Abstract
Although the control of malaria epidemics has been a priority for the World Health Organization and other agencies for many years, surprisingly little is known about their public health burden. Here we evaluate the available evidence on the morbidity and mortality impacts of individual epidemics in Africa and examine the problems involved in using these data to estimate average annual burden of epidemics at national and continental scales. We argue that conventional approaches to assessing epidemic burden are inadequate and outline the future steps required to produce more accurate estimates.
THE BURDEN OF MALARIA EPIDEMICS IN AFRICA
Recent years have seen renewed efforts to understand the determinants, epidemiology and public health impact of Plasmodium falciparum malaria epidemics in Africa. Commitment to tackle this major public health problem has been reaffirmed by the Roll Back Malaria (RBM) partnership, which has adopted epidemic detection and response as one of the four principal pathways through which its mandate to reduce the global burden of malaria risk, morbidity and mortality by half by 2010 can be achieved [1]. Reducing the impact of epidemics remains a prominent goal in RBM’s revised strategy for 2005-2015 [2].
Malaria control in epidemic-prone areas represents a different challenge to that in endemic settings [3]. In Africa, populations exposed to the risks of epidemics reside in areas of unstable malaria transmission, commonly along the fringes of stable, endemic malaria transmission. These populations have little or no acquired functional clinical immunity and the consequences of rapid expansion of parasite transmission can have potentially devastating public health impacts. The challenges of detecting sudden upsurges in transmission, mobilizing resources and rapidly deploying interventions to mitigate the public health impact of epidemics cannot be met by routinely applying prevention and control tools from endemic settings. In particular, effective response to epidemics can only be achieved through precise and accurate targeting of interventions (typically indoor residual spraying or mass drug administration) in space and time – a fact which places special emphasis on disease surveillance systems.
There have been two systematic attempts to describe the malaria disease burden resulting from epidemics in Africa. Snow et al. [4] reviewed the available literature to estimate the average clinical attack and mortality rates associated with individual epidemics and went on to derive average annualized estimates of morbid and fatal outcomes using combinations of assumptions regarding epidemic duration, periodicity and populations potentially at risk of unstable transmission derived from climate-based models of malaria risk. Worrall et al. [5] undertook a similar analysis using different approaches to estimating populations at risk and different parameter estimates of the average clinical attack rate, case-fatalities and epidemic periodicity. Neither approach has been validated and the sensitivities of the implicit assumptions have not been tested.
BURDEN OF INDIVIDUAL EPIDEMICS
Attempts to estimate the disease burden associated with individual epidemics have been hampered in two ways. First, the term ‘epidemic’ has been used to refer to a wide variety of scenarios ranging from ‘classic’ large epidemics in non-immune populations to unusual seasonal rises in malaria cases within semi-immune populations6 – a situation that is further complicated by the many and various ecological and anthropogenic determinants of epidemics [7, 8]. Lack of clarity in definitions of epidemics makes comparisons between epidemic events difficult and therefore reduces the representativeness of ‘aggregated’ sets of epidemic data. Second, and more fundamentally, few reliable data exist on the risk of morbidity and mortality in epidemic situations. In Africa, for example, the most exhaustive review of data on malaria burden to date was carried out by Snow et al. [4], who found only 15 reports on morbidity and mortality risks during epidemics for the period 1929-1988 (of which half referred to events occurring before 1960). To our knowledge the only reliable estimates of epidemic-related mortality since that time have come from retrospective surveys carried out by the World Health Organization (WHO) [9] and Epicentre/Médecins Sans Frontières (MSF) [10, 11].
Combining Snow et al.’s dataset with these more recent data for Africa provides a total of 12 records of all-cause mortality during malaria epidemics and ten records of malaria-specific mortality (although the reliability and appropriateness of this distinction is moot). Summary statistics from these records indicate median mortality rates of 2.3 and 1.6 deaths per 10,000 population per day for all-cause and malaria-specific estimates respectively. Where all-cause or malaria specific mortality rates among children are reported, these tend to be higher than corresponding rates in the general population. Data on clinical attack rates from 11 reports indicate an overall median morbidity rate of 32.2 per 10,000 population per day (Table 1). Eight reports contained estimates of the case fatality rate (data not shown in table), either among in-patients attending fixed or mobile clinics or based on comparisons of community estimates of morbid cases and deaths (range = 3-21%; median = 6%). These relatively high rates are usually thought to reflect a high occurrence of acute, life-threatening disease (and cerebral malaria in particular), as well as the relatively wide range of ages affected during epidemics, but could result in equal measure from poor access to health services, inadequate case-management, overwhelmed health services, poor immunological competence due to malnutrition, a general disruption to livelihoods due to often associated flooding, or any combination of these factors.
Table 1.
Key indicators of burden for individual malaria epidemic events.
| Locality | Dates | Malaria morbidity (cases/10000/day) |
All-cause crude mortality (deaths/10000/day) |
Malaria mortality (deaths/ 10000/day) |
Reference | ||
|---|---|---|---|---|---|---|---|
| All ages | All ages | <5s | All ages | <5s | |||
| Kitale, Kenya | 1929 | 4.1 | 39 | ||||
| Mupumulo, South Africa | 1931-1932 | 5.2 | 40 | ||||
| Eshowe, South Africa | 1931-1932 | 3.4 | 40 | ||||
| Mtunzini, South Africa | 1931-1932 | 2.0 | 40 | ||||
| Nkandah, South Africa | 1931-1932 | 2.5 | 40 | ||||
| Kericho, Kenya | 1942-1947 | 8.9 | 41 | ||||
| Lake Tana area (site 1), Ethiopia | 1958 | 61.6 | 10.3 | 13 | |||
| Lake Tana area (site 2), Ethiopia | 1958 | 26.8 | 1.6 | 13 | |||
| Lake Tana area (site 3), Ethiopia | 1958 | 62.8 | 4.0 | 13 | |||
| Lake Haik , Ethiopia | 1958 | 28.2 | 6.0 | 13 | |||
| Ganje District, Ethiopia | 1958 | 8.9 | 13 | ||||
| Manarintsoa, Madagascar | 1988 | 32.2 | 3.9 | 42 | |||
| Balcad | 1988 | 3.8 | 43 | ||||
| Ziway, Ethiopia | 1992 | 2.7 | 4.3 | 9 | |||
| Wajir, Kenya | 1998 | 9.4 | 28.4 | 44 | |||
| Nshamba, Tanzania | 1998 | 1.4 | 4.2 | 10 | |||
| Karuzi | 2000 | 0.9 | 3.1 | 11 | |||
| Kayanza, Burundi | 2000-2001 | 35.3 | 1.0 | 3.6 | 0.8 | 2.3 | 11 |
| Karuzi | 2001 | 107.3 | 1.1 | 3.0 | 0.6 | 1.6 | 11 |
| Ngozi | 2000-2001 | 32.9 | 1.8 | 5.0 | 1.0 | 2.6 | 11 |
| Damot Gale | 2003-2004 | 2.1 | 3.4 | 1.5 | 2.8 | 11 | |
|
| |||||||
| Median | 32.2 | 2.3 | 3.6 | 1.6 | 2.6 | ||
| Interquartile range | 17.8-48.4 | 1.6-3.5 | 3.2-4.7 | 1.1-5.5 | 2.3-2.8 | ||
The daily disease rates shown in Table 1 are unquestionably higher than one would expect among populations exposed to stable Plasmodium falciparum transmission [12]. In Africa, the most notable epidemic event occurred in Ethiopia in 1958 and was responsible for an estimated 150,000 deaths among a largely non-immune population with little access to curative services [13]. The data in Table 1 illustrate the very high levels of malaria mortality experienced at four sites during this epidemic, in contrast to the lower levels of mortality recorded in highland malaria epidemics in Madagascar [42], Burundi [11] and Ethiopia [9, 11] from the late 1980s onwards. The lower rates of mortality experienced during these more recent episodes are unlikely to be the result of lower intensities of malaria transmission, as the rates of morbidity associated with these events were significant, even by historical standards (Table 1). It is more probable that they reflect both an improvement in access to emergency care, including antimalarial drugs, and comparatively high levels of pre-existing clinical immunity resulting from previous parasite exposure. Since the 1980s there have been numerous reports of increased malaria transmission in areas of the African highlands previously considered free of infection or subject to very low/sporadic levels or transmission [14-19]. Although the drivers behind the changing epidemiology of malaria transmission in these areas have been the subject of much debate [17, 20, 21], less disputed is the fact that areas once subject to infrequent, ‘classic’ epidemics now experience more frequent transmission and acutely seasonal clinical surges of disease incidence. This has manifested a change in the age-specific patterns of disease suggesting age-acquired clinical immunity following repeated annual parasite exposures [22]. Data from a number of recent epidemics show relatively high rates of malaria-specific mortality among children – not the predominantly flat age profile that would be expected in populations lacking any immunity [9, 11, 23].
Although mortality rates experienced during recent malaria epidemics have been markedly lower than those reported in Ethiopia in 1958, associated disease burdens may still be substantial, not least because of the large populations at risk in densely inhabited highland areas. A serious epidemic in Burundi in 2000-2001, for example, affected nine of the country’s 16 provinces and resulted in around 3.5 million malaria cases [23]. Results from retrospective mortality surveys carried out in the area indicated that during the epidemic more than 12,000 malaria-attributable deaths occurred within a population of around 1.1 million, representing a cumulative mortality rate of 91 deaths per 10,000 population over the survey periods (average 120 days) [11]. A similar survey carried out during an epidemic in Damot Gale district (Ethiopian highlands) in 2003-2004 indicated around 5,000 excess deaths within a population of 290,000, at a cumulative rate of 173 deaths per 10,000 over 125 days [11]. In highland areas, the high burden of recent epidemics therefore appears to be the product of relatively long-lived events (lasting 30 weeks or longer [23]) affecting large populations, rather than particularly high mortality rates per se. This is in marked contrast to an epidemic that occurred in the lowland, desert fringe district of Wajir, Kenya, in 1998. Here, mortality rates were extremely high (see Table 1), but the overall effect of the epidemic was limited by the relatively small size of the affected population and by the comparatively short duration of the epidemic [23].
The key characteristics of epidemics therefore – their causality, presentation, evolution and impact – vary markedly between epidemic-prone sites [8] and over time at individual localities. While this brings into question the sense of previous attempts to derive average rates for morbidity and mortality across all types of epidemic event, there may be scope for developing a ‘typology’ of epidemic types based on, for example, the principal determining factors involved, the ecology of the area affected, the timing of the epidemic and the characteristics of affected populations [8]. Given the paucity of the existing data record, attributing meaningful epidemiological characteristics to such a typology will not be a straightforward exercise; nevertheless it is doubtful whether current estimates of epidemic burden can be significantly improved without this change.
ANNUALIZED ESTIMATES OF EPIDEMIC BURDEN
Scaling up locality-based estimates of epidemic burden to the national-, regional- or continental-scale requires knowledge of the distribution of populations at risk from epidemics (PAR). To be able to articulate epidemic risk in terms of an average annualized burden, we also need to make basic assumptions regarding the frequency and length of epidemic events and estimate parameters for the shape of the epidemic curve and the distribution of incidence between age groups.
DEFINING POPULATIONS AT RISK
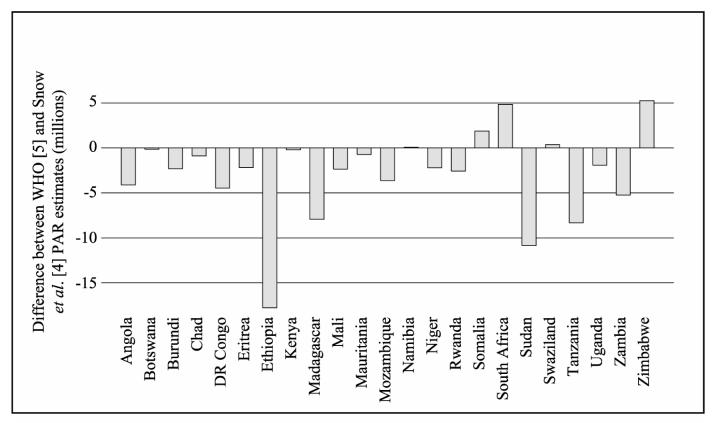
The first estimates of epidemic PAR for Africa were produced by the WHO in 1996 and subsequently used by Worrall et al. [5]. These estimates were derived for 26 countries and were based on local expert opinion regarding the percentage of the national population deemed to be at risk of epidemics. An alternative approach, adopted by Snow et al. [4], used models of climate suitability for malaria transmission [24] to define geographical risk zones for epidemics. These zones were then overlaid with data on human population distribution within a geographical information system to derive PAR. Given the very different methodologies involved, PAR estimates derived from these two approaches differ significantly at both continental and national scales (Table 2 and Figure 1). The quantitative climate model used by Snow uses climate suitability limits to define epidemic-prone regions that were not validated against empirical or expert opinion information. The methodology used by WHO, which relied upon expert national opinion, historical incidence data and knowledge of risk factors [25] is, by its very nature, subjective and not reproducible. Clearly both approaches suffer from a lack of any standardized definition of what constitutes epidemic risk, and neither take into account variations in the aetiologies of epidemics. Any future work aimed at defining PAR of epidemics should use combinations of quantitative and qualitative risk definitions that are subjected to rigorous validation and include an appropriate sensitivity analysis.
Table 2.
Available estimates for populations at risk of malaria epidemics in Africa in 2005.
| Source of estimate/ reference |
Number of countries included in estimate |
Year of original estimate |
Population at risk for 2005 |
|---|---|---|---|
| WHO [5] | 26 | 2001 | 131,905,028 a |
| Snow et al. [4] | 29 | 1995 | 76,143,097 b |
The WHO’s PAR estimates were originally calculated on the basis of 2001 population data. This figure was projected to 2005 using published information in the UN Population Prospects database (http://esa.un.org/unpp).
Estimated by repeating original analysis using Gridded Population of the World (v2.0) [45] data to generate population distribution maps for Africa for the year 2000. Per-country urban-rural growth rates from the UN Population Prospects database were then used to extrapolate PARs to 2005.
Figure 1.
National estimates of population at risk for Africa, 2005: differences between the estimates of WHO [5] and Snow et al. [4]
a Where values are negative, estimates from [5] exceed those of [4]; where they are positive estimates from [4] exceed those [5]. Note that WHO PAR estimates exceed those of Snow et al. in 18 of 23 instances. For a description of data sources see Table 1.
DEFINING ‘TYPICAL’ EPIDEMIC CHARACTERISTICS
Even if it were possible to derive reliable estimates of epidemic PAR, a number of other parameters require quantification before the average annualized burden of epidemics can be calculated. At the most basic level, information is needed on epidemic periodicity and the average duration of epidemic events; the immunological and environmental drivers of which are still only rudimentarily understood [26]. In terms of periodicity it appears to be generally accepted that recurring, climate-driven epidemics occur every 2-7 years [8], but the evidence base for this appears to be limited and there has not been any concerted effort to refine or validate this estimate for the purposes of estimating epidemic burden. In the case of the length of epidemic events, Snow et al. [4] assumed a typical epidemic duration of 12 weeks, but in reality this period is likely to be highly variable and epidemic periods of 15-36 months have been reported [23]. Within this epidemic period the exact shape of the epidemic curve will also have a direct impact on the associated public health burden, and may similarly show substantial spatial and temporal variation.
The sensitivity of estimated burden to variations in these assumptions becomes apparent if we follow the approach used to calculate current WHO burden estimates. Here an epidemic periodicity of five years is assumed, together with an average attack rate (during epidemic years) of 0.5 [5]. On the basis of these assumptions the annual number of epidemic malaria cases for Africa in 2005 could have been as low as 7.6 million or as high as 13.2 million depending on which estimate of PAR is used in the calculations (Table 3).
Table 3.
Calculations for estimated average annual burden of malaria epidemics in Africa expressed as total number of clinical malaria episodes, severe cases and deaths, and derived using two alternative PAR estimatesa
| A. Using assumed attack rate |
B. Using documented attack rate |
|||
|---|---|---|---|---|
| PAR estimate from WHO |
PAR estimate from Snow et al. |
PAR estimate from WHO |
PAR estimate from Snow et al. |
|
| Scenario 1 | ||||
| Average annual number of malaria episodes | 13,190,503 | 7,614,310 | 7,650,492 | 1,921,851 |
| Average annual number of severe cases | 659,525 | 380,715 | 382,525 | 96,093 |
| Average annual number of deaths (range) | 164,881–329,763 | 95,179–190,358 | 95,631–191,262 | 24,023–48,046 |
| Scenario 2 b | ||||
| Average annual number of malaria episodes | 10,792,230 | 6,229,890 | 6,259,493 | 1,572,424 |
| Average annual number of severe cases | 485,650 | 280,345 | 281,677 | 70,759 |
| Average annual number of deaths (range) | 109,271–218,543 | 63,078–126,155 | 63,377–126,755 | 15,921–31,842 |
| Scenario 3 c | ||||
| Average annual number of malaria episodes | 15,247,000 | 9,306,379 | 9,350,601 | 2,348,929 |
| Average annual number of severe cases | 838,585 | 511,851 | 514,283 | 129,191 |
| Average annual number of deaths (range) | 230,611–461,222 | 140,759–281,518 | 141,428– 282,856 | 35,528– 71,055 |
Calculations are repeated using (A) the assumed average annual attack used by Worrall et al. [5], and (B) the median average annual attack rate reported in this review. Scenario 1 uses similar assumptions to those used by WHO for its own burden calculations. These assumptions have been altered in Scenarios 2 and 3 to reflect more ‘optimistic’ and ‘pessimistic’ situations by altering only the frequency of epidemics and the clinical attack rate associated with them.
Scenario 2 incorporates a 10% decrease in epidemic frequency and clinical attack rate over scenario 1
Scenario 3 incorporates a 10% increase in epidemic frequency and clinical attack rate over scenario 1
Estimates of total burden are particularly sensitive to the attack rate parameter. Based on reports included in Table 1, the overall ‘documented’ unweighted median attack rate is 0.29 (assuming, in the absence of data on epidemic duration in many of these reports, a typical epidemic duration of 12 weeks). If, for the purpose of illustration this lower rate is applied to the WHO calculations, projected caseloads are significantly reduced (Table 3). However, it should be recognised that these attack rate estimates include data derived from health centre-based surveillance, and most probably suffer from significant under-detection bias, due to low access to formal health care in many of the remote, impoverished communities where epidemics have taken place. Antimalarial treatment coverage is mostly poor wherever it is measured [27]: for example, only 7% of childhood fevers were treated promptly in Kenya [28], and coverages of 33% and 47% were estimated in Tanzania and The Gambia, respectively [29, 30]. More favourable results (>60% access to treatment) were found in the Ugandan highlands – to our knowledge, the only estimate of treatment access from an epidemic setting [31]. Estimation of attack rate is further complicated by the low specificity of diagnosis, especially when, as in all of the cited studies, cases are treated presumptively (which would lead to a probable overestimation of attack rate). More reliable estimates are needed of true attack rates during epidemics, based on community surveillance and parasitological diagnosis.
The uncertainty in our burden estimates will increase with the number of steps (and thus assumptions) involved in their derivation. Worrall et al. [5], for example extended their basic morbidity calculations to generate estimates of the average annual number of cases of severe malaria and malaria-related deaths, using the assumption that 5% of malaria cases develop acute symptoms, and that of these between 25 and 50% die. In this scenario, the average number of deaths could be anywhere between about 24,000 and 330,000, depending on which estimates of PAR and attack rates are used as inputs (Table 2). These estimates are also sensitive to any changes we make to our assumptions. For example, if we generated a more ‘optimistic’ scenario by reducing by 10% both the frequency of epidemics and the clinical attack rate associated with them (while keeping constant our assumptions concerning rates of severe disease and mortality), the average annual number of deaths associated with epidemics would fall to as few as 16,000 or 110,000, again depending on which PAR and attack rates are used (Table 3). Conversely in a 10% more ‘pessimistic’ scenario, annual deaths calculated on the basis of the WHO’s PAR figure could be almost as high as 0.5 million, which would represent a significant proportion of existing estimates of total annual malaria deaths in Africa across all endemicities [32].
UNCERTAINTY IN BURDEN ESTIMATES
The findings presented here demonstrate that there is still a huge amount of uncertainty surrounding current methods and estimates of malaria epidemic burden at regional level. This reflects a basic lack of good quality epidemiological data for past epidemics and for epidemic-prone localities more generally, as well as the technical difficulties of producing meaningful summary indicators of burden for a wide range of epidemiological scenarios (which all, nevertheless, come under the heading of ‘epidemic’ or ‘unstable’). Table 1, for example, while representing a useful summary of some key indicators of burden across of range of epidemics (and a timely reminder of the potentially devastating effects of individual outbreaks), is nevertheless an unsound basis for producing scaled-up estimates of burden for epidemic-prone regions, as it is unlikely that the records included are fully representative of the ‘epidemic-prone’ epidemiological stratum. Indeed, it could be argued that any attempt to come up with some sort of ‘headline’ figures on epidemic burden are flawed in that a single epidemic stratum is largely illusory; the characteristic of epidemic-prone localities and the aetiologies of epidemics themselves being too diverse and dynamic to allow simple aggregation. Derivative estimates of average annualized burden of epidemics should perhaps be viewed with even greater caution, as the burden parameters used in their calculation (clinical attack rate, rates of severe disease and mortality) are unlikely to apply equally to all the epidemiological scenarios that correspond to the diverse types of area where populations at risk are predicted to live (whether that be by expert opinion, or through some more objective means).
IMPLICATIONS OF UNCERTAINTY IN BURDEN ESTIMATES
Clearly current estimates of epidemic burden are a suboptimal basis for generating general policy in the area of epidemic prevention and control. For Africa, this is evident at the continental level, but is especially apparent at national level (Figure 1), where the design of more specific policy measures is required. The current lack of credible burden data is particularly unfortunate given recent initiatives in some countries to strengthen disease surveillance or epidemic early warning systems [33, 34] and/or to re-introduce malaria prevention strategies in epidemic-prone areas [35]. In the absence of accurate data on burden, evidence based planning of malaria control and rational targeting of epidemic prevention and control measures is difficult to achieve and the basis for evaluating the cost-effectiveness of such measures is limited.
THE WAY FORWARD
The problem of generating more accurate estimates of epidemic burden is not intractable, but a number of basic conditions will need to be met before significant progress in this area can be made. First, and most fundamentally, better epidemiological data (for morbidity and mortality risks) over a range of epidemic settings are needed. This paper does not claim to be an exhaustive review and it is probable that relevant epidemic data exist that have not been included in Table 1 – however, it seems unlikely that a significant number of such records exist and arguably it would be more productive to focus efforts on ensuring that reliable information from future epidemics are captured routinely at country level. Methods to estimate all-cause mortality through retrospective surveys or through prospective surveillance are already well established, but require timely intervention by experienced teams of epidemiologists with considerable resources [36]. Alternative, indirect approaches to estimating mortality (for example through simple prospective community surveillance systems), may be less costly overall but are also associated with significant human resource costs. Obtaining high quality, representative data therefore hinges as much on the willingness of policy makers and the research community to invest in better evidence gathering as on developing appropriate protocols for data collection during and after epidemic events.
Second, a better empirical basis for estimating typology-specific parameters for epidemic periodicity, length, shape and age distribution of incidence is also required. The levels of evidence required here are probably less demanding than those required to obtain community-level estimates of morbidity and mortality risks and substantial advances could be made by reviewing passive case detection data, supplemented with a full systematic review of published and unpublished records.
Third, better PAR estimates require both high fidelity population estimates – often a particular problem in rainfall limited epidemic prone areas characterized by nomadic and transhumant populations [37] – and an objective evidence-based stratification of malaria endemicity that includes epidemic risk [38]. The Malaria Atlas Project (MAP, http://www.ox.ac.uk) is committed to mapping the distribution of malaria endemicity globally [38]. A huge logistical investment is underway, developing a global database of contemporary parasite rate data and ancillary environmental data with which to make extensive malaria endemicity maps. This effort has been extended to recording the distribution of the dominant anopheline malaria vector species globally to inform this endemicity mapping process. In addition, population distribution information is being searched, archived and forwarded to collaborators to help make higher fidelity population maps. The exact project scope and the protocol by which MAP will develop these new global malaria endemicity map and thereafter revised PAR estimates globally is detailed elsewhere [38]. Thus better defining the population at risk of epidemic malaria is integral to the wider effort to refine estimate of the total global malaria burden.
Finally, these uncertainties must all be compounded to provide realistic confidence intervals around the PAR and related burden estimates provided to international agencies, donors and national governments.
ACKNOWLEDGEMENTS
JC is funded by the U.K. Department for International Development through the TARGETS consortium and, together with TAA, is also supported by the Bill and Melinda Gates Foundation through the Gates Malaria Partnership. SIH is funded by a Senior Research Fellowship from the Wellcome Trust (#079091). RWS is a Wellcome Trust Principal Research Fellow (#079080) and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). This paper is published with the permission of the director of KEMRI. This work is an output of the HIMAL project (http://www.himal.uk.net) and also forms part of the activities of the Malaria Atlas Project (MAP, http://www.map.ox.ac.uk) principally funded by the Wellcome Trust, U.K. The authors would like to acknowledge the valuable input of Jean-Paul Guthmann during the development of this paper.
GLOSSARY OF TERMS
- Epidemic burden
Sum of the public health consequences of the epidemic within the population affected by it, and over its entire duration. This includes at a minimum morbidity and mortality in excess of the expected, but, ideally, other indirect consequences, such as long-term sequelae of infection, increased rates of anaemia and malnutrition, economic losses, decreased educational achievement, etc.
- (Clinical) incidence rate
Number of new or recrudescent episodes of malaria occurring within a defined population at risk, per person per unit time. The term ‘clinical’ indicates that the episode was symptomatic, and that the diagnosis was established presumptively, without parasitological confirmation
- (Clinical) attack rate
Proportion of the population that experiences an episode of malaria per unit time
- All-cause mortality (death) rate
Number of deaths per population per unit time, due to any cause. In emergencies this is usually per 10,000 persons per day and may be age-stratified
- Malaria-specific mortality rate
Mortality rate attributed to malaria
- Average annualized estimate (burden) of morbidity and mortality
Mean burden of malaria epidemics per year, expressed either as total episodes and/or deaths, or in terms of attack rate or cumulative mortality rate per population at risk
- Case-fatality (rate or ratio, CFR)
Proportion of episodes that results in death
- Epidemic periodicity
Average frequency with which epidemics occur in a given region or population
- Duration of epidemic events
Time elapsed between the onset and the end of the epidemic
- Epidemic stratum
Sub-region or sub-population within the entire population characterised by specific aetiology, demography and pattern of disease transmission
- Passive case detection
Detection of malaria cases based on the spontaneous presentation of ill patients to fixed health services
Footnotes
TEASER SENTENCE: Here we provide a critical review of the evidence on the morbidity and mortality toll of individual malaria epidemic events and examine the underlying assumptions required to define an annual estimate of malaria epidemic disease burden for Africa.
REFERENCES
- 1.WHO . Malaria early warning systems - concepts, indicators and partners. A Framework for Field Research in Africa. World Health Organization/Roll Back Malaria; 2001. (WHO/CDS/RBM/2001.32) [Google Scholar]
- 2.WHO . Roll Back Malaria global strategic plan 2005-2015. World Health Organization; 2005. 43. [ http://www.rollbackmalaria.org/forumV/docs/gsp_en.pdf] [Google Scholar]
- 3.Hay SI, et al. Malaria early warning in Kenya. Trends Parasitol. 2001;17:95–99. doi: 10.1016/s1471-4922(00)01763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snow RW, et al. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull. W.H.O. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- 5.Worrall E, et al. The burden of malaria epidemics and cost-effectiveness of interventions in epidemic situations in Africa. Am. J. Trop. Med. Hyg. 2004;71:136–140. [PubMed] [Google Scholar]
- 6.Hay SI, et al. Defining and detecting malaria epidemics in the highlands of western Kenya. Emerg. Infect. Dis. 2002;8:555–562. doi: 10.3201/eid0806.010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiszewski AE, Teklehaimanot A. A review of the clinical and epidemiologic burdens of epidemic malaria. Am. J. Trop. Med. Hyg. 2004;71:128–135. [PubMed] [Google Scholar]
- 8.Nájera JA, et al. Malaria epidemics, detection and control, forecasting and prevention. Division of Control of Tropical Diseases/ World Health Organization; 1998. (WHO/MAL/98.1084) [Google Scholar]
- 9.Bosman A, et al. Malaria epidemic in Ziway area. WHO; 1993. (WHO Mission Report; M2/370/23 ETH) [Google Scholar]
- 10.Garay J. Epidemiological survey and situation analysis in Muleba District (Nshamba Division) MSF-Tanzania: 1998. [Google Scholar]
- 11.Guthmann J-P, et al. Death rates from malaria epidemics, Burundi and Ethiopia. Emerg. Infect. Dis. 2007;13:140–143. doi: 10.3201/eid1301.060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snow RW, et al. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontaine RE, et al. The 1958 malaria epidemic in Ethiopia. Am. J. Trop. Med. Hyg. 1961;10:795–803. doi: 10.4269/ajtmh.1961.10.795. [DOI] [PubMed] [Google Scholar]
- 14.Loevinsohn ME. Climatic warming and increased malaria incidence in Rwanda. Lancet. 1994;343:714–718. doi: 10.1016/s0140-6736(94)91586-5. [DOI] [PubMed] [Google Scholar]
- 15.Shanks GD, et al. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans. R. Soc. Trop. Med. Hyg. 2000;94:253–255. doi: 10.1016/s0035-9203(00)90310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindblade KA, et al. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Trop. Med. Int. Health. 2000;5:263–274. doi: 10.1046/j.1365-3156.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 17.Hay SI, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepers JP, et al. Reappearance of falciparum malaria in central highland plateaux of Madagascar. Lancet. 1988;331:586. doi: 10.1016/s0140-6736(88)91375-x. [DOI] [PubMed] [Google Scholar]
- 19.Bødker R, et al. Resurgence of malaria in the Usambara mountains, Tanzania, an epidemic of drug-resistant parasites. Glob. Change and Hum. Health. 2000;1:134–153. [Google Scholar]
- 20.Hay SI, et al. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 2002;18:530–534. doi: 10.1016/s1471-4922(02)02374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patz JA, et al. Climate change - Regional warming and malaria resurgence. Nature. 2002;420:627–628. doi: 10.1038/420627a. [DOI] [PubMed] [Google Scholar]
- 22.Hay SI, et al. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg. Infect. Dis. 2002;8:543–548. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Checchi F, et al. Malaria epidemics and interventions, Kenya, Burundi, southern Sudan and Ethiopia, 1999-2004. Emerg. Infect. Dis. 2006 doi: 10.3201/eid1210.060540. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig MH, et al. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today. 1999;15:105–111. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 25.WHO . Malaria epidemics: forecasting, prevention, early detection and control: from policy to practice. World Health Organization; 2004. (WHO/HTM/MAL/2004.1098) [Google Scholar]
- 26.Hay SI, et al. Etiology of interepidemic periods of mosquito-borne disease. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9335–9339. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams HA, Jones COH. A critical review of behavioral issues related to malaria control in sub-Saharan Africa: what contributions have social scientists made? Soc. Sci. Med. 2004;59:501–523. doi: 10.1016/j.socscimed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt HL, Snow RW. The management of fevers in Kenyan children and adults in an area of seasonal malaria transmission. Trans. R. Soc. Trop. Med. Hyg. 2004;98:111–115. doi: 10.1016/s0035-9203(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 29.von Seidlein L, et al. Treatment uptake by individuals infected with Plasmodium falciparum in rural Gambia, West Africa. Bull. W.H.O. 2002;80:790–796. [PMC free article] [PubMed] [Google Scholar]
- 30.Alilio MS, et al. Malaria control at the district level in Africa: the case of the muheza district in northeastern Tanzania. Am. J. Trop. Med. Hyg. 2004;71:205–213. [PubMed] [Google Scholar]
- 31.Lindblade KA, et al. Treatment for clinical malaria is sought promptly during an epidemic in a highland region of Uganda. Trop. Med. Int. Health. 2000;5:865–875. doi: 10.1046/j.1365-3156.2000.00651.x. [DOI] [PubMed] [Google Scholar]
- 32.Snow RW, et al. The public health burden of Plasmodium falciparum malaria in Africa: Deriving the numbers. Fogarty International Center, National Institutes of Health; 2003. Working Paper No. 11, Disease Control Priorities Project. [Google Scholar]
- 33.Abeku TA, et al. Malaria epidemic early warning and detection in African highlands. Trends Parasitol. 2004;20:400–405. doi: 10.1016/j.pt.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson MC, et al. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am. J. Trop. Med. Hyg. 2005;73:214–221. [PubMed] [Google Scholar]
- 35.The US President’s Malaria Initiative. Lancet. 2006;368:1. doi: 10.1016/S0140-6736(06)68939-3. Anonymous. [DOI] [PubMed] [Google Scholar]
- 36.Checchi F, Roberts L. Humanitarian Practice Network Paper. Overseas Development Institute; 2005. Interpreting and using mortality data in humanitarian emergencies. A primer for non-epidemiologists. [Google Scholar]
- 37.Hay SI, et al. The accuracy of human population maps for public health application. Trop. Med. Int. Health. 2005;10:1073–1086. doi: 10.1111/j.1365-3156.2005.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay SI, Snow RW. The Malaria Atlas Project (MAP): developing global maps of malaria risk. PLoS Med. 2006;3:e473. doi: 10.1371/journal.pmed.0030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farnsworth-Anderson TF. Report on an investigation of health conditions on farms in the Trans Nzoia, with special reference to malaria. Kenya and East Afr. Med. J. 1930;6:274–308. [Google Scholar]
- 40.Le Sueur D, et al. Historical perspective of the malaria problem in Natal with emphasis on the period 1928-1932. South Afr. J. Sci. 1983;89:232–239. [Google Scholar]
- 41.Strangeways-Dixon D. Paludrine (proguanil) as a malaria prophylactic amongst African labour in Kenya. East Afr. Med. J. 1950;27:125–130. [PubMed] [Google Scholar]
- 42.Lepers JP, et al. Newly transmitted Plasmodium falciparum malaria in the central highland plateaux of Madagascar: assessment of clinical impact in a rural community. Bull. W.H.O. 1990;68:217–222. [PMC free article] [PubMed] [Google Scholar]
- 43.Warsame M. An epidemic of Plasmodium falciparum malaria in Balcad, Somalia, and its causation. Trans. R. Soc. Trop. Med. Hyg. 1995;89:142–145. doi: 10.1016/0035-9203(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 44.Brown V, et al. Epidemic of malaria in north-eastern Kenya. Lancet. 1998;352:1356–1357. doi: 10.1016/s0140-6736(05)60747-7. [DOI] [PubMed] [Google Scholar]
- 45.Deichmann U, et al. Transforming population data for interdisciplinary usages: from census to grid. The Center for International Earth Science Information Network (CIESIN); 2001. [ http://sedac.ciesin.columbia.edu/gpw-v2/GPWdocumentation.pdf] [Google Scholar]