Letter
The article by De Paiva et al.1 provides additional evidence that interleukin-17 (IL-17) is associated with disruption of corneal epithelial barrier function, which is the most sight-threatening complication of dry eye disease (DE), arguably the most common ophthalmologic condition. It reports increased expression of Th17 cell inducers including IL-6, TGF-β, IL-23 and IL-17A on the ocular surface of DE patients as well as increased concentration of IL-17 in tears and number of Th17 cells on the ocular surface of experimental animals with DE. In addition, authors show that IL-17 induced secretion of MMP-3 and -9 by epithelial cells causes a significant increase in corneal epithelial permeability which is ameliorated by in-vivo neutralization of IL-17.
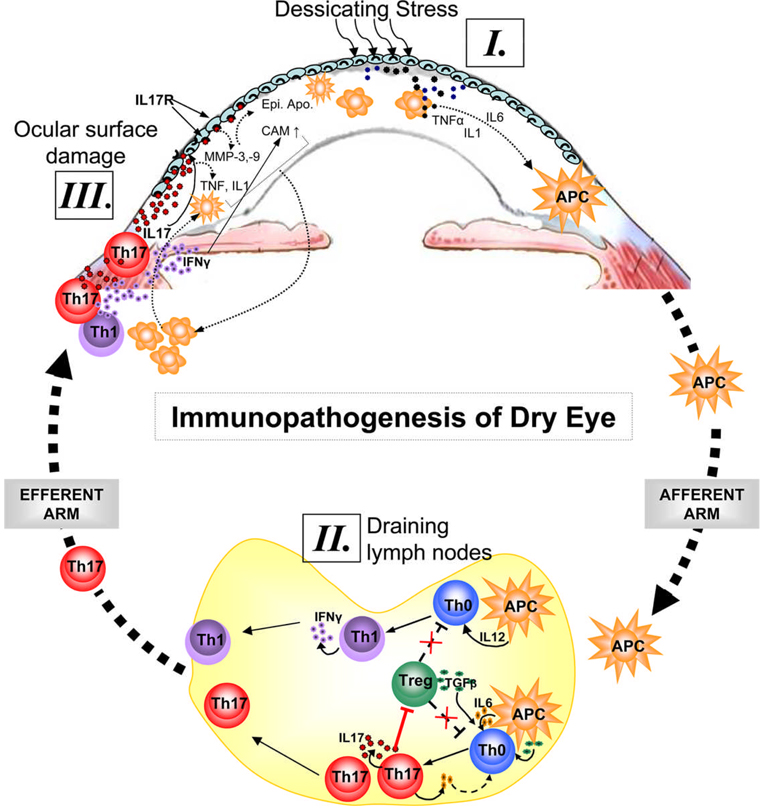
However, the precise etiopathogenesis of DE, particularly the nature of ocular surface autoantigen(s), and the site and mechanism of generation of these self-reactive T cells is still not very clear2–4. In a recent article5, we have demonstrated not only the involvement of Th17 cells in dry eye pathogenesis but also the mechanism of induction of autoimmunity in the draining lymph nodes (LN) using a mouse model of DE. In addition to the expression of Th17 profile on the ocular surface, we showed increased frequency of Th17 cells in the draining LN where these cells exhibit specific resistance to regulatory-T cell (Treg) mediated suppression. In agreement with De Pavia et al.1, the functional relevance of Th17 cells in the pathogenesis of DE was fundamentally confirmed by demonstrating that in-vivo neutralization of IL-17 results in a markedly attenuated induction and severity of disease. Interestingly, DE amelioration was paralleled by a reduction in the expansion of Th17 cells and prevention of the loss of Treg function in the draining LN suggesting that Th17 cell-secreted-IL-17 antagonizes the Treg function, which in turn further unleashes Th17 and Th1 cells to expand, migrate to ocular surface and cause epithelial damage. Together, these studies suggest that Th17 cells are the dominant pathogenic effectors in DE. Based on current1,2 and previously published3–5 data, an overarching hypothesis of the mechanism(s) involved in the pathogenesis of DE is illustrated in Figure-1. DE affects visual performance and the quality of life of >10 million people in the United States alone. The current demonstration that IL-17 blockade leads to diminished disease severity suggests potential novel approaches for the treatment of DE.
Figure 1. Immunopathogenesis of dry eye disease.
Ocular surface inflammation in DE is sustained by ongoing activation and infiltration of pathogenic immune cells, primarily CD4+ T cells in the conjunctiva and CD11b+ monocytic cells in the cornea1–5. [I.] Dessicating stress induces secretion of inflammatory cytokines especially IL-1, TNFα and IL-6 by ocular surface tissues which facilitate activation and migration of resident antigen presenting cells (APC) toward the regional draining lymph nodes (LN). [II.] In the LN, these APCs stimulate cognate naïve T cells (Th0) leading to the expansion of IL-17 secreting Th17 cells and IFN-γ secreting Th1 cells. IL-17 in turn antagonizes the Treg function by facilitating further expansion of Th17 cells which may compete with Tregs for TGF-β available in the milieu. [III.] Once these effectors are generated in the LN, they migrate to the ocular surface and secret effector cytokines. Interaction of IL-17 with its receptors on ocular surface leads to epithelial damage via increase secretion of matrix metalloproteinases (MMP) and inflammatory cytokines. In addition to apoptosis and metaplasia of ocular surface epithelia, IFN-γ causes upregulation of chemokine ligands/receptor and adhesion molecules (CAM) which facilitate increased ingress of immune cells to the ocular surface tissues.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health Grant NEI R01-12963.
REFERENCES
- 1.De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, III, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccating stress. Mucosal. Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea. 2008;27 Suppl 1:S9–S11. doi: 10.1097/ICO.0b013e31817f4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Report of the International Dry Eye WorkShop. Ocul. Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. No authors listed. [DOI] [PubMed] [Google Scholar]
- 4.Barabino S, Dana MR. Dry eye syndromes. Chem. Immunol. Allergy. 2007;92:176–184. doi: 10.1159/000099268. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]