1. Introduction
Approximately one million children die of severe malaria each year in sub-Saharan Africa(Breman 2001). Metabolic acidosis, clinically presenting as respiratory distress, is common in severe childhood malaria, and is strongly associated with death (Taylor, Borgstein et al. 1993; Marsh, Forster et al. 1995; Allen, O'Donnell et al. 1996; English, Sauerwein et al. 1997). For children who get to hospital, most deaths occur within 12 to 24 hours of admission(Krishna, Waller et al. 1994; Marsh, Forster et al. 1995; Allen, O'Donnell et al. 1996). For this reason, it is argued that even modest improvements in management during the first 24 hours, such as introducing specific treatment for acidosis, may improve survival(Newton and Krishna 1998).
In severe childhood malaria, metabolic acidosis is associated with a raised blood lactate, although in up to a third of cases lactate is not raised (English, Sauerwein et al. 1997). A phase II clinical trial of dichloroacetate (a lactate lowering drug) as specific treatment for lactic acidosis of severe childhood malaria has shown promising results(Agbenyega, Planche et al. 2003). However, the drug did not reduce mortality in critically ill adults with severe lactic acidosis in a large clinical trial(Stacpoole, Wright et al. 1992). To develop and test other specific treatment options for metabolic acidosis in these children, further characterisation of metabolic acidosis aimed at the identification of underlying pathophysiological mechanisms is needed.
Traditionally, clinical assessment of acid-base status is based on the Henderson-Hasselbalch equation (see appendix). Respiratory acidosis is associated with raised arterial partial pressure of carbon dioxide, and the level of carbon dioxide tension directly reflects the severity of acidosis. Metabolic acidosis results from the presence in plasma of excess nonvolatile acids, which are otherwise, absent in health and is characterized by a low plasma bicarbonate. These acids may be organic, inorganic, endogenous or exogenous. They are thought to consume bicarbonate in the process of buffering. However, metabolic acidosis can also result from the loss of bicarbonate. The common routes through which bicarbonate may be lost include the gut (diarrhoea) and the kidney (renal tubular acidosis).
In the traditional approach, therefore, a decrease in bicarbonate concentration is taken to be a consequence of metabolic acidosis. And the degree of severity is quantified using the base excess (quantity of base that must be added to a sample of whole blood in vitro in order to restore its pH to 7.40 while the pCO2 is held at 40 mmHg)(Siggaard-Andersen 1962). The anion gap (see appendix) is calculated to work out whether metabolic acidosis is due to the presence of nonvolatile acids or bicarbonate loss. A high anion gap (> 13mEq/L)(Winter, Pearson et al. 1990; Lolekha, Vanavanan et al. 2001; Lolekha, Vanavanan et al. 2003) indicates the presence of nonvolatile acids whereas a normal anion gap suggests bicarbonate loss. However, this approach does not consider the role of plasma non-bicarbonate buffers, notably albumin and inorganic phosphate. Indeed, the value of the anion gap is strongly influenced by the concentration of plasma albumin(Figge, Rossing et al. 1991) and, this approach can miss or underestimate the presence of excess nonvolatile acids when serum albumin is low (Fencl, Jabor et al. 2000). A correction factor for plasma albumin, however, improves the accuracy of the anion gap (Figge, Jabor et al. 1998).
Stewart's application of physical-chemical principles of aqueous solutions to blood plasma(Stewart 1983), has lead to a new approach to acid-base disorders. In this physical-chemical approach, only three variables: pCO2, strong ion difference (SID), the net charge of all strong ions present in plasma; and the total amount of plasma weak acids independently determine plasma pH (acid-base status). And metabolic acidosis can result from either a decrease in SID or an increase in the total plasma weak acid. A decrease in SID produces an electrochemical force, which increases the dissociation of plasma water. This generates free H+ and acidosis develops (Kellum 2000). The presence in plasma of excess strong anions such as lactate, ketones, chloride, or sulfate is associated with a decrease in SID and thus, acidosis. A low SID may also result from the loss of cations (e.g. diarrhea), mishandling of anions (e.g. renal tubular acidosis), or the addition of exogenous anions (e.g. poisoning). Using the Stewart's approach, the behavior of blood plasma as a physical-chemical system can be quantitatively explored. This is possible through mathematical models that are developed from equations expressing the principles of electroneutrality, dissociation equilibrium, and the conservation of mass(Figge, Rossing et al. 1991). The strong anion gap (SIG), a measure of the concentration of unmeasured strong ions in plasma, is used to characterise metabolic acidosis (see appendix). This is free from the limitations of the anion gap, and is more specific for detecting excess strong anions (Constable 1999). In addition, the acid-base contribution of individual strong anions (if measured), and even that of unidentified anions can be explored.
Studies of acidosis in African children have been limited to severe malaria. And the few that have characterised acidosis(English, Sauerwein et al. 1997) applied only the traditional approach. The observation of chronic salicylate poisoning in some Kenyan children with severe malaria and acidosis (English, Marsh et al. 1996) highlighted the possibility for multiple aetiological factors including inappropriate treatment of childhood malaria fever episodes in the home with aspirin or other non-aspirin salicylates (Slutsker, Chitsulo et al. 1994; Marsh, Mutemi et al. 1999) The aim of this study was to characterise childhood malarial acidosis using the Stewart's and traditional approach, and to investigate the role of lactate, ketones and salicylates. We extended the scope of previous investigations by including children with non-severe falciparum malaria, and those with a range of severe childhood illnesses to investigate which characteristics of malarial acidosis are specific or more pronounced.
2. Materials and methods
2.1 Study site and patients
The study was done at the Kenya Medical Research Institute (KEMRI) Center for Geographic Medicine Research-Coast at Kilifi District Hospital, after approval from the National Research and Ethics Committees. The hospital draws most of its admissions from a population of about 213,000 people the majority of whom live in rural, subsistence-farming households. Consecutive admissions to the pediatric ward were eligible for inclusion if they were aged between one-month and 13 years. Written informed consent was obtained from the parent or accompanying guardian at the time of enrolment. Children admitted primarily due to surgical conditions (trauma, burns, and elective surgery) were excluded. Also excluded, were children whose parent or guardian did not give consent; those without an indication for routine blood sampling at admission; those being re-admitted with the same problem within two weeks after discharge; and those admitted with known chronic disease.
2.2 Procedures
On admission, a research clinician reviewed the child and collected data on clinical symptoms and signs using a pre-coded questionnaire. Children were reviewed daily and treated according to local inpatient guidelines and their subsequent outcome and discharge diagnosis were recorded. Disease severity on admission was classified using previously identified clinical criteria: presence of prostration (inability to sit without support or breast feed if aged less than one year), coma (Blantyre coma score ≤ 2), or respiratory distress (Marsh, Forster et al. 1995; Berkley, Ross et al. 2003). An extra admission-venous-blood sample (2ml) for purposes of this study was taken at the same time as the routine admission blood sampling. Aliquots of this sample were used for blood gas and blood lactate. Plasma was separated from the remaining sample and analysed for sodium, potassium, creatinine, chloride, albumin, inorganic phosphorous, 3-hydroxybutyrate, and salicylate.
Aliquots (0.5ml) for blood gas were collected in heparinised syringes and immediately analyzed (IL 1620 Blood Gas System, Instrumentation Laboratories, Milano Italy). Aliquots for blood chemistry were collected in heparin-tubes, centrifuged immediately and plasma separated. An aliquot of plasma (60μl) was analysed for sodium and potassium (614 ISE Na+/K+ analyser, Chiron Diagnostics Limited, Essex, England), and creatinine (Creatinine analyser 2, Beckman Coulter Inc., Fullerton, CA, USA). The remaining plasma was stored at −70 °C until analysed for chloride (Chloride Analyser 925, Ciba Corning Diagnostics Limited, Essex, England), albumin and inorganic phosphorus (Photometer 5010, Boehringer Mannheim GmbH, Mannheim, Germany), 3-hydroxybutyrate, and salicylate.
2.2.1 Lactate measurements
Aliquots of blood for lactate were collected in tubes containing heparin, sodium nitrite, and fluoride, and were stored at −20 °C before analysis within 24 hours of collection (GM 7 analyser, Analox Instruments Ltd, Hammersmith, London, UK). The analyser was calibrated daily (lactate standard, Analox Instruments Ltd, Hammersmith, London, UK), and a quality control serum (Analox Instruments Ltd, Hammersmith, London, UK) analysed before test samples. In addition, the quality control serum was analysed after every 10 test samples.
2.2.2 3-hydroxybutyrate measurements
Plasma 3-hydroxybutyrate measurement was performed using an enzymatic reaction based on the method first described by Williamson and others(Williamson, Mellanby et al. 1962). Procedure No. 310-UV (Sigma Diagnostics, St. Louis, Missouri USA) was used and photometry reading at 340nm (Photometer 5010, Boehringer Mannheim GmbH, Mannheim, Germany) performed before and after incubating reagent, sample and enzyme. We validated this method in our laboratory by preparing a standard calibration curve, which we checked against a standard (Sigma Diagnostics, St. Louis, Missouri USA), and against quality control samples from a different source (Randox Laboratories Ltd, Crumlin Co. Antrim, UK). The standard and quality control samples supplied by the manufacturer (Sigma Diagnostics, St. Louis, Missouri USA) together with the quality control samples from Randox (Randox Laboratories Ltd, Crumlin Co. Antrim, UK) were included on each run of test samples.
Briefly, to appropriately labeled Pyrex tubes containing 1.5ml reagent at 37.0 °C was added 50μl of deionised water (blank), standard (calibrator), controls and samples and the mixture vortexed before initial absorbency (340nm) was read. Enzyme (Sigma Diagnostics, St. Louis, Missouri USA) was added, the mixture vortexed and incubated for 10 minutes at 37.0 °C before final absorbency (340nm) was read. 3-hydroxybutyrate (mmol/l) was calculated according to the manufacture's instructions. The change in absorbency of the reagent blank was subtracted from the change in absorbency of the sample and the result was multiplied by the molecular weight of 3-hydroxybutyrate (104) and a conversion factor (0.096).
2.2.3 Salicylate measurements
Plasma salicylate measurements were performed by fluorescence polarization immunoassay using the Abbott TDx analyser (Abbott Diagnostics, Abbott Park, IL, USA). This method has been validated against well-established HPLC methods(Karnes and Beightol 1985). Briefly, standard calibration curves were generated by loading calibrators with known salicylate concentration (Abbott Diagnostics, Abbott Park, IL, USA) and running the calibration assay. The analyser generated a standard calibration curve and applied calibration curve acceptance criteria. The curve was checked against quality control samples from the manufacturer (Abbott Diagnostics, Abbott Park, IL, USA) and from a different source (Randox Laboratories Ltd, Crumlin Co. Antrim, UK). Patient samples were analysed together with the quality control samples from the manufacturer, and the analyzer was re-calibrated whenever any of the controls was outside the specified range.
2.2.4 Parameters for characterisation of metabolic acidosis
For the purpose of this study, metabolic acidosis was defined a priori as an admission base excess of ≤ −10mmol/l. Although this definition is indicative of a more marked acidosis than in other studies (English, Sauerwein et al. 1997; Day, Phu et al. 2000; Moviat, van Haren et al. 2003), this level was selected as it is close to a readily clinically identifiable threshold (English, Waruiru et al. 1996) and it unequivocally identifies children with a significant metabolic acidosis.
The formulae used for calculating the classic anion gap (AG), albumin corrected anion gap (AGc), the apparent strong ion difference (SIDapp) and the effective strong ion difference (SIDeff) are provided in the appendix (Fencl and Leith 1993). Since calcium and magnesium concentrations were not measured, the lower limit of standard reference ranges in our laboratory (1.75mmol/l and 0.63mmol/l respectively), were assigned to every patient for the calculation of the apparent strong ion difference. Imputing these low values in each case is a conservative approach likely to underestimate the mean strong ion difference in each group(Maitland, Pamba et al. 2005). SIG (strong ion gap) was calculated by subtracting effective SID (SIDeff) from apparent SID (SIDapp). Including lactate and 3-hydroxybutyrate concentrations in the calculation of the apparent strong ion difference (see appendix) allowed estimation of unidentified strong anions.
2.3 Statistical analysis
Data were analyzed using STATA version 8.0 (Stata Corporation, USA). Non-normally distributed data were transformed prior to analyses. Proportions were compared using Chi-square test, and forward stepwise regression models were used to identify biochemical parameters associated with acidosis.
3. Results
A total of 1 902 children were admitted during the study period. Two hundred and fifty-nine did not meet the inclusion criteria. Complete data were available for 1375/1644 (84 %) of the total eligible children. The most common reason for failure to recruit children was the temporary unavailability of blood gas analysis in the laboratory. Despite the limited study period and minor problems with recruitment, we feel the population studied is representative of admissions at Kilifi district hospital. Baseline clinical and biochemical characteristics of children recruited in the study are shown in tables 1 and 2 respectively.
Table 1.
Baseline clinical characteristics of children admitted with malaria, LRTI and severe malnutrition
| Malaria (n = 624) | GE (n = 127) | LRTI (n = 184) | Severe malnutrition (n = 95) | |
|---|---|---|---|---|
| Median age in months (IQR) | 25 (13, 42) | 12 (8, 18) | 13 (5, 23) | 24 (15, 36) |
| Male [n (%)] | 317 (51) | 74 (58) | 92 (50) | 48 (51) |
| Median weight in kg (IQR) | 9.9 (7.7, 12.3) | 7.5 (6.2, 8.8) | 7.9 (6.1, 9.4) | 6.6 (5.7, 8.7) |
| Mean pulse rate (95 % CI) | 156 (154, 159) | 146 (140, 151) | 160 (155, 165) | 141 (135, 147) |
| Reduced skin turgor [n (%)] | 6 (1) | 37 (29) | 4 (2) | 35 (37) |
| Prolonged capillary refill [n (%)] | 94 (18)b | 32 (29)c | 26 (17)d | 25 (28)e |
| Mean weight for age Z score (95 % CI) | −1.7 (−1.8, −1.6) | −1.9 (−2.2, −1.8) | −1.6 (−1.8, 1.4) | −4.0 (−4.3, −3.8) |
| Mean haemoglobin in g/dl (95 % CI) | 7.7 (7.5, 7.9) | 9.6 (9.3, 9.9) | 9.0 (8.7, 9.3) | 7.6(7.2, 8.1) |
| Severe disease [n (%)] | 89 (14) | 7 (6) | 7 (4) | 6 (6) |
| History of seizures (current illness) [n (%)] | 223 (36) | 5 (4) | 12 (7) | 1 (1) |
| Mortality [n (%)] | 9 (1) | 2 (2) | 4 (2) | 18 (19) |
Measured using a pulse oximeter
Malaria (n = 531)
GE (n = 115)
LRTI (n = 156)
Severe malnutrition (n = 90)
Table 2.
Baseline biochemical characteristics [mean (95 % CI)] of children admitted with malaria, GE, LRTI and severe malnutrition
| f Sodium | fPotassium | Creatinine (μmol/l) |
f Lactate | pH | pCO2 (kPa) | f Bicarbonate | |
|---|---|---|---|---|---|---|---|
| Malaria | |||||||
| Acidotic (n = 117) | 133 (132, 134) | 4.6 (4.4, 4.8) | g74 (68, 80) | g2.9 (2.5, 3.4) | 7.24 (7.22, 7.27) | 3.2 (3.0, 3.5) | 10.6 (10.0, 11.3) |
| Non-aciotic (n = 507) | 13.9 (133, 34) | 4.1 (4.0, 4.2) | g47 (45, 48) | g2.1 (1.9, 2.2) | 7.37 (7.37, 7.38) | 4.2 (4.1, 4.2) | 18.2 (18.0, 18.5) |
| GE | |||||||
| Acidotic (n = 77) | 136 (135, 138) | 3.5 (3.2, 3.7) | g65 (60, 71) | g1.6 (1.4, 1.8) | 7.24 (7.22, 7.26) | 3.1 (2.9, 3.2) | 10.2 (9.7, 10.8) |
| Non-acidotic (n = 50) | 135 (133, 136) | 3.8 (3.6, 4.1) | g54 (48, 59) | g1.9(1.6, 2.3) | 7.36 (7.35, 7.38) | 4.0 (3.8, 4.2) | 17.6 (16.9, 18.3) |
| LRTI | |||||||
| Acidotic (n = 26) | 136 (134, 137) | 4.3 (3.9, 4.7) | g52 (41, 65) | g1.9 (1.5, 2.5) | 7.23 (7.18, 7.28) | 3.4 (2.9, 3.9) | 10.8 (9.4, 12.2) |
| Non-acidotic (n = 158) | 136 (135, 137) | 4.4 (4.3, 4.5) | g38 (35, 40) | g1.8 (1.7, 2.0) | 7.36 (7.36, 7.37) | 4.4 (4.2, 4.5) | 19.0 (18.6, 19.5) |
|
Severe malnutrition |
|||||||
| Acidotic (n = 23) | 133 (130, 135) | 3.4 (2.9, 3.8) | g58 (46, 73) | g1.9 (1.4, 2.6) | 7.27 (7.22, 7.31) | 2.8 (2.5, 3.2) | 9.9 (8.7, 11.2) |
| Non-acidotic (n = 72) | 133 (132, 134) | 3.6 (3.4, 3.9) | g31 (26, 36) | g2.0 (1.8, 2.3) | 7.39 (7.38, 7.40) | 4.0 (3.9, 4.2) | 18.6 (17.9, 19.3) |
mmol/l
geometric mean
3.1 Prevalence of metabolic acidosis in pediatric admissions
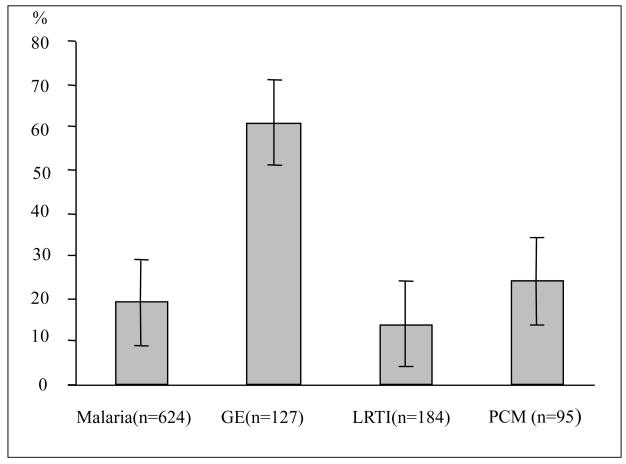
Two hundred and eighty-five of the 1375 with complete data (21 %) were acidotic, and gastroenteritis had the highest percentage of children with acidosis (fig 1). In malaria acidosis was observed to be more common in children meeting our definition of severe disease than in those not meeting this definition (57 % versus 12 % P <0.001). In other diagnostic groups the prevalence of severe disease according to these criteria was low. ,
Fig 1.
Percentage of children with acidosis (whiskers, 95 % CI)
GE Acute gastroenteritis
LRTI Lower Respiratory Tract Infection
PCM Severe malnutrition (Weight for Age Z score ≤ −3)
3.2 Characterisation of metabolic acidosis
We selected frozen samples for further assay from all cases of malaria (n= ), LRTI (n= ), gastroenteritis (n= ) and severe malnutrition (n= ) that met our pre-defined criterion of metabolic acidosis. As the total numbers of non-acidotic gastroenteritis (n= ) and severe malnutrition (n= ) cases were relatively small we selected all of these for further assay. However, to reduce the total number of assays to be performed while still permitting within group comparisons of reasonable precision we selected sub-samples of frozen specimens from non-acidotic malaria and LRTI cases for further assay. To do this we listed the unique case record numbers for non-acidotic malaria patients in the study and randomly selected a 25% sub-sample (using STATA version 8.0, Stata Corporation, USA). The procedure was repeated for non-acidotic LRTI cases although in this case a 50% sub-sample was selected. This resulted in 127 non-acidotic malaria cases and 79 non-acidotic LRTI cases being identified to compare with acidotic cases with the same diagnosis In total characterisation of acidosis and its determinants was therefore, limited to a population of 571 cases: 243 acidotic, and 328 non-acidotic children, for whom complete data were present in 91 %.
Albumin-corrected anion gap and strong ion gap was higher than normal (> 13mmol/l and positive respectively) in most children with acidosis in malaria, GE, LRTI and severe malnutrition. Mean albumin-corrected anion gap (95 % CI) was 22 (20 – 23), 18 (17 – 20), 21 (18 – 23), and 21 (17 – 24), and mean strong ion gap was 15 (14 –17), 10 (9 – 12), 14(11 – 16), 13(10 – 17) mmol/l respectively. This suggested a role for excess anions other than chloride in all four diagnostic categories. When all deaths were pooled, the mean strong ion gap (95 % CI) was significantly higher in those with fatal outcome compared with survivors, 14.8 (11.2 – 18.4) and 9.7 (9.1 – 10.4) mmol/l respectively.
Clinically significant unidentified anions or tissue acids (> 5mmol/L)(Durward, Skellett et al. 2001) were present in 51/81 (63 %), 27/55 (49 %), 17/21(81 %) and 11/18(61 %) of acidotic children with malaria, gastroenteritis, lower respiratory tract infection, and severe malnutrition respectively (P = 0.076). And the mean (95 % CI) concentration of unidentified anions was 8 (7,10), 5 (3,7), 10 (7,13) and 10mmo/l (6,23) for malaria, GE, LRTI and severe malnutrition respectively.
3.3 Determinants of metabolic acidosis
Overall, hyperlactataemia (blood lactate ≥ 5mmol/l) was more common in children with acidosis than in those without 40/223 (18 %) versus 20/306 (7 %), P <0.001. However, hyperlacatataemia was significantly more common in children with malaria than those with the other three conditions, 30/106(28 %) compared with 4/71 (6 %) in GE, 3/23 (13 %) in LRTI and 3/23 (13 %) in severe malnutrition (P= 0.001). Similarly, overall, ketosis (plasma 3-hydroxybutyrate ≥4mmol/l) was more common in children with acidosis than those without (39 % versus 18 %, P <0.001). In this case, however, there was no significant difference in the percentage of acidotic children with elevated 3-hydroxybutyrate across the four diagnostic categories, 46 %, 35 %, 26 %, 26 % for malaria, GE, LRTI and severe malnutrition respectively, P = 0.112.
Likewise, hyperchloraemia (plasma chloride > 107mmol/L) was more common in acidotic than in nonacidotic children (56/223, 25 % versus 53/306, 17 % P=0.029). However, it was significantly more common in children with GE than in the other three diseases, 36 % versus 18 %, 22 %, 26 % in malaria, LRTI and severe malnutrition respectively (P = 0.045).
Plasma salicylate data were available for 475 (82 %) of the target 581 children (276 non-acidotic and 199 acidotic children). Fifty (11 %) had detectable salicylate in plasma (≥ 5mg/l), the limit of detection for the assay method. There was no difference overall, in prevalence of salicylate detection in acidotic and non-acidotic children, 22/199 (11 %) versus 28/276 (10 %), P = 0.75. In malaria, the largest clinical subgroup, there was similarly no difference in salicylate detection between children with acidosis and those without acidosis, 13/82 (14 %) versus 18/103 (17 %), P = 0.46. Overall however, after adjusting for the presence of malaria, an association between salicylate detection and the presence of signs indicative of severe illness on admission was observed (OR, 2.11, 95% CI 1.1, 4.2)
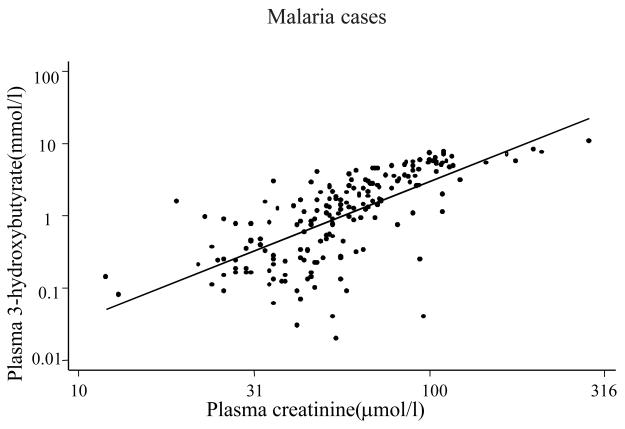
In univariable regression analyses for malaria cases only, log10 plasma creatinine (coefficient = −14.66, P <0.001), log10 blood lactate (coefficient = −6.55, P <0.001) and log10 plasma 3-hydroxybutyrate (coefficient = −4.0, P <0.001) were significant predictors of base excess (acidosis). Other significant predictors were age (coefficient = 0.05, P = 0.004) and plasma inorganic phosphorus (coefficient = −2.877, P <0.001). Sodium, albumin, and chloride were not significant predictors. In a forward stepwise regression model, log10 creatinine, log 10 lactate and inorganic phosphorus levels were the best independent predictors of acidosis together accounting for about 40 % of variation in base excess (adjusted R2 = 0.397). The inclusion of plasma 3-hydroxybutyrate in the model did not improve the fit because of the strong correlation between plasma 3-hydroxybutyrate and creatinine (fig. 2).
Fig 2.
Scatter plot showing the correlation between (log) plasma 3-hydroxybutyrate and creatinine in children with malaria (r = 0.68, P = 0.001)
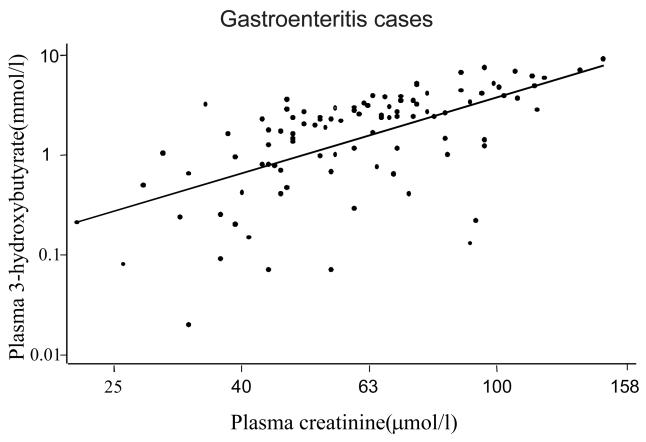
When the same approach was applied in gastroenteritis cases only, log10 3-hydroxybutyrate (coefficient = −4.8, P <0.001), chloride (coefficient = −0.2, P<0.001) and albumin (coefficient −0.256, P = 0.006) were significant predictors of acidosis. There was also a strong positive correlation between plasma creatinine and 3-hydroxy butyrate levels in children with gastroenteritis (fig. 3). In a forward stepwise regression model, log10 3-hydroxybutyrate, chloride, and albumin levels accounted for 38 % of variation in base excess (adjusted R2 = 0.3834). In lower respiratory tract infection and severe malnutrition, log 10 plasma creatinine and log 10 plasma 3-hydroxybutyrate were the only significant predictors of acidosis accounting for 20 % and 28 % of base excess variability (adjusted R2 = 0.20, and 0.276 respectively).
Fig 3.
Scatter plot showing the correlation between (log) plasma 3-hydroxybutyrate and creatinine in children with gastroenteritis (r = 0.60, P = 0.001)
4. Discussion
Acidosis is a common complication of serious illness in children, occurring in 20% of all admitted children even using a conservative definition. Although in our setting malaria is numerically the commonest cause acidosis is by no a unique presentation of severe malaria, and in fact, the prevalence is highest in children admitted with acute gastroenteritis. Improved approaches to recognizing and managing acidotic children might therefore have benefits beyond malaria.
Furthermore, our data show that metabolic acidosis in malaria, GE, LRTI and severe malnutrition share a common underlying mechanism, the presence in plasma of strong anions (non-volatile acids) which are otherwise absent in health. We measured lactate, ketones and salicylate but in malaria these did not account for the entire measured strong ion gap indicating considerable amounts of unidentified anions. The presence and prognostic significance of unidentified anions has recently been reported in Thai adults with severe malaria(Dondorp, Chau et al. 2004). In our study, the number of deaths was small but when all deaths were pooled the mean strong ion gap was higher in those with a fatal outcome than in survivors. Establishing the identity of these acids may therefore have therapeutic implications.
Experience from critical care units suggest that the choice of specific treatment for non-toxin associated acidosis depends on the nature of acids involved (Levraut and Grimaud 2003). There are suggestions that optimum treatment of the underlying disease (e. g. malaria) in combination with supportive care aimed at normalising blood volume, cardiac output and tissue oxygenation such as intravenous fluids may be the best option for treating metabolic acidosis associated with the presence of organic acids (e. g. lactate and ketone) (Levraut and Grimaud 2003). Organic acids are metabolisable and the acidosis would resolve with time provided the underlying cause is adequately treated and life is supported. On the other hand, alkali therapy may be of benefit in the treatment of acidosis associated with the presence of non-metabolisable acids (e. g. acids associated with renal failure) including chloride (Rehm and Finsterer 2003). Conversely the benefit of alkali therapy is doubtful (Lever and Jaspan 1983; Morris, Murphy et al. 1986; Cooper, Walley et al. 1990; Okuda, Adrogue et al. 1996) and in fact, may be harmful in organic acidosis (Hale, Crase et al. 1984).
Our data also demonstrate the important association between plasma creatinine and acidosis in malaria. This association, in large part may result from the strong correlation between creatinine and ketones (represented by plasma 3-hydroxybutyrate). Two explanations for this association are possible. Firstly a longer period of severe illness prior to admission may result in ketoacidosis and an elevated creatinine as a result of catabolism and mild to moderate dehydration, the latter potentially reducing excretion of ketoacids. Alternatively, or in addition, there may be a direct effect of malaria to impair renal function resulting in an elevation in creatinine and a reduced capacity to excrete ketoacids and other unidentified acids. The latter suggests that the unidentified anions might be mineral acids associated with renal impairment (e. g. sulfate, urate, hippurate)(Niwa 1996). Although frank acute renal failure is rare in African children with severe malaria (Waller, Krishna et al. 1995), significantly reduced glomerular filtration rates have been documented(English, Waruiru et al. 1996).
Salicylate exposure did not commonly underlie acidosis in this series allaying previous concerns that chronic salicylate poisoning or exposure may be common, important co-factors in the development of acidosis in severe malaria. However, occasional, inadvertent salicylate toxicity does occur(English, Marsh et al. 1996) (and personal observation of the authors) and still causes diagnostic confusion with severe malaria. Interestingly post hoc analysis in this study did suggest an association between salicylate exposure and the presence of severe illness. However, the numbers of cases included in these analyses were small and further research is needed to address this question specifically.
Traditionally, metabolic acidosis in gastroenteritis is of normal anion gap and characterised by a raised plasma chloride concentration (hyperchloraemic acidosis) (Hill, Morris et al. 1971). Surprisingly our data suggest that the presence of strong anions other than chloride underlie acidosis in African children with GE. According to the traditional approach, loss of bicarbonate in stool is associated with a shift of chloride ions from the intracellular to the extra-cellular compartment (to maintain electroneutrality) leading to increased plasma chloride concentration. Whereas by the Stewart's approach, loss of cations in stool rather than bicarbonate, is responsible for acidosis (decrease in SID) and chloride shift (Kellum 2000). Diarrhoea is associated with dehydration, which causes haemo-concentration leading to an increase in plasma albumin concentration, and thus an increase in total plasma weak acid. According to the Stewart's theory, this has an acidifying effect. Therefore, our finding that plasma chloride and albumin are significant determinants of acidosis in gastroenteritis but not in the other three diseases was expected. Additionally however, ketones (3-hydroxybutyrate) but not lactate were associated with acidosis in this group of children and unidentified anions were significant common. This implies that in African children with gastroenteritis, the accumulation of acids in plasma is a more pronounced mechanism than bicarbonate or cation loss in the development of acidosis.
We conclude that acidosis is a much more common problem amongst pediatric admissions in Africa than has previously been recognised and that it is associated most commonly with the presence in plasma of organic acids and currently unidentified strong anions. In malaria cases, lactate and ketoacids (3-hydroxybutyrate) make a significant contribution to this acidosis. However, a substantial proportion of anions remain unidentified. Exposure to salicylates or chronic salicylate poisoning does not seem to be a common cofactor in the development of acidosis as previously hypothesized.
Acknowledgements
This study is published with the permission of the Director of the Kenya Medical Research Institute (KEMRI). A preliminary report was presented at the 3rd MIM meeting in Arusha Tanzania, November 2002. The study was supported by a Research Capacity Strengthening Grant from W.H.O (TDR/MIM Grant No. 980074) to Professor Gilbert Kokwaro. Dr. Philip Sasi is a trainee in Clinical Pharmacology supported by MIM and the KEMRI/Wellcome Trust Collaborative Research Programme. The authors are grateful to the staff of Kilifi District Hospital, and the KEMRI Center for Geographic Medicine Research-Coast and the Wellcome Trust Research Laboratories, for their valuable assistance during this study. We also gratefully acknowledge the support from Prof. Gilbert Kongola, Prof. Mary Temu, Dr. Gerald Rimoy and Dr. Jane Sayi of the Muhimbili University College of Health Sciences, Dar es Salaam, Tanzania. The authors also appreciate the technical laboratory assistance from Mr. Martin Mwakala, and the help of Prof. R.D. Cohen. Consent was obtained from the parents/guardians of children before enrollment in this study to whom the authors are greatly indebted.
APPENDIX
The Henderson-Hasselbalch equation
pH = pK × log[HCO3−/(0.003 × pCO2)]
Formula 1: Anion gap
Formula 2: Albumin-corrected Anion gap
*40g/l was used, the median value for the reference range for plasma albumin in our laboratory (36 – 45g/l)
Formula 3: Apparent Strong Ion Difference
Formula 4: Effective Strong Ion Difference
References
- Agbenyega T, Planche T, et al. Population kinetics, efficacy, and safety of dichloroacetate for lactic acidosis due to severe malaria in children. J Clin Pharmacol. 2003;43(4):386–96. doi: 10.1177/0091270003251392. [DOI] [PubMed] [Google Scholar]
- Allen SJ, O'Donnell A, et al. Severe malaria in children in Papua New Guinea. Qjm. 1996;89(10):779–88. doi: 10.1093/qjmed/89.10.779. [DOI] [PubMed] [Google Scholar]
- Berkley JA, Ross A, et al. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. Bmj. 2003;326(7385):361. doi: 10.1136/bmj.326.7385.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64(1-2 Suppl):1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- Constable PD. Clinical assessment of acid-base status. Strong ion difference theory. Vet Clin North Am Food Anim Pract. 1999;15(3):447–71. doi: 10.1016/s0749-0720(15)30158-4. [DOI] [PubMed] [Google Scholar]
- Cooper DJ, Walley KR, et al. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study. Ann Intern Med. 1990;112(7):492–8. doi: 10.7326/0003-4819-112-7-492. [DOI] [PubMed] [Google Scholar]
- Day NP, Phu NH, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000;28(6):1833–40. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Chau TT, et al. Unidentified acids of strong prognostic significance in severe malaria. Crit Care Med. 2004;32(8):1683–8. doi: 10.1097/01.ccm.0000132901.86681.ca. [DOI] [PubMed] [Google Scholar]
- Durward A, Skellett S, et al. The value of the chloride: sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. 2001;27(5):828–35. doi: 10.1007/s001340100915. [DOI] [PubMed] [Google Scholar]
- English M, Marsh V, et al. Chronic salicylate poisoning and severe malaria. Lancet. 1996;347(9017):1736–7. doi: 10.1016/s0140-6736(96)90809-0. [DOI] [PubMed] [Google Scholar]
- English M, Sauerwein R, et al. Acidosis in severe childhood malaria. Qjm. 1997;90(4):263–70. doi: 10.1093/qjmed/90.4.263. [DOI] [PubMed] [Google Scholar]
- English M, Waruiru C, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55(5):521–4. doi: 10.4269/ajtmh.1996.55.521. [DOI] [PubMed] [Google Scholar]
- English MC, Waruiru C, et al. Hyponatraemia and dehydration in severe malaria. Arch Dis Child. 1996;74(3):201–5. doi: 10.1136/adc.74.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fencl V, Jabor A, et al. Diagnosis of metabolic acid-base disturbances in critically ill patients. Am J Respir Crit Care Med. 2000;162(6):2246–51. doi: 10.1164/ajrccm.162.6.9904099. [DOI] [PubMed] [Google Scholar]
- Fencl V, Leith DE. Stewart's quantitative acid-base chemistry: applications in biology and medicine. Respir Physiol. 1993;91(1):1–16. doi: 10.1016/0034-5687(93)90085-o. [DOI] [PubMed] [Google Scholar]
- Figge J, Jabor A, et al. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807–10. doi: 10.1097/00003246-199811000-00019. [DOI] [PubMed] [Google Scholar]
- Figge J, Rossing TH, et al. The role of serum proteins in acid-base equilibria. J Lab Clin Med. 1991;117(6):453–67. [PubMed] [Google Scholar]
- Hale PJ, Crase J, et al. Metabolic effects of bicarbonate in the treatment of diabetic ketoacidosis. Br Med J (Clin Res Ed) 1984;289(6451):1035–8. doi: 10.1136/bmj.289.6451.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LL, Morris CR, et al. Role of tissue hypoxia and defective renal acid excretion in the development of acidosis in infantile diarrhea. Pediatrics. 1971;47((1): Suppl 2):246+. [PubMed] [Google Scholar]
- Karnes HT, Beightol LA. Evaluation of fluorescence polarization immunoassay for quantitation of serum salicylates. Ther Drug Monit. 1985;7(3):351–4. doi: 10.1097/00007691-198507030-00021. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Determinants of blood pH in health and disease. Crit Care. 2000;4(1):6–14. doi: 10.1186/cc644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Waller DW, et al. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg. 1994;88(1):67–73. doi: 10.1016/0035-9203(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Lever E, Jaspan JB. Sodium bicarbonate therapy in severe diabetic ketoacidosis. Am J Med. 1983;75(2):263–8. doi: 10.1016/0002-9343(83)91203-2. [DOI] [PubMed] [Google Scholar]
- Levraut J, Grimaud D. Treatment of metabolic acidosis. Curr Opin Crit Care. 2003;9(4):260–5. doi: 10.1097/00075198-200308000-00002. [DOI] [PubMed] [Google Scholar]
- Lolekha PH, Vanavanan S, et al. Update on value of the anion gap in clinical diagnosis and laboratory evaluation. Clin Chim Acta. 2001;307(1-2):33–6. doi: 10.1016/s0009-8981(01)00459-4. [DOI] [PubMed] [Google Scholar]
- Lolekha PH, Vanavanan S, et al. Reference ranges of electrolyte and anion gap in venous whole blood and plasma of healthy school children. Clin Chim Acta. 2003;331(1-2):167–9. doi: 10.1016/s0009-8981(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Maitland K, Pamba A, et al. Perturbations in electrolyte levels in kenyan children with severe malaria complicated by acidosis. Clin Infect Dis. 2005;40(1):9–16. doi: 10.1086/426022. [DOI] [PubMed] [Google Scholar]
- Marsh K, Forster D, et al. Indicators of life-threatening malaria in African children [see comments] N Engl J Med. 1995;332(21):1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- Marsh VM, Mutemi WM, et al. Changing home treatment of childhood fevers by training shop keepers in rural Kenya. Trop Med Int Health. 1999;4(5):383–9. doi: 10.1046/j.1365-3156.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Morris LR, Murphy MB, et al. Bicarbonate therapy in severe diabetic ketoacidosis. Ann Intern Med. 1986;105(6):836–40. doi: 10.7326/0003-4819-105-6-836. [DOI] [PubMed] [Google Scholar]
- Moviat M, van Haren F, et al. Conventional or physicochemical approach in intensive care unit patients with metabolic acidosis. Crit Care. 2003;7(3):R41–5. doi: 10.1186/cc2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79(1):1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Niwa T. Organic acids and the uremic syndrome: protein metabolite hypothesis in the progression of chronic renal failure. Semin Nephrol. 1996;16(3):167–82. [PubMed] [Google Scholar]
- Okuda Y, Adrogue HJ, et al. Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. J Clin Endocrinol Metab. 1996;81(1):314–20. doi: 10.1210/jcem.81.1.8550770. [DOI] [PubMed] [Google Scholar]
- Rehm M, Finsterer U. Treating intraoperative hyperchloremic acidosis with sodium bicarbonate or tris-hydroxymethyl aminomethane: a randomized prospective study. Anesth Analg. 2003;96(4):1201–8. doi: 10.1213/01.ANE.0000048824.85279.41. table of contents. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. The pH-log pCO2 blood acid-base nomogram revised. Scand J Lab Invest. 1962;14:598–604. doi: 10.1080/00365516209051290. [DOI] [PubMed] [Google Scholar]
- Slutsker L, Chitsulo L, et al. Treatment of malaria fever episodes among children in Malawi: results of a KAP survey. Trop Med Parasitol. 1994;45(1):61–4. [PubMed] [Google Scholar]
- Stacpoole PW, Wright EC, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. N Engl J Med. 1992;327(22):1564–9. doi: 10.1056/NEJM199211263272204. [DOI] [PubMed] [Google Scholar]
- Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61(12):1444–61. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Taylor TE, Borgstein A, et al. Acid-base status in paediatric Plasmodium falciparum malaria. Q J Med. 1993;86(2):99–109. [PubMed] [Google Scholar]
- Waller D, Krishna S, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21(3):577–87. doi: 10.1093/clinids/21.3.577. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Mellanby J, et al. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962;82:90–6. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SD, Pearson JR, et al. The fall of the serum anion gap. Arch Intern Med. 1990;150(2):311–3. [PubMed] [Google Scholar]