Abstract
This study was designed to investigate the beneficial effects of combination therapy of simvastatin and marrow stromal cells (MSCs) in improving functional outcome after traumatic brain injury (TBI) in rats. Adult female Wistar rats (n = 72 and 8, per group) were injured with controlled cortical impact and treated either with monotherapy of MSCs or simvastatin or a combination therapy of these two agents. Different combination doses were tested, and nine groups of animals were studied. Neurological function was evaluated using Modified Neurological Severity Score (MNSS), and animals were sacrificed 3 months after injury. Coronal brain sections were stained with standard hematoxylin and eosin immunohistochemistry. Our results showed that, though functional improvement was seen with monotherapies of MSCs and simvastatin, the combination therapy when used in optimal doses was significantly better in improving functional outcome. This improvement was long lasting and persisted until the end of the trial (3 months). The optimum combination dose was 0.5 mg of simvastatin combined with 2×106 MSCs. Post mortem analysis showed the presence of donor MSCs within the injured cortex. Endogenous cellular proliferation induced by the neurorestorative treatments was also observed in the lesion boundary zone. Our data show that MSCs and simvastatin have a synergistic effect in improving functional outcome after TBI.
Key words: combination therapy, marrow stromal cells (MSCs), simvastatin, traumatic brain injury (TBI)
Introduction
Marrow stromal cells (MSCs) and statins have both shown promise in improving functional outcome in experimental studies of traumatic brain injury (TBI) (Lu et al., 2001a,b, 2003, 2004a,b,c, 2007; Mahmood et al., 2001, 2003, 2005). Treatment with MSCs as well as statins have shown an improvement in neuromotor function and spatial learning after TBI (Lu et al., 2001a,b, 2003, 2004a,b,c, 2007; Mahmood et al., 2001, 2002, 2003, 2005, 2006). The histological studies have shown that these agents induce endogenous cellular proliferation and enhance neural plasticity after TBI (Lu et al., 2003, 2007; Mahmood et al., 2004). It was intuitively challenging to investigate if combination therapy of statins and MSCs is superior to monotherapies of these agents as they may have a synergistic effect on neural plasticity. Previously, we conducted a pilot study combining MSCs with atorvastatin which showed a super additive benefit using these agents (Mahmood et al., 2007). However, this was a short-term trial, and there was a compelling need to conduct long-term studies to confirm that the benefit persisted over time. Therefore, we designed a 3-month study to examine multiple combination doses of MSCs and statin. The rationale for this was twofold: first to determine the optimum combination dose of MSCs and statins, and second to observe the combination effects of MSCs and statins in a large cohort of animals. We had initially used atorvastatin but we subsequently shifted to another statin (i.e., simvastatin) for this combination treatment design. Simvastatin has been shown to possess superior neuroprotective and neurorestorative properties when compared to atorvastatin (Lu et al., 2007). Simvastatin and atorvastatin are both lipophilic; however, they have different molecular structure which determines their tissue deposition (Ballantyne et al., 2004). Atorvastatin is deposited on endothelial cells and is unable to cross the blood-brain barrier (BBB), but simvastatin can cross the BBB, which leads to a higher concentration of simvastatin in brain tissue (Zacco et al., 2003).
Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the Henry Ford Health System.
Preparation of rat marrow stromal cells
Male Wistar bone MSCs were prepared, frozen in liquid nitrogen, and transported to our laboratory by the Cognate Company (Sunnyvale, CA). MSCs were restored, and a small sample was selected for cell counting by using trypan blue stain (0.4%). Nucleated marrow cells were counted using a cytometer to ensure adequate cell number for transplantation. The viability rate was 77–90%, and the injected cell number excluded dead cells. Male Wistar MSCs suspended in phosphate-buffered saline (PBS) were injected into female rats via the tail vein. This male-to-female transplantation was performed to identify donor MSCs by localizing the Y chromosomes within them.
Animal model and injection of MSCs
A controlled cortical impact (CCI) model in rat was used (Dixon et al., 1991; Postmantur et al., 1997). Female Wistar rats were anesthetized intraperitoneally (i.p.) with 350 mg/kg body weight chloral hydrate. Rectal temperature was controlled at 37°C by using a feedback-regulated water-heating pad. A CCI device was used to induce the injury. Rats were placed in a stereotactic frame. The dura was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm-diameter tip at the rate of 4 m/sec and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Treatment of TBI rats with MSCs and simvastatin
Rats were treated with simvastatin and MSCs, alone or in combination, and nine groups were studied. Two doses of simvastatin were tested: 0.5 or 1.0 mg/kg. Simvastatin was mixed with water and administered directly into the esophagus using a lavage tube for 14 days starting 1 day after TBI. MSCs were also administered in two doses: 1 × 106 and 2 × 106. For MSC administration, rats were anesthetized i.p. with 350 mg/kg body weight chloral hydrate on day 7 after TBI and MSCs were injected via tail vein. These doses of simvastatin and MSCs were selected based on our previous experiences (Lu et al., 2001b, 2004a,c, 2007; Mahmood et al., 2002, 2003, 2004, 2007). A control group received PBS orally for 14 days starting 1 day after TBI as well as injected with PBS on day 7 after TBI. All animals were injected i.p. with bromodeoxyuridine (BrdU) for 10 days starting 1 day after TBI. We used different combination doses of simvastatin and MSCs to determine the optimum combination dose, and animals were divided into nine experimental groups, as follows:
Group 1 (n = 8): TBI + 0.5 mg/kg of simvastatin for 14 days
Group 2 (n = 8): TBI + 1.0 mg/kg of simvastatin for 14 days
Group 3 (n = 8): TBI + 2× 106 MSCs + 0.5 mg/kg of simvastatin for 14 days
Group 4 (n = 8): TBI + 2× 106 MSCs + 1.0 mg/kg of simvastatin for 14 days
Group 5 (n = 8): TBI + 1 × 106 MSCs + 0.5 mg/kg of simvastatin for 14 days
Group 6 (n = 8): TBI + 1 × 106 MSCs + 1.0 mg/kg of simvastatin for 14 days
Group 7 (n = 8): TBI + 1 × 106 MSCs
Group 8 (n = 8): TBI + 2 × 106 MSCs
Group 9 (n = 8): TBI + PBS intravenously as well as orally
All rats were sacrificed 3 months after TBI.
Brain sample preparation
Brain tissues from each group were processed for preparation of paraffin-embedded sections which were employed for the histological evaluation and immunohistochemical staining.
In situ hybridization
Female rat brains were removed and stored in 4% buffered paraformaldehyde for 48–72 h. Standard 2-mm-thick blocks of the rat brains were cut on a rodent brain matrix (a total of seven blocks from A to G) and embedded with paraffin. A series of 6-μm-thick sections were cut. Three sections with 50-μm intervals from Block E or F were dewaxed and rehydrated with xylene and graded ethanol, and subsequently digested with proteinase K (100 μg/ml) for 15 min at 37°C. The oligonucleotide probe (5′-AGATCTTGATTTTTAGTGTTC-3′) for the Sry gene of the murine sex-determining region carried on the Y chromosome was labeled at the 3′-end with digoxigenin (DIG)-ddUTP using a DIG oligonucleotide 3′-end labeling kit (Roche Pharmaceuticals, Nutley, NJ). After denaturing the tissue sample, hybridization was performed in a mixture consisting of 50% deionized formamide, 10% salmon test deoxyribonucleic acid, 10% dextran sulfate, 10% 50× Denhardt's solution, 10% 20× standard saline citrate, and 500 ng of DIG-labeled probe at 42°C overnight. The DIG-labeled Y chromosome was visualized using a fluorescent antibody enhancer set (Boehringer Mannheim GmbH, Penzburg, Germany) under fluorescent microscopy, which resulted in fluorescein isothiocyanate fluorescence (green). The slides were then counterstained with 10 ng/ml of propidium iodide (red) for nuclear staining and mounted with antifade solution and coverslips (Mahmood et al., 2001). Negative control sections from each animal received identical staining preparation, except that the probe or the antidigoxigenin antibodies were omitted.
Immunohistochemistry staining was performed on coronal cerebral samples.
Bromodeoxyuridine immunohistochemical staining
BrdU is incorporated into the newly formed deoxyribonucleic acid and is a marker of newly generated endogenous cells (Cameron et al., 2001). Single staining with DAB was performed to identify BrdU-labeled cells and to detect the distribution of these newly generated endogenous cells. For single staining, brain sections were deparaffinized and incubated in 50% formamide-2× SSC at 60°C for 30 min, treated with 2N HCI at 37°C for 10 min to denature the deoxyribonucleic acid, and then incubated in 0.1 mol/L boric acid at room temperature for 3 min to neutralize the residual acid. After blocking in normal serum, sections were incubated overnight with mouse anti-BrdU antibody (Calbiochem, San Diego, CA) diluted at 1:100 in PBS at 4°C. After sequential incubation with biotin-conjugated anti-mouse immunoglobulin G (dilution, 1:100; Dakopatts), the sections were treated with an avidin-biotin-peroxidase system (ABC kit; Vector Laboratories, Inc., Burlingame, CA). DAB was used as a sensitive chromogen for light microscopy. An average number of three equally spaced slides (approximate interval, 100 μm) were obtained from brain blocks E and F, which contained lesion boundary zone. The number of BrdU-positive cells was counted in the ipsilateral hemisphere using the light fraction of microscope BH-2 (Olympus Optical Co., Tokyo, Japan).
Neurological functional evaluation
Neurological motor measurement was performed using the Modified Neurological Severity Score (mNSS). The test is sensitive to unilateral cortical injury because it reflects multiple asymmetries, including postural, sensory, and forelimb and hind limb use asymmetries. A detailed description of this functional test has been previously published (Li et al., 2002; Shohami et al., 1995). These tests were performed on all rats 1 day before TBI, and after TBI on days 1, 4, 7, 14, and biweekly thereafter. All measurements were performed by observers blinded to individual treatment.
Statistical analysis
Rats with TBI were enrolled into one of the two treatment combination groups (simvastatin and MSCs) with three doses for each treatment, including zero dose (PBS) and a total of nine groups as detailed in Methods, with eight rats per group. Rats were evaluated by the mNSS assessment for neurological deficits at day 1 before TBI, and on days 1, 4, 7, 14 after TBI and biweekly thereafter until 3 months after TBI. The purpose of the study was to identify an optimal combination dose which improved the TBI recovery, measured by mNSS reduction at 3 months. mNSS data were evaluated for normality. The ranked data were evaluated because the data were not normal. Two-way analysis of variance (ANOVA) was used for testing the three-dose simvastatin and MSC interactions, followed by testing the synergistic effects on each combination of a simvastatin dose with MSCs if the simvastatin dose by MSC interaction was detected at the 0.05 level. The mean and standard deviation of original mNSS by groups were presented as data illustration.
Results
Modified Neurological Severity Scores
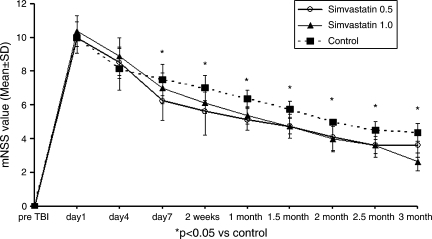
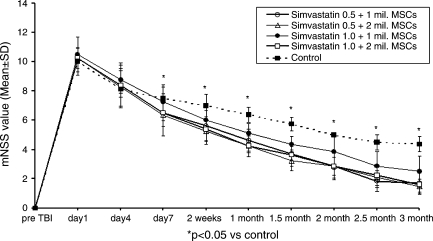
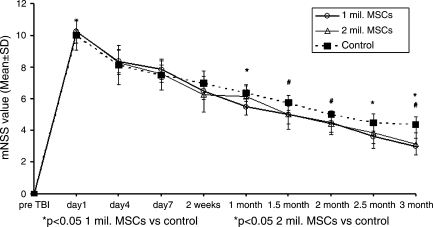
Improvement was seen using monotherapies as well as combination therapies (Figs. 1–3), though combination therapy was superior to monotherapies. Simvastatin had consistent and significant treatment effects on mNSS at early and later time points compared to control and though 0.5 mg/kg dose seemed to be better than 1.0 mg/kg, the difference was not significant (Fig. 1). The therapeutic effect of MSCs on mNSS was significant at later time points (i.e., 1 month and beyond). There was no significant difference in mNSS between the two MSC doses (Fig. 2). All combination therapy groups also showed significant improvement compared to control (Fig. 3), but when compared to monotherapies the 0.5 mg/kg simvastatin + 2 × 106 MSCs group showed synergistic effect, i.e., the mNSS of this group were significantly better (p = 0.039) than the monotherapy groups at three months. The other combination groups had subadditive effects compared to monotherapy groups.
FIG. 1.
Plot shows Modified Neurological Severity Score (mNSS) of different simvastatin-treated groups. *p < 0.05, compared to the control.
FIG. 3.
Plot shows Modified Neurological Severity Score (mNSS) of different groups treated with different combination doses of simvastatin and marrow stromal cells (MSCs). *p < 0.05 compared to the control.
FIG. 2.
Plot shows Modified Neurological Severity Score (mNSS) of different marrow stromal cell (MSC)–treated groups. *p < 0.05, 1 × 106 MSCs versus control; #p < 0.05, 2 × 106 MSCs versus control.
Distribution and identification of MSCs in the rat brain
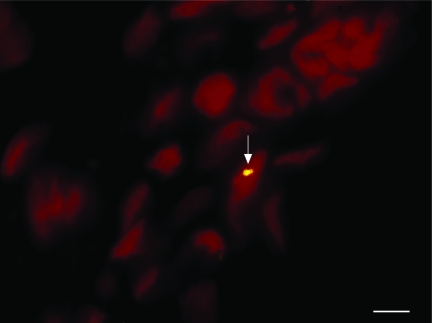
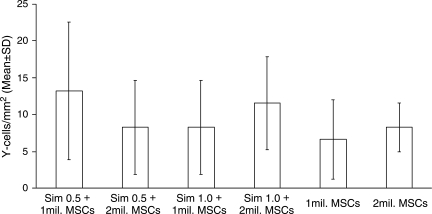
Donor MSCs were detected in the brains of female recipient rats receiving MSCs and a combination of MSCs and sim-vastatin by localizing Y chromosomes within them by using fluorescence in situ hybridization (Fig. 4). MSCs were seen in the lesion boundary zone, although some were also found in other areas of both the ipsilateral and contralateral cortices. There was no significant difference in the number of MSCs among the different treatment groups (Fig. 5).
FIG. 4.
Microphotograph shows Y chromosome-positive marrow stromal cell (MSC; yellow) in the lesion boundary zone of animals treated with 0.5 mg/kg simvastatin + 2 × 106 MSCs. Original magnification, × 400. Scale bar = 25 μm.
FIG. 5.
Bar graph shows the number of Y chromosome–positive cells (marrow stromal cells [MSCs])/mm2 in different treatment groups. There was no significant difference in the number of Y chromosome–positive cells in different treatment groups.
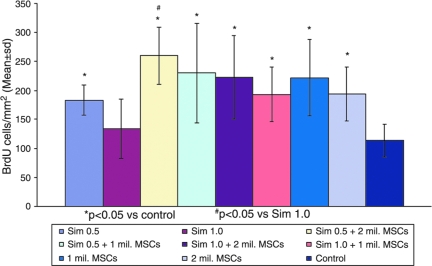
BrdU-positive cell identification
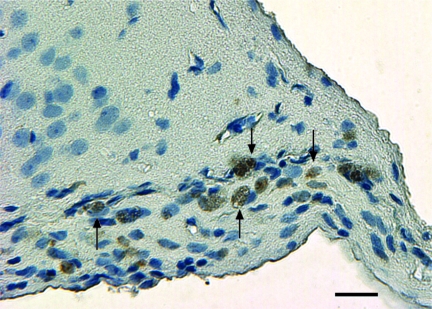
Treatment with a combination of simvastatin and MSCs or MSC and simvastatin monotherapies significantly increased the number of BrdU-labeled cells in the lesion boundary zone (Fig. 6) compared with treatment with control (Fig. 7). There was no difference in number of BrdU-labeled cells among the different combination therapy groups as well between the combination therapy groups and MSC-alone treated groups. However, there was a significant difference between the group receiving optimal combination dose (0.5 mg/kg simvastatin + 2 × 106 MSCs) and the group receiving 1 mg/kg simvastatin alone. All BrdU-labeled cells were Y chromosome-negative, indicating their origin from endogenous cells and not from male donor cells.
FIG. 6.
Coronal brain section shows newly regenerating bromodeoxyuridine (BrdU)–positive cells (brown) in the lesion boundary zone of animals treated with 0.5 mg/kg simvastatin + 2 × 106 marrow stromal cells (MSCs). Original magnification, ×200. Scale bar = 50 μm.
FIG. 7.
Bar graph shows the number of bromodeoxyuridine (BrdU)–positive cells/mm2 in different treatment groups. *p < 0.05 versus control; #p < 0.05 versus 1 mg/kg simvastatin.
Discussion
The results of this study showed that a combination of 2 × 106 MSCs and 0.5 mg/kg simvastatin had synergistic effects in improving functional outcome after TBI, that is their effects were super additive regarding their influence on the functional outcome. Also, the benefit observed was long lasting in that it persisted until 3 months (end of trial). The optimum combination dose was 0.5 mg/kg simvastatin and 2 × 106 MSCs. Other combination doses though showed significant improvement in functional outcome when compared to controls, but their effects were subadditive when compared to monotherapies. Also, when used as a monotherapy, 0.5 mg/kg simvastatin seemed more effective than 1.0 mg/kg simvastatin, though the difference was not statistically significant. Similarly, when used as a monotherapy 2 × 106 MSCs had a greater effect than 1 × 106 MSCs, but again the difference was not statistically significant.
We have used sequential rather than concurrent treatment of simvastatin and MSCs. The rationale behind this was that statins have a distinctive effect in promoting angiogenesis, restoring the integrity of injured microvasculature, and improving blood flow after TBI (Lu et al., 2004a,b,c, 2007). This would subsequently lead to increased access of MSCs to the target zone. There is severe disruption of cerebral microvasculature in the lesion boundary zone and hippocampus (Lu et al., 2004a,b,c) observed 1 day after injury along with diffuse, intravascular microthrombi. However, after 1 week of statin treatment, this vascular disruption is significantly restored in treated animals (Lu et al., 2004a,c). For this reason, this combination treatment was administered sequentially with simvastatin administered one week prior to MSC administration. MSCs have been shown to be still effective when administered 1 week after TBI (Mahmood et al., 2006). In addition to increasing delivery of MSCs to the injury zone, angiogenesis induced by simvastatin can promote the survival of MSCs and increase their efficacy. This may be more important than simply increasing delivery of MSCs to the injury zone since our studies have shown that even after MSCs are directly implanted adjacent to the injury site by intracerebral transplantation only a small percentage survive 1 week after transplantation (Mahmood et al., 2002). Combining MSCs with statins (atorvastatin) enhances the angio-genic effect of statins, and vessel-to-tissue ratio is significantly increased in animals treated with a combination therapy compared to those receiving MSC or statin alone (Mahmood et al., 2007).
Newly proliferating vascular endothelial cells are known to produce an array of growth factors (Leventhal et al., 1999), which likely promote the survival of MSCs. It has been well recognized that trophic factors enhance the survival and subsequent differentiation of transplanted stem cells into neurons and glia (Yurek et al., 1996). Our previous studies have shown that culturing MSCs with trophic factors increases the expression of neurotrophic receptors (i.e., trk A and trk B) on the MSCs (Mahmood et al., 2002), thereby making them more receptive to trophic factor stimulation. Essentially, the statin treatment may prime the cerebral microvasculature and microenvironment to be receptive to the exogenously administered MSCs. In the present study, MSCs were still visible in the recipient brain 3 months after the transplantation; however, their number was small and there was no significant difference among different groups, irrespective if MSCs were administered alone or with simvastatin. We believe this lack of difference is merely a consequence of a small total number of MSCs. In our previous studies also, the number of MSCs was far less in post mortem analysis performed at 3 months compared to earlier time points (Mahmood et al., 2005, 2006). However, MSC-induced neurorestorative functions are still active at 3 months even though most of the MSCs themselves have not survived by that time.
Endogenous cellular proliferation was studied using BrdU immunohistochemical staining, and our data revealed that combination treatment as well as MSC and simvastatin monotherapies significantly increased the newly proliferating cells in the injured brain. The maximum number of newly proliferating cells was seen in the group receiving the optimal combination dose (0.5 mg/kg simvastatin + 2 × 106 MSCs), and this was significantly more than the simvastatin-alone group, which received 1 mg/kg of simvastatin. However, there was no significant difference between combination therapy groups and MSC-alone treated groups. We appreciate that it is hard to determine how functionally active these newly proliferating cells are, and we are not directly addressing the question if functional improvement seen by MSC and simvastatin treatments is secondary to this endogenous cellular proliferation. Nonetheless, this endogenous cellular proliferation provides a histological basis of induction of neural plasticity by neurorestorative treatments (Mahmood et al., 2004).
Our study represents a marriage between cell therapy and pharmacotherapy aimed at restoring neurological function after TBI. Though these treatment modalities have shown efficacy individually in improving outcome after TBI, this is the first study which shows that they can be combined to produce long-lasting superadditive benefit after TBI. However, detailed genetic, biochemical, and molecular studies are needed to gain insight into the mechanisms of this synergistic effect.
In summary, combination therapy of MSCs and simvasatin produces a long-lasting improvement in the functional outcome of rats after TBI. Their synergistic effect increases the clinical relevance of both of these agents and brings us one step closer in initiating clinical trials using these neurorestorative treatments.
Author Disclosure Statement
No competing financial interests exist.
References
- Ballantyne C.M. Blazing M.A. King T.R. Brady W.E. Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am. J. Cardiol. 2004;93:1487–1494. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]
- Cameron H.A. McKay R.D.G. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Leventhal C. Rafii D. Shahar A. Goldman S.A. Endothelial trophic support of neuronal production of recruitment from the adult mammalian subependyma. Mol. Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li Y. Chen J. Chen X.G. Wang L. Gautam S.C. Xu X.Y. Katakowski M. Zhang L.J. Lu M. Janakiraman N. Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Lu D. Li Y. Wang L. Chen J. Mahmood A. Chopp M. Intraarterial administration of marrow stromal cells in rat model of traumatic brain injury. J. Neurotrauma. 2001a;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Li Y. Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001b;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Chopp M. Biological transplantation and neurotrophic induced neuroplasticity after traumatic brain injury. J. Head Trauma Rehabil. 2003;18:357–376. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Lu D. Goussev A. Chen J. Pannu P. Li Y. Mahmood A. Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma. 2004a;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Goussev A. Qu C. Zhang Z.G. Chopp M. Delayed thrombosis after traumatic brain injury in rats. J. Neurotrauma. 2004b;21:1756–1766. doi: 10.1089/neu.2004.21.1756. [DOI] [PubMed] [Google Scholar]
- Lu D. Mahmood A. Goussev A. Schallert T. Qu C. Zang Z.G. Li Y. Lu M. Chopp M. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J. Neurosurg. 2004c;101:819–827. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- Lu D. Qu C. Goussev A. Jiang H. Lu C. Schallert T. Mahmood A. Chen J. Li Y. Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Wang L. Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1204. [PubMed] [Google Scholar]
- Mahmood A. Lu D. Li Y. Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J. Neurotrauma. 2002;19:1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–703. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation with the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Chopp M. Human marrow stromal cell treatment provides long lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J. Neurosurg. 2006;104:272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Chopp M. Treatment of traumatic brain injury in rats with a combination therapy of marrow stromal cells (MSCs) and atorvastatin. Neurosurgery. 2007;60:546–553. doi: 10.1227/01.NEU.0000255346.25959.99. [DOI] [PubMed] [Google Scholar]
- Postmantur R. Kampfl A. Simon R. Liu J. Zhao X. Clifton G.L. Hayes R.L. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–888. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- Shohami E. Novikov M. Bass R. Long term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- Yurek D.M. Lu W. Hipkens S. Wiegand S.J. BDNF enhances the functional reinervation of the striatum by grafted fetal dopamine neurons. Exp. Neurol. 1996;137:105–118. doi: 10.1006/exnr.1996.0011. [DOI] [PubMed] [Google Scholar]
- Zacco A. Togo J. Spence K. Ellis A. Lloyd P. Furlong S. Pisa T. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J. Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]