Abstract
Objective
Interleukin-8 (IL-8) is a soluble human-specific chemokine implicated in the development of the chronic inflammatory disease atherosclerosis. Recently, we showed that atheroprone hemodynamics induced IL-8 secretion from endothelial cells (ECs) concurrent with increased EC/smooth muscle cell (SMC) VCAM-1 expression in a human hemodynamic coculture model. Despite an IL-8 association with inflammation, we show here that blocking IL-8 activity during atheroprone flow resulted in increased levels of EC/SMC VCAM-1 expression. We tested the hypothesis that IL-8 limits SMC VCAM-1 expression in response to inflammatory stimuli, either atheroprone flow or cytokine interleukin-1β (IL-1β) addition.
Methods and Results
Atheroprone flow increased monocyte adhesion in both EC/SMCs, concurrent with the induction of VCAM-1 protein. VCAM-1 antisera attenuated this response. IL-1β upregulated VCAM-1 in SMCs by 3-fold, a response inhibited by the addition of IL-8 at 24 hours. Neither IL-1β nor IL-8 induced proliferation or migration. Neutralization of the IL-8 receptor, CXCR2, further induced VCAM-1 in the presence of IL-1β, and phospho-p38 was required for NF-κB activation and VCAM-1 expression. Additionally, IL-8 reduced p38 activation and NF-κB activity induced by IL-1β alone.
Conclusions
Together, these findings provide evidence for a novel role whereby IL-8 limits the inflammatory response in ECs/SMCs via VCAM-1 modulation. (Arterioscler Thromb Vasc Biol. 2009;29:725-731.)
Keywords: endothelial, smooth muscle, interleukin-8, VCAM-1, hemodynamics
Initiating phases of atherosclerosis in large arteries are marked by chronic inflammation and are localized to hemodynamically defined geometries where blood flow is both compromised and disturbed. Chemokines and cytokines are locally secreted in “atheroprone” regions and are known to play important roles in driving the inflammatory response (for reviews see Tedgui and Mallat1 and von der Thu¨sen et al2). Endothelial cells (ECs) and smooth muscle cells (SMCs) can both contribute and respond to cytokine production, thus promoting atherosclerosis. Recently, we demonstrated using a novel in vitro EC/SMC coculture hemodynamic flow system that the human chemokine interleukin-8 (IL-8/CXCL8) is secreted at higher levels by ECs exposed to an atheroprone flow environment, compared to atheroprotective flow.3 Also, atheroprone flow caused ECs/SMCs to undergo inflammatory priming, whereby the adhesion molecule, vascular cell adhesion molecule-1 (VCAM-1), was upregulated in both cell types compared to atheroprotective flow.
VCAM-1 expression, often resulting from NF-κB signaling, is observed in intimal SMCs in humans, in vivo animal models and cultured SMCs by inflammatory cytokines, including IL-1β.4–10 A recent study examined VCAM-1 expression in mouse intimal cells in the lesser curvature (ie, atheroprone) of the aortic arch and determined that VCAM-1 levels were significantly higher compared to the greater curvature (ie, atheroprotective) region.11 Cai et al showed leukocytes bound to SMCs were more antiapoptotic attributable in part to SMC VCAM-1 expression.12 SMC VCAM-1 expression may be important for trapping monocytes within a developing lesion.13,14 Furthermore, the inflammatory SMC phenotype is differentially regulated by specific collagen matrix components,11,15 and SMC VCAM-1 expression has been linked to proliferation16 and migration.17
IL-8 is an ELR (glutamic acid-leucine-arginine) CXC chemokine, expressed in humans, but not rodents, and is known to activate neutrophils.18 IL-8 is implicated in atherosclerosis via leukocyte attraction and induction of firm adhesions of monocytes to endothelium localized to developing lesions.19–21 Production of IL-8 in ECs occurs under inflammatory conditions, including stimulation with potent inflammatory mediators such as IL-1β, TNF-α, and exposure to “atheroprone” shear stresses.20,22–25 IL-8 induction is associated with proinflammatory stimuli and disease processes in vivo; however, direct localized inflammatory effects of this molecule on the vasculature are largely unexplored.26,27 Because rodents do not express IL-8, further understanding its role in atherosclerosis has been challenging.
We currently demonstrate that blocking IL-8 activity in our hemodynamic coculture system further enhances human EC/SMC VCAM-1 protein during atheroprone flow and that IL-1β–induced VCAM-1 in monocultured SMCs can be inhibited by costimulation with IL-8. Contrary to our hypothesis that IL-8 has proinflammatory effects on the vasculature, we show herein that IL-8 limits SMC VCAM-1 expression in response to inflammatory stimuli by reducing activation of p38 and NF-κB, and thereby decreasing VCAM-1 expression and suppressing monocyte adhesion.
Methods
Please see http://atvb.ahajournals.org for expanded Methods section.
Human Cell Culture and Hemodynamic Flow Model
Human EC/SMC cell culture and coculture plating conditions were used for flow experiments and performed as previously described.3 The two hemodynamic flow patterns were run in parallel for each EC/SMC subpopulation, or identical flow patterns were run in parallel in the presence or absence of a treatment condition.
IL-8 Inhibition During Flow
Cocultured ECs/SMCs were incubated with IL-8 antisera (1:300) raised in rabbit, donated by R.M. Strieter, MD (University of Virginia, Charlottesville) or with normal rabbit serum control, 10 minutes before the onset of atheroprone or atheroprotective flow and throughout the duration of the experiment. IL-8 siRNA (ON-TARGETplus SMARTpool, Thermo Scientific Dharmacon) was optimized in HUVECs (EC-siIL8) using Oligofectamine Reagent (Invitrogen) and Optimem Media.
Results
Human Coronary Arteries Express VCAM-1 and CXCR2 in All Vessel Layers
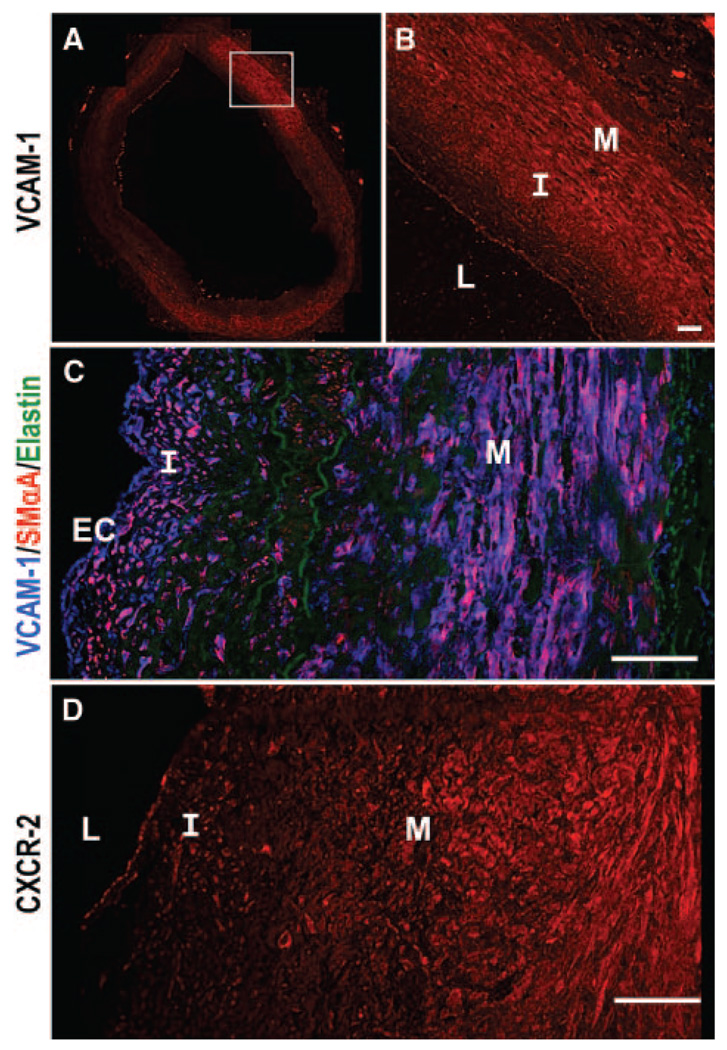
Whereas previous literature established the general presence of SMC VCAM-1 expression in human atherosclerotic tissue,7 we wanted to focus on the correlation between coexpression of VCAM-1 and SMCs in the neointima and medial layers as a function of intimal thickness. Cross-sections of human coronary arteries (n=10 patient samples) were analyzed for VCAM-1 expression in ECs and in intimal/medial layers. Distinct VCAM-1 expression was observed in ECs and intimal and medial regions (Figure 1A and 1B). In general, VCAM-1 was regionally heterogeneous across the tissue and each vascular cell type. In regions where SMCs expressed higher levels of VCAM-1, intimal thickening was present. Similarly, when the intima remained thin, VCAM-1 was not expressed in the deeper layers of the vessel wall. Smooth muscle α-actin (SMαA) staining demonstrated that SMCs were present in the intima, in addition to the media and this SMαA expression colocalized with VCAM-1 (Figure 1C). To understand how IL-8 signaling may be influencing VCAM-1, expression of CXCR2, a receptor which binds IL-8, was also identified in ECs and intima/media layers of the vessel wall (Figure 1D); however, no correlation between intimal thickness and CXCR2 expression patterns was observed. See supplemental figure for additional images (supplemental Figures I through IV). Data suggest that local secretion and signaling of IL-8, rather than differential CXCR2 expression, may regulate the differential VCAM-1 expression.
Figure 1.
VCAM-1 expression is heterogeneous, correlating with SMαA localization. Coronary artery cross-sections show VCAM-1 (A and B) white box highlights detail (B). Images costained for VCAM-1 (blue), SMαA (red), and elastin (green) indicate expression overlap (C). CXCR2 expression was also examined (D). Patient A08.4. (L indicates lumen; I, intima, M, media). Bars=50 µm.
Atheroprone Hemodynamics Cause Increased Monocyte Binding to ECs/SMCs
Our previous work showed that atheroprone flow induced the expression of VCAM-1 in both ECs and SMCs, relative to atheroprotective flow.3 VCAM-1 is known to play a role in monocyte adhesion.29,30 To demonstrate a functional consequence of this response, monocyte adhesion studies were performed after exposure of the EC/SMC coculture to 24 hours of flow. After cessation of shear stress, labeled MM6 monocytes were seeded on to the EC or SMC layer and examined for number of bound monocytes. Atheroprone flow exhibited 2-fold greater monocyte adhesion to ECs and SMCs compared to atheroprotective flow (Figure 2A and 2B), a response attenuated on incubation with a VCAM-1 blocking antibody (Figure 2B). Although it is possible that cessation of flow caused VCAM-1 degradation and thus reduced monocyte adhesion, it is unlikely since the half-life of VCAM-1 is on the order of hours.33–35 This is the first model to directly compare this functional consequence after atheroprone and atheroprotective flow on cocultured ECs/SMCs.
Figure 2.
Hemodynamics influences monocyte adhesion to ECs/SMCs. Atheroprone/protective flow was applied to EC/SMC cocultures for 24 hours, then monocytes were seeded onto ECs or SMCs (A and B). Adhered monocytes were quantified and a minimum of 9 fields per experiment were analyzed. (Mean±SE; n=4; +P<0.10; *P<0.05). Bars=50 µm.
Blocking Interleukin-8 During Atheroprone Flow Enhances SMC VCAM-1 Expression
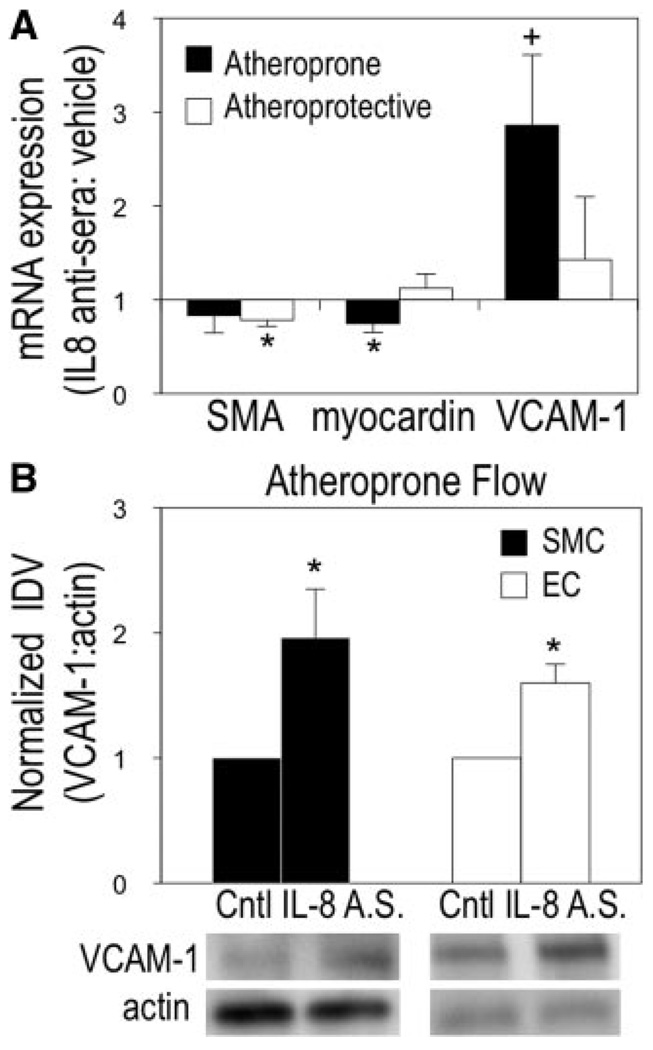
Previous work further established greater amounts of EC-secreted IL-8 at 24 hours of atheroprone relative to atheroprotective flow.3 To test whether atheroprone flow–induced secretion of IL-8 is regulating vascular cell VCAM-1 expression, flow was applied to the coculture system for 24 hours in the presence of IL-8 antisera or control serum. The IL-8 antisera during atheroprone flow enhanced VCAM-1 mRNA by 2.86-fold (Figure 3A). Myocardin was reduced by 25% because of IL-8 antisera and atheroprone conditions, but SMαA was not significantly downregulated (Figure 3A). Myocardin is a critical transcription factor required for serum response factor (SRF)-dependent transcription of SMC differentiation markers containing CArG cis elements in their promoters.36 Changes in myocardin suggest that cell remodeling is still occurring at this time point. In contrast, no change was observed for SMC myocardin or VCAM-1 expression in response to atheroprotective flow with IL-8 antisera, though SMαA was downregulated (Figure 3A). The discrepancy between changes in SMαA and myocardin are unexpected, but could be attributable to other factors inhibiting or competing against myocardin binding to SRF, (ie, KLF-4, Elk-1). Interestingly, blocking IL-8 activity during atheroprone flow further enhanced VCAM-1 protein expression in both ECs and SMCs, indicating that IL-8 may be serving to limit the extent of inflammation (Figure 3B).
Figure 3.
Effects of IL-8 inhibition on SMC phenotype during atheroprone flow. Atheroprone flow was applied to EC/SMC cocultures in the presence of IL-8 antisera or control serum. SMC SMA, myocardin, and VCAM-1 mRNA (A) and SMC/EC VCAM-1 protein (B) expression changes were analyzed. Representative blots shown. (Mean±SE, n=4; A.S. indicates antisera; *P<0.05, +P<0.10).
Interleukin-8 Limits the Interleukin-1β–Induced SMC Inflammatory Response
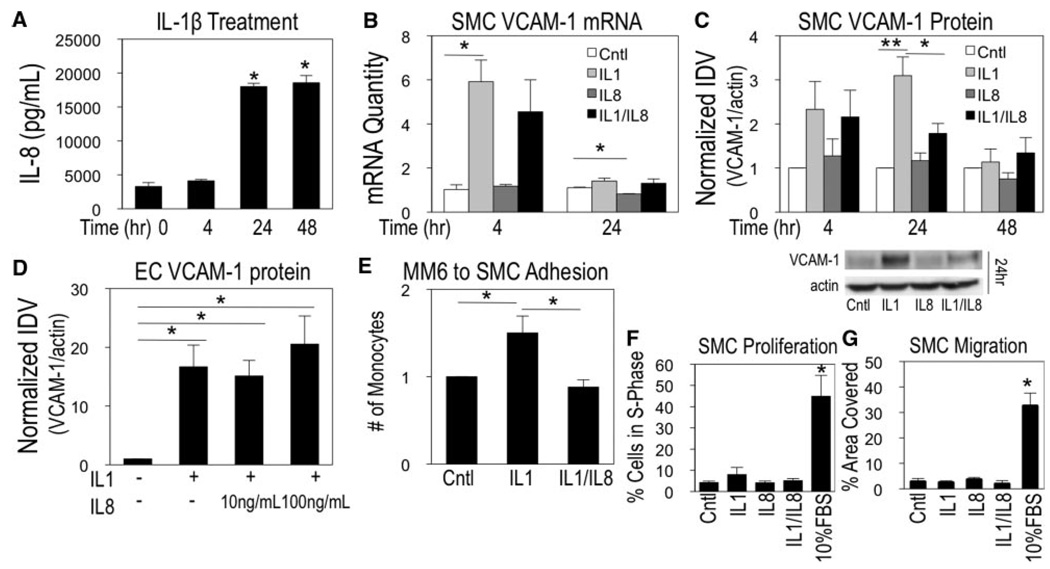
To understand how IL-8 might be influencing the SMC VCAM-1 expression, studies were performed with human SMC monocultures. Previous work demonstrated that IL-1β induced VCAM-1 expression in vascular SMCs14 and caused IL-8 secretion in airway SMCs.37 We examined the release profile of IL-1β–treated human vascular SMCs to establish the amount and timing of IL-8 intervention. ELISA analysis showed IL-8 secretion at levels 5.5-fold greater than control after 24 hours, with sustained levels at 48 hours (Figure 4A).
Figure 4.
Combinatorial response of IL-1/IL-8 in SMCs. SMCs were treated with IL-1β and analyzed for IL-8 secretion (A). SMC VCAM-1 mRNA (B) and SMC/EC protein (C and D) changes were examined for each condition. SMC monocyte adhesion (E), proliferation (F), and migration (G) were assayed. (Mean±SE, n=3, *P<0.05).
To determine the effect of IL-8 on cytokine stimulated SMCs, cells were treated with IL-1β, IL-8, or the combination to understand the temporal regulation of VCAM-1 expression. At the mRNA level, IL-1β caused significant VCAM-1 upregulation by 4 hours. At 24 hours, VCAM-1 mRNA returned to basal levels (Figure 4B). IL-8 alone did not induce VCAM-1 expression. Changes in myocardin and SMαA were also analyzed, where only myocardin was significantly reduced by 39% at 4 hours after IL-1β treatment (supplemental Figure V).
At the protein level, 4 hours of treatment with IL-1β and IL-1β/IL-8 induced VCAM-1 expression, by 2.33 and 2.16-fold, respectively; however, no significant differences were observed between the conditions (Figure 4C). The most dramatic response was observed after 24 hours of IL-1β/IL-8 treatment, which reduced VCAM-1 levels by 42.6% relative to IL-1β treatment alone (Figure 4C). By 48 hours, VCAM-1 expression returned to basal levels for all conditions. Analysis in ECs showed no change in VCAM-1 expression for the IL-1β/IL-8 condition compared to IL-1β alone after 24 hours at the same and higher (100 ng/mL) concentrations of IL-8, indicating SMC-specific responsiveness (Figure 4D). SMCs exposed to IL-1β increased monocyte adhesion, inhibited by the presence of exogenous IL-8 (Figure 4E), demonstrating a functional role for IL-8 to reduce monocyte adhesion to SMCs in the presence of inflammatory mediators.
Interleukin-8 and Interleukin-1β Do Not Promote SMC Proliferation or Migration
Because IL-8 has a role in resolving the SMC inflammatory response, proliferation and migration resulting from IL-8 combinatorial signaling were also investigated. Growth arrested SMCs were treated with 10%FBS, IL-1β, IL-8, or IL-1β/IL-8 for 24 hours. Cells were analyzed for proliferative responses via flow cytometry or for chemotactic responses via Boyden Chamber assays. Cells treated with 10% FBS both displayed strong induction of proliferation and migration at this time point; however, the effects of IL-1β, IL-8, or IL-1β/IL-8 were modest and remained insignificant, near control levels (Figure 4F and 4G).
CXCR2 Signaling Promotes IL-8 Resolution of VCAM-1
IL-8 binds to 2 G protein–coupled receptors, CXCR1 and CXCR2,38 both expressed by airway SMCs.27 We tested the hypothesis that blocking CXCR2 will potentiate the IL-1β– induced expression of VCAM-1 (ie, block the IL-1β/IL-8 reduction in VCAM-1). Blocking CXCR2 via neutralization abolished IL-8-induced ERK phosphorylation, an established response to IL-8 (Figure 5A). SMCs were treated with IL-1β and CXCR2 antibody for 24 or 48 hours. Blocking CXCR2 in the presence of IL-1β further enhanced VCAM-1 expression by 24 hours compared to IL-1β treatment alone (Figure 5B). This is in direct contrast to the response of IL-1β and IL-8, where VCAM-1 was reduced by 42.6% compared to IL-1β alone, as previously shown above in Figure 4C. Further, SMCs were treated with the CXCR2 antibody in the presence of IL-1β/IL-8. This combinatorial response no longer reduced VCAM-1 expression under these conditions, and instead caused a 1.76-fold induction from IL-1β (Figure 5C). We tested the specificity of the CXCR2 receptor for the observed responses using a different CXCR2-specific ligand, Gro-α. Of interest, the combination of Gro-α and IL-1β did not show a reduction in VCAM-1 expression by 24 hours (Figure 5D). Data show CXCR2 can attenuate IL-1β–mediated VCAM-1 expression modulated by IL-8.
Figure 5.
SMC VCAM-1 reduction is attributable to CXCR2 and phospho-p38 signaling. Phospho-ERK protein was examined in the presence of a CXCR2Ab/IL-8 (A). SMCs were treated as indicated and analyzed for VCAM-1 protein changes (B, C, D, and E). Phospho-p38 (F) and NF-κB activity (G) were measured after treatment. (Mean±SE, n=3, *P<0.05).
IL-8 Attenuates IL-1β–Induced p38 and NF-κB Activation
IL-1β upregulates VCAM-1 in part, because of activation of MAPK signaling molecule p38, shown previously in human tracheal SMCs.4 In the current monoculture system with vascular SMCs, p38 inhibition (SB202190) reduced VCAM-1 expression in response to IL-1β (Figure 5E). We hypothesized IL-8 may be blocking IL-1β–induced VCAM-1 expression by reducing p38 activation. Combined effects of IL-1β and IL-8 yielded a 25% reduction in phospho-p38, compared to IL-1β alone (Figure 5F), which is on the same order of reduction of VCAM-1 expression attributable to IL-1β/IL-8 treatment. Additionally, as IL-1β is known to activate the inflammatory transcription factor NF-κB, SMCs treated with IL-1β/IL-8 reduced Ad-NF-κB-luc activity after 9 hours, as did SB202190, consistent with the downstream changes in VCAM-1 (Figure 5G). Further, blocking CXCR2 with a neutralizing antibody in the presence of IL-1β/IL-8 inhibited the reduction in NF-κB activity as seen with the IL-1β/IL-8 reduced NF-κB activity.
Knockdown of IL-8 in Endothelial Cells Enhances Atheroprone-Induced VCAM-1 Expression
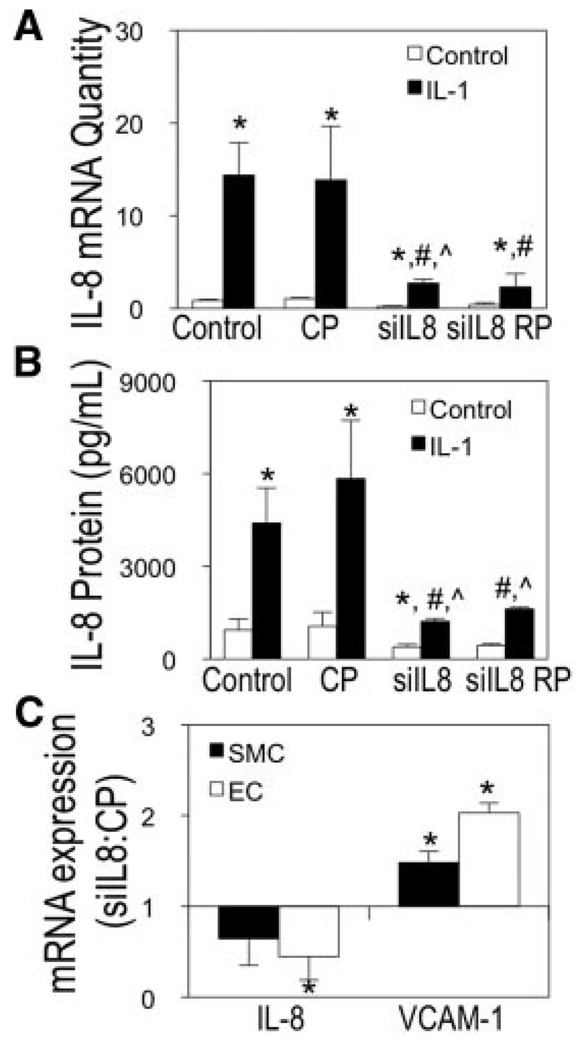
To determine whether EC-derived IL-8 caused atheroprone regulation of SMC VCAM-1 expression, EC expression of IL-8 was knocked down using IL-8 specific siRNA oligonucleotides. IL-8 siRNA was optimized at the mRNA and protein levels by treating transfected ECs with IL-1β and comparing to nonstimulated controls. Figure 6A shows a 65% reduction of IL-8 mRNA, and Figure 6B shows a 72% knockdown of secreted IL-8 in response to IL-1β stimulation for 4 hours compared to control.
Figure 6.
IL-8 EC silencing induces VCAM-1 during atheroprone flow. EC-siIL8 was optimized via RT-PCR (A) and ELISA (B). SMCs/ECs were examined for mRNA changes after atheroprone flow for EC-siIL8 or EC-CP (C). (Mean±SE; n=3; *P≤0.05 to untreated; #P<0.05 to IL-1β–treated; P<0.05 to IL-1β–treated CP; CP indicates control pool; RP, replated).
Optimal EC-siIL8 conditions were used in the EC/SMC coculture model. EC-siIL8 exposed to atheroprone flow upregulated VCAM-1 1.6-fold and 2-fold in SMCs and ECs, respectively, compared to control (Figure 6C). Downregulation of IL-8 by ECs showed transfection efficiency maintained throughout the flow experiment, which was on average 65% knockdown of expression. EC-siIL8 did not significantly influence SMC IL-8 mRNA levels (P=0.35).
Discussion
During atherogenesis, EC/SMC cross-communication via secreted paracrine factors is proposed to promote the chronic inflammatory response localized to hemodynamically compromised regions of arteries. As shown previously, VCAM-1 is expressed in human SMCs in proximity to human ECs exposed to atheroprone flow3 and IL-8 was upregulated and secreted at higher levels by ECs exposed to atheroprone flow.3 The functional role of IL-8 in regulating vascular cell phenotype is unknown. A major challenge for the field is that the cytokine IL-8 is not expressed in rodents. Thus, we used a model that recalibrates human in vivo vascular cell phenotypes in vitro to unmask a novel role for IL-8 in regulating SMC phenotype. Herein, we show that IL-8 attenuates a proinflammatory SMC phenotype by reducing VCAM-1 expression via CXCR2, p38, and NF-κB mechanisms. We also demonstrate that atheroprone flow induces VCAM-1–dependent monocyte adhesion.
The proinflammatory phenotype promoted during atheroprone flow translated to observations of VCAM-1 in human coronary artery tissue. Strong expression of VCAM-1 was confined to intimal/medial regions of human coronary arteries, coinciding with intimal thickening in arteries of patients.
The current study is, to our knowledge, the only one showing distinct heterogeneous VCAM-1 expression in entire crosssections of human intimal and medial layers, colocalizing with SMαA-positive cells.
To understand the role of IL-8 on VCAM-1 expression in SMCs, we performed monoculture experiments where cells were activated via IL-1β. IL-8 decreased IL-1β–induced VCAM-1 protein more quickly than when cells are treated with IL-1β alone. Further, VCAM-1 downregulation occurred via CXCR2 and reduced p38 activation. This is the first report of an IL-8 role in influencing adhesion molecule expression in any cell type. Unmasking the role of IL-8 in atherogenesis is experimentally challenging because mice do not express IL-8. Results herein support the hypothesis that upregulation of IL-8 serves, in part, to maintain overall lower levels of SMC VCAM-1 via reduced p38 and NF-κB activation, thus reducing monocyte adhesion. IL-1β is a wellestablished activator of NF-κB, and thus VCAM-1, in SMCs; however, it was previously unknown that IL-8 can reduce NF-κB activity when combined with an inflammatory stimulus. Others have reported that inhibition of p38 activation can reduce NF-κB activity in human tracheal SMCs.4,39 This response in our monoculture system further supports this mechanism by which IL-8 is acting to reduce VCAM-1 expression.
SMC phenotypic modulation in response to shear stress exposure on ECs has not been widely explored.3,40 One recent study analyzed SMC paracrine effects on EC adhesion molecule expression, where ECs presheared with high steady flow (12 dynes/cm2) for 24 hours were subsequently cocultured with SMCs after cessation of flow. Authors concluded E-selectin expression induced by culturing SMCs with ECs occurred via IL-6- and IL-1β–dependent mechanisms.40 Although these findings are interesting, this model used methods with potentially confounding factors, including a lack of physiological recapitulation, no serum withdrawal conditions for SMCs, and short time between plating and experimentation. On examination of IL-1β secretion by ELISA in our flow system (data not shown), we observed no detectable levels of the cytokine, which we suggest is attributable to a more quiescent SMC phenotype.
The effects of IL-8 on EC adhesion molecule expression in the context of inflammation were also examined. We observed similar results where blocking IL-8 activity (IL-8 antisera) or attenuating its secretion (EC-siIL8) enhanced EC VCAM-1 during atheroprone flow. This was interesting because the EC response to IL-1β/IL-8 treatments under static conditions had no effect in comparison, revealing the significance of studying EC/SMC communication in the presence of physiologically relevant hemodynamics. We anticipate similar mechanisms exist for EC/SMC responses to flow-induced inflammation, demonstrating that IL-8 may play a more important role than previously appreciated.
Interestingly, despite its association with inflammation, IL-8 protective effects have been described in several studies, specifically examining the response of IL-8 on neutrophil adhesion to ECs.22,41,42 IL-8 reduces neutrophil binding to IL-1β activated ECs, thus promoting antiinflammatory effects,41 and inhibition of neutrophil adhesion could be explained by an IL-8 role to reduce adhesion molecules on ECs. The cytokine IL-6, often upregulated simultaneously with IL-8, has been shown to be atheroprotective. IL-6+/− and IL-6−/− mice crossed with ApoE+/− mice resulted in increased lesion formation, coinciding with increased macrophages and reduced SMCs.43 Increased serum levels of soluble VCAM-1 were detected in ApoE+/−-IL-6−/−. Clearly, the role of IL-8 may be dual in its capabilities of promoting and protecting against inflammation, and the current study further emphasizes the complexity of this chemokine.
The hemodynamic environment is known to prime ECs/ SMCs toward an inflammatory phenotype, but we now understand a previously unknown compensatory mechanism involved in limiting inflammation via paracrine effects between these 2 cell types. We have described a novel role for IL-8 to limit IL-1β–induced VCAM-1 expression via reduction of p38 and NF-κB activation, without alteration of contractile, migratory, or proliferative phenotypes. We speculate IL-8 induction is one of many pathways that has evolved to maintain homeostasis in the blood vessel wall, and such pathways will continue to unfold as we further our understanding of human EC/SMC communication in systems that recreate critical components of the human vasculature, including EC/SMC spatial orientation and critical hemodynamic environments. In conclusion, the induction of IL-8 in response to atheroprone hemodynamics may maintain lowered levels of VCAM-1, preventing further inflammation localized to the vessel wall during atherogenesis.
Supplementary Material
Acknowledgments
The authors acknowledge Andy Pryor for cell culture work and staining assistance and Oana Nicoara, MD, Clay Cauthen, MD, and Robin LeGallo, MD for providing human coronary artery sections.
Sources of Funding
Funding was provided by the Vice President for Research Studies Fund for Excellence in Science and Technology and the Partner’s Fund from the University of Virginia, B.R.B. and B.R.W., NIH HL081682 and AHA SDG to B.R.W., NIH RO1 HL082836, B.R.B., and the Basic Cardiovascular Research Training Grant, NIH ST32HL0084, N.E.H., R.E.F., M.Y.L.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 2.von der Thusen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–C1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 4.Wang CC, Lin WN, Lee CW, Lin CC, Luo SF, Wang JS, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK, and NF-kappaB in IL-1beta-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L227–L237. doi: 10.1152/ajplung.00224.2004. [DOI] [PubMed] [Google Scholar]
- 5.Jang Y, Lincoff AM, Plow EF, Topol EJ. Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol. 1994;24:1591–1601. doi: 10.1016/0735-1097(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 6.Duplaa C, Couffinhal T, Dufourcq P, Llanas B, Moreau C, Bonnet J. The integrin very late antigen-4 is expressed in human smooth muscle cell. Involvement of alpha 4 and vascular cell adhesion molecule-1 during smooth muscle cell differentiation. Circ Res. 1997;80:159–169. doi: 10.1161/01.res.80.2.159. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronaryatheroscleroticplaquesImplications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oguchi S, Dimayuga P, Zhu J, Chyu KY, Yano J, Shah PK, Nilsson J, Cercek B. Monoclonal antibody against vascular cell adhesion molecule-1 inhibits neointimal formation after periadventitial carotid artery injury in genetically hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2000;20:1729–1736. doi: 10.1161/01.atv.20.7.1729. [DOI] [PubMed] [Google Scholar]
- 9.Fyfe AI, Qiao JH, Lusis AJ. Immune-deficient mice develop typical atherosclerotic fatty streaks when fed an atherogenic diet. J Clin Invest. 1994;94:2516–2520. doi: 10.1172/JCI117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 11.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Q, Lanting L, Natarajan R. Interaction of monocytes with vascular smooth muscle cells regulates monocyte survival and differentiation through distinct pathways. Arterioscler Thromb Vasc Biol. 2004;24:2263–2270. doi: 10.1161/01.ATV.0000146552.16943.5e. [DOI] [PubMed] [Google Scholar]
- 13.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun M, Pietsch P, Schror K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/s0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- 15.Orr AW, Lee MY, Lemmon JA, Yurdagul A, Schoppee Bortz P, Wamhoff BR. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29:225–231. doi: 10.1161/ATVBAHA.108.178749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun-Dullaeus RC, Mann MJ, Sedding DG, Sherwood SW, von der Leyen HE, Dzau VJ. Cell cycle-dependent regulation of smooth muscle cell activation. Arterioscler Thromb Vasc Biol. 2004;24:845–850. doi: 10.1161/01.ATV.0000125704.28058.a2. [DOI] [PubMed] [Google Scholar]
- 17.Petersen EJ, Miyoshi T, Yuan Z, Hirohata S, Li JZ, Shi W, Angle JF. siRNA silencing reveals role of vascular cell adhesion molecule-1 in vascular smooth musclecellmigration. Atherosclerosis. 2008;198:301–306. doi: 10.1016/j.atherosclerosis.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard EJ, Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]) Am J Respir Cell Mol Biol. 1990;2:479–486. doi: 10.1165/ajrcmb/2.6.479. [DOI] [PubMed] [Google Scholar]
- 19.Boisvert WA. Modulation of atherogenesis by chemokines. Trends Cardiovasc Med. 2004;14:161–165. doi: 10.1016/j.tcm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 21.Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis. 2002;160:91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA, Jr., Obin MS, Brock AF, Luis EA, Hass PE, Hebert CA, Yip YK, Leung DW, Lowe DG, Kohr WJ, et al. Endothelial interleukin-8: a novel inhibitor of leukocyte-endothelial interactions. Science. 1989;246:1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- 23.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117:113–122. doi: 10.1007/s00418-001-0368-9. [DOI] [PubMed] [Google Scholar]
- 25.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue TL, Wang X, Sung CP, Olson B, McKenna PJ, Gu JL, Feuerstein GZ. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994;75:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol. 2006;291:C957–C965. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 28.Deleted in proof.
- 29.Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 30.Thorne SA, Abbot SE, Stevens CR, Winyard PG, Mills PG, Blake DR. Modified low density lipoprotein and cytokines mediate monocyte adhesion to smooth muscle cells. Atherosclerosis. 1996;127:167–176. doi: 10.1016/s0021-9150(96)05948-5. [DOI] [PubMed] [Google Scholar]
- 31.Deleted in proof.
- 32.Deleted in proof.
- 33.Swerlick RA, Lee KH, Li LJ, Sepp NT, Caughman SW, Lawley TJ. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992;149:698–705. [PubMed] [Google Scholar]
- 34.Braun M, Pietsch P, Felix SB, Baumann G. Modulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on human coronary smooth muscle cells by cytokines. J Mol Cell Cardiol. 1995;27:2571–2579. doi: 10.1006/jmcc.1995.0044. [DOI] [PubMed] [Google Scholar]
- 35.Pober JS, Gimbrone MA, Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- 36.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 37.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1023–L1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 38.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 39.Lin WN, Luo SF, Lee CW, Wang CC, Wang JS, Yang CM. Involvement of MAPKs and NF-kappaB in LPS-induced VCAM-1 expression in human tracheal smooth muscle cells. Cell Signal. 2007;19:1258–1267. doi: 10.1016/j.cellsig.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tasi MC, Lin CW, Usami S, Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007 doi: 10.1182/blood-2006-08-040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luscinskas FW, Kiely JM, Ding H, Obin MS, Hebert CA, Baker JB, Gimbrone MA., Jr In vitro inhibitory effect of IL-8 and other chemoat-tractants on neutrophil-endothelial adhesive interactions. J Immunol. 1992;149:2163–2171. [PubMed] [Google Scholar]
- 42.Hechtman DH, Cybulsky MI, Fuchs HJ, Baker JB, Gimbrone MA., Jr Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991;147:883–892. [PubMed] [Google Scholar]
- 43.Madan M, Bishayi B, Hoge M, Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. 2008;197:504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.