholesterol varies in the range of 10 – 50 mol% in a variety of different biological membranes (Pasenkiewicz-Gierula et al., 2000). Cholesterol is needed for proper cell growth, function and stability (Yeagle, 1985; Kurad et al., 2004). It is also implicated in many diseases like heart disease, stroke, and Alzheimer disease etc. (Borroni et al., 2003; Mirnov et al., 2000). Therefore, studies focusing on cholesterol-lipid interactions are needed to better understand the effect of cholesterol on the organization of the membrane. Different techniques have been employed in the past to study the effect of cholesterol on model membrane systems such as electron paramagnetic resonance (EPR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, Fluorescence, X-ray diffraction, differential scanning caloriemetry (DSC), Fourier transform infrared (FTIR) spectroscopy, neutron diffraction and thermal analysis etc. (Yeagle, 1985; Kurad et al., 2004; Nussair and Lorigan, 2005; Lu et al., 2004; Dave et al., 2005; Aussenac et al., 2003; McMullen et al., 1993; Leonard et al., 2001; Rowe and Neal, 2003; McMullen et al., 2003).
Magnetically aligned phospholipid bilayers are an excellent model membrane system for NMR and EPR spectroscopic studies (Nussair and Lorigan, 2005; Lu et al., 2004; Aussenac et al., 2003). Aligning bicelles in the magnetic field offers several advantages to increase both the spectral resolution and the signal-to-noise ratio revealing unique structural and dynamical information when compared to unoriented samples (Aussenac et al., 2003). Bicelles are formed by mixing a long-chain phospholipid, such as dimyristoyl phosphatidylcholine (DMPC) with short chain detergent such as dihexanoyl phosphatidylcholine (DHPC) (Vold and Prosser, 1996). The magnetic alignment of the bicelles depends on several factors such as the magnetic susceptibility anisotropy tensor (Δχ) of the phospholipids, the strength of the magnetic field, the molar ratio of the long and short chain phospholipids (q-ratio), the temperature of the system and the types of the lanthanide ions used (Prosser et al., 1998). The sign and magnitude of Δχplays a major role in the alignment of the bicelles (Sanders et al., 1994). Normally the bicelles align with their bicelles normal perpendicular to the direction of the static magnetic field due to their negative Δχ value. However, at lower magnetic fields used in X-band and even for Q-band EPR, the phospholipid bilayers do not fully align at the perpendicular orientation without the addition of alignment reagents. The addition of Dy3+which has a large negative Δχ value is needed for perpendicular alignment. Conversely, the addition of Tm3+ or Yb3+ with a large positive Δχ value flips the bicelle by 90º, such that the membrane normal is parallel to the direction of the static magnetic field (Prosser et al., 1998; Cardon et al., 2001).
Previous studies in our lab have shown that magnetically aligned phospholipid bilayers doped with either Dy3+, Tm3+ or Yb3+ have been successfully aligned at perpendicular and parallel orientations with respect to the magnetic field (Nussair and Lorigan, 2005; Lu et al., 2004; Cardon et al., 2001; Inbaraj et al., 2004; Tiburu et al., 2004). Nussair et al. and Lu et al. studied the effect of cholesterol on bicelles using EPR spectroscopy with a series of 5-, 7-, 12-and 16-doxyl stearic acid and cholestane spin probes and also with solid-state 2H NMR spectroscopy (Nussair and Lorigan, 2005; Lu et al., 2004). Both of these EPR studies by Nussair et al. and Lu et al. were carried out at X-band.
The purpose of this paper is to have a comparative study of the effects of cholesterol on the bicelle model membrane systems using magnetically alignable DMPC/DHPC phospholipids at both X-band (9.5 GHz, 0.35 T) and Q-band (35 GHz, 1.25 T). EPR spectroscopy is highly sensitive to the rate of motion and degree of organization of the phospholipids due to exact matching of characteristic time scale of the nitroxide spin-label to the rates of molecular rotation of the lipids within the membranes (Moser et al., 1989). This paper attempts to effectively characterize a cholesterol-bicelle system using the corresponding molecular order parameters, hyperfine splitting values, and the change in the spectral line shapes of cholestane containing bicelles at different temperatures. The X-band versus Q-band experimental results will be compared to better understand the effectiveness of the two magnetic fields and frequencies.
2. Materials and Methods
2.1. Materials
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC), and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N- [methoxy-(polyethylene glycol)-2000] (PEG2000PE) were purchased from Avanti Polar Lipids (Alabaster, AL). The cholesterol was obtained from Avocado Research Chemicals (Ward Hill, MA). Thulium (III) chloride hexahydrate, dysprosium (III) chloride hexahydrate, 3β-doxyl-5-α-cholestane [cholestane] and N- [2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid](HEPES) were obtained from Sigma-Aldrich (St. Louis, MO). Deuterium-depleted water was obtained from Isotec (Miamisburg, OH). All phospholipids were dissolved in chloroform and stored at −20°C prior to use. Aqueous solutions of (N-2 [Hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid]) (HEPES) buffer and lanthanide ions in deuterium-depleted water were prepared fresh each day.
2.2 Sample Preparation
The standard bicelle sample consists of 25% (w/w) DMPC/DHPC phospholipids (molar ratio 3.5:1) in HEPES buffer along with PEG200-PE, cholestane, and various amounts of cholesterol (0, 5, 10, 15 and 20 mol% with respect to DMPC). For bicelles with 10 mol% cholesterol, DMPC, DHPC, PEG2000-PE, cholesterol and the spin-label cholestane were mixed together in a flask at the molar ratios of 3.5/1/0.035/0.35/0.0196 respectively. Cholesterol concentrations were used from 0 to 20 mol% in 5 mol% increments. The chloroform in the flask was evaporated under a stream of nitrogen gas and the flask was placed under high vacuum overnight. The following day, 100 mM HEPES buffer at pH 7 was added to the flask so that the amount of lipid in the sample was 25% (wt%). The sample was vortexed and chilled on ice bath periodically until all of the lipids were solublized. In contrast, for the samples containing high cholesterol content (15 and 20 mol%) samples were vortexed at room temperature without ice and then kept in ice bath for 20 minutes until the sample became clear and homogeneous. All samples were then sonicated with a Fisher Scientific FS30 bath sonicator (Florence, KY) for about 30 min with the heater turned off and ice added to the bath. The sample was subjected to 4 freeze thaw (at 77 K in liquid nitrogen) cycles at room temperatures to homogenize the sample and remove air bubbles if any. Finally, at 0°C (ice bucket), a 20 mol% (molar ratio to DMPC) of either DyCl3 or TmCl3 was added and mixed into the EPR sample. The total mass of each sample was approximately 200 mg. For X-band EPR experiments, 50 μL of sample was drawn into a 1 mm inner diameter capillary tube via a syringe. Both ends of the capillary tube were sealed with a Cristoseal (Fisher Scientific) and placed inside standard quartz EPR tubes (Wilmad, 707-SQ-250M) filled with the mineral oil. For Q-band measurements, the bicelles samples were placed in quartz capillaries, with an inner diameter of 0.3 mm (CV3040) from Vitro Com (Mountain Lakes, NJ). The ends of the capillaries were sealed with Cristoseal. The capillary with the sample was introduced into a Vitro Com quartz tube with a 1.5mm i.d. (CV1518Q) and sealed at one end. The typical sample volume inside the Q-band EPR tubes was about 3–5μL.
2.3. EPR spectroscopy
For X-band EPR studies, experiments were carried out on a Bruker EMX CW-EPR spectrometer consisting of and ER041xG microwave bridge and ER4119-HS cavity coupled with a BVT 3000 nitrogen gas temperature controller (temperature stability ± 0.2 K). Each spin labeled EPR spectrum was acquired by taking a 42-s field scan with a center field of 3370 G, a sweep width of 100 G, a microwave frequency of 9.35 GHz, a modulation frequency of 100 kHz, a modulation amplitude of 1 G, and a microwave power of 2 mW.
For Q band EPR studies, spectra were recorded at a microwave frequency of 34.18 GHz on a Bruker EMX Q-band EPR spectrometer consisting of an ER051QG microwave bridge and a TE01X-mode cylindrical Q-band cavity resonator (ER 5106) coupled with a CF935 dynamic continuous flow cryostat. Each spin-labeled EPR spectrum was acquired by taking a 42-s field scan with a center field of 12,120 G, a sweep width of 100 G, a modulation frequency of 100 kHz, and a microwave power of 2 mW.
2.4. Molecular order parameter (Smol) calculations
The EPR spectra of a nitroxide spin label consists of three lines as a result of an unpaired S=1/2 electron coupled to a 14N (I=1) nucleus. In a Cartesian coordinate system, the magnetic principal axes have the x-axis along the nitroxide N-O bond, the z-axis is along the 2p π orbital of the nitrogen and the y-axis is perpendicular to the xz plane. The order parameter S33 can be determined by measuring the resultant hyperfine splitting of the aligned spectra using the following equation:
| (1) |
Where A|| and A⊥ are the observed hyperfine splitting values measured between the MI = +1 and 0 spectral lines from the parallel and perpendicular oriented EPR spectra, respectively. The tensoral values Axx = 6 G and Ayy = 6 G and Azz = 32 G were taken from a spectrum previously reported for the cholestane spin label (Sanders et al., 1994; Moser et al., 1989). aN = (Axx+Ayy+Azz)/3 represents the isotropic hyperfine splitting constant and is sensitive to solvent polarity. aN′= (A||+ 2A⊥)/3 is the solvent polarity correction factor for the hyperfine splitting. The molecular order parameter (Smol) corresponding to the long molecular axis can be calculated from the following equation:
| (2) |
Where θ denotes the angle between the long molecular axis and the 2p π orbital of the nitrogen. For the cholestane spin label, θ = 90°and Smol = −2S33
3. Results and Discussions
EPR spectra of randomly dispersed bicelles are needed to directly compare the subsequent parallel and perpendicular aligned bicelle EPR spectra. The orientation and motional behavior of the spin labeled bicelles in EPR spectroscopy can be defined with a simple molecular coordinate system. In an aligned spectrum, the orientation that the spin label makes with respect to the magnetic field and the motion about its molecular axis will determine the corresponding hyperfine splitting. The principal hyperfine tensors for the cholestane spin probe are Axx = 6 G, Ayy = 6 G and Azz = 32 G (Lapper et al., 1972; Smith and Butler, 1976). At the parallel orientation, the normal to the bicelles and the long molecular axis of cholestane are parallel to the static magnetic field and the EPR spectrum consists of 3 lines separated by 6 G (Ayy). If the bicelles are aligned with their bilayer normal perpendicular to the magnetic field, the hyperfine splitting should equal the average of the value of Azz and Axx, [(Azz+Axx)/2 = 19 G].
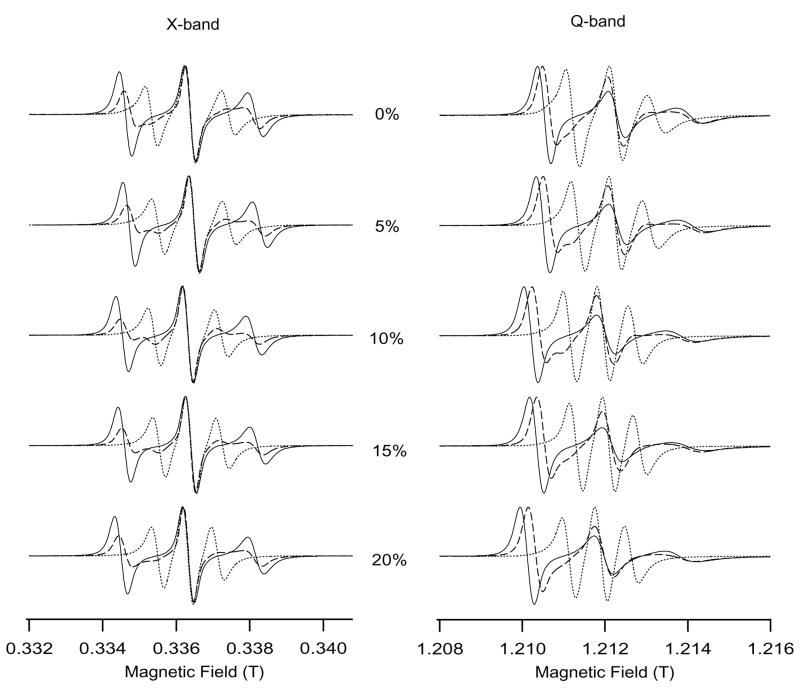
Figure 1 shows the superimposed EPR spectra of bicelles of varying cholesterol concentration (0, 5, 10, 15 and 20 mol%) in the liquid crystalline phase at 318 K (dashed line: unaligned bicelles, solid line: Dy3+- doped perpendicular aligned bicelles and dotted line: Tm3+-doped parallel aligned bicelles). The corresponding intensities of the EPR spectra were normalized to 1. As shown in Figure 1, there are variations in the line shapes and hyperfine splitting values of bicelle spectra with different amounts of cholesterol.
Figure 1.
A comparison between X-band and Q-band EPR spectra of cholestane incorporated into magnetically aligned bicelles at 318 K as a function of cholesterol mol% (0,5,10,15,20) with respect to DMPC. For the superimposed EPR spectra, the dashed line represents the randomly dispersed spectra (without lanthanides), solid line represents the Dy3+-doped perpendicular-aligned spectra and the dotted line shows the Tm3+-doped parallel-aligned spectra with respect to the static magnetic field.
The cholesterol-bicelle spectra at two frequencies (9.5 GHz at X-band and 35 GHz at Q-band) look fairly similar in parallel orientation; however, they appear quite different in perpendicular orientation. At X-band, the MI = −1 linewidth is the broadest followed by the MI = +1 and MI = 0 lines. However, at Q-band, the spectral linewidth increases according to the following: MI = +1 < MI = 0 < MI = −1. The asymmetric line broadening at Q-band results from relaxation theory parameters associated with the cross product of the g-tensor and hyperfine tensor anisotropies (Kivelson 1960; Stone et. al. 1965; Lapper et al., 1972). At the perpendicular orientation, the rotation of the cholestane spin label about its long steroid axis involves large modulation of the g-value at Q-band unlike at X-band where the hyperfine anisotropy tensor has the major modulating effect. This indicates that any factors that decrease the rotational correlation rate of the spin label about its molecular axis would result in large asymmetric line widths.
The average rotational correlation times calculated from the best-fit experimental simulations (Budil et al., 1996) were 1.8 ns and 3.0 ns at X-band and 2.0 ns, 3.6 ns at Q-band for 0 mol % and 20 mol % cholesterol concentration respectively. These values of rotational correlation times are comparable to Smith and Butler 1976 which reports that the addition of 30 mol% of cholesterol to hydrated egg lecithin film increased the correlation time characteristic of the probe motion from 1.8 ns to 3.8 ns. We observe a decrease in the rotational motion (increased rotational correlation time) as the cholesterol concentration increases. For the same composition of cholesterol-bicelle system, the rotational correlation times at Q-band were longer than at X-band.
Lapper et al, 1972 and Smith and Butler, 1976 have reported that increasing cholesterol content in multibilayers increases the steric hindrance and slows down the amplitude as well as the rotational rate of the random walk motion of the cholestane spin label. This leads to increased rotational correlation time and hence results the line broadening effect as observed in Figure 1. The decreased rotational rate due to added cholesterol manifests a major effect on the line shapes of the perpendicular aligned samples (more asymmetric line broadening effect) and a relatively smaller effect on the line shapes of parallel aligned samples (less line broadening effect). For perpendicular oriented egg-lecithin multibilayers, Lapper et al., 1972 have shown that the amplitude of the random walk can vary from 46° at 0 mol % cholesterol to 17° at 55 mol% cholesterol, whereas in the parallel direction, the random walk amplitude limits only to a very narrow range (<10°). This is the reason why the lineshapes of the spin label incorporated into parallel aligned bicelles look fairly similar at both X-band and Q-band unlike the perpendicular aligned samples.
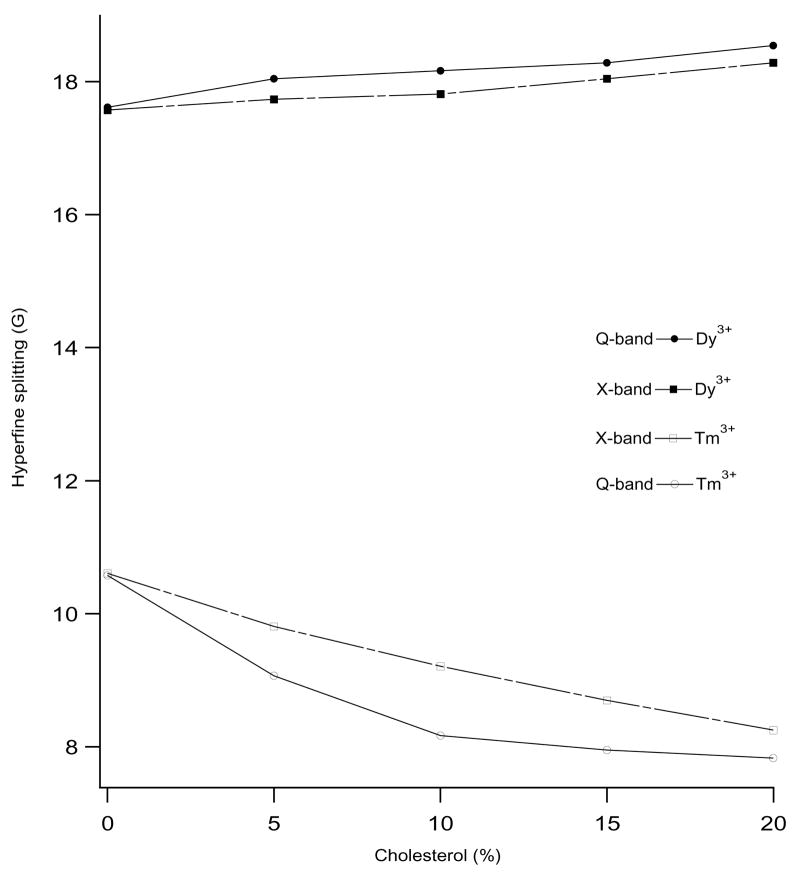
Figure 2 shows the variation of hyperfine splitting as a function of cholesterol concentration in bicelles at 318 K from the EPR spectra in Figure 1. The hyperfine splitting values of Dy3+-doped perpendicular aligned spectra increases from 17.58 G to 18.29 G at X-band and from 17.62 G to 18.55 G at Q-band as the cholesterol content in bicelles increase from 0 to 20 mol%. Conversely, the hyperfine splitting values of Tm3+-doped bicelles decreases from 10.61 G to 8.25 G at X-band and from 10.58 G to 7.83 G at Q-band for the same increase of cholesterol mol%. The rigid limit hyperfine splitting values for perpendicular and parallel orientations are 19 G and 6 G respectively for the cholestane spin probe (Cardon et al., 2001; Lapper et al., 1972; Smith and Butler, 1976). If the hyperfine splitting values are closer to these rigid limit values, then it is an indication of more ordering and better overall bicelle alignment. Thus, in both cases, (Tm3+-doped parallel aligned and Dy3+-doped perpendicular aligned bicelles), the results show that the hyperfine splitting values at Q-band are closer to the rigid limit values suggesting that the bicelle ordering and alignment in the Q-band is much more effective than the corresponding X-band. The difference in the alignment between X-band and Q-band data is due to the increase in the magnetic field strength at Q-band (1.25 T) when compared to X-band (0.35 T), since the bicelle alignment depends upon the square of the magnetic field (Mangels et al., 2001; Sanders and Prosser, 1998). The experimental hyperfine splitting for the perpendicular aligned bicelle matches very closely with the rigid limit values, but there is a slight deviation in the experimental hyperfine splitting values (slightly higher than the rigid limit values) for the parallel-aligned spectra. This may be due to two reasons: the long molecular axis of the cholestane spin label may not be exactly parallel to the bicelle normal and the spin label undergoes a random walk motion perpendicular to the long molecular axis within the limit of a cone (Lapper et al., 1972; Mailer et al., 1974).
Figure 2.
Diagram showing the dependence of hyperfine splitting values on the concentration of cholesterol in the DMPC/DHPC phospholipids bilayers at 318 K. The filled circles and squares represent the hyperfine splitting values of Dy3+-doped perpendicular aligned bicelles spectra and open circles and squares represent the hyperfine splitting values of Tm3+-doped parallel aligned bicelles spectra. The solid lines and broken lines represent the hyperfine splitting values measured at Q-band and X-band, respectively.
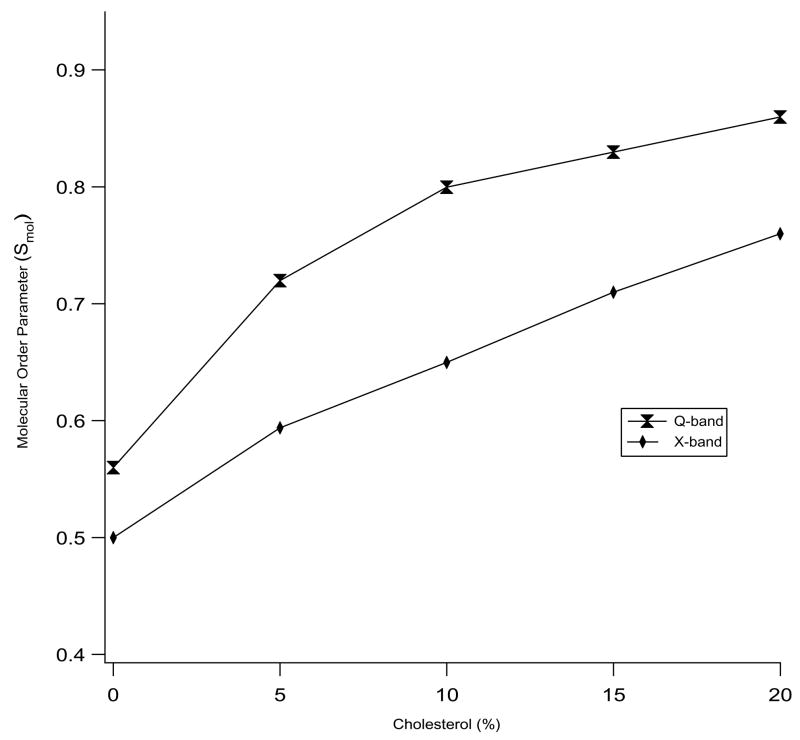
The interaction between the cholesterol and the phospholipids can be defined in terms of a molecular order parameter (Smol). An ensemble of molecules produces Smol = 0 for unrestricted motions of every individual molecule whereas Smol = 1 implies that all the molecules are perfectly aligned and motionally restricted to only one direction. Figure 3 displays the corresponding molecular order parameters (Smol) of the bicelles with different amounts of cholesterol at 318 K. The ordering of the bicelle increases as the cholesterol concentration is raised from 0 to 20 mol %. The increase in the order parameter is more pronounced at Q-band (increases from 0.56 to 0.86) than at X-band (increases from 0.50 to 0.76) as the cholesterol concentration is raised from 0 to 20 mol%. The increase in the bicelles ordering indicates that the rigid sterol ring of cholesterol limits the motion of the lipid molecules in the membrane by replacing chain-chain interactions of phospholipids with the chain-sterol interaction (Schreier-Muccillo et al., 1973). Molecular dynamics simulations study of DMPC-cholesterol bilayers containing 22 mol% cholesterol in the liquid crystalline phase by Róg and Pasenkiewicz-Gierula (2001) showed an overall increase in the molecular order parameter and a decrease in the number of gauche rotamers/chain for DMPC’s located near the cholesterol molecules.
Figure 3.
Molecular order parameter (Smol) values of X-band and Q-band EPR spectra at 318 K as a function of cholesterol concentration in bicelles.
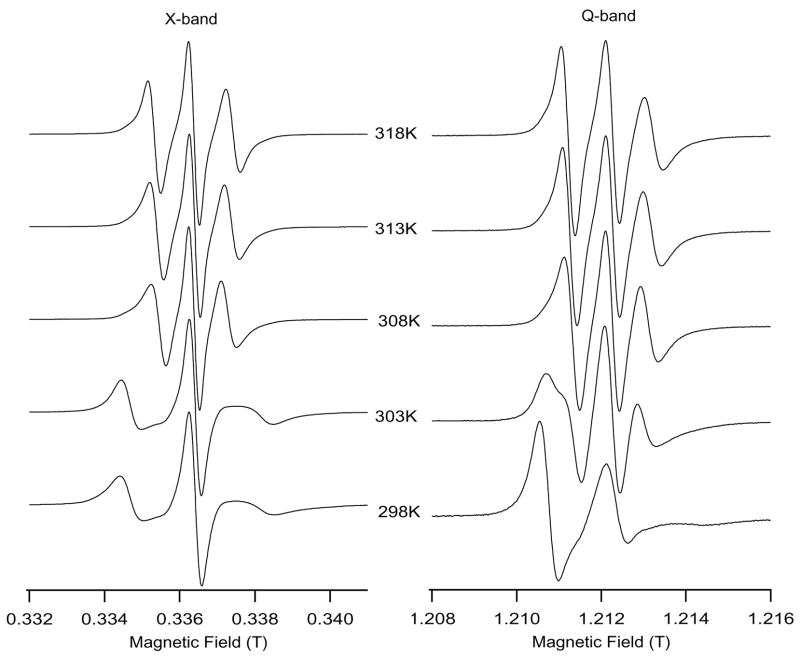
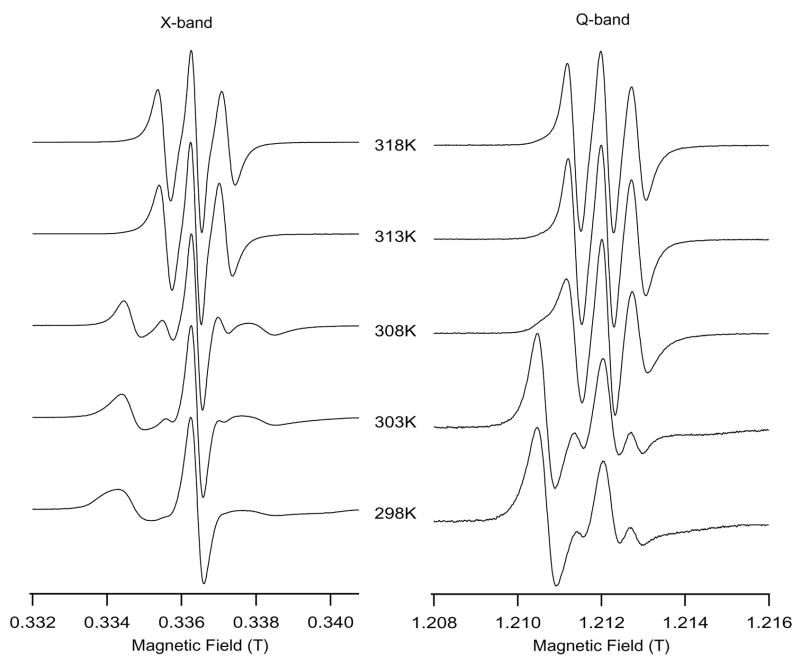
Besides increasing the molecular ordering of the phospholipid acyl chains, cholesterol also increases the minimum alignment temperature of the DMPC/DHPC bicelles and the gel to liquid crystalline phase-transition temperature. Figure 4A reveals Tm3+-doped perpendicular aligned bicelle spectra at X-band and Q-band without cholesterol. Figure 4A clearly shows that Tm3+-doped bicelles without cholesterol are well aligned at the temperature of 308 K and above at X-band. However, the same bicelle sample at Q-band is nearly aligned at 303 K (hyperfine splitting = 10.01 G, much lower than the isotropic hyperfine splitting value, 14.67 G). The lower temperature alignment of the bicelles at Q-band is attributed to the high magnetic field effect of Q-band (1.25 T) when compared to X-band (0.35 T). The alignment temperature at 308 K at X-band is consistent with earlier results in the literature (Cardon et al., 2003); which reports that bicelles start to align in the nematic liquid crystalline phase at 307 K. Similarly nearly aligned spectra at 303 K at Q-band is in agreement with the report of Inbaraj et al., (2004).
Figure 4.
Figure 4A: A comparison of Tm3+-doped bicelle EPR spectra at X-band and Q-band without cholesterol. The bicelle sample temperature was ramped from 298 K to 318 K for 16 minutes time in the static magnetic field (0.64 T for X-band and 1.25 T for Q-band) and allowed to equilibrate for 10 minutes at 318 K. The bicelle spectra were first recorded at 318 K. Then the temperature was lowered to 313, 308, 303 and 298 K, and the spectra were taken after a 5 minutes equilibration time in each step.
Figure 4B: X-band and Q-band EPR spectra of Tm3+-doped bicelles containing 15 mol% cholesterol as a function of temperature
Figure 4B shows the Tm3+-doped perpendicular aligned spectra of bicelles containing 15 mol% cholesterol. The Tm3+-doped bicelles with 15 mol% cholesterol starts to align partially at 308 K and aligns completely at 313 K at X-band, whereas at Q-band partial alignment starts at 303 K and alignment is complete at 308 K. Aligned EPR bicelle spectra are observed in the liquid crystalline phase and not in the gel phase (Cardon et al., 2003). An increase in bicelle alignment temperature observed in Fig. 4B when compared to Fig. 4A is due to the increased ordering of the bicelles with added cholesterol (15 mol% as compared to 0 mol%). Thus, cholesterol increases the bicelle alignment temperature and phase transition temperature from the gel to nematic liquid crystalline phase. The bicelles alignment temperature increased to 318 K at X-band and 313 K at Q-band for Tm3+-doped bicelles sample with 20 mol% cholesterol (Data not shown).
Differential scanning calorimetry studies by Ohvo-Rekilä et al. (2002) on cholesterol/phosphocholine mixtures report that cholesterol increases the phase transition temperature (Tm) if the saturated phosphocholine hydrocarbon chain length is equal to 16 or less and decreases the (Tm) if the chain length is 18 and longer (Ohvo-Rekilä et al., 2002). The data indicate that cholesterol interacts to a greater extent with phosphocholines of the same hydrocarbon chain length. Thus, cholesterol acts more specifically as a membrane stabilizing/organizing agent (McMullen et al., 1993; Ohvo-Rekilä et al., 2002) and its stabilizing effect is uniform throughout the DMPC hydrocarbon chain (Lu et al., 2004). Solid-state 2H NMR and EPR spectral studies utilizing the cholestane and the 5-, 7-, 12- and 16-doxyl stearic acid spin-label in the cholesterol-bicelle system also showed an increase in the phase transition temperature with the addition of cholesterol (Dave et al., 2003; Lu et al., 2004; Nussair and Lorigan, 2005). Our EPR bicelle results agree well with the results of these studies.
A potential drawback in working at X-band is the use of high concentration of the lanthanide ions that are necessary to magnetically align the phospholipids bilayers at the low magnetic fields used on a X-band EPR spectrometer. This high lanthanide concentration may result in protein-lanthanide interaction, possibly causing paramagnetic line broadening (Prosser et al., 1998). Inbaraj et al. (2004) compared the minimum amounts of lanthanide ions required to magnetically align bicelles at X and Q-band fields. This report suggests that at Q-band, 2.5 mol% of Dy3+ and 5 mol% of Tm3+ with respect to DMPC is enough for proper bicelle alignment, whereas at X-band 10 mol% Dy3+ and 20 mol% of Tm3+ is required for the complete alignment in perpendicular and parallel orientations with respect to the static magnetic field.
The use of high magnetic field has two major advantages for studying aligned membrane systems. First, membranes are well aligned and therefore, provide resonant peaks with higher signal to noise ratios and better spectral resolution (Freed, 2000). Second, for a given diffusion rate of the cholestane spin label, two different frequencies at X-band and Q-band respond with different averaging effect. Therefore, high frequency ESR spectra acts as faster snapshot of the dynamics (Borbat et al., 2001, Gaffney et al., 1998), which yields better information on the motion and the environment of the spin label. Besides, the orientational resolution of the nitroxide spectrum is significantly improved at the higher magnetic field.
In conclusion, we found that Q-band EPR spectroscopy is more useful for studying the cholesterol-bicelle membrane system when compared to X-band. Due to higher magnetic field effects at Q-band, lipids were better aligned and the cholestane spin label possessed a higher degree of ordering. We observed an increase in the molecular order parameter (Smol) of the cholestane spin label at Q-band when compared to X-band at all concentrations of the cholesterol-bicelle system studied in our experiments. The resulting hyperfine splitting values of the parallel or perpendicular aligned spectra matched closer to the rigid limit hyperfine splitting values for aligned spectra at Q-band than at X-band. Additionally, bicelles were found to align even at lower temperatures at Q-band. Increasing cholesterol concentration increased the membrane stabilizing effect as observed by the higher molecular order parameter values. At 318 K, Smol value changed from 0.56 to 0.86 at Q-band, whereas it changed from 0.51 to 0.76 at X-band when the cholesterol concentration increased from 0 mol% to 20 mol% in the bicelle.
Acknowledgments
We greatly appreciate the financial support from National Institute of Health Grant GM60259-01 and a National Science Foundation Award CHE-0645709 for this work
Abbreviations
- DMPC
1,2 Dimyristoyl-sn-glycero-3-phosphocholine
- DHPC
1,2-dihexanoyl-sn-glycero-3-phosphocholine
- HFS
hyperfine splitting
- cholestane
3-β-doxyl-5-α-cholestane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aussenac F, Laguerre M, Schmitter JM, Dufourc EJ. Detailed structure and dynamics of bicelle phospholipids using selectively deuterated and perdeuterated labels. 2H NMR and Molecular Mechanics Study. Langmuir. 2003;19:10468–10479. [Google Scholar]
- Borbat PP, Costa-Filho AJ, Earle KA, Moscicki JK, Freed JH. Electron Spin Resonance in Studies of Membranes and Proteins. Science. 2001;291:266–269. doi: 10.1126/science.291.5502.266. [DOI] [PubMed] [Google Scholar]
- Borroni B, Pettenati C, Bordonali T, Akkawi N, Luca MD, Padovani A. Serum cholesterol levels modulate long-term efficacy of cholinesterase inhibitors in Alzherimer disease. Neuroscience Lett. 2003;343:213–215. doi: 10.1016/s0304-3940(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Budil DE, Lee S, Saxena S, Freed JH. Nonlinear-least-square analysis of slow motion EPR spectra in one and two dimensions using a modified Levenberg-Marquardt Algorithm. J Magn Reson Series A. 1996;120:155–189. [Google Scholar]
- Cardon TB, Tiburu EK, Lorigan GA. Magnetically aligned phospholipid bilayers in weak magnetic fields: optimization, mechanism, and advantages for X-band EPR studies. J Magn Reson. 2003;161(1):77–90. doi: 10.1016/s1090-7807(02)00109-x. [DOI] [PubMed] [Google Scholar]
- Cardon TB, Tiburu EK, Padmanabhan A, Howard KP, Lorigan GA. Magnetically aligned phospholipid bilayers at the parallel and perpendicular orientations for X-band spin-label EPR studies. J Am Chem Soc. 2001;123:2913–2914. doi: 10.1021/ja005574i. [DOI] [PubMed] [Google Scholar]
- Chiu SW, Jakobsson E, Mashl J, Scott HL. Cholesterol-induced modifications in lipid bilayers: a simulation study. Biophys J. 2002;83:1842–1853. doi: 10.1016/S0006-3495(02)73949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong PL. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc Natl Acad Sci USA. 1994;91:10069–10073. doi: 10.1073/pnas.91.21.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MI, Goni FM, Alonso A, Marsh D. Domain formation in sphingomyelin/cholesterol mixed membranes studied by spin-label electron spin resonance spectroscopy. Biochemistry. 2005;44:4911– 4918. doi: 10.1021/bi0474970. [DOI] [PubMed] [Google Scholar]
- Dave PC, Tiburu EK, Nusair NA, Lorigan GA. Calculating order parameter profiles utilizing magnetically aligned phospholipid bilayers for 2H solid-state NMR studies. Solid State Nucl Magn Reson. 2003;24:137–149. doi: 10.1016/S0926-2040(03)00052-3. [DOI] [PubMed] [Google Scholar]
- Dave PC, Nusair NA, Inbaraj JJ, Lorigan GA. Electron paramagnetic resonance studies of magnetically aligned phospholipids bilayers utilizing a phospholipid spin label: the effect of cholesterol. Biochem Biophys Acta. 2005;714:41– 51. doi: 10.1016/j.bbamem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Dzikovski B, Earle K, Pachtchenko S, Freed J. High-field ESR on aligned membranes: A simple method to record spectra from different membrane orientations in the magnetic field. J Magn Reson. 2006;179:273–279. doi: 10.1016/j.jmr.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Freed JH. New Technologies in Electron Spin Resonance. Annu Rev Phys Chem. 2000;51:655–689. doi: 10.1146/annurev.physchem.51.1.655. [DOI] [PubMed] [Google Scholar]
- Gaffney BJ, Marsh D. High-frequency spin-label EPR of nonaxial lipid ordering and motion in cholesterol-containing membranes. Proc Natl Acad Sci USA. 1998;95:2940–12943. doi: 10.1073/pnas.95.22.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsäβ C, Lindahl E, Edholm O. Molecular dynamics simulations of phospholipid bilayer with cholesterol. Biophys J. 2003;84:2192–2206. doi: 10.1016/S0006-3495(03)75025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KP, Opella SJ. High-resolution solid-state NMR spectra of integral membrane proteins reconstituted into magnetically oriented phospholipd bilayers. J Magn Reson B. 2000;112:7052–7058. doi: 10.1006/jmrb.1996.0116. [DOI] [PubMed] [Google Scholar]
- Inbaraj JJ, Nusair NA, Lorigan GA. Investigating magnetically aligned phospholipid bilayers with EPR spectroscopy at Q-band (35 GHz): optimization and comparison with X-band (9 GHz) J Magn Reson. 2004;171(1):71–79. doi: 10.1016/j.jmr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Jedlovszky P, Mezei M. Effect of cholesterol on the properties of phospholipid membranes. 1 structural features. J Phys Chem B. 2003;107:5311–5321. doi: 10.1021/jp051832s. [DOI] [PubMed] [Google Scholar]
- Kessel A, Ben-Tal N, May S. Interactions of cholesterol with lipid bilayers: the preferred configuration and fluctuations. Biophys J. 2001;81:643–658. doi: 10.1016/S0006-3495(01)75729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelson D. Theory of ESR linewidths of Free Radicals. J Chem Phys. 1960;33:1094–1106. [Google Scholar]
- Kurad D, Jeschke G, Marsh D. Lateral ordering of lipid chains in cholesterol-containing membranes: high-field spin-label EPR. Biophys J. 2004;86:264–271. doi: 10.1016/S0006-3495(04)74102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper RD, Paterson SJ, Smith ICP. A spin label study of the influence of cholesterol on egg lecithin multibilayers. Can J Biochem. 1972;50:969–981. [PubMed] [Google Scholar]
- Leonard A, Escrive C, Laguerre M, Pebay-Peyroula E, Neri WPT, Katsaras J, Dufourc EJ. Location of cholesterol in DMPC membranes. A comparative study by neutron diffraction and molecular mechanics simulation. Langmuir. 2001;17:2019–2030. [Google Scholar]
- Lu JX, Caporini MA, Lorigan GA. The Effects of Cholesterol on Magnetically Aligned Phospholipid Bilayers: A Solid-State NMR and EPR Spectroscopy Study. J Magn Reson. 2004;168:18–30. doi: 10.1016/j.jmr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Mangels ML, Harper AC, Smirnov AI, Howard KP, Lorigan GA. Investigating magnetically aligned phospholipid bilayers with EPR spectroscopy at 94 GHz. J Magn Reson. 2001;151(2):253–259. doi: 10.1006/jmre.2001.2368. [DOI] [PubMed] [Google Scholar]
- McMullan RK, McElhaney RN. Physical studies of cholesterol-phospholipid interactions. Curr Opin Colloid Interface Sci. 1996;1:83–90. [Google Scholar]
- McMullen TP, Lewis RN, McElhaney RN. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993;32:516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- McMullen TP, Lewis RN, McElhaney RN. Comparative differential scanning calorimetric and FTIR and 31P-NMR spectroscopic studies of the effects of cholesterol and androstenol on the thermotropic phase behavior and organization of phosphatidylcholine bilayers. Biophys J. 1994:741–752. doi: 10.1016/s0006-3495(94)80850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov VS, Galyametdinov YG, Ceulemans A, Binnemans K. On the magnetic anisotropy of lanthanide-containing metallomesogens. J Chem Phys. 2000;113(22):10293–10303. doi: 10.1002/1439-7641(20011119)2:11<680::AID-CPHC680>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Moser M, Marsh D, Meier P, Wassmer KH, Kothe G. Chain configuration and flexibility gradient in phospholipid membranes. Comparison between spin-label electron spin resonancee and deuteron nuclear magnetic resonance, and identification of new conformations. Biophys J. 1989;55:111–123. doi: 10.1016/S0006-3495(89)82784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusair NA, Lorigan GA. Investigating the structural and dynamic properties of n-doxylstearic acid in magnetically-aligned phospholipid bilayers by X-band EPR spectroscopy. Chem Phys Lipids. 2005;133(2):151–164. doi: 10.1016/j.chemphyslip.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Ohvo-Rekilä H, Ramstedt B, Leppimäki P, Slotte JP. Cholesterol interactions with phospholipids in membranes. Prog in Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- Pasenkiewicz-Gierula M, Rog T, Kitamura K, Kusumi A. Cholesterol effects on the phosphatidylcholine bilayer polar region: a molecular simulation study. Biophys J. 2000;78:1376–1389. doi: 10.1016/S0006-3495(00)76691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RS, Hwang JS, Vold RR. Magnetically aligned phospholipid bilayers with positive ordering: a new model membrane system. Biophys J. 1998;74:2405–2418. doi: 10.1016/S0006-3495(98)77949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RS, Volkov VB, Shiyanovskaya IV. Novel chelate-induced magnetic alignment of biological membranes. Biophys J. 1998;75(5):2163–2169. doi: 10.1016/S0006-3495(98)77659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róg T, Pasenkiewicz-Gierula M. Cholesterol effects on the phosphatidylcholine bilayer nonpolar region: a molecular simulation study. Biophys J. 2001;81:2190–2202. doi: 10.1016/S0006-3495(01)75867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe BA, Neal SL. Fluorescence Probe Study of Bicelle Structure as a Function of Temperature: Developing a practical bicelle structure model. Langmuir. 2003;19:2039–2048. [Google Scholar]
- Sanders CR, Hare BJ, Howard KP, Prestegard JH. Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules. Prog in NMR Spectrosc. 1994;26:421–444. [Google Scholar]
- Sanders CR, Prosser RS. Bicelles: a model membrane system for all seasons? Structure. 1998;6(10):1227–1234. doi: 10.1016/s0969-2126(98)00123-3. [DOI] [PubMed] [Google Scholar]
- Sanders R, Schonek JP. Characterization of magnetically orientable bilayers in mixtures of dihexanoylphosphatidylcholine and dimyristoylphosphatidicholine by solid-state NMR. Biochemistry. 1992;31:8898–8905. doi: 10.1021/bi00152a029. [DOI] [PubMed] [Google Scholar]
- Schreier-Muccillo S, Marsh D, Duas H, Schneider H, Smith ICP. A spin probe study of the influence of cholesterol on motion and orientation of phospholipids in oriented multibilayers and vescles. Chem Phys Lipids. 1973;10:11–27. doi: 10.1016/0009-3084(73)90037-6. [DOI] [PubMed] [Google Scholar]
- Seelig J. Anisotropic motion in liquid crystalline structures. In: Berliner LJ, editor. Spin Labeling Theory and Applications. Academic Press; New York: 1976. pp. 373–410. [Google Scholar]
- Seelig J, Niederberger W. Two pictures of a lipid bilayer. A comparison between deuterium label and spin-label experiments. Biochemistry. 1974;13(8):1585–1588. doi: 10.1021/bi00705a005. [DOI] [PubMed] [Google Scholar]
- Shin YK, Moscicki JK, Freed JH. Dynamics of phosphatidylcholine-cholesterol mixed model membranes in the liquid crystalline state. Biophys J. 1990;57:445–459. doi: 10.1016/S0006-3495(90)82561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ICP, Butler KW. Oriented lipid systems as model membranes 1976. In: Berliner LJ, editor. Spin Labeling Theory and Applications. Academic Press; New York: pp. 411–453. [Google Scholar]
- Smondyrev AM, Berkowits ML. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: molecular dynamics simulation. Biophys J. 1999;77:2075–2089. doi: 10.1016/S0006-3495(99)77049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TJ, Buckman T, Nordio PL, McConnel HM. Spin-Labeled Biomolecules. Proc Natl Acad Sci, US A. 1965;54:1010–1017. doi: 10.1073/pnas.54.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburu EK, Dave PC, Lorigan GA. Solid-state 2H NMR studies of the effects of cholesterol on acyl chain dynamics of magnetically aligned phospholipid bilayers. Magn Reson Chem. 2004;42:132–138. doi: 10.1002/mrc.1324. [DOI] [PubMed] [Google Scholar]
- Vold RR, Prosser RS. Magnetically oriented phospholipid bilayered micelles for structural studies of polypeptides. Does the ideal bicelle exist? J Magn Reson B. 1996;113:267–271. [Google Scholar]
- Yamaji T, Noda Y, Yamauchi S, Yamauchi J. Multi-frequency ESR study of the polycrystalline phenoxyl radical of α-(3,5-Di-tert-butyl-4-hydroxyphenyl)-N-tert-butylnitrone in the diamagnetic matrix. J Phys Chem A. 2006;110:1196–1200. doi: 10.1021/jp054358z. [DOI] [PubMed] [Google Scholar]
- Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]